SUMMARY
Post-mastectomy immediate breast reconstruction in the US continues to experience an upward trend owing to heightened awareness, innovations in reconstructive technique, growing evidence of improved patient reported outcomes and shifts in mastectomy patterns. Women with unilateral breast cancer are increasingly electing to undergo contralateral prophylactic mastectomy, instead of unilateral mastectomy or opting for breast conservation. The ascent in prophylactic surgeries correlates temporally to a shift towards prosthetic methods of reconstruction as the most common technique. Factors associated with the choice for implants include younger age, quicker recovery time, along with documented safety and enhanced aesthetic outcomes with newer generations of devices. Despite advances in autologous transfer, its growth is constrained by the greater technical expertise required to complete microsurgical transfer and potential barriers such as poor relative reimbursement. The increased use of radiation as an adjuvant treatment for management of breast cancer has created additional challenges for plastic surgeons who need to consider the optimal timing and method of breast reconstruction to perform in these patients.
Keywords: Autologous breast reconstruction, Breast reconstruction, Immediate Breast Reconstruction, Post-mastectomy breast reconstruction, Trends, Implants
EPIDEMIOLOGY AND CHANGES IN MASTECTOMY PATTERNS
Breast cancer is the most common malignancy in women worldwide as reported by the World Health Organization contributing over 25% to new cancer cases diagnosed in 2012(excluding non-melanoma skin cancer) [1]. For the current year 2017, the North American Association of Central Cancer Registries American Cancer Society estimates 252,710 new invasive cancer cases, 63,410 cases of carcinoma in situ, and 40,610 breast cancer-specific deaths [2]. Breast cancer incidence is projected to exceed all cancers by 2020, according to a recent population-based National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database study [3]. With increasing numbers of women diagnosed with and surviving breast cancer, it is critical to stay acquainted with current trends in surgical care including breast conservation surgery, mastectomy and reconstruction.
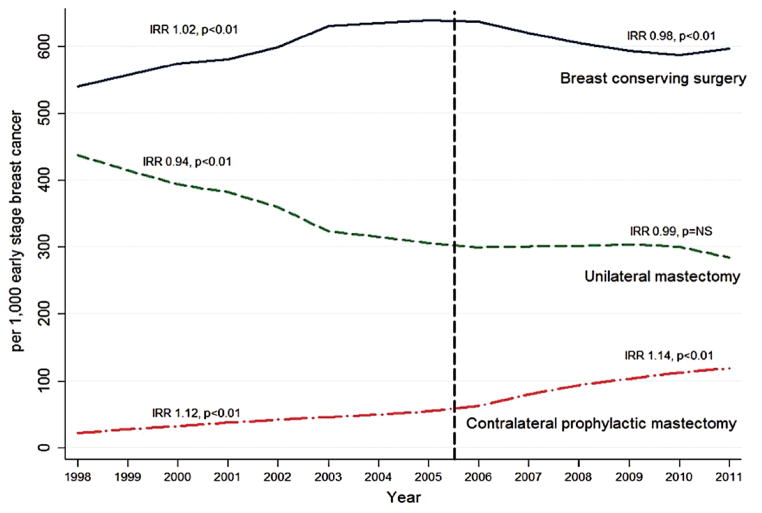
Breast conservation surgery (BCS) with organ preservation, compared to the alternative of mastectomy, gained momentum following the results of large clinical trials which demonstrated equivalent long-term survival [4–6] albeit with a higher local recurrence[7]. More recently, over the last 5–10 years, however, there has actually been a relative decline in the number of lumpectomies performed. Interestingly, the decline in lumpectomy rates correlates with an increase in the number of bilateral, not unilateral mastectomies [Figure-1][8]. In a National Cancer Database (NCDB) analysis, the annual rate of contralateral prophylactic mastectomy (CPM) increased by 14 % with a decline in BCS by 2% per year from 2005–2011[8]. Although some women with a strong family history or genetic predisposition (e.g. BRCA1/2) do benefit from bilateral prophylactic mastectomy (BPM)[9], this oncologic benefit does not extend to the majority of women with unilateral breast cancer who opt for CPM [10] [11–21].
Figure 1.
Temporal Trends of Surgical Treatment in Patients with Early Stage Breast Cancer from 1998–2011 Using National Cancer Database, IRR: Incidence Rate Ratio; NS: Not significant; Adapted from Albornoz et al. 2015 Source: Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer: The Role of Breast Reconstruction. Plastic and Reconstructive Surgery. 135(6): 1518–1526, June 2015.
The choice between BCS or unilateral mastectomy (UM) versus CPM is complex; studies have tried to explain the rationale and to elicit the drivers of the trend toward CPM. Treatment factors including MRI at diagnosis, increase in the frequency of prior negative breast biopsies, and availability of immediate breast reconstruction (IBR) are associated with CPM [22]. Younger age, White or non-Black ethnic origin, and private health insurance are also commonly cited [23, 24].
Position statements as well as campaigns by the Society of Surgical Oncology, and regulatory guidelines by American Society of Breast Surgeons (ASBrS) have been created to address the increasing rate of CPM. For example, in 2016, the ASBrS issued an evidence-based consensus statement, which recommends BCS in the setting of all appropriately eligible patients and to consider neoadjuvant therapy and or oncoplastic approaches to preserve the native breast whenever possible [25].
In an effort to better understand why women increasingly choose CPM, healthcare providers are interested in better understanding its impact on health-related quality of life (HR-QoL). The Mastectomy Reconstruction Outcomes Consortium (MROC) carried out a multicenter prospective cohort study, which assessed patient-reported outcome along with the post-operative morbidity in women of 18 and older with a diagnosis of unilateral in situ or invasive breast cancer. The results revealed that patients choosing CPM with bilateral implant-based reconstruction experienced significantly improved satisfaction with breasts as measured by the BREAST-Q along with reductions in anxiety and worry about future cancer episodes on the GAD-7 and PROMIS-29 anxiety inventories. Not surprisingly, CPM was associated with greater rates of complications whether a patient received prosthetic or autologous reconstruction[26] [27]. In a separate study looking at the contribution of various factors, young age and availability of IBR, explained the greatest variation in receipt of CPM with the coefficient of determination being 32% and 29% respectively[8]. Other reasons invoked by patients who choose CPM pertain to psychological aspects of cancer such as fear of additional cancer episodes, peace of mind, and avoidance of future surveillance imaging[28, 29].
Lastly, the increased utilization of CPM raises ethical issue for physicians. In the current patient-centered model of health care advocated by the Institute of Medicine, physicians are increasingly faced with the tension between honoring the patient’s autonomy to choose CPM and the goal to “first do no harm”. Studies have shown discordance between patients’ and physicians’ beliefs about the benefits of CPM [30–34]. In some instances, surgical oncologists may be hesitant to even offer or discuss CPM, causing a breakdown in the shared decision-making process with their patients [35].
THE RATE OF BREAST RECONSTRUCTION
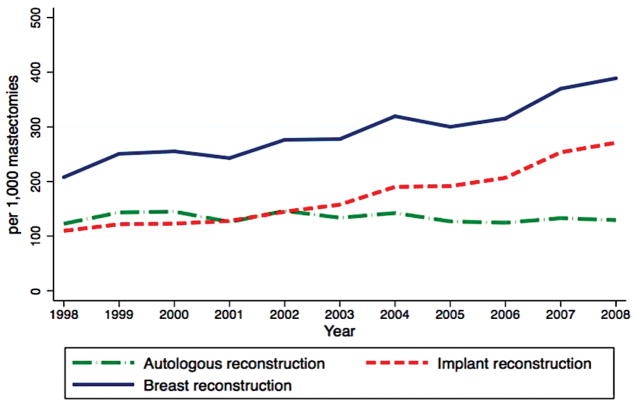
The United States has experienced a gradual rise in both immediate and delayed breast reconstruction over the past few decades [Figure-2] [14, 36]. A variety of factors are contributory. Perhaps, the most important was implementation of the Women’s Health & Cancer Rights Act in 1998, in which payers were required to provide benefits for mastectomy-related services including, but not limited to, all stages of reconstruction and surgeries aimed to restore symmetry between the breasts. Recent information on disparities in reconstruction rates across the nation highlighted the fact that many women were unaware about breast reconstruction or insurance benefit coverage [37, 38]. As a result, individual states, such as NY, now have legislation which mandates information be given to women about breast reconstruction, or have a consultation with a plastic surgeon after diagnosis with breast cancer [14, 37]. Multiple advocacy groups and campaigns through social media have also increased the national presence of breast disease, and have heightened awareness about the improved HR-QoL benefits associated with breast reconstruction. Jointly, these factors contributed to the upsurge in the proportion of women obtaining breast reconstruction with rates as low as 8% in 1995 to recently published rates of about 41% in 2013 based on an NCDB analysis [39, 40]. The latest rate of IBR for 2014 using the American College of Surgeons National Surgical Quality Improvement Program data was reported to be as high as 54% of invasive cancer cases and 63% of ductal carcinoma in situ cases [41].
Figure 2.
Temporal Trends of Reconstructive Methods in Patients who underwent Mastectomy from 1998–2008, Using Nationwide Inpatient Sample Database IRR: Incidence Rate Ratio; NS: Not significant; Adapted from Albornoz et al. 2013 Source: A Paradigm Shift in U.S. Breast Reconstruction: Increasing Implant Rates. Plastic and Reconstructive Surgery. 131(1): 15–23, January 2013.
THE PARADIGM SHIFT TOWARDS IMPLANT-BASED RECONSTRUCTION
A multitude of factors have contributed to a growth in the number of implant-based breast reconstructions performed in the US. One reason is reversal of the moratorium on use of silicon implants for reconstruction by the US Food and Drug Administration in 2006 along with greater long-term data on safety [42]. The second explanation is the increase in number of bilateral mastectomies. Women, who chose bilateral mastectomy (BM), compared to UM, not only have higher reconstruction rates, but also are more likely to opt for prosthetic techniques. In a longitudinal trend analysis of the NIS database from 1998–2008, the rate of implant reconstructions increased 11% yearly, with a greater increase in the setting of BM compared to UM (22% vs. 6%). As of 2002, implants surpassed autologous techniques as the most common method of breast reconstruction performed in the US [Figure-2][14].
Several additional elements may have secondarily contributed to expanded implant use. For instance, advancement in mastectomy techniques including nipple-sparing procedures allow for improved aesthetic benefits. Silicone implants are also now available in greater varieties of sizes and shapes (e.g. anatomical/teardrop,) allowing for enhanced and individualized outcomes. Introduction of acellular dermal matrices, although unproven in long-term patient outcomes and satisfaction, have also led to progress in creating a subjectively more natural appearing lower pole to the breast mound[43]. Adjunctive procedures such as fat grafting are increasingly popular in the setting of implants, as greater technical modifications have been made to make this a more reliable adjunctive technique. As elicited in an MROC study, the role of patient-centric drivers such as psychological benefits of eliminated surveillance consequent to CPM, and enhanced aesthetic outcomes due to implant-based reconstruction cannot be understated, especially in young patients [44]. Furthermore, factors such as decreased operative hours as well as post-operative recovery period may incentivize both surgeons’ and patients’ decision towards implants.
CURRENT STATUS OF AUTOLOGOUS BREAST RECONSTRUCTION
Despite mounting evidence of superior long-term satisfaction and improved HR-QoL associated with autologous techniques [45–48], the US has paradoxically experienced a relative decline in the proportion of patients undergoing this method of reconstruction [Figure-2][14]. Although the advent of perforator flaps such as DIEP, SIEA SGAP, PAP, TUG, would seemingly offer women greater choice and opportunity to minimize donor-site morbidity, microvascular transfer is inherently complex with increased demands placed on practitioners as well as the healthcare system.
When compared to the historic gold standard of pedicle TRAM flaps, the time it takes to complete a single autologous transfer, in most surgeons’ hands, is longer when using microsurgical techniques. Overall this may limit the number of procedures accomplished. There is also likely an insufficient supply of surgeons to perform free flaps, creating a disparity for ABR, restricting its availability to high volume academic microsurgical centers. Moreover, lack of residents in non-academic medical centers to assist intra-operatively or to help with postoperative care is another reason why some hospitals may not offer autologous transfer [49]. The relatively limited volume of tissue provided by non-abdominal donor sites has also had other downstream effects that make ABR ever more challenging. For example, stacked flap techniques using two separate free flaps for a single breast reconstruction are now commonplace. Lastly, plastic surgery trainees increasingly seek additional fellowship training to master the nuances of microvascular tissue transfer and to practice it in a time efficient manner before entering the workforce. All of the aforementioned aspects of ABR are reflected in a recent survey of members of the American Society for Reconstructive Microsurgery, reported a significant decline in the microsurgical proportion of their practice with career advancement [50].
Another potential impediment to autologous transfer is physician payment. Medicare reimbursement for tissue-based procedures significantly declined during 2000–2010, whereas that for implants remained relatively stable [51]. According to a nationwide survey of 312 members from American Society of Plastic Surgeons, 95% of the reconstructive surgeons enjoyed technical aspects of performing ABR and found it personally rewarding, but nearly half of them reported having reduced their reconstruction volume over the previous year, due to poor reimbursement. In a multivariable analysis, longer years of practice and perceived financial constraint independently predicted lower volume of reconstructive surgery [49]. When comparing rates of reimbursement per operation room (OR) utilization hour in a single institutional study, the average physician payment for IBR using flaps compared to implants was lower by 55% in 2006 ($322. vs. $587 per OR hour). This disparity grew even further in 2011, free flaps included, to 66% ($535 vs. $1622 per OR hour)[52]. The differential physician compensation favoring implants may have played a role in the lack of rise in ABR. These financial constraints necessitate furthering understanding along with integration of value, into the reimbursement metric. Healthcare payment legislation, such as Medicare Access and Children’s Health Insurance Program Reauthorization Act, which places increased emphasis on outcomes, represents a shift towards value, as opposed to volume.
RADIOTHERAPY AND IMMEDIATE BREAST RECONSTRUCTION
Although the rate of reconstruction is lower in the setting of post-mastectomy radiation therapy (PMRT), compared to non-radiated patients, PMRT appears to be a diminishing relative contraindication to IBR [53–55] [56–58]. A population-based SEER database study of 5481 radiated patients reported that the proportion of IBRs almost doubled (14% to 25%) over the decade of 2000 [59]. Interestingly, the annual increase in the rates of reconstruction among radiated patients was steeper than that in non-radiated ones for both implants (15% versus 11%), as well as flap-based reconstructions (8% versus 6%). By 2008, implant-based reconstruction surpassed ABR as the more common method of reconstruction in the setting of PMRT [39].
Several factors likely explain these trends. Traditional indications of radiotherapy included tumor size of 5 cm or larger, involvement of four or more lymph nodes, and extension to skin or muscle; however, increasing numbers of patients are offered irradiation by many institutions including those with fewer lymph nodes and smaller tumor size [60–62]. It is not uncommon for a woman with a single involved lymph node to receive PMRT in our center. Also, early breast mound replacement provides psychological benefit to women as they receive adjuvant treatments for their cancer. Studies report improved HR-QoL among radiated patients who undergoing immediate compared with delayed breast reconstruction [53, 54, 63–66]. As such, plastic surgeons must increasingly balance their reconstruction planning along with the anticipated need for adjuvant treatments [60, 67, 68]. That said, a well-defined algorithm for breast reconstruction in the setting of PMRT is lacking.
An important question to consider, while planning treatment, is whether or not outcomes are acceptable with radiation to flaps, tissue expanders or permanent implants. A single institutional longitudinal study of patients treated from 2003–2012 by a single surgeon compared outcomes in patients who received radiation to permanent implants versus tissue expanders [69]. Patients with radiated expanders experienced significantly higher six-year implant failure rate compared to those with radiated implants (32% vs.16% respectively; p<0.01). The finding that radiotherapy to the expander may compromise long-term outcomes has been supported by other studies including a meta-analysis [70, 71]. Nonetheless, a significantly greater proportion of patients with radiated expanders reported higher satisfaction and lower rate of grade-IV capsular contracture compared to their counterparts. Patients requiring radiation and opting for implant-based reconstruction must weigh the trade-offs associated with the varying timing of radiotherapy.
The high failure rate associated with implant-based reconstruction in the setting of PMRT has lead some plastic surgeons to favor autologous techniques. A systemic review of 25 observational studies of patients who underwent ABR demonstrated comparable rates of complication and revisionary surgery in both radiated and non-radiated groups; however, radiated patients were significantly more likely to have fat necrosis [72]. This result must be interpreted with caution due to limitations inherent in a dataset pooled from multiple retrospective non-randomized studies and limited follow-up time.
Still another option, is the one called delayed immediate approach, which can be performed in one of two permutations. One selection is to place an expander in any potential candidate for radiation with a re-evaluation once final pathology has been determined. For those patients who do not require PMRT, the expander is replaced with an autologous flap within approximately two weeks [73]. A variant on this approach would include radiation to the tissue expander with conversion to an autologous flap six months after radiotherapy completion. The argument, for use of the tissue expander in this instance, as opposed to a delayed autologous reconstruction altogether, is the preservation of expanded chest wall skin with less of a patch like appearance upon completion of the reconstruction. A shared-decision making process involving breast surgery, plastic surgery, and radiation oncology clearly benefits patients in this distinct group [74].
SUMMARY STATEMENT
With increasing numbers of both tissue- and implant-based options available, patients and their reconstructive surgeons will need to collaborate in a shared decision making process to identify the best reconstructive while incorporating oncologic factors.
Acknowledgments
This research was funded in part though the NIH/NCI Cancer Center Support Grant P30 CA008748
Financial disclosure statement:
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
ABBREVIATIONS
- ABR
Autologous Breast Reconstruction
- ASBrS
American Society of Breast Surgeons
- BCS
Breast Conservation Surgery
- BPM
bilateral prophylactic mastectomy
- CHIP
Children’s Health Insurance Program
- CPM
contralateral prophylactic mastectomy
- DIEP
Deep inferior epigastric artery perforator
- GAD-7
Generalized Anxiety Disorder 7 Scale
- HR-QoL
Health-Related Quality of Life
- IBR
Immediate Breast Reconstruction
- MACRA
Medicare Access and CHIP Reauthorization Act
- NACCR
North American Association of Central Cancer Registries
- PAP
Profunda Artery Perforator
- PROMIS
Patient Reported Outcome Measurement Information System
- SEER
Surveillance, Epidemiology, and End Results
- SGAP
Superior Gluteal Artery Perforator
- SIEA
Superficial Inferior Epigastric Artery
- TUG
Transverse Upper Gracilis
References
- 1.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society, I., Surveillance Research. Cancer Facts & Figures 2017. 2017 [cited 2017 05/25/2017]; Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf.
- 3.Weir HK, et al. Heart Disease and Cancer Deaths - Trends and Projections in the United States, 1969–2020. Prev Chronic Dis. 2016;13:E157. doi: 10.5888/pcd13.160211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665–73. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305(1):6–11. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 7.Morrow M, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–6. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albornoz CR, et al. Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer. Plastic and Reconstructive Surgery. 2015;135(6):1518–1526. doi: 10.1097/PRS.0000000000001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boughey JC, et al. Contralateral prophylactic mastectomy is associated with a survival advantage in high-risk women with a personal history of breast cancer. Ann Surg Oncol. 2010;17(10):2702–9. doi: 10.1245/s10434-010-1136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen PP, et al. Contralateral breast carcinoma: an assessment of risk and prognosis in stage I (T1N0M0) and stage II (T1N1M0) patients with 20-year follow-up. Surgery. 1989;106(5):904–10. [PubMed] [Google Scholar]
- 11.Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 12.Howell A, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–2. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 13.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 14.Albornoz CR, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 15.Lostumbo L, et al. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2004;(4):CD002748. doi: 10.1002/14651858.CD002748.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Arrington AK, et al. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16(10):2697–704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 17.Jones NB, et al. Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Ann Surg Oncol. 2009;16(10):2691–6. doi: 10.1245/s10434-009-0547-9. [DOI] [PubMed] [Google Scholar]
- 18.Tuttle TM, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–9. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 19.Hoskin TL, et al. Use of immediate breast reconstruction and choice for contralateral prophylactic mastectomy. Surgery. 2016;159(4):1199–209. doi: 10.1016/j.surg.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010;(11):CD002748. doi: 10.1002/14651858.CD002748.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Pesce C, et al. Contralateral Prophylactic Mastectomy Provides No Survival Benefit in Young Women with Estrogen Receptor-Negative Breast Cancer. Annals of Surgical Oncology. 2014;21(10):3231–3239. doi: 10.1245/s10434-014-3956-3. [DOI] [PubMed] [Google Scholar]
- 22.King TA, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158–64. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 23.Albornoz CR, et al. Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer: The Role of Breast Reconstruction. Plast Reconstr Surg. 2015;135(6):1518–26. doi: 10.1097/PRS.0000000000001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alderman AK, et al. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer. 2008;112(3):489–94. doi: 10.1002/cncr.23214. [DOI] [PubMed] [Google Scholar]
- 25.Boughey JC, et al. Contralateral Prophylactic Mastectomy (CPM) Consensus Statement from the American Society of Breast Surgeons: Data on CPM Outcomes and Risks. Ann Surg Oncol. 2016;23(10):3100–5. doi: 10.1245/s10434-016-5443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkins EG, et al. Complications in Postmastectomy Breast Reconstruction: One-year Outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study. Ann Surg. 2016 doi: 10.1097/SLA.0000000000002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Momoh AO, et al. Tradeoffs Associated With Contralateral Prophylactic Mastectomy in Women Choosing Breast Reconstruction: Results of a Prospective Multicenter Cohort. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg SM, et al. Perceptions, Knowledge, and Satisfaction With Contralateral Prophylactic Mastectomy Among Young Women With Breast Cancer. Annals of Internal Medicine. 2013;159(6):373. doi: 10.7326/0003-4819-159-6-201309170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawley ST, et al. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA Surgery. 2014;149(6):582. doi: 10.1001/jamasurg.2013.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung A, et al. Comparison of Patient Characteristics and Outcomes of Contralateral Prophylactic Mastectomy and Unilateral Total Mastectomy in Breast Cancer Patients. Annals of Surgical Oncology. 2012;19(8):2600–2606. doi: 10.1245/s10434-012-2299-1. [DOI] [PubMed] [Google Scholar]
- 31.Yi M, et al. Factors Affecting the Decision of Breast Cancer Patients to Undergo Contralateral Prophylactic Mastectomy. Cancer Prevention Research. 2010;3(8):1026–1034. doi: 10.1158/1940-6207.CAPR-09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arrington AK, et al. Patient and Surgeon Characteristics Associated with Increased Use of Contralateral Prophylactic Mastectomy in Patients with Breast Cancer. Annals of Surgical Oncology. 2009;16(10):2697–2704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 33.Musiello T, Bornhammar E, Saunders C. Breast surgeons’ perceptions and attitudes towards contralateral prophylactic mastectomy. ANZ Journal of Surgery. 2012;83(7–8):527–532. doi: 10.1111/j.1445-2197.2012.06209.x. [DOI] [PubMed] [Google Scholar]
- 34.Silva AK, et al. The Effect of Contralateral Prophylactic Mastectomy on Perioperative Complications in Women Undergoing Immediate Breast Reconstruction: A NSQIP Analysis. Ann Surg Oncol. 2015;22(11):3474–80. doi: 10.1245/s10434-015-4628-7. [DOI] [PubMed] [Google Scholar]
- 35.Bellavance E, et al. Surgeons’ Perspectives of Contralateral Prophylactic Mastectomy. Ann Surg Oncol. 2016;23(9):2779–87. doi: 10.1245/s10434-016-5253-9. [DOI] [PubMed] [Google Scholar]
- 36.Cemal Y, et al. A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg. 2013;131(3):320e–6e. doi: 10.1097/PRS.0b013e31827cf576. [DOI] [PubMed] [Google Scholar]
- 37.Garfein ES. The privilege of advocacy: legislating awareness of breast reconstruction. Plast Reconstr Surg. 2011;128(3):803–4. doi: 10.1097/PRS.0b013e3182221501. [DOI] [PubMed] [Google Scholar]
- 38.Yang RL, et al. Trends in immediate breast reconstruction across insurance groups after enactment of breast cancer legislation. Cancer. 2013;119(13):2462–8. doi: 10.1002/cncr.28050. [DOI] [PubMed] [Google Scholar]
- 39.Razdan SN, et al. National Breast Reconstruction Utilization in the Setting of Postmastectomy Radiotherapy. J Reconstr Microsurg. 2017 doi: 10.1055/s-0037-1598201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrow M, et al. Factors influencing the use of breast reconstruction postmastectomy: a National Cancer Database study. J Am Coll Surg. 2001;192(1):1–8. doi: 10.1016/s1072-7515(00)00747-x. [DOI] [PubMed] [Google Scholar]
- 41.Kamali P, et al. Differences in the Reporting of Racial and Socioeconomic Disparities among Three Large National Databases for Breast Reconstruction. Plast Reconstr Surg. 2017;139(4):795–807. doi: 10.1097/PRS.0000000000003207. [DOI] [PubMed] [Google Scholar]
- 42.Administration., U.S.F.a.D. Regulatory History of Breast Implants in the U.S. 2006. 2013 Sep 25; [cited 2017 05/01/2017]; Available from: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm064461.htm.
- 43.Zenn M, et al. Optimizing Outcomes of Postmastectomy Breast Reconstruction With Acellular Dermal Matrix: A Review of Recent Clinical Data. Eplasty. 2017;17:e18. [PMC free article] [PubMed] [Google Scholar]
- 44.Koslow S, et al. Long-term patient-reported satisfaction after contralateral prophylactic mastectomy and implant reconstruction. Ann Surg Oncol. 2013;20(11):3422–9. doi: 10.1245/s10434-013-3026-2. [DOI] [PubMed] [Google Scholar]
- 45.Hu ES, et al. Patient-reported aesthetic satisfaction with breast reconstruction during the long-term survivorship Period. Plast Reconstr Surg. 2009;124(1):1–8. doi: 10.1097/PRS.0b013e3181ab10b2. [DOI] [PubMed] [Google Scholar]
- 46.Yueh JH, et al. Patient satisfaction in postmastectomy breast reconstruction: a comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast Reconstr Surg. 2010;125(6):1585–95. doi: 10.1097/PRS.0b013e3181cb6351. [DOI] [PubMed] [Google Scholar]
- 47.Macadam SA, et al. Quality of Life and Patient-Reported Outcomes in Breast Cancer Survivors: A Multicenter Comparison of Four Abdominally Based Autologous Reconstruction Methods. Plast Reconstr Surg. 2016;137(3):758–71. doi: 10.1097/01.prs.0000479932.11170.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pusic AL, et al. Patient-Reported Outcomes 1 Year After Immediate Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium Study. J Clin Oncol. 2017:JCO2016699561. doi: 10.1200/JCO.2016.69.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alderman AK, et al. Patterns and correlates of postmastectomy breast reconstruction by U.S. Plastic surgeons: results from a national survey. Plast Reconstr Surg. 2011;127(5):1796–803. doi: 10.1097/PRS.0b013e31820cf183. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen PD, et al. Career satisfaction and burnout in the reconstructive microsurgeon in the United States. Microsurgery. 2015;35(1):1–5. doi: 10.1002/micr.22273. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez-Boussard T, et al. Breast reconstruction national trends and healthcare implications. Breast J. 2013;19(5):463–9. doi: 10.1111/tbj.12148. [DOI] [PubMed] [Google Scholar]
- 52.Sando IC, et al. Comprehensive breast reconstruction in an academic surgical practice: an evaluation of the financial impact. Plast Reconstr Surg. 2014;134(6):1131–9. doi: 10.1097/PRS.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clemens MW, Kronowitz SJ. Current perspectives on radiation therapy in autologous and prosthetic breast reconstruction. Gland Surg. 2015;4(3):222–31. doi: 10.3978/j.issn.2227-684X.2015.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124(2):395–408. doi: 10.1097/PRS.0b013e3181aee987. [DOI] [PubMed] [Google Scholar]
- 55.Chetta MD, et al. Reconstruction of the Irradiated Breast: A National Claims-Based Assessment of Postoperative Morbidity. Plast Reconstr Surg. 2017;139(4):783–792. doi: 10.1097/PRS.0000000000003168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albornoz CR, et al. Implant breast reconstruction and radiation: a multicenter analysis of long-term health-related quality of life and satisfaction. Ann Surg Oncol. 2014;21(7):2159–64. doi: 10.1245/s10434-014-3483-2. [DOI] [PubMed] [Google Scholar]
- 57.Albornoz CR, et al. Diminishing relative contraindications for immediate breast reconstruction. Plast Reconstr Surg. 2014;134(3):363e–369e. doi: 10.1097/PRS.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 58.Albornoz CR, et al. Diminishing relative contraindications for immediate breast reconstruction: a multicenter study. J Am Coll Surg. 2014;219(4):788–95. doi: 10.1016/j.jamcollsurg.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Agarwal S, et al. Immediate Reconstruction of the Radiated Breast: Recent Trends Contrary to Traditional Standards. Ann Surg Oncol. 2015;22(8):2551–9. doi: 10.1245/s10434-014-4326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frasier LL, et al. Temporal Trends in Postmastectomy Radiation Therapy and Breast Reconstruction Associated With Changes in National Comprehensive Cancer Network Guidelines. JAMA Oncol. 2016;2(1):95–101. doi: 10.1001/jamaoncol.2015.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Recht A, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1539–69. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- 62.Moo TA, et al. Selection criteria for postmastectomy radiotherapy in t1–t2 tumors with 1 to 3 positive lymph nodes. Ann Surg Oncol. 2013;20(10):3169–74. doi: 10.1245/s10434-013-3117-0. [DOI] [PubMed] [Google Scholar]
- 63.Al-Ghazal SK, et al. The psychological impact of immediate rather than delayed breast reconstruction. Eur J Surg Oncol. 2000;26(1):17–9. doi: 10.1053/ejso.1999.0733. [DOI] [PubMed] [Google Scholar]
- 64.Chao LF, et al. Monitoring patient-centered outcomes through the progression of breast reconstruction: a multicentered prospective longitudinal evaluation. Breast Cancer Res Treat. 2014;146(2):299–308. doi: 10.1007/s10549-014-3022-7. [DOI] [PubMed] [Google Scholar]
- 65.Elder EE, et al. Quality of life and patient satisfaction in breast cancer patients after immediate breast reconstruction: a prospective study. Breast. 2005;14(3):201–8. doi: 10.1016/j.breast.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Teo I, et al. Body image and quality of life of breast cancer patients: influence of timing and stage of breast reconstruction. Psychooncology. 2015 doi: 10.1002/pon.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carlson RW, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7(2):122–92. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 68.Yao K, et al. Increased utilization of postmastectomy radiotherapy in the United States from 2003 to 2011 in patients with one to three tumor positive nodes. J Surg Oncol. 2015;112(8):809–14. doi: 10.1002/jso.24071. [DOI] [PubMed] [Google Scholar]
- 69.Cordeiro PG, et al. What Is the Optimum Timing of Postmastectomy Radiotherapy in Two-Stage Prosthetic Reconstruction: Radiation to the Tissue Expander or Permanent Implant? Plast Reconstr Surg. 2015;135(6):1509–17. doi: 10.1097/PRS.0000000000001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plast Reconstr Surg. 2013;132(3):511–8. doi: 10.1097/PRS.0b013e31829acc41. [DOI] [PubMed] [Google Scholar]
- 71.Nava MB, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128(2):353–9. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- 72.Schaverien MV, Macmillan RD, McCulley SJ. Is immediate autologous breast reconstruction with postoperative radiotherapy good practice?: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2013;66(12):1637–51. doi: 10.1016/j.bjps.2013.06.059. [DOI] [PubMed] [Google Scholar]
- 73.Kronowitz SJ. Delayed-immediate breast reconstruction: technical and timing considerations. Plast Reconstr Surg. 2010;125(2):463–74. doi: 10.1097/PRS.0b013e3181c82d58. [DOI] [PubMed] [Google Scholar]
- 74.Disa JJ, Matros E. Discussion: Reconstruction of the Irradiated Breast: A National Claims-Based Assessment of Postoperative Morbidity. Plast Reconstr Surg. 2017;139(4):793–794. doi: 10.1097/PRS.0000000000003169. [DOI] [PubMed] [Google Scholar]