Abstract
Models of preclinical Alzheimer’s disease (AD) propose that cerebral amyloidosis leads to neurodegeneration and subsequent cognitive decline. This study investigated whether APOE genotype is related to β-amyloid (Aβ) burden in brain regions preferentially affected by AD and whether Aβ burden is associated with gray matter fraction (as a marker of neurodegeneration) and episodic memory performance in cognitively normal middle-aged individuals at varying genetic risk for AD. Three groups of cognitively normal participants aged 50–65 with a first-degree family history of AD [APOE genotype ε4ε4 (n=15), ε3ε4 (n=15), and ε3ε3 (n=15)], underwent [11C]PiB PET scans to quantify cortical Aβ, brain MRI and neuropsychological testing. APOE ε4ε4 participants demonstrated significantly higher cortical Aβ burden than APOE ε3ε3 (p<0.001). Furthermore, cortical Aβ burden was inversely associated with cortical gray matter fraction (p=0.017), but not episodic memory performance. In cognitively normal, middle-aged individuals, Aβ burden is significantly associated with gray matter fraction but not episodic memory performance. These findings are consistent with models of preclinical AD in which neurodegeneration occurs before manifest cognitive decline.
Keywords: Amyloid-β, Apolipoprotein E, Alzheimer’s disease, [11C]PiB, PET, Preclinical Alzheimer’s disease
1. Introduction
In recent years, Alzheimer’s disease (AD) has been conceptualized as a continuum of disease, beginning with a preclinical stage in which symptoms are not yet present (Sperling et al., 2011). The preclinical stage is postulated to involve accumulating brain pathology followed by very subtle cognitive decline. This stage may precede Mild Cognitive Impairment (MCI) by several years and represents an important stage for early intervention and symptom prevention. Most research in preclinical AD and prevention has focused on the 65+ population. The study of this older age group offers a high likelihood of capturing cases of preclinical AD, as the percentage of individuals with cerebral β-amyloid (Aβ) pathology increases from about 20% at age 65 to about 40% at age 85 (Jansen et al., 2015). AD prevention studies are already targeting this age group, which confers greater practical power to test treatments (Sperling et al., 2014).
Work to operationalize the new NIA-Alzheimer’s Association diagnostic criteria has thus largely focused on adults over 65 years of age (Jack et al., 2012). Moreover, convergent evidence suggests that elderly individuals with brain Aβ accumulation demonstrate subtle cortical thinning (Dickerson et al., 2009), greater rates of cortical and hippocampal atrophy (Andrews et al., 2013; Chetelat et al., 2012; Schott et al., 2010), and disruption of functional connectivity (Hedden et al., 2009; Mormino et al., 2011; Sheline et al., 2010). Finally, a number of studies have reported that individuals with elevated brain Aβ perform worse on neuropsychological tests (Hedden et al., 2012; Rentz et al., 2010; Rodrigue et al., 2012; Sperling et al., 2013) and are at increased risk for cognitive decline and progression to MCI and AD dementia (Ellis et al., 2013; Knopman et al., 2012; Lim et al., 2013b; Morris et al., 2009).
However, an equally important but comparatively neglected population is that of middle-aged (50–65) individuals with preclinical AD. This younger age group may present an earlier stage of disease pathogenesis and thus a greater opportunity for early intervention. Studies of preclinical AD in the 50–65-year-old population require the use of risk factors (family history and APOE ε4) to enrich samples for the presence of AD pathogenesis. Studies of cognitively normal individuals at high genetic risk of AD (i.e., APOE ε4 homozygotes and heterozygotes, compared to non-carriers, mean age 64 years) have reported that cortical fibrillar Aβ burden is significantly associated with APOE ε4 carrier status and APOE ε4 dose (Reiman et al., 2009). In cognitively normal subjects enriched for parental family history and APOE ε4 enrolled in the Wisconsin Registry for AD Prevention (mean age 60–61 years) Aβ status has not been associated with lower GM volume (Johnson et al., 2014), but has been linked to thinning of entorhinal cortex (Doherty et al., 2015). Moreover, elevated brain Aβ has not been associated with cognition cross-sectionally but confers an increased risk of MCI longitudinally and steeper rates of cognitive decline (Clark et al., 2016).
In the present study, our over-arching goal was to utilize a middle-aged sample that is highly enriched—both for family history and genetic risk of AD—to address three major hypotheses: 1) that APOE genotype was related to global fibrillar Aβ burden as measured by [11C]PiB) in cognitively normal first-degree relatives; 2) that fibrillar Aβ burden was associated with gray matter fraction (as a marker of neurodegeneration) in brain regions known to be preferentially affected by AD; and 3) that fibrillar Aβ burden was associated with episodic memory (and neuropsychological test) performance. The primary analyses were performed with partial volume corrected (PVC) PET data, but we also studied the effect of PVC on the results.
2. Methods
2.1. Participants
Cognitively normal adults aged 50–65 with a positive family history for probable AD (in at least one first-degree relative) were invited to participate. After signing informed consent, as approved by the Yale Human Investigation Committee, potentially eligible participants underwent an initial Screening Genetic Evaluation that involved a medical history questionnaire and confidential APOE genotyping [as previously described (van Dyck et al., 1998)] of 454 individuals. Participants were selected from each of three APOE genotype groups (ε4ε4, ε3ε4, and ε3ε3) and individually matched for age (±2 years) and sex for further study. These individuals underwent an additional Screening Diagnostic Evaluation to ensure eligibility. Assessments included ECG, physical examination, screening laboratory studies including chemistry profile, CBC, thyroid function studies, B12, and urinalysis, as well as Mini Mental Status Examination (MMSE) (Folstein et al., 1975), Clinical Dementia Rating Scale (CDR) (Morris, 1993), Logical Memory II (Wechsler, 1987), and the Geriatric Depression Scale. Participants were excluded for possible or probable AD (McKhann et al., 1984), or MCI (Petersen, 2004) [as evidenced by CDR>0 (Morris, 1993) and abnormal memory function documented by scoring 1.5 SD below education adjusted cutoff on Logical Memory II subscale from Wechsler Memory Scale – Revised (Wechsler, 1987)], or has a score on MMSE (Folstein et al., 1975) <27. In addition, they were excluded for any significant neurologic disease, unstable medical condition, history of alcohol or substance abuse/dependence within the past 5 years, major psychiatric disorder, use of medications with central nervous system activity within 4 weeks, pregnancy, or contraindications to magnetic resonance imaging (MRI).
Participants then underwent a detailed neuropsychological test battery (Supplementary Table 2) to enable analysis of neuropsychological correlates of [11C]PiB binding. PET and MRI scanning were then scheduled within one month. Neuropsychological testing and brain image analyses were performed by investigators blind to participant genotype.
2.2. Magnetic Resonance Imaging
MRI was performed on a 3T Trio Scanner: 1) to exclude any structural abnormalities; and 2) to co-register the PET and MRI images for image analysis and PVC. A sagittal 3D-MPRAGE-FSPGR pulse sequence with an IR prep of 300ms (TE=3.34ms, TI=1100ms, TR=2500ms, flip angle=7, slice thickness=1.0mm, 176 slices, matrix=256×256) was utilized for delineating gray matter (GM), white matter (WM), and CSF boundaries, and the small voxel size (0.98×1.00×0.98mm) provided high-resolution volumetric images.
2.3. [11C]PiB PET imaging
Participants underwent [11C]PiB PET scanning with the ECAT HR+ (Siemens) operating in 3D mode producing 63 slices with slice separation of 2.4mm and final image resolution of ~6mm. A transmission scan was acquired for attenuation correction. Then 15 mCi of [11C]PiB was administered by iv injection, followed by dynamic scanning for 90 min.
2.4. Image analyses
2.4.1. Reconstruction/Registration
PET images were reconstructed into 27 frames, containing 63 axial slices of 128×128 voxels (2.1×2.1×2.4mm). Reconstruction included corrections for attenuation, randoms, scatter, and deadtime. Motion correction was applied to the dynamic images, using a mutual-information algorithm (FSL-FLIRT, FSL 3.2; Analysis Group, FMRIB, Oxford, UK) by frame-by-frame registration to a summed image (0–10 min postinjection). A summed image was then created from the motion-corrected data, and registered to the participant’s MR anatomical image (6-parameter affine registration, FSL-FLIRT), which was then registered to the Montreal Neurological Institute (MNI) MR template(Holmes et al., 1998) using a nonlinear transform (BioImage Suite version 2.5; www.bioimagesuite.com).
2.4.2. Regions of Interest (ROIs)
The Anatomical Automatic Labeling (AAL) algorithm for SPM2 (Tzourio-Mazoyer et al., 2002) was used to define ROIs for both PET images and MRI. For our primary outcome measure, a mean cortical ROI consisting of frontal, posterior cingulate, precuneus, lateral parietal, and lateral temporal ROIs was used. Based on the published list of AAL regions (Tzourio-Mazoyer et al., 2002), the frontal ROI included frontal, anterior cingulate, middle cingulate, and insular cortex regions 3–34; the posterior cingulate ROI regions 35–36, the precuneus ROI regions 67–68; the lateral temporal ROI regions 79–90; the lateral parietal ROI regions 59–66; and the cerebellar reference ROI regions 91–115.
2.4.3. MRI Segmentation
Prior to transformation to MNI template space, MR images were segmented into GM, WM, and CSF using FAST— FMRIB’s Automated Segmentation Tool (The Analysis Group, FMRIB, Oxford, UK). To quantify cortical GM within each ROI while accounting for intersubject variability in brain size, we applied inverse transformations of the AAL from MNI to subject space and calculated GM fraction as the number of voxels segmenting as GM divided by the total number of voxels in a region. GM fractions (ranging between 0.00 and 1.00) were thus generated for the mean cortical ROI and component ROIs.
2.4.4. Partial Volume Correction (PVC)
Some studies of cognitively intact adults have shown GM reduction in APOE carriers relative to non-carriers (Wishart et al., 2006). Therefore, we applied PVC to PET images and also analyzed the effects of PVC. In this approach (Muller-Gartner et al., 1992), binary mask images of GM and WM were smoothed to the system resolution (~6mm). For each dynamic PET frame, GM voxels were corrected for spill-in and spill-out of activity, assuming activity in CSF was zero and WM activity was uniform and was estimated from each image time frame.
2.4.5. Tracer Kinetic Modeling
Parametric images of BPND (the ratio at equilibrium of specifically bound radioligand to that of nondisplaceable radioligand in tissue (Innis et al., 2007)) were generated using SRTM2 (Wu and Carson, 2002) using whole cerebellum as reference region for both uncorrected and partial volume corrected PET scans. BPND was calculated so that a value of 0 reflects no specific binding, i.e., tracer uptake no greater than that in the reference region. This is directly related to the distribution volume ratio (DVR) reported by other investigators (Reiman et al., 2009), in that DVR=BPND+1. BPND images were created from both original, uncorrected data, and from partial-volume corrected image data. Three sets of BPND values were extracted: i) uncorrected BPND from the full AAL region, ii) uncorrected BPND from the AAL regions masked to only include GM voxels, and iii) PVC-BPND images, again with GM masking. In secondary analyses, we investigated the effect of PVC on measurement of Aβ binding. Unless otherwise noted, BPND refers to the calculation using PVC images.
2.5. Statistical analyses
Differences among genotype groups were assessed using ANOVA for continuous variables and χ2 tests for categorical variables. To investigate the association between APOE genotype and Aβ burden, a multivariable generalized linear model was fit for mean cortical BPND with APOE genotype as the main explanatory variable and age and sex as covariates. A natural logarithm link was used for the right-skewed outcomes. In addition, to account for clustering introduced by matching on age and sex, a robust variance estimator was used by invoking the quasi-likelihood estimation method of generalized estimating equations. Secondary analyses examined the association between APOE genotype and BPND for each of the five component ROIs.
The association between Aβ burden and cortical GM fraction within the mean cortical ROI was assessed using a general linear model with mean cortical BPND as the main explanatory variable and age and sex as covariates. For the association between Aβ burden and episodic memory performance, an episodic memory score was calculated (by averaging the z-scores for California Verbal Learning Test free delayed recall and Logical Memory II) and was assessed using the same general linear model as for GM fraction. Secondary analyses explored the association between either APOE genotype or mean cortical Aβ burden and performance on each of the neuropsychological tests. Since individual test scores were not all normally distributed, the Kruskal-Wallis test and Spearman’s rank correlation were utilized.
Finally, the effect of PVC on mean cortical Aβ values was explored. Spearman’s rank correlation coefficient was used to assess the association between cortical GM fraction and the change in BPND from uncorrected to PVC. In addition, model fits of BPND were examined using separate general linear models with APOE genotype as the main explanatory variable, as well as age and sex covariates. Williams t-test was used to compare the differences in correlation between GM fraction and BPND values for the uncorrected, GM masked, and PVC conditions (Weaver and Wuensch, 2013).
P-values <0.05 for two-sided tests were interpreted as statistically significant. Analyses used either SPSS version 21.0 (IBM Corp.) or Matlab R2015a Statistics Toolbox (Mathworks, Inc.).
3. Results
3.1. Participant characteristics
The final study sample consisted of 45 cognitively normal FDRs who were enrolled in three APOE genotype groups: ε3ε3 (n=15), ε3ε4 (n=15), and ε4ε4 (n=15). Table 1 summarizes the demographics and clinical characteristics of each group. As they were individually matched for age and sex, the APOE groups did not differ with respect to these variables. In addition, they did not differ in education, WRAT-3 Reading subtest standard score as an estimate of premorbid intelligence, MMSE, full scale IQ score, or GDS score.
Table 1.
Participant demographics and characteristics by APOE genotype
| APOE Genotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| ε3,ε3 (n=15) | ε3,ε4 (n=15) | ε4,ε4 (n=15) | ||||||
|
| ||||||||
| Mean/Count | ±SD/% | Mean/Count | ±SD/% | Mean/Count | ±SD/% | F/χ2a | p-value | |
| Demographic | ||||||||
| Age (y) | 59.1 | ±4.4 | 59.2 | ±4.6 | 59.1 | ±5.4 | .00 | .99 |
| Female Sex | 8 | 53 | 8 | 53 | 8 | 53 | .00 | 1.00 |
| Education (y) | 16.5 | ±1.6 | 16.3 | ±2.3 | 16.3 | ±1.8 | .06 | .95 |
| Clinical | ||||||||
| MMSE | 29.7 | ±0.5 | 29.3 | ±0.8 | 29.1 | ±1.2 | 1.43 | .25 |
| WAIS-III FSIQ | 113.5 | ±14.4 | 118.8 | ±14.8 | 112.9 | ±10.5 | .90 | .42 |
| WRAT-3 Reading | 109.4 | ±8.7 | 109.2 | ±9.4 | 109.8 | ±11.3 | .01 | .99 |
| Geriatric Depression Scale | 0.7 | ±0.7 | 0.4 | ±0.6 | 1.1 | ±1.9 | 1.1 | .35 |
F statistics and p-values for means are from ANOVA significance tests; χ2 statistics and p-values for counts are from chi-square significance tests. SD=standard deviation.
3.2. Association between APOE Genotype and Cortical Aβ Burden (BPND)
As depicted in Figure 1, APOE ε4ε4 participants had significantly higher mean cortical BPND (0.455) than those with ε3ε3 (0.187) (p<0.001), and ε3ε4 participants (0.346) were marginally higher than those with ε3ε3 (p=0.125), but ε4ε4 and ε3ε4 groups did not differ (p = 0.317). A significant linear trend (p < 0.005) indicated that as the ε4 allele number increased, cortical Aβ burden increased proportionally. Results for the individual brain ROIs that comprise the mean cortical ROI are presented in Supplementary Table 1.
Figure 1. Box plots of mean cortical Aβ burden (BPND) by APOE genotype.
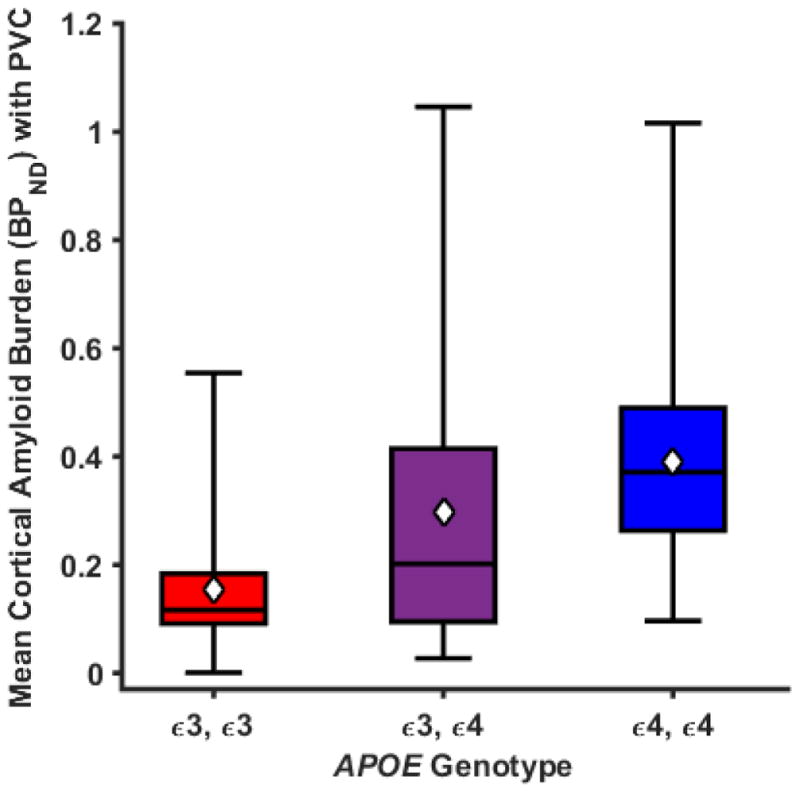
Boxes define the interquartile range. The black horizontal lines indicate medians and the white diamonds indicate the means. Plotted values are unadjusted means and statistical analysis was performed using a generalized linear model adjusted for age and sex. The APOE genotype variable is significant with p = 0.003 (n =15 per group).
3.3. Association between Cortical Aβ Burden (BPND) and Cortical Gray Matter Fraction
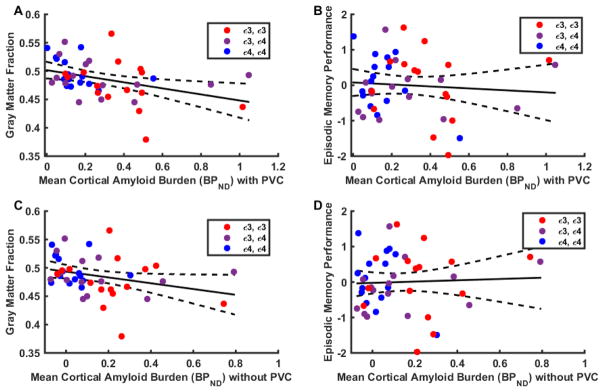
The unadjusted association between mean cortical BPND and cortical GM fraction values within the mean cortical ROI in the overall sample is displayed in Figure 2A by a linear regression line and its 95% confidence interval. Mean cortical BPND was inversely associated with GM fraction (regression coefficient = −0.046, p = 0.017) in a general linear model with adjustments for age and sex covariates. Post hoc analysis showed that this association was also present prior to PVC for GM masked mean cortical BPND (regression coefficient = −0.046, p = 0.048, Figure 2C).
Figure 2. Association between cortical Aβ burden (BPND) and cortical gray matter (GM) fraction (A, C) or episodic memory performance (B, D).
Plotted values are partial volume corrected BPND for the mean cortical ROI (A) and unadjusted cortical gray matter fraction (regression coefficient = −0.046, p = 0.017) or (B) episodic memory performance (regression coefficient = −0.317, p = 0.558). Gray matter masked BPND without partial volume correction for the mean cortical ROI (C) and unadjusted cortical gray matter fraction (regression coefficient = −0.046, p = 0.048) or (D) episodic memory performance (regression coefficient = 0.1271, p = 0.846) are also shown. Episodic memory performance is the average of z-scores for CVLT free delayed recall and Logical Memory II. The figure displays unadjusted linear regression lines with their 95% confidence intervals. Statistical analysis was performed using general linear models adjusted for age and sex.
3.4. Association between Cortical Aβ Burden (BPND) and Episodic Memory Performance
The unadjusted association between BPND and episodic memory performance values within the mean cortical ROI in the overall sample is displayed in Figure 2B by a linear regression line and its 95% confidence interval. There was no statistically significant association between mean cortical BPND and episodic memory performance (regression coefficient = −0.317, p = 0.558) in a general linear model with adjustments for age and sex. Post hoc analysis showed that this association was also non-significant prior to PVC for GM masked mean cortical BPND (regression coefficient = 0.127, p = 0.846, Figure 2D).
Exploratory analysis was performed using the larger neuropsychological battery. APOE genotype was not associated with any of the neuropsychological test performance measures (Supplementary Table 2). In addition, there were no significant correlations between any neuropsychological test and mean cortical Aβ burden (BPND) in the overall sample (Supplementary Table 3).
3.5. Effect of Partial Volume Correction on BPND
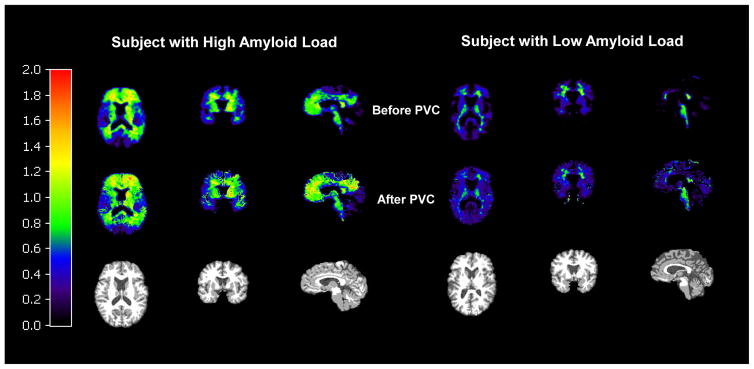
As expected, PVC tended to increase values of BPND in the overall participant sample (N=45). Figure 3 demonstrates the effect of PVC on BPND images of participants with high and low Aβ load. Values of mean cortical BPND increased from 0.10 ± 0.18 uncorrected, to 0.13 ± 0.20 GM masked, to 0.28 ± 0.24 with PVC. Not surprisingly, the increase in mean cortical BPND produced by PVC (from uncorrected to PVC-corrected) was highly inversely associated with the cortical GM fraction (Spearman r = −0.46, p < 0.002). I.e., uncorrected analyses may underestimate Aβ burden to a greater extent in individuals with lower cortical GM fraction. Although APOE ε4 allele number is associated with increased cortical amyloid even prior to PVC, PVC improved the goodness of fit (R2) for the overall model (Table 2). In addition, the association between cortical GM fraction and cortical Aβ burden is significantly greater when PVC is performed compared with GM mask correction alone (Williams t-score = 2.15, p = 0.04) or with no correction (Williams t-score = 3.04, p = 0.004).
Figure 3. Effect of partial volume correction on BPND images with [11C]PiB.
Images are for cognitively normal, first-degree relatives of AD patients with high Aβ load and low Aβ load. Mask images of gray and white matter were smoothed to the system resolution (~6mm). For each dynamic PET frame, GM voxels were corrected for spill-in and spill-out of activity, assuming activity in CSF was zero and WM activity was uniform and was estimated from the centrum semiovale. Finally, SRTM2 was re-applied to the PVC-images to produce PVC-corrected BPND values. In the resulting PVC images, values in white matter voxels are not corrected.
Table 2.
Effect sizes of overall models* and APOE genotype for mean cortical Aβ burden (BPND) (N=45)
| Overall Model R2 | APOE Genotype Bias-Corrected Semi-Partial Correlation Ratio† | APOE Genotype Correlation Ratio 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| BPND-Uncorrected | 0.327 | 0.127 | 0.012, 0.303 | 0.013 |
| BPND-Gray Matter Masked | 0.368 | 0.134 | 0.015. 0.308 | 0.009 |
| BPND-Partial Volume Corrected | 0.393 | 0.128 | 0.011, 0.300 | 0.009 |
These statistics are obtained from separate general linear models in which APOE genotype is the main explanatory variable and age and sex are covariates.
This ratio represents the proportion of total variation accounted for by the allele group factor.
4. Discussion
We studied cognitively normal middle-aged individuals with a first-degree family history of AD and varying APOE risk to investigate the relationship between Aβ deposition, GM fraction, and neuropsychological test performance in this unique population. APOE ε4ε4 participants demonstrated significantly higher mean cortical Aβ burden (PVC BPND) than ε3ε3 participants, with ε3ε4 marginally higher than ε3ε3 participants. In the overall sample, Aβ burden was inversely associated with GM fraction within the mean cortical ROI. Reported results show no statistically significant association between mean cortical Aβ burden and episodic memory performance. PVC increased values of BPND and led to a statistically significant increase in the association between cortical Aβ burden and GM fraction.
4.1. Comparison with In Vivo Imaging Studies
Our results corroborate and extend observations by Reiman et al (Reiman et al., 2009), but with a somewhat reduced APOE ε4 effect on Aβ deposition in this younger sample (mean age 59 vs. 64). Our findings are also consistent with those of Johnson et al. (Johnson et al., 2014) and demonstrate that in high-risk individuals, considerable Aβ deposition begins by the early 50s, and earlier than has generally been reported in large series of cognitively normal participants (Morris et al., 2010; Rowe et al., 2010).
Neuropsychological test results confirmed the full cognitive “normality” of many at risk participants with considerable fibrillar Aβ burden. Mean cortical BPND was unrelated to an index of episodic memory performance for the pooled sample of 45 participants. These results agree with those of Johnson et al. (Johnson et al., 2014), who also observed no relationship between Aβ status and any of several cognitive measures in an at-risk middle aged sample (mean age 60.1 years). In other Aβ imaging studies of cognitively normal elderly individuals (generally > age 65), Aβ binding has been shown to be associated with cognitive performance in some (Hedden et al., 2012; Rentz et al., 2010; Rodrigue et al., 2012; Sperling et al., 2013), but not all (Harrington et al., 2013) previous studies. In some studies, an association has been observed with episodic memory only in females (Pike et al., 2011) or in APOE ε4 carriers (Lim et al., 2013a).
Our finding of an inverse association between Aβ deposition and GM fraction within the Aβ susceptible brain regions—in the absence of a cognitive association—is somewhat unexpected. Previous studies have demonstrated that cognitively normal elderly individuals with brain Aβ accumulation demonstrate subtle cortical thinning (Dickerson et al., 2009), greater rates of cortical and hippocampal atrophy (Andrews et al., 2013; Chetelat et al., 2012; Schott et al., 2010), and disruption of functional connectivity (Hedden et al., 2009; Mormino et al., 2011; Sheline et al., 2010). However, these studies generally involve the older age range (>65 years), in which Aβ burden has also been associated with cognitive function. Our results differ from those of Johnson et al. (Johnson et al., 2014), who observed no association between Aβ status and GM volume in subjects of similar age. This divergence may relate to different methods of evaluating gray matter (GM fraction within the Aβ susceptible ROIs versus voxel based morphometry) or our analysis of Aβ deposition as a continuous rather than categorical variable.
A major difference between our subject sample and those of most of the other referenced studies is that our participants are significantly younger and largely non-overlapping in age with other samples. They may simply fall at an earlier stage of preclinical disease without “subtle cognitive decline” (Sperling et al., 2011). Alternatively, they may exhibit lesser aging effects in comparison to the older participants in other studies. Age-related atrophy occurs partly in AD-specific brain regions and may have additive effects with AD-related neurodegeneration (Bakkour et al., 2013). Thus, our middle-aged subjects may have greater “brain reserve” compared to older preclinical samples and may not manifest cognitive effects of Aβ deposition and early neurodegeneration. The preclinical stage of AD has been theorized to include sub-stages of Aβ deposition with and without neuronal damage and subtle cognitive symptoms (Sperling et al., 2011). Thus our findings are fully consistent with this theoretical model. They suggest that future prevention studies may benefit from intervention at a younger age and an earlier preclinical stage than the current A4 study (Sperling et al., 2014). Enrichment of samples through family history and APOE genotyping will likely be necessary.
4.2. Implications for Early Intervention
Our finding that Aβ burden is inversely associated with GM fraction prior to an association with any cognitive measures has particular implications for future prevention strategies. A limitation of the present study is its cross-sectional design, which cannot fully establish the temporal relationship implicated in the association of Aβ burden with GM losses. Longitudinal studies that span the emergence of Aβ pathogenesis may be necessary to establish whether the early steps in this process are already associated with GM reductions and other markers of neuronal injury. However, the definition of this stage will be critical to determine the optimal period for intervention strategies. A broad range of potential interventions—including anti-Aβ strategies or attempts to repair structural defects of APOE ε4 (Mahley and Huang, 2012)—should ultimately be implemented prior to earliest neurodegeneration. It presently remains unclear whether an early stage of Aβ pathogenesis fully precedes neuronal injury and thus whether detection of fibrillar Aβ deposition in the brain by a screening test is a sufficient condition for identifying candidates for early intervention studies.
4.3. Importance of Partial Volume Correction
Finally, our results underscore the importance of PVC for Aβ PET imaging, even in the preclinical stages of AD. The fact that Aβ burden is inversely associated with GM fraction at a preclinical stage inherently entails that uncorrected analyses will underestimate Aβ burden in the very individuals with the greatest burden. Indeed, our additional analyses confirm that the increase in mean cortical BPND yielded by PVC is highly inversely associated with the fraction of GM within the mean cortical ROI. Additional support for performing PVC in the present study is provided by the improved overall model fit of BPND values vs. APOE genotype (Table 2), i.e., PVC enlarges group differences in Aβ burden. Although PVC has not been widely employed in Aβ PET imaging—and has been essentially absent from preclinical investigations—these results highlight the advantages of correcting for partial volume effects when performing Aβ imaging in the preclinical stages.
Recently, interest has mounted in performing PVC for Aβ PET (Gonzalez-Escamilla et al., 2017; Shidahara et al., 2017). The optimal method of PVC for PET imaging remains a topic of investigation (For review, see (Erlandsson et al., 2012)), with alternatives including region-based methods such as the regional spread function (RSF) method (Rousset et al., 2008), as well as voxel-based methods such as the Muller-Gartner algorithm used here. The selection of a PVC method is based on several factors, including the system point-spread function and its spatial variation, assumptions about uniformity of tracer uptake within regions, and the accuracy of registrations and segmentations. As an example, Su et al. (Su et al., 2015) compared 2 PVC methods, the 2-compartment image-based method (Meltzer et al., 1990) which corrects for GM loss but not for spill-in between WM and GM, and RSF, and concluded that RSF performed better. However, this conclusion was based in part on a simulation study, for which the validity may be limited to the chosen simulation conditions. Here, we used the Muller-Gartner method, which is of intermediate complexity between the methods compared by Su et al. This method can account for the time-varying spill-in of activity from WM to GM, which is particularly important for Aβ PET due to the high WM uptake.
4.4. Conclusion
In cognitively normal, middle-aged individuals at varying genetic risk for AD, Aβ burden is significantly associated with gray matter fraction but not episodic memory performance. These findings are consistent with models of preclinical AD in which neurodegeneration occurs before manifest cognitive decline.
Supplementary Material
Highlights.
Cognitively normal adults with APOE ε4ε4 genotype had higher Aβ burden than ε3ε3.
Cortical Aβ burden was inversely associated with gray matter fraction.
There was no significant association of cortical Aβ burden with episodic memory.
Acknowledgments
The authors wish to thank staff of the Yale PET Center for their excellent technical assistance. This research was supported by the Alzheimer’s Association [IIRG-07-60026]; the National Institute on Aging [P50-AG047270 and P30-AG021342]; and the National Institute of Mental Health [R25-MH071584].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews KA, Modat M, Macdonald KE, Yeatman T, Cardoso MJ, Leung KK, Barnes J, Villemagne VL, Rowe CC, Fox NC, Ourselin S, Schott JM. Atrophy rates in asymptomatic amyloidosis: implications for Alzheimer prevention trials. PLoS One. 2013;8(3):e58816. doi: 10.1371/journal.pone.0058816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Wolk DA, Dickerson BC. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage. 2013;76:332–344. doi: 10.1016/j.neuroimage.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Villemagne VL, Villain N, Jones G, Ellis KA, Ames D, Martins RN, Masters CL, Rowe CC. Accelerated cortical atrophy in cognitively normal elderly with high beta-amyloid deposition. Neurology. 2012;78(7):477–484. doi: 10.1212/WNL.0b013e318246d67a. [DOI] [PubMed] [Google Scholar]
- Clark LR, Racine AM, Koscik RL, Okonkwo OC, Engelman CD, Carlsson CM, Asthana S, Bendlin BB, Chappell R, Nicholas CR, Rowley HA, Oh JM, Hermann BP, Sager MA, Christian BT, Johnson SC. Beta-amyloid and cognitive decline in late middle age: Findings from the Wisconsin Registry for Alzheimer’s Prevention study. Alzheimers Dement. 2016;12(7):805–814. doi: 10.1016/j.jalz.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BM, Schultz SA, Oh JM, Koscik RL, Dowling NM, Barnhart TE, Murali D, Gallagher CL, Carlsson CM, Bendlin BB, LaRue A, Hermann BP, Rowley HA, Asthana S, Sager MA, Christian BT, Johnson SC, Okonkwo OC. Amyloid burden, cortical thickness, and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. Alzheimers Dement (Amst) 2015;1(2):160–169. doi: 10.1016/j.dadm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KA, Lim YY, Harrington K, Ames D, Bush AI, Darby D, Martins RN, Masters CL, Rowe CC, Savage G, Szoeke C, Villemagne VL, Maruff P. Decline in cognitive function over 18 months in healthy older adults with high amyloid-beta. J Alzheimers Dis. 2013;34(4):861–871. doi: 10.3233/JAD-122170. [DOI] [PubMed] [Google Scholar]
- Erlandsson K, Buvat I, Pretorius PH, Thomas BA, Hutton BF. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Physics in medicine and biology. 2012;57(21):R119–159. doi: 10.1088/0031-9155/57/21/R119. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, et al. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Escamilla G, Lange C, Teipel S, Buchert R, Grothe MJ Alzheimer’s Disease Neuroimaging I. PETPVE12: an SPM toolbox for Partial Volume Effects correction in brain PET - Application to amyloid imaging with AV45-PET. Neuroimage. 2017;147:669–677. doi: 10.1016/j.neuroimage.2016.12.077. [DOI] [PubMed] [Google Scholar]
- Harrington KD, Lim YY, Ellis KA, Copolov C, Darby D, Weinborn M, Ames D, Martins RN, Savage G, Szoeke C, Rowe C, Villemagne VL, Masters CL, Maruff P. The association of Abeta amyloid and composite cognitive measures in healthy older adults and MCI. Int Psychogeriatr. 2013;25(10):1667–1677. doi: 10.1017/S1041610213001087. [DOI] [PubMed] [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(46):16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29(40):12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Rocca WA, Boeve BF, Petersen RC. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Annals of neurology. 2012;71(6):765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Visser PJ. Amyloid Pathology, Cognitive Impairment, and Alzheimer Disease Risk--Reply. JAMA. 2015;314(11):1177–1178. doi: 10.1001/jama.2015.9719. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, Hillmer AT, Wooten DW, Murali D, Barnhart TE, Hall LT, Racine AM, Klunk WE, Mathis CA, Bendlin BB, Gallagher CL, Carlsson CM, Rowley HA, Hermann BP, Dowling NM, Asthana S, Sager MA. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiol Aging. 2014;35(3):576–584. doi: 10.1016/j.neurobiolaging.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Boeve BF, Petersen RC. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78(20):1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Ellis KA, Ames D, Darby D, Harrington K, Martins RN, Masters CL, Rowe C, Savage G, Szoeke C, Villemagne VL, Maruff P. Abeta amyloid, cognition, and APOE genotype in healthy older adults. Alzheimers Dement. 2013a;9(5):538–545. doi: 10.1016/j.jalz.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Lim YY, Pietrzak RH, Ellis KA, Jaeger J, Harrington K, Ashwood T, Szoeke C, Martins RN, Bush AI, Masters CL, Rowe CC, Villemagne VL, Ames D, Darby D, Maruff P. Rapid decline in episodic memory in healthy older adults with high amyloid-beta. J Alzheimers Dis. 2013b;33(3):675–679. doi: 10.3233/JAD-2012-121516. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76(5):871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Leal JP, Mayberg HS, Wagner HN, Jr, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr. 1990;14(4):561–570. doi: 10.1097/00004728-199007000-00011. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, Janabi M, Baker SL, Yen IV, Madison CM, Miller BL, Jagust WJ. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cerebral cortex. 2011;21(10):2399–2407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J, Roe C, Xiong C, Fagan A, Goate A, Holtzman D, Mintun M. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Gartner H, Links J, Prince J, Bryan R, McVeigh E, Leal J, Davatzikos C, Frost J. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Pike KE, Ellis KA, Villemagne VL, Good N, Chetelat G, Ames D, Szoeke C, Laws SM, Verdile G, Martins RN, Masters CL, Rowe CC. Cognition and beta-amyloid in preclinical Alzheimer’s disease: data from the AIBL study. Neuropsychologia. 2011;49(9):2384–2390. doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Reiman E, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder S, Langbaum J, Alexander G, Klunk W, Mathis C, Price J, Aizenstein H, Dekosky S, Caselli R. Fibrillar amyloid-{beta} burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78(6):387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset OG, Collins DL, Rahmim A, Wong DF. Design and implementation of an automated partial volume correction in PET: application to dopamine receptor quantification in the normal human striatum. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2008;49(7):1097–1106. doi: 10.2967/jnumed.107.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe C, Ellis K, Rimajova M, Bourgeat P, Pike K, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters C, Ames D, Villemagne V. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Schott JM, Bartlett JW, Fox NC, Barnes J. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Abeta1-42. Annals of neurology. 2010;68(6):825–834. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidahara M, Thomas BA, Okamura N, Ibaraki M, Matsubara K, Oyama S, Ishikawa Y, Watanuki S, Iwata R, Furumoto S, Tashiro M, Yanai K, Gonda K, Watabe H. A comparison of five partial volume correction methods for Tau and Amyloid PET imaging with [18F]THK5351 and [11C]PIB. Ann Nucl Med. 2017;31(7):563–569. doi: 10.1007/s12149-017-1185-0. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, Sadowsky CH, Carpenter A, Davis MD, Lu M, Flitter M, Joshi AD, Clark CM, Grundman M, Mintun MA, Skovronsky DM, Pontecorvo MJ. Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging. 2013;34(3):822–831. doi: 10.1016/j.neurobiolaging.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, Aisen P. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):228fs213. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Blazey TM, Snyder AZ, Raichle ME, Marcus DS, Ances BM, Bateman RJ, Cairns NJ, Aldea P, Cash L, Christensen JJ, Friedrichsen K, Hornbeck RC, Farrar AM, Owen CJ, Mayeux R, Brickman AM, Klunk W, Price JC, Thompson PM, Ghetti B, Saykin AJ, Sperling RA, Johnson KA, Schofield PR, Buckles V, Morris JC, Benzinger TL Dominantly Inherited Alzheimer N. Partial volume correction in quantitative amyloid imaging. Neuroimage. 2015;107:55–64. doi: 10.1016/j.neuroimage.2014.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Gelernter J, MacAvoy MG, Avery RA, Criden M, Okereke O, Varma P, Seibyl JP, Hoffer B. The absence of an apolipoprotein E ε4 allele is associated with increased parietal rCBF asymmetry in Alzheimer’s disease. Arch Neurology. 1998;55:1460–1466. doi: 10.1001/archneur.55.11.1460. [DOI] [PubMed] [Google Scholar]
- Weaver B, Wuensch KL. SPSS and SAS programs for comparing Pearson correlations and OLS regression coefficients. Behav Res Methods. 2013;45(3):880–895. doi: 10.3758/s13428-012-0289-7. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-R Wechsler Memory Scale - Revised Manual. The Psychological Corporation, Harcourt Brace Jovanovich, Inc; New York: 1987. [Google Scholar]
- Wishart H, Saykin A, McAllister T, Rabin L, McDonald B, Flashman L, Roth R, Mamourian A, Tsongalis G, Rhodes C. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67(7):1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.