Abstract
Managed bees are critical for crop pollination worldwide. As the demand for pollinator-dependent crops increases, so does the use of managed bees. Concern has arisen that managed bees may have unintended negative impacts on native wild bees, which are important pollinators in both agricultural and natural ecosystems. The goal of this study was to synthesize the literature documenting the effects of managed honey bees and bumble bees on wild bees in three areas: (1) competition for floral and nesting resources, (2) indirect effects via changes in plant communities, including the spread of exotic plants and decline of native plants, and (3) transmission of pathogens. The majority of reviewed studies reported negative effects of managed bees, but trends differed across topical areas. Of studies examining competition, results were highly variable with 53% reporting negative effects on wild bees, while 28% reported no effects and 19% reported mixed effects (varying with the bee species or variables examined). Equal numbers of studies examining plant communities reported positive (36%) and negative (36%) effects, with the remainder reporting no or mixed effects. Finally, the majority of studies on pathogen transmission (70%) reported potential negative effects of managed bees on wild bees. However, most studies across all topical areas documented the potential for impact (e.g. reporting the occurrence of competition or pathogens), but did not measure direct effects on wild bee fitness, abundance, or diversity. Furthermore, we found that results varied depending on whether managed bees were in their native or non-native range; managed bees within their native range had lesser competitive effects, but potentially greater effects on wild bees via pathogen transmission. We conclude that while this field has expanded considerably in recent decades, additional research measuring direct, long-term, and population-level effects of managed bees is needed to understand their potential impact on wild bees.
Introduction
The status of bees worldwide is currently a topic of research and conservation concern [1–5]. There are approximately 20,000 species of bees worldwide, and these insects are arguably the most important pollinators for both crop and wild plants [6–8]. Numerous factors may be threatening bees including habitat loss and fragmentation, pesticides, and disease [3, 9–10]. In addition, the increasingly widespread use of managed bees may have negative effects on wild bee populations (reviewed by [11, 12]). Managed bees, including honey bees, bumble bees, and some solitary bees, have become an integral component of agriculture due to a rising demand for pollinator-dependent crops (e.g., almonds, tree fruits, berries), and without which many farms would likely experience pollination deficits [13–14]. However, the use of managed bees may negatively affect wild bee abundance or diversity, which could in turn impact food production since a diverse wild bee community has been found to increase pollination rates and subsequent crop yields even when managed bees are present [15–19]. Furthermore, in natural habitats, a diverse wild bee community is integral for maintaining plant diversity and ecosystem function [20–21]. Thus, identifying and quantifying the factors that affect wild bees is essential for bee conservation and to ensure pollination services within both managed and natural habitats.
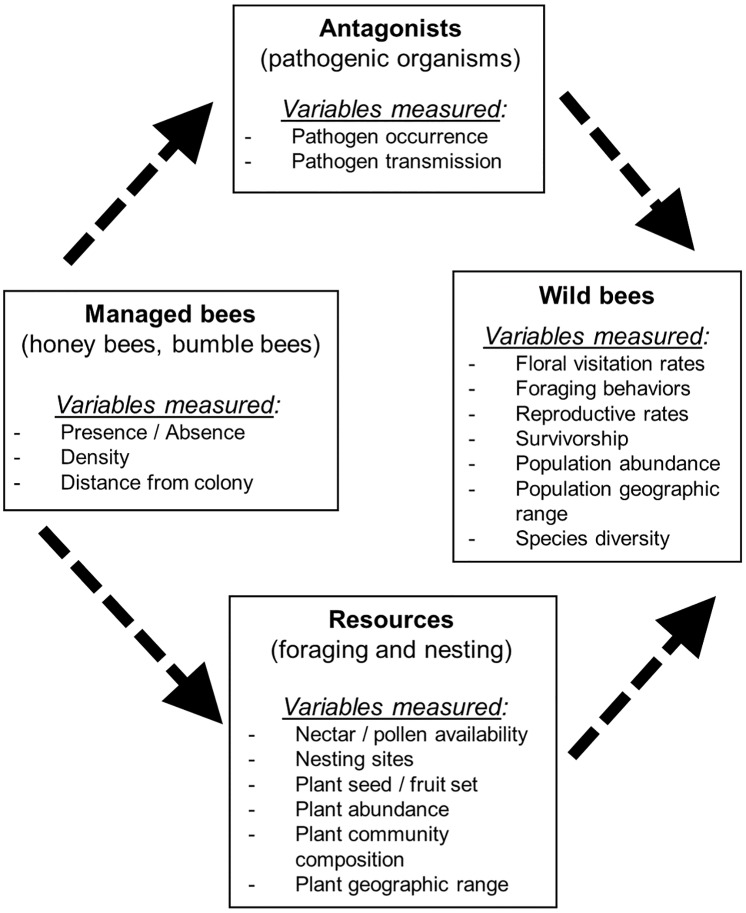
There are several ways in which managed bees could affect wild bees including through competition over finite resources such as nectar, pollen, or nesting habitat (Fig 1). Competition with managed bees for pollen and nectar may induce changes in wild bee floral use and niche breadth, with potential consequences for bee fitness. While the majority of wild bees are polylectic and potentially able to modify foraging behaviors in the presence of honey bees, competition could still have negative effects if wild bees are forced to forage on less nutritious plants, spend more time searching for flowers that are unoccupied or whose resources have not yet been depleted, or forage further from their nests [22–26]. Additionally, in regions where managed bees escape and establish in the wild, they could compete with wild bees for nesting sites such as tree or ground cavities [27]. The extent of competitive effects, however, could depend on many factors including overall resource availability, the degree of niche overlap between managed and wild bee species, and densities of both managed and wild bees.
Fig 1. Hypothesized interactions between managed bees and wild bees.
Wild and managed bees may interact indirectly (dashed lines) through either bottom-up effects on shared resources (including pollen, nectar, and nesting sites), or by altering top-down interactions through shared antagonists such as pathogenic organisms.
Managed bees could also affect resource availability for wild bees by changing plant community composition. Previous studies have shown that in some regions where managed bees are exotic, they preferentially forage on exotic plants [28–30]. These foraging preferences can form invasive mutualisms whereby exotic pollinators and plants facilitate each other’s spread in non-native regions, subsequently reducing populations of native plants [29]. The decline of native plants could then disrupt plant-pollinator networks, potentially leading to a loss of native bee species [1, 31]. However, while some bee species are specialists and may therefore be sensitive to the loss of native plants, the majority of wild bees are generalists and may therefore be resilient to changes in plant community composition [32–34].
Finally, managed bees may affect wild bees through shared antagonists, specifically pathogenic organisms. Most managed bees, including honey bees and bumble bees, are social species and occur in high densities, potentially making them more likely to harbor pathogens than their solitary wild counterparts [35–36]. The movement of these managed bees across large regions for crop pollination could increase their potential to spread such pathogens to wild bees. Furthermore, managed bees are often deployed outside of their native ranges, and can thus introduce novel, invasive pathogens [11, 28, 37]. Transmission of infectious agents by managed bees to wild bees can occur via contaminated pollen [38], feces [39], or through contact on shared foraging resources [40]. Shared pathogens have been found between managed and wild bees of the same species, closely related species, and distantly related species, suggesting that transmission of antagonists is possible and has the potential to affect a broad wild bee community [41–46]. The extent to which managed bees transmit pathogens to wild bees, and the effects of such antagonists on wild bee fitness, is likely to vary with the density and health of managed bees as well as the type of pathogen.
Two previous review papers by Goulson [11] and Paini [12] on this general topic found much circumstantial evidence for competition between managed and wild bees, but very little evidence that such competition has population-level or long-term effects on wild bees. Additionally, Goulson [11] concluded that exotic managed bees negatively affect plant community composition through the pollination of invasive exotic weeds, but the effects of native managed bees on plant communities were not addressed [11]. Furthermore, the effect of managed bees on wild bees via the transmission of natural enemies, including pathogens and parasites, was not well-covered in either review because there were few studies to date on that topic. Since the publication of these reviews in 2003 and 2004, no systematic review has been conducted on the overall effects of managed bees on wild bees. And with the increasing use of managed bees to meet agricultural demand [13], the effects of managed bees on wild flora and fauna is a mounting issue. Managed bees may be necessary in agricultural landscapes as crop pollinators, and may also benefit from supplemental foraging in natural habitats. Thus, this topic is relevant not only for growers, beekeepers, and the commercial bee industry, but for public land managers who may be considering the placement of managed bees within conservation areas or other public lands.
In this paper, we synthesize the literature on the effects of managed bees, here restricted to honey bees Apis spp. and bumble bees Bombus spp., on wild bees. Though there are other species of managed bees, honey bees and bumble bees are the most commonly used worldwide and relatively well researched. We searched for and synthesized papers that fell into three broad topical areas by which managed bees can affect wild bees: 1) competition for shared resources; 2) changes in plant community composition, specifically an increase in exotic plants and a subsequent decline in native plants, which is both a conservation concern in itself and has the potential to negatively affect native wild bees, and 3) the transmission of shared pathogens. While there may be other pathways by which managed bees affect wild bees, such as interspecific mating [47], these three topical areas are relatively well-studied and encompass those covered in earlier reviews [11–12]. Our findings have implications for the management of pollinators in natural and agricultural systems and for the conservation of wild bees.
Materials and methods
We performed a systematic search of the literature using Web of Knowledge/Web of Science (ISI Thompson-Reuters, webofknowledge.com) to identify studies that examined the effects of managed bees on wild bees via competition, changes in plant communities, and transmission of pathogens. Due to the broad nature of our focal question, we synthesized the literature with a systematic review as opposed to a meta-analysis. In addition, the studies in our review measured different metrics associated with both managed bees and wild bees (e.g., bee visitation rates, abundance, diversity, reproductive rates) that would have been difficult to standardize in a meta-analysis (Fig 1). Instead, as part of our systematic review, we used a vote-counting analysis to quantify the variables measured, and results found, across studies. We focused our review on the most common and widely used managed bees, honey bees and bumble bees. The use of other managed bees, including the orchard mason bee Osmia lignaria Say and alfalfa leafcutter bee Megachile rotundata Fabricius, is more limited to specific crops and geographic regions, resulting in fewer studies on these bees, and thus we excluded them from this systematic review.
To search for the effects of managed honey bees on wild bees via competition, changes in plant communities, and transmission of pathogens, including pathogenic parasites, we used the search terms: (“Apis mellifera” OR “honey bee” OR honeybee) AND (competition OR disease OR pathogen OR (pollin* AND (exotic OR invasive))). To identify studies that examined the effects of managed bumble bees, we used the search terms: (Bombus OR “bumble bee” OR bumblebee) AND (competition OR disease OR pathogen OR (pollin* AND (exotic OR invasive))). We additionally conducted a more general search to find studies that were not identified by the previous searches using the terms: “managed bee” AND (competition OR disease OR pathogen OR (pollin* AND (exotic OR invasive))). We included all papers returned by these searches beginning in 1900 and through the end of 2016. We additionally reviewed all articles that were cited by the two older non-systematic reviews on this topic [11–12], and searched for all recent articles that cited these two reviews [11–12].
We evaluated every article returned by our searches for whether or not it broadly addressed one of our three topical areas: competition between managed and wild bees, effects of managed bees on plant communities (natives vs. exotics), and transmission of pathogens, including pathogenic parasites, from managed to wild bees. Studies that did not broadly fall into the three topical areas, as well as review papers, were excluded. Additionally, we excluded papers that were not peer-reviewed (e.g. theses, conference proceedings) and papers not available in English. Furthermore, to be included in our review, studies needed to measure some response metric of either wild bees or plants (dependent variables, e.g., foraging behavior, abundance, reproductive rates) and relate that to a measured or assumed aspect of managed bee “intensity” (independent variable, e.g., presence/absence, before/after introduction, distance from colony, abundance). A study measuring pathogen presence in only managed bees, for example, would not be included if it did not also measure a wild bee response, regardless of any implications for wild bees discussed within the paper. For all studies, we recorded which topical area was addressed, the managed bee species examined and whether it was native to the study region, the wild bee taxa examined, the location and context of the study (e.g. field vs. lab), the independent managed bee variables measured, the dependent response variables measured (i.e. wild bee or plant metrics), and any additional explanatory or mechanism variables measured. We found a variety of independent and dependent variables across studies, and we did not discriminate among these variables for inclusion in this study. Furthermore, while we noted mechanistic or explanatory variables, studies did not need to measure such variables for inclusion in the study.
We additionally scored each article for whether the authors reported negative, positive, mixed, or no effects of managed bees. Consistent across all three topical areas, scores are from the perspective of native wild bees or native plants, where a negative score means that some measure of their performance decreases with managed bees, and a positive score means that performance improves with managed bees. Specifically, for competitive effects of managed bees on wild bees, “negative” (-) means that managed bees compete with wild bees and/or increased intra- or interspecific competition among wild bees, “no effect” (0) means that managed bees did not compete with wild bees and/or had no competitive effect on wild bees, and "mixed effects" means that responses varied across different wild bee species or different measures of competition. While we did not specifically search for studies examining mutualism or commensalism, a “positive” effect (+) in this area would include studies examining potential competitive effects but finding positive relationships between managed and wild bees (e.g. a positive correlation between abundances or visitation rates of managed and wild bees).
For the effects of managed bees on plant communities, “negative” (-) means that managed bees had a negative effect on native plants (e.g., decreased plant abundance) and/or a positive effect on exotic plants (e.g., increased plant abundance), “positive” (+) means that managed bees had a positive effect on native plants and/or a negative effect on exotic plants, “no effect” (0) means that managed bees had no effect on plant communities, and "mixed effects" means that responses varied by plant species or across different plant variables measured. Increases in native plants and/or decreases in exotic plants was considered to be a positive response because restoring native plant communities, a common bee conservation goal, is often associated with increases in native wild bees [48–49].
For evaluating the potential effects of managed bees on wild bees via pathogens, "negative” (-) means that managed bees increased pathogen occurrence in wild bees or that managed bee pathogens had a negative effect on wild bees including on fitness, abundance, diversity, etc., “no effect” (0) means that managed bees had no effect on the occurrence of pathogens in wild bees, or that managed bee pathogens had no effect on wild bees, and “mixed effects" means that effects varied across wild bee species, pathogens, or response variables examined. As it is unlikely that managed bees could have a positive effect on wild bees in this area (e.g. decrease pathogen occurrence), and pathogens by definition do not have a positive effect on their host, there were no positive effects found in this category.
Results
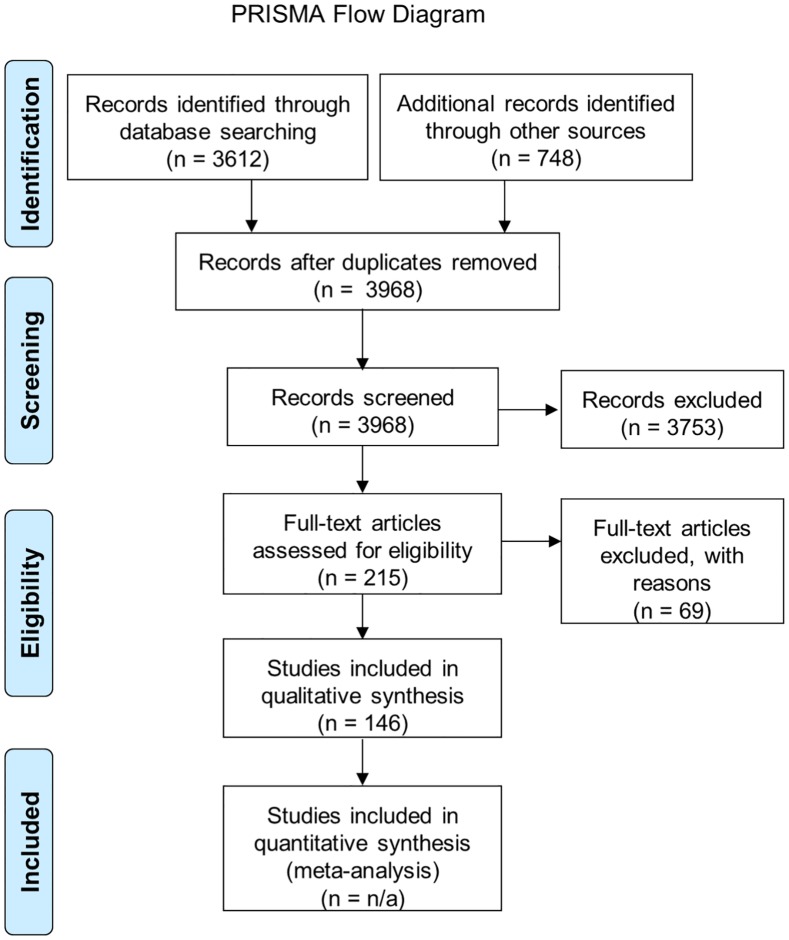
Our search of the literature identified 146 studies that fit our inclusion criteria and broadly addressed the effects of managed bees on wild bees via competition, changes in plant communities (specifically changes in exotic and native plant populations), or transmission of pathogens (Fig 2, S1 References). Of these studies, 72 addressed competition, 41 addressed plant communities, 6 studied both competition and plant communities, and 27 addressed pathogens. The majority of studies examining competition and plant communities focused on managed honey bees Apis spp. (number of studies, n = 59 and 36, respectively) with fewer studies on managed bumble bees (n = 17 and 6, respectively) or on both (n = 2 and 5, respectively) (Tables 1 and 2). However, studies on pathogens were more evenly split between those studying managed honey bees (n = 15) and managed bumble bees (n = 10) (Table 3).
Fig 2. PRISMA flow diagram.
A flow diagram showing the process for a systematic review including the number of studies processed, reviewed, and analyzed at each step in the review process.
Table 1. Studies published from 1900–2016 examining potential competitive effects of managed bees on wild bees.
For all studies, we recorded the species of managed and wild bees, and indicated whether managed bees were native or exotic to the study region, the location (continent and country) and context of the study including field (natural, semi-natural, developed, agricultural, or experimental plot), lab, or greenhouse, and all variables measured, including the managed bee metric (independent variable), wild bee metric (dependent variable), and any explanatory or mechanistic variables. The overall competitive effect of managed bees on wild bees, as reported by the study, is also recorded and noted as positive (+), neutral (0), negative (-), or mixed.
| Reference | Managed bee species (* indicates exotic range) | Wild bee species | Location | Context | Managed bee metric (independent variable) | Explanatory mechanism variable | Wild bee metric (dependent variable) | Reported effect |
|---|---|---|---|---|---|---|---|---|
| Abe et al. 2010 | Apis mellifera* | Xylocopa Ogasawarensis & endemic small bees | Asia (Japan) | field (natural) | honey bee presence/absence and/or abundance | none | distribution | 0 |
| Aizen & Feinsinger 1994 | A. mellifera* | many | South America (Argentina) | field (natural) | visitation rates | none (different responses to forest fragmentation speculated) | visitation rates | - |
| Aizen et al. 2011 | Bombus ruder-atus* | Bombus dahlbomii | South America (Argentina) | field (natural) | foraging behavior (floral preferences, nectar removal), visitation rates | nectar availability | foraging behavior (floral preferences, nectar removal), visitation rates | 0 |
| Badano & Vergara 2011 | A.mellifera* | many | North America (Mexico) | field (agricultural) | abundance | none | diversity | - |
| Balfour et al. 2013 | A.mellifera | Bombus terrestris/lucorum, Bombus pascuorum, Bombus lapidarius | Europe (UK) | field (experiment plots) | visitation rates, foraging behavior (handling times, number of floral probes) | tongue length | visitation rates, foraging behavior (handling time, number of floral probes) | 0 |
| Balfour et al. 2015 | A.mellifera | Bombus spp. | Europe (UK) | field (experiment plots) | visitation rates, foraging behavior (search time, extraction time, etc.) | nectar volume & sugar concentration, energetic returns per flower | visitation rates, foraging behavior (search time, extraction time, etc.) | 0 |
| Batra 1999 | A. mellifera* | many | North America (USA) | field (semi-natural) | visitation rates | none | visitation rates | 0 |
| Cane & Tepedino 2017 | A. mellifera* | many (average-sized solitary bees) | North America (USA) | lab | amount of pollen collected per colony | none | amount of pollen needed to produce one offspring | - |
| Carneiro & Martins 2012 | A. mellifera scutellata* | many | South America (Brazil) | field (natural) | visitation rates | pollen depletion | visitation rates | - |
| Connor & Neumeier 1995 | A. mellifera* | many | North America (USA) | field (natural) | visitation rates | none | visitation rates | - |
| Dohzono et al. 2008 | B. terrestris* | Bombus ardens, Bombus hypocrita | Asia (Japan) | field (natural) | presence/absence | nectar robbing & collection | visitation rates | - |
| Dupont et al. 2004 | A. mellifera* | Anthophora alluaudi, Eucera gracilipes | Canary Islands | field (natural) | abundance | nectar depletion | visitation rates | - |
| El Shafie et al. 2002 | Apis florea* | A. mellifera sudanensis | Africa (Sudan) | field (agricultural) | foraging behavior (types of pollen collected), visitation rates | none (niche partitioning implied) | foraging behavior (type of pollen collected), visitation rates | 0 |
| Elbgami et al. 2014 | A. mellifera* | B. terrestris 1 | Europe (UK) | field (agricultural) | distance from apiary | none | individual bee weight & reproductive success | - |
| Esterio et al. 2013 | B. terrestris* | B. dahlbomii | South America (Chile) | field (natural) | visitation rates, foraging behavior (number of pollen grains carried & deposited) | none | visitation rates, foraging behavior (number of pollen grains carried & deposited) | 0 |
| Forup & Memmot 2005 | A. mellifera | Bombus spp. | Europe (UK) | field (natural) | abundance, foraging behavior (diet breadth) | tongue length | abundance, diversity, foraging behavior (diet breadth) | -/0 |
| Franco et al. 2009 | A. mellifera* | Bombus atratus | South America (Brazil) | field (natural) | foraging behavior (plant use, diet breadth) | niche overlap | foraging behavior (plant use, diet breadth) | -/0 |
| Ginsberg 1983 | A. mellifera* | many | North America (USA) | field (semi-natural) | foraging behavior (plant preferences & foraging period) | niche overlap | foraging behavior (plant preferences & foraging period) | -/0 |
| Goras et al. 2016 | A. mellifera | many | Europe (Greece) | field (natural) | hive density | none | visitation rates, foraging behavior (visit duration) | 0 |
| Goulson & Sparrow 2009 | A. mellifera | B. pascuorum, B. lucorum, B. lapidarius, B. terrestris | Europe (UK) | field (semi-natural) | presence/absence | none | thorax width | - |
| Goulson et al. 2002 | A. mellifera*, Bombus terrestris* | many | Australia | field (natural, semi-natural, & developed) | presence/absence | niche overlap | abundance, diversity, & foraging behavior (floral preference) | -/0 |
| Gross 2001 | A. mellifera* | many | Australia | field (natural) | abundance, visitation rates | none | abundance, visitation rates | - |
| Gross & Mackay 1998 | A. mellifera* | many | Australia | field (natural) | visitation rates | direct displacement interactions | visitation rates | - |
| Herbertsson et al 2016 | A. mellifera | Bombus spp. | Europe (Sweden) | field (agricultural) | presence/absence | tongue length, thorax width | density | -/0 |
| Hingston & McQuilan 1998 | B. terrestris* | many | Australia | field (natural) | foraging behavior (diet breadth) | niche overlap | foraging behavior (diet breadth) | - |
| Hingston & McQuilan 1999 | B. terrestris* | Chalicodoma spp. | Australia | field (natural) | presence/absence | none (nectar availability implied) | visitation rates, foraging behavior (foraging time) | - |
| Holmes 1964 | A. mellifera* | Bombus spp. | North America (USA) | field (developed) | visitation rates | none | visitation rates | - |
| Horskins & Turner 1999 | A. mellifera* | many | Australia | field (natural) | foraging behavior (temporal foraging patterns, stigma contact, nectar vs. pollen collecting trips) | nectar availability | foraging behavior (temporal foraging patterns, stigma contact, nectar vs. pollen collecting trips) | 0 |
| Hudewenz & Klein 2013 | A. mellifera | many | Europe (Germany) | field (natural) | distance to hive, presence/absence | none | visitation rates, number of nests | - |
| Hudewenz & Klein 2015 | A. mellifera | Osmia bicornis | Europe (Germany) | field (experiment plots) | abundance | interspecific displacement, visitation rates, niche breadth & overlap | number of nests & brood cells | - |
| Inari et al. 2005 | B. terrestris* | B. ardens | Asia (Japan) | field (agricultural & semi-natural) | abundance, distance from greenhouse | none | abundance | - |
| Ings et al. 2006 | B. terrestris dalmatinus* | B. terrestris audax | Europe (UK) | field (natural) | foraging behavior, visitation rates, production of new queens & males | none | foraging behavior, visitation rates, production of new queens & males | - |
| Inoue & Yokoyama 2010 | B. terrestris* | B. hypocrita sapporoensis, Bombus schrencki albidopleuralis, Bombus pseudobaicalensis, Bombus diversus tersatus | Asia (Japan) | field (natural) | foraging behavior (diet breadth), reproductive capacity, temporal changes in abundance | niche overlap | temporal changes in abundance, foraging behavior (diet breadth) | - |
| Inoue et al. 2010 | B. terrestris* | Bombus ignitus | Asia (Japan) | field (experiment plot) | foraging behavior (foraging load, foraging efficiency) | tongue length | foraging behavior (foraging load, foraging efficiency) | - |
| Ishii et al. 2008 | B. terrestris* | B. diversus tersatus, B. pseudobaicalensis, B. hypocrita sapporoensis | Asia (Japan) | field (agricultural & natural) | habitat occupancy, foraging behavior (floral preferences) | flower morphology & tongue length | habitat occupancy, foraging behavior (floral preferences) | - |
| Kajobe 2007 | A. mellifera | Meliponula bocandei, Meliponula nebulata | Africa (Uganda) | field (natural) | foraging behavior (diversity of pollen collected) | bee body & colony size | foraging behavior (diversity of pollen collected) | -/0 |
| Kato & Kawakita 2004 | A. mellifera* | many | New Caledonia | field (natural) | foraging behavior (plant use) | none | foraging behavior (plant use) | - |
| Kato et al. 1999 | A. mellifera* | many | Bonin Islands | field (natural) | relative abundance | none | relative abundance | - |
| Kuhn et al. 2006 | A. mellifera | Megachile lapponica | Europe (Germany) | field (natural) | density | none | visitation rates, foraging behavior (duration of foraging flights), brood cell construction | 0 |
| Lindstrom et al. 2016 | A. mellifera | many | Europe (Sweden) | field (agricultural) | presence/absence, density | none | density | - |
| Lye et al. 2011 | B. terrestris* | many | North America (USA) | field (agricultural) | presence/absence | none | visitation rates | 0 |
| Martins 2004 | A. mellifera | many | Africa (Kenya) | field (natural) | visitation rates, foraging behavior (temporal foraging patterns, plant use) | direct displacement, nectar & pollen removal/depletio-n | visitation rates, foraging behavior (temporal foraging patterns, plant use) | - |
| Menezes et al. 2007 | A. mellifera* | Scaptotrigona spp. | South America (Brazil) | field (experiment plot) | presence/absence | none (resource partitioning implied) | visitation rates, foraging behavior (floral preference) | - |
| Morales et al. 2013 | Bombus ruderatus*, B. terrestris* | B. dahlbomii | South America (Chile) | field (natural) | temporal trends in regional abundance, geographic distribution | none | temporal trends in regional abundance, geographic distribution | - |
| Nagamitsu et al. 2007a | B. terrestris* | B. ardens, B. hypocrita | Asia (Japan) | field (experiment plot) | presence/absence | nectar availability | queen body mass, colony mass | 0 |
| Nagamitsu et al. 2007b | B. terrestris* | B. hypocrita, B. ardens, B. diversus | Asia (Japan) | field (natural) | abundance | tongue length | abundance, body size | 0 |
| Nagamitsu et al. 2010 | B. terrestris* | B. ardens, B. hypocrita | Asia (Japan) | field (natural) | presence/absence | tongue length | abundance, worker body size | - |
| Nakamura 2014 | B. terrestris* | B. pseudobaicalensis, B. hypocrita sapporoensis | Asia (Japan) | field (developed) | visitation rates, foraging behavior (pollen type & diversity on body) | niche overlap | visitation rates, foraging behavior (pollen type & diversity on body) | 0/- |
| Neumayer 2006 | A. mellifera | many | Europe (Austria) | field (natural) | distance from hive, presence/absence | nectar availability | visitation rates/local abundance | - |
| Nielsen et al. 2012 | A. mellifera | many | Europe | field (natural) | visitation rates | none | visitation rates | -/0/+ |
| Nishikawa & Shimamura 2015 | B. terrestris* | B. hypocrita, Bombus deuteronymus | Asia (Japan) | field (natural) | visitation rates | tongue length, head width, niche overlap | visitation rates | 0 |
| Paini & Roberts 2005 | A. mellifera* | Hylaeus alcyoneus | Australia | field (natural) | presence/absence | none | fecundity (number of nests, number of eggs per nest, progeny mass) | - |
| Paini et al. 2005 | A. mellifera* | Megachile spp. | Australia | field (natural) | presence/absence | none (temperature adaptations implied) | reproductive success | 0 |
| Pedro & Camargo 1991 | A. mellifera* | many | South America (Brazil) | field (semi-natural) | relative abundance, foraging behavior (floral preference) | none | relative abundance, foraging behavior (floral preference) | 0 |
| Pick & Schlindwein 2011 | A. mellifera* | Melitoma segmentaria, Melitoma osmioides, Melitomella murihir, Lithurgus huberi | South America (Brazil) | field (natural) | foraging behavior (floral preferences), visitation rates | pollen removal | foraging behavior (floral preferences), visitation rates | 0 |
| Pinkus-Rendon et al. 2005 | A. mellifera* | Peponapis limitaris, Partamona bilineata | North America (Mexico) | field (agricultural) | visitation rates, foraging behavior (plant use) | niche overlap, direct displacement interactions | visitation rates, foraging behavior (plant use) | - |
| Pleasants 1981 | A. mellifera* | Bombus spp. | North America (USA) | field (experiment plots) | presence/absence | tongue length | abundance | - |
| Rogers et al. 2013 | A. mellifera* | Bombus impatiens | North America (USA) | field (experiment plots) | response to intra & interspecific physical encounters at flowers | none | response to intra & interspecific physical encounters at flowers | - |
| Roubik 1978 | A. mellifera* | many | South America (French Guiana) | field (natural) | presence/absence | none | flower visitation rates, foraging behavior (duration of floral visits) | -/0 |
| Roubik 1980 | A. mellifera* | Melipona spp., Trigona spp. | South America (French Guiana) | field (natural) | visitation rates to feeders | partitioning & displacement interactions at feeders | visitation rates to feeders | 0/- |
| Roubik 1983 | A. mellifera* | Melipona favosa, Melipona fulva | South America (French Guiana) | field (natural) | presence/absence, number of hives, amounts of brood, honey, & pollen in hive | none | amounts of brood, honey, & pollen in nest | 0 |
| Roubik et al. 1986 | A. mellifera* | many | North America (Panama) | field (natural) | rate of forager return, foraging behavior (type, quantity, & quality of pollen & nectar gathered) | niche overlap | rate of forager return, foraging behavior (type, quantity & quality of pollen & nectar gathered) | -/0 |
| Roubik & Villanueva-Gutierrez 2009 | A. mellifera* | many | North America (Mexico) | field (natural) | presence/absence, foraging behaviors (plant use) | niche overlap | abundance, foraging behavior (pollen identity & diversity) | 0 |
| Roubik & Wolda 2001 | A. mellifera* | many | North America (Panama) | field (natural) | presence/absence, abundance | none | abundance | 0 |
| Schaffer et al. 1979 | A. mellifera* | Bombus sonorous, Xylocopa arizonensis | North America (USA) | field (natural) | visitation rates, foraging behavior (resource collection) | none | visitation rates, foraging behavior (resource collection) | - |
| Schaffer et al 1983 | A. mellifera* | many | North America (USA) | field (natural) | presence/absence | nectar standing crop | visitation rates | - |
| Semida & Elbanna 2006 | A. mellifera | many | Africa (Egypt) | field (natural) | visitation rates | none | visitation rates | -/0 |
| Shavit et al. 2009 | A. mellifera | many | Asia (Israel) | field (natural) | presence/absence | none | foraging behavior (temporal foraging patterns, plant use), visitation rates | -/0 |
| Smith-Ramirez et al. 2014 | A. mellifera*, B. terrestris* | many | South America (Chile) | field (natural) | visitation rates | none | visitation rates | - |
| Sugden & Pyke 1991 | A. mellifera* | Exoneura asimillima | Australia | field (natural) | presence/absence | none | colony survival, developmental stage & sex ratios, relative frequency of founder vs. established colonies | - |
| Steffan-Dewenter & Tscharntke 2000 | A. mellifera | many | Europe (Germany) | field (natural) | density, visitation rates | niche overlap | abundance, diversity, number of nests, number of brood cells, visitation rates | 0 |
| Tepedino et al. 2007 | A. mellifera* | many | North America (USA) | field (agricultural) | visitation rates, distance from hive | none | visitation rates | 0 |
| Thomson 2004 | A. mellifera* | B. occidentalis | North America (USA) | field (natural) | distance from hive | foraging effort devoted to pollen collection | foraging behavior (pollen vs. nectar collection, forager return rates), reproductive success | - |
| Thomson 2006 | A. mellifera* | Bombus spp. | North America (USA) | field (natural) | foraging behavior (plant use), visitation rates, distance from hive | niche overlap | foraging behavior (plant use), visitation rates, abundance | -/0 |
| Thomson 2016 | A. mellifera* | Bombus spp. | North America (USA) | field (natural) | density | niche overlap | densities | - |
| Torne-Noguera et al. 2016 | A. mellifera | many | Europe (Spain) | field (natural) | distance to apiary, visitation rate | resource consumption (nectar & pollen consumption) | visitation rate, wild bee biomass | - |
| Walther-Hellwig et al. 2006 | A. mellifera | Bombus spp. | Europe (Germany) | field (agricultural) | density | tongue length | visitation rates/local abundance | -/0 |
| Wilms & Weichers 1997 | A. mellifera* | Melipona bicolor, Melipona quadrifasciata | South America (Brazil) | field (natural) | foraging behavior (types & amount of pollen & nectar collected) | niche overlap | foraging behavior (types & amount of pollen & nectar collected) | - |
1 Commercial bumble bee colonies were used as indicators for conspecific wild bumble bees
* Indicates managed bee species that were used outside of their native range
Table 2. Studies published from 1900–2016 examining the potential effect of managed bees on wild bees through changes in plant communities, including the spread of exotic plants.
For all studies, we recorded the species of managed and wild bees, and indicated whether managed bees were native or exotic to the study region, the location (continent and country) and context of the study including field (natural, semi-natural, developed, agricultural, or experimental plot), lab, or greenhouse, and all variables measured, including the managed bee metric (independent variable), plant metric (dependent variable), and any explanatory or mechanistic variables. The overall effect of managed bees on plant communities, as reported by the study, is also recorded and noted as positive (+), neutral (0), negative (-), or mixed.
| Reference | Managed bee species (* indicates exotic range) | Wild bee species | Location | Context | Managed bee metric (independent variable) | Explanatory mechanism variable | Plant metric (dependent variable) | Reported effect |
|---|---|---|---|---|---|---|---|---|
| Abe et al. 2011 | Apis mellifera* | Xylocopa ogasawarensis & others | Asia (Japan) | field (natural) | visitation rates | pollen limitation | fruit set | - |
| Aslan et al. 2016 | A. mellifera* | many | North America (USA) | field (natural) | visitation rates | none | none | +/0 |
| Barthell et al. 2001 | A. mellifera* | many | North America (USA) | field (natural) | visitation rates | none | seed set | - |
| Beavon & Kelly 2012 | A. mellifera*, Bombus spp.* | many | New Zealand | field (natural) | visitation rates, presence/absence | none | fruit set, seed set, fruit size, germination success | - |
| Bruckman & Campbell 2014 | A. mellifera* | many | North America (USA) | field (natural) | visitation rates, foraging behavior (pollen deposition) | pollinator importance (visitation rates x conspecific pollen deposition) | seed set | +/0 |
| Carbonari et al. 2009 | A. mellifera* | none | South America (Brazil) | field (natural) | foraging behavior (frequency of nectar robbing) | occurrence of illegitimate visits | floral abortion | - |
| Cayuela et al. 2011 | A. mellifera | none | Europe (Spain) | field (natural) | distance from apiary | none | fruit set | +/0 |
| Chamberlain & Schlising 2008 | A. mellifera* | many | North America (USA) | field (natural) | visitation rates | none | seed set | + |
| Descamps et al. 2015 | A. mellifera | many | Europe (France) | field (natural) | visitation rates | none | none | + |
| Dick 2001 | A. mellifera scutellata* | many | South America (Brazil) | field (natural) | visitation rates | none | seed set, genetic diversity, gene flow | + |
| Dohzono et al. 2008 | Bombus terrestris* | Bombus ardens, Bombus hypocrita | Asia (Japan) | field (natural) | presence/absence | occurrence of illegitimate visits | fruit & seed set | - |
| Dupont et al. 2004 | A. mellifera* | many | Canary Islands | field (natural) | abundance | foraging behavior (visitation length, foraging preferences) | seed set & viability | 0 |
| Esterio et al. 2013 | B. terrestris* | many | South America (Chile) | field (natural) | visitation rates, foraging behavior (pollen collection, pollen deposition) | none | none | 0 |
| Faria & Araujo 2015 | A. mellifera* | Augochloropsis spp. | South America (Brazil) | field (natural) | pollinator effectiveness (fruit set per visit) | none | fruit set | + |
| Faria & Araujo 2016 | A. mellifera* | many | South America (Brazil) | field (natural) | visitation rates | none | none | + |
| Gilpin et al. 2014 | A. mellifera* | many | Australia | field (natural) | visitation rates, foraging behavior (inter & intra-plant movements, pollen diversity on body) | pollinator fidelity | relative plant distribution | + |
| Goulson & Derwent 2004 | A. mellifera* | many | Australia | field (natural), greenhouse | abundance, visitation rates, presence/absence | none | fruit set, seed set | - |
| Goulson & Rotheray 2012 | A. mellifera*, B. terrestris* | many | Tasmania | field (natural) | visitation rates | none | population size, seed set | -/0 |
| Gross 2001 | A. mellifera* | many | Australia | field (natural) | abundance, visitation rates, foraging behavior (handling time) | none | pollen limitation, fruit set | + |
| Gross & Mackay 1998 | A. mellifera* | many | Australia | field (natural) | visitation rates | pollen deposition & removal per visit | fruit & seed set | - |
| Gross et al. 2010 | A. mellifera* | many | Australia | field (semi-natural, experiment plots) | visitation rates, abundance, presence/absence, foraging behavior (foraging time, number of probes per flower head, etc.) | none | abundance, seed set | - |
| Hanna et al. 2013 | A. mellifera* | many | Hawaii | field (natural, experiment plots) | visitation rates, presence/absence | none | fruit set | + |
| Hingston 2005 | B. terrestris* | none | Australia | field (garden) | visitation rates, foraging behavior (floral preferences) | none | none | 0 |
| Hermansen et al. 2014 | A. mellifera* | many | Australia | field (natural) | visitation rates, foraging behavior (pollen load diversity, pollen removal & deposition) | none | none | + |
| Horskins & Turner 1999 | A. mellifera* | many | Australia | field (natural) | foraging behavior (temporal foraging patterns, stigma contact, nectar vs. pollen collection, pollen load diversity) | none | none | + |
| Junker et al. 2010 | A. mellifera* | Hylaeus spp. | Hawaii | field (natural) | presence/absence, visitation rates, foraging behavior (foraging trip duration, stigma contacts, resource collection) | pollinator effectiveness | fruit set | + |
| Kaiser-Bunbury & Müller 2009 | A. mellifera* | many | Mauritius | field (experiment plots) | visitation rates | none | fruit set, seed set, fruit size & weight | + |
| Kaiser-Bunbury et al. 2011 | A. mellifera* | many | Seychelles | field (natural) | visitation rates | none | plant reproductive success, fruit set | -/0 |
| Kenta et al. 2007 | Bombus terrestris* | Bombus spp. | Asia (Japan) | greenhouse | presence/absence | rate of legitimate floral visits | fruit set, fruit quality | - |
| Liu et al. 2013 | A. mellifera* | many | Asia (China) | field (natural, experiment plots) | visitation rates | pollen transfer & deposition | fruit & seed set | - |
| Liu et al. 2006 | A. mellifera* | many | North America (USA) | field (natural) | visitation rates | none | fruit set | - |
| Lomov et al. 2010 | A. mellifera* | many | Australia | field (natural) | presence/absence, visitation rates, foraging behavior (contact with stigma & anthers) | pollen count per stigma, presence/absence of germinated pollen | fruit & seed set | 0 |
| Madjidian et al. 2008 | Bombus ruderatus* | Bombus dahlbomii | South America (Argentina) | field (natural) | visitation rates, foraging behavior (time spent per flower, pollen deposition) | pollinator effectiveness (efficiency per visit*visitation frequency) | seed set | + |
| McGregor et al. 1959 | A. mellifera* | many | North America (USA) | field (natural) | visitation rates, foraging behavior | none | none | +/0 |
| Miller et al. 2015 | A. mellifera* | Hylaeus spp. | Hawaii | field (natural/ semi-natural) | visitation rates, foraging behavior (pollen quantity, type & diversity on body) | none | none | - |
| Montalva et al. 2011 | B. terrestris*, B. ruderatus* | B. dahlbomii, Bombus funebris | South America (Chile) | field (natural) | distribution, foraging behavior (floral association) | none | distribution | - |
| Morandin & Kremen 2013 | A. mellifera* | many | North America (USA) | field (natural) | abundance, foraging behavior (floral preference) | none | none | +/0 |
| Ott et al. 2016 | A. mellifera* | Bombus vosnesenskii, Xylocopa spp. | North America (USA) | field (natural) | visitation rates, foraging behavior (handling time, contact with pollen/stigma, nectar intake), body size | none | none | 0 |
| Richardson et al. 2016 | A. mellifera* | many | North America (USA) | field (natural) | visitation rates, foraging behavior (number of floral visits per plant, plant preferences) | none | numbers of seed capsules, intact seeds, & total seeds | + |
| Sanguinetti & Singer 2014 | A. mellifera*, B.terrestris*, B. ruderatus* | many | South America (Argentina) | field (natural) | visitation rates, pollinator behavior (time per flower, number of flowers visited) | none | fruit set | + |
| Simpson et al. 2005 | A. mellifera* | many | Australia | field (natural) | presence/absence, visitation rates, foraging behavior (flower tripping) | pollinator efficacy (fruit set per single visit) | seed set, fruit set | - |
| Stout et al. 2002 | A. mellifera*, B. terrestris* | many | Tasmania | field (natural) | visitation rates | none | seed set, number of ovules fertilized per flower | - |
| Sun et al. 2013a | A. mellifera*, B. terrestris* | many | Asia (China) | field (natural) | visitation rates, foraging behavior (resource collection, number of flower visits per foraging bout, pollen removal & deposition) | pollination efficacy (combinations of all bee variables) | fruit & seed set | + |
| Sun et al. 2013b | A. mellifera* | many | Asia (China) | field (natural, experiment plots) | presence/absence, visitation rate, foraging behavior (number of capitula visited per plant, pollen load diversity) | none | seed set | +/0 |
| Taylor & Whelan 1988 | A. mellifera* | many | Australia | field (natural) | visitation rate, foraging behavior (nectar vs. pollen collection, pollen deposition, pollen type & diversity) | none | none | - |
| Woods et al. 2012 | A. mellifera* | many | North America (USA) | field (natural) | visitation rate, foraging behavior | none | none | - |
| Xia et al. 2007 | A. mellifera*, Apis cerana | Bombus richardsi, Bombus. atrocinctus | Asia (China) | field (natural) | presence/absence, abundance, visitation rate, foraging behaviors (intra- & inter- plant movement) | none | outcrossing rates, fruit & seed set | + |
* Indicates managed bee species that were used outside of their native range
Table 3. Studies published from 1900–2016 examining the potential transmission of pathogens from managed to wild bees.
For all studies, we recorded the species of managed and wild bees, and indicated whether managed bees were native or exotic to the study region, the location (continent and country) and context of the study including field (natural, semi-natural, developed, agricultural, or experimental plot), lab, or greenhouse, and all variables measured, including the managed bee metric (independent variable), wild bee metric (dependent variable), and any explanatory or mechanistic variables. The overall effect of managed bees on wild bees via pathogens, as reported by the study, is also recorded and noted as positive (+), neutral (0), negative (-), or mixed.
| Reference | Managed bee species (* indicates non-native) | Wild bee species | Location | Context | Managed bee metric (independent variable) | Explanatory mechanism variable | Wild bee metric (response variable) | Reported effect |
|---|---|---|---|---|---|---|---|---|
| Arbetman et al. 2013 | Bombus ruderatus*, Bombus terrestris* | Bombus dahlbomii | South America (Argentina) | field (natural) | presence/absence | none (transmission implied) | parasite presence/absence | - |
| Cameron et al. 2016 | Bombus spp. | Bombus spp. | North America (USA) | lab | before/after pathogen introduction from commercial colonies | none (transmission implied) | pathogen prevalence, pathogen genetic variation | - |
| Colla et al. 2006 | Bombus impatiens | Bombus spp. | North America (Canada) | field (semi-natural) | distance to commercial greenhouses | none (transmission implied) | pathogen prevalence | - |
| Dolezal et al. 2016 | Apis mellifera* | many | North America (USA) | field (natural, agriculture), lab | pathogen prevalence, viral load | none | pathogen prevalence, pathogen load, lethality to bees | -/0 |
| Forsgren et al. 2015 | A. mellifera* | Apis cerana | Asia (Vietnam & China) | field | pathogen prevalence | none | pathogen prevalence | 0 |
| Fürst et al. 2014 | A. mellifera | Bombus spp. | Europe (UK) | field, lab | pathogen prevalence | transmission | pathogen susceptibility/ infectivity, pathogen prevalence | - |
| Genersch et al. 2006 | A. mellifera | B. terrestris, Bombus pascuorum | Europe (Germany) | field | presence | none (transmission implied) | pathogen occurrence | - |
| Gilliam et al. 1994 | A. mellifera*1 | Xylocopa californica arizonensis | North America (USA) | field (natural) | none | none (transmission implied) | pathogen occurrence | - |
| Graystock et al. 2013 | A. mellifera 1 | Bombus spp. | Europe (UK) | field, lab | none | none (transmission implied) | pathogen prevalence & infectivity | - |
| Graystock et al. 2014 | Bombus spp., A. mellifera | Bombus spp. | Europe (UK) | field (agricultural) | presence/absence, distance from apiary | none | pathogen/parasite prevalence & richness | - |
| Hoffmann et al. 2008 | A. mellifera* | B. impatiens 2 | North America (USA) | greenhouse, lab | parasite host preference & host shifting | none | parasite host preference & host shifting, bee defense behavior | - |
| Koch & Strange 2012 | Bombus spp. 1 | Bombus occidentalis, Bombus moderatus | North America (USA) | field (natural) | none | none (transmission implied) | bee distribution & relative abundance, pathogen prevalence | 0 |
| Kojima et al. 2011 | A. mellifera* | A. cerana | Asia (Japan) | field | infection frequency | none (implied transmission) | infection frequency | -/0 |
| Levitt et al. 2013 | A. mellifera* | many | North America (USA) | field (natural) | pathogen presence | none (implied transmission) | pathogen presence | - |
| Li et al. 2011 | A. mellifera*1 | Bombus huntii | North America (USA) | field, lab | none | none | pathogen infectivity | - |
| Maharramov et al. 2013 | B. terrestris*, B. ruderatus*, A. mellifera* | B. dahlbomii | South America (Argentina) | field (natural) | genetic description of parasite | none (implied transmission) | genetic description of parasite | - |
| McMahon et al. 2015 | A. mellifera | Bombus spp. | Europe (UK) | field | abundance (estimated), pathogen prevalence, pathogen load | none | pathogen prevalence, pathogen load | - |
| Murray et al. 2013 | B. terrestris | Bombus spp. | Europe (Ireland) | field (agricultural) | pathogen prevalence | foraging behavior | pathogen prevalence | - |
| Niwa et al. 2004 | B. terrestris* | Bombus hypocrita, Bombus diversus | Asia (Japan) | lab | pathogen prevalence | none | pathogen infectivity | - |
| Otterstater et al. 2008 | B. impatiens | Bombus spp. | North America (Canada) | field (agricultural), lab | presence/absence, distance from greenhouse | none (implied transmission) | pathogen prevalence | - |
| Peng et al. 2011 | A. mellifera*1 | B. huntii | North America (USA) | field, lab | none | none | pathogen infectivity | - |
| Plischuk & Lange 2009 | Bombus terrestris* | Bombus atratus, Bombus morio, Bombus bellicosus, Bombus opifex, Bombus. tucumanus | South America (Argentina) | field (natural) | pathogen prevalence | none (implied transmission risk) | pathogen prevalence | 0 |
| Plischuk et al. 2009 | A. mellifera*1 | B. atratus, B. morio, B. bellicosus | South America (Argentina) | field (natural) | none | none (implied transmission risk) | pathogen presence | -/0 |
| Ravoet et al. 2014 | A. mellifera | Osmia spp., Andrena spp., Heriades truncorum | Europe (Belgium) | field (developed) | pathogen presence | none | pathogen presence | - |
| Singh et al. 2010 | A. mellifera* | many | North America (USA) | field (natural, agricultural), lab | pathogen presence | transmission | pathogen presence | - |
| Szabo et al. 2012 | B. terrestris* | Bombus affinis, Bombus terricola, Bombus pensylvanic-us | North America | field (natural) | density of vegetable greenhouses | none (implied transmission) | bee geographic range (historic/current) | -/0 |
| Whitehorn et al. 2013 | B. terrestris, B. terrestris audax | B. pascuorum, Bombus pratorum, Bombus lapidarius | Europe (UK) | field (agricultural) | presence/absence | none (implied transmission) | pathogen prevalence & abundance | 0 |
1 No measurement of managed bees taken; pathogen examined known to be specific to a managed bee species
2 Commercial bumble bee colonies were used as indicators for conspecific wild bumble bees
* Indicates managed bee species that were used outside of their native range
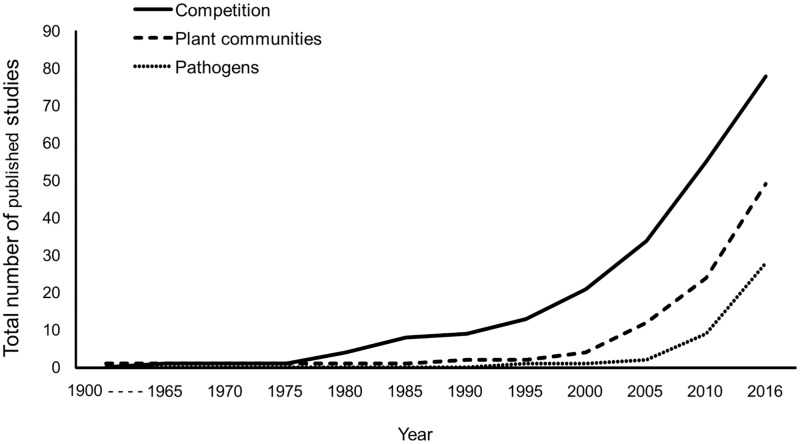
Most competition studies were done in North America (n = 19) and Europe (n = 17), followed by South America (n = 14) and Asia (n = 12), with fewer done in Australia (n = 9), Africa (n = 4) or on smaller islands (n = 3) (Table 1). In contrast, of studies done on plant communities, the majority were conducted in Australia (n = 11) and North America (n = 10), followed by islands (n = 9), South America (n = 8), and Asia (n = 7), with few conducted in Europe (n = 2) and none in Africa (Table 2). Studies on pathogens were done primarily in North America (n = 12) and Europe (n = 8), with few in South America (n = 4) and Asia (n = 3), and none in Africa, Australia, or on smaller islands (Table 3). The vast majority of competition and plant studies were conducted in the field, specifically in natural/semi-natural habitats (69% and 85%, respectively, Tables 1 and 2). Pathogen studies were more variable, with many conducted in managed habitats including agricultural systems, or across multiple habitat types, or within the lab (Table 3). Studies on competition were published earlier and with greater frequency as compared to the other topical areas; competition studies began to be published at increasing rates around 1975, while studies on plant communities increased around 2000, and studies on pathogens were not published in notable numbers until 2005 (Fig 3).
Fig 3. Publication trends.
The total number of published studies over time from 1900–2016 that examined the effects of managed bees on wild bees via three reviewed mechanisms: competition for resources, changes in plant communities (specifically native and exotic plant populations), and transmission of pathogens. While the literature search began in 1900, the first publication within these topical areas did not occur until 1964.
Competition
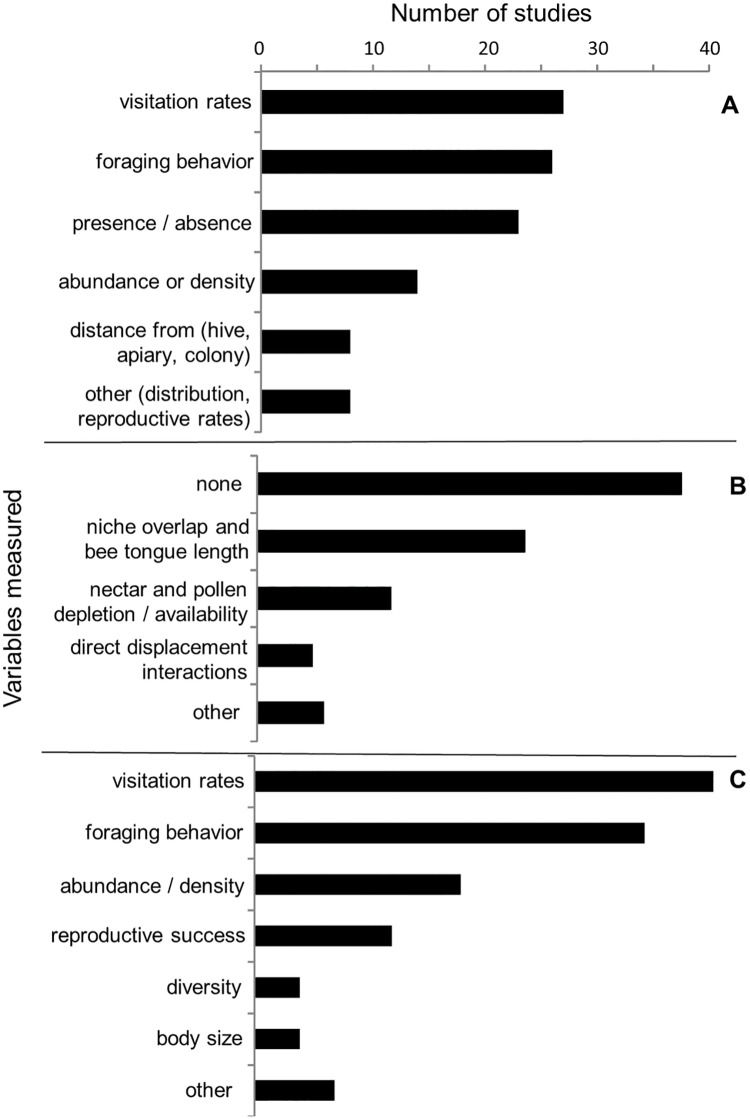
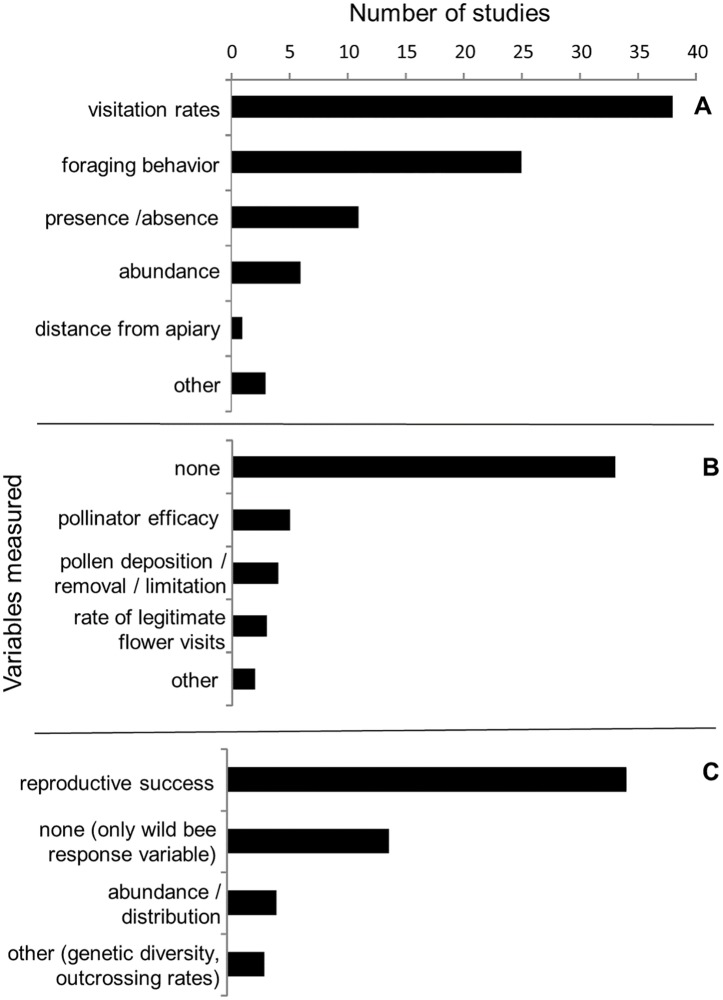
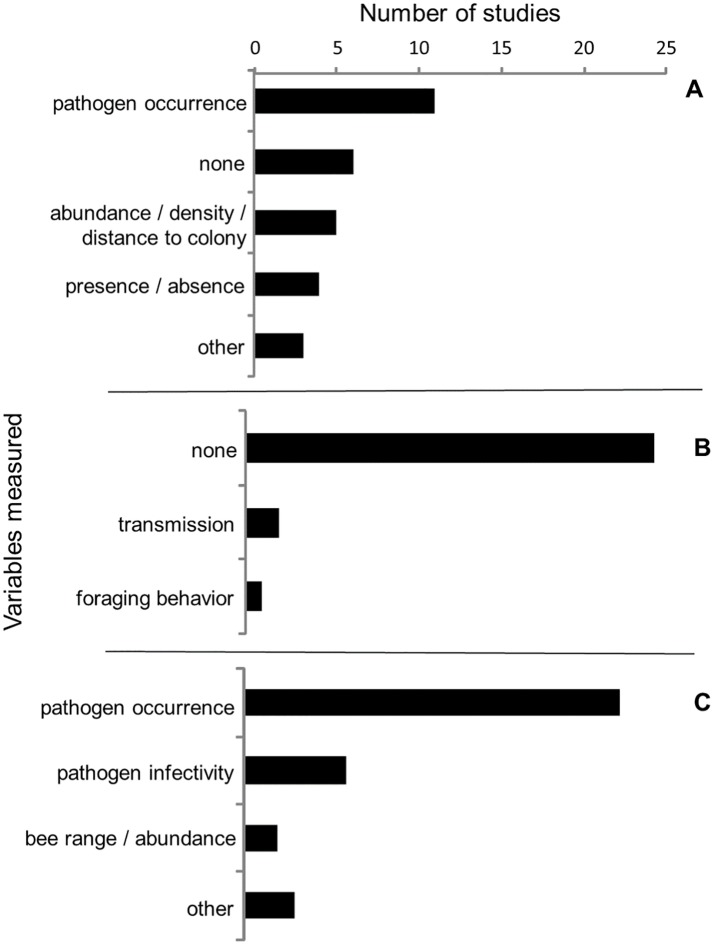
Of the studies that examined competition between managed bees and wild bees, the most commonly measured independent variables associated with managed bees were visitation rates (n = 27) and various aspects of foraging behaviors such as handling time, pollen vs. nectar collection, or nectar robbing (n = 26), followed by presence/absence (n = 23), and abundance or density (n = 14). Fewer studies analyzed competition as a function of the distance from managed bee colonies (n = 8). The most commonly examined wild bee responses to managed bees were visitation rates to flowers (n = 40) and other aspects of bee foraging behaviors (n = 34), with fewer studies examining bee abundance or density (n = 18), bee reproductive success (n = 12), or bee diversity (n = 4) as a function of managed bees. The majority of studies (n = 38) did not measure explanatory variables, or potential mechanisms for the observed results, though some looked at the degree of niche overlap between managed and wild bees (n = 24), depletion or availability of nectar and pollen (n = 12), or direct displacement interactions between managed and wild bees (n = 5) (Fig 4A–4C).
Fig 4. Reviewed effects of managed bees on wild bees through competition for shared resources.
Variables reported by studies examining the competitive effects of managed bees on wild bees including (A) managed bee metrics (independent variables), (B) potential mechanisms (explanatory variables), and (C) wild bee responses (dependent variables).
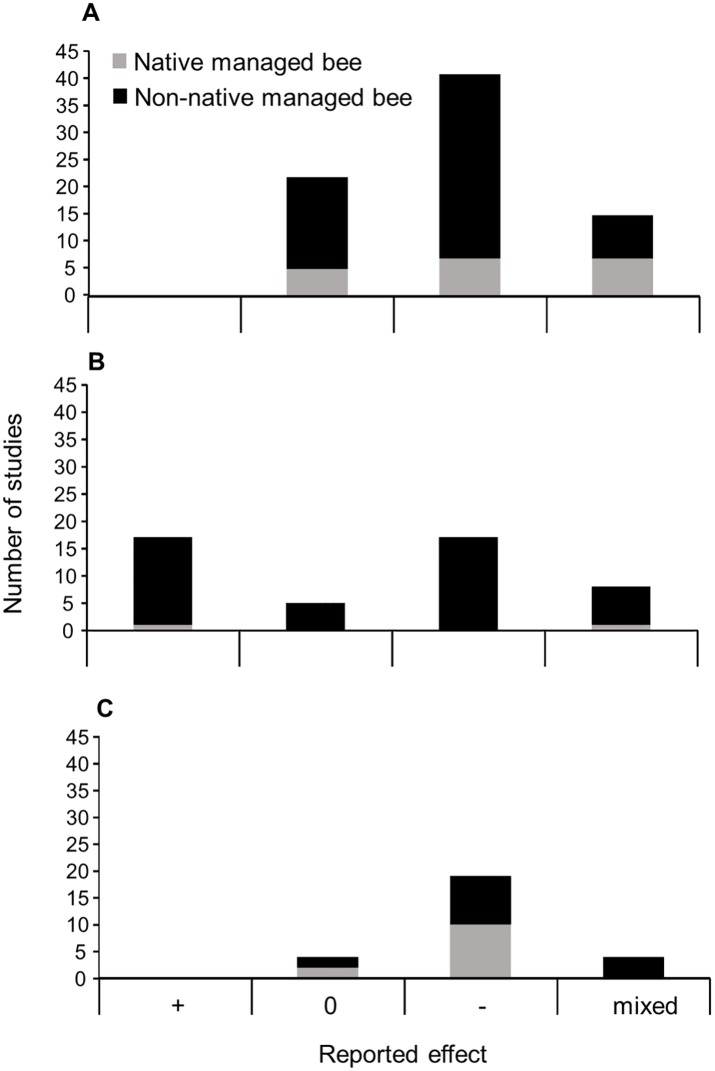
Fifty-three percent of studies reported a negative effect of managed bees on wild bees via competition for shared resources while 28% reported no effect and 19% reported mixed effects (Fig 5A). Though no studies reported entirely positive effects, some positive effects were included in studies reporting mixed effects (Table 1). Negative effects were more common with managed bees outside of their native range (58% of studies) as compared to managed bees within their native range (37%), indicating that the use of managed bees outside of their native range is more likely to have negative competitive effects on wild bees (Fig 5A).
Fig 5. Reported results from reviewed studies on the effects of managed bees on wild bees.
The total number of reviewed studies that found positive (+), neutral (0), negative (-), or mixed effects of managed bees on wild bees through (A) competition for shared resources, (B) changes in plant communities, and (C) transmission of pathogens. Studies within each category are divided into those that examined managed bees within their native range, and those that studied managed bees within their introduced range.
Plant communities
Among studies examining the potential effects of managed bees on wild bees through changes in plant communities, floral visitation rates were the most commonly measured independent variable associated with managed bees (n = 38) followed by other aspects of bee foraging behaviors (n = 25). Few studies examined plant responses as a function of managed bee presence/absence (n = 11), abundance (n = 6), or distance to managed bee colonies (n = 1). The majority of studies (n = 32) measured individual-level reproductive success of native or exotic plants as the response variable, such as fruit or seed set, while few studies (n = 4) examined population-level responses such as plant abundance or distribution. Most studies did not measure an explanatory or mechanistic variable, though a few studies measured pollen deposition or removal from managed bee visits (n = 4), or calculated pollinator efficacy (n = 5), a metric combining bee visitation rates, various aspects of bee foraging behavior, and/or plant reproductive success per pollinator visit (Fig 6A–6C).
Fig 6. Reviewed effects of managed bees on wild bees through changes in plant communities.
Variables reported by studies examining the effects of managed bees on plant communities including (A) managed bee metric (independent variable), (B) potential mechanism (explanatory variable), and (C) plant responses (dependent variable).
An equal number of studies reported positive (36%) and negative (36%) effects of managed bees on native plant communities, with the remainder reporting mixed effects (17%) or no effects (11%) (Fig 5B). The vast majority of studies examined managed bees outside of their native range; only two studies examined managed bees within their native range, and these studies found positive or mixed effects of managed bees on native plant communities (Fig 5B).
Pathogens
Among studies examining the effect of managed bees on wild bees via transmission of pathogens, the occurrence of pathogens within managed bee populations was the most commonly measured independent variable, including the presence/absence of pathogens, frequency of pathogen detection within a population, and pathogen load or diversity per individual (n = 11). Fewer studies examined the effects of managed bees as a function of their abundance or density (n = 5) or presence/absence (n = 4). Furthermore, many studies did not measure any independent variable associated with managed bees (n = 6). That is, managed bees were assumed to occur in the study area or assumed to have a certain pathogen previously documented in other studies. The most commonly measured response variable was pathogen occurrence in wild bees (n = 22), followed by pathogen infectivity within wild bees (i.e. the ability of a pathogen to establish an infection) (n = 6), and with few studies measuring wild bee population-level responses such as wild bee abundance or geographic range (n = 2). The majority of studies (n = 24) did not measure potential mechanisms to explain their study findings and few (n = 2) documented transmission of pathogens from managed bees to wild bees (Fig 7A–7C).
Fig 7. Reviewed effects of managed bees on wild bees through transmission of pathogens.
Variables reported by studies examining the effects of managed bees on wild bees through pathogens including (A) managed bee metric (independent variable), (B) potential mechanisms (explanatory variable), and (C) wild bee responses (dependent variable).
The majority of studies (70%) reported negative effects of managed bees on wild bees via pathogen transmission, with 15% reporting no effects and an additional 15% reporting mixed effects. As compared to the other topical areas, studies on pathogen transmission more frequently examined managed bees within their native ranges. Of studies done with managed bees in their native ranges, a greater proportion found negative effects (83%) as compared to studies done with managed bees outside of their native ranges (60%), indicating that pathogen transmission and subsequent negative effects on wild bees may be as or more likely with managed bees used in their native ranges (Fig 5C).
Discussion
In recent years, concern that managed bees have negative effects on wild bees has grown [3, 11, 12, 50], however no recent study has synthesized the research that examines these potential impacts. We found that across three mechanisms by which managed bees can affect wild bees (competition, changes in plant community composition, and pathogen transmission), the majority of studies concluded that managed bees have the potential to negatively affect wild bees. These conclusions may support the use of the precautionary principle when employing managed bees, particularly in or near areas with species of conservation concern. However, most of these studies did not measure wild bee fitness, population, or community-level responses including reproductive rates, survival, abundance, or diversity, making it difficult to draw long-term or broad-scale conclusions about the effects of managed bees. Furthermore, some studies found positive effects of managed bees, particularly on native plant communities, indicating that in some contexts, managed bees may aid in restoration or conservation efforts. These findings suggest that even after several decades of research on these topics, there remains some uncertainty as to the magnitude of the effects that managed bees have on wild bees.
Our review reaches some of the same conclusions as previous reviews on this topic, particularly with regards to competition, though our conclusions differ on other points due to both the expansion of the literature in recent years as well as our systematic approach to reviewing studies. Like the previous reviews [11, 12], we conclude that there is evidence for the presence of competition between managed bees and wild bees, though there is little evidence that this competition can lead to wild bee population declines. For instance, the majority of competition studies examined how managed bees affect wild bee foraging behaviors, in particular visitation rates to different flowers. How changes in wild bee foraging behaviors translate to variation in wild bee abundance or diversity was rarely studied. Since many bees are generalist flexible foragers and can partition resources in the presence of other bee species [23–24, 51], changes in foraging behaviors may not necessarily have population-level effects. In order to fully assess the effects of competition on wild bee populations, more studies that include measures of wild bee reproductive success or abundance as a function of managed bees are needed. While it may be more challenging to document long-term or direct effects of competition on wild bees, relatively recent studies provide good examples of how wild bee fitness or population-level responses can be evaluated [52–57].
Furthermore, the degree of competition and the subsequent effects on wild bee populations is likely to vary with the density of managed bees [58], which was not manipulated or observed in most studies (but see [52, 57, 59–62]). Studies that examined competition as a function of inferred managed bee density (e.g. variable distances from managed bee nests), found that competitive effects were strongest close to managed bee colonies, generally under 800 m, with reduced or no effects at increasing distances up to 1200 m suggesting that the impact of managed bees may be relatively local (< 1 km from the managed bee source) [52, 57, 60–61]. Additionally, the degree of competition may depend on overall resource availability, having significant effects on wild bees in contexts where resources are scarce, such as homogeneous landscapes, but insignificant effects during periods of high resource availability or in heterogeneous landscapes [57, 63, but see 76]. Therefore, while there is evidence that managed bees compete with wild bees for shared resources, in contexts with abundant resources, both managed and wild bee populations may be able to coexist.
While a previous review [11] concluded that the effects of managed bees on native plant communities were generally negative, we found an equal number of studies showing managed bees to be important pollinators of native plants as those that showed them to pollinate exotic invasive plants. However, as in the studies on competition, most plant community studies showed potential effects, both positive and negative, but did not show direct or long-term effects of managed bees on plant community composition. For example, some studies compared managed bee and wild bee foraging behaviors, in particular their preferences for native vs. exotic plants, but did not measure the effects of such preferences on plant reproduction, abundance, or diversity. Even among studies that measured plant reproductive output as a function of managed bees, individual-level responses such as fruit and seed set were not followed to population-level responses such as plant abundance or geographic range expansion (e.g., [29–30, 64–67], but see [68]). Furthermore, while it was generally outside the scope of these studies, the consequences of such changes in plant community composition for wild bees has not been well examined, and will likely vary across plant communities and bee species, especially between generalists and specialists [1, 69]. Thus, based on the literature we reviewed, the overall effects of managed bees on wild bees via changes in plant communities remains speculative.
Since the publication of previous reviews, research on pathogen transmission from managed bees to wild bees has increased rapidly, and with it, a greater focus on managed bumble bees in addition to managed honey bees. The conclusions reached by these studies primarily indicate negative effects of managed bees. However, these studies have similar limitations to those on the other topics, including that they do not show direct, long-term, or population and community-level effects of managed bees on wild bees. In particular, most studies documented the presence of shared pathogens in populations of managed and wild bees, but did not measure the effects of such pathogens on wild bees. Of the few studies that measured pathogen disease symptoms, infectivity, survival or fitness within wild bees, results varied across pathogens and were furthermore specific to controlled laboratory conditions [41, 70, 71]. Additional studies showed correlations between pathogen presence and wild bee species decline, however, in these cases, the origin of the pathogen is unclear and may not have come from managed bees [37, 72–73]. Furthermore, few studies documented transmission directionality making it unclear whether pathogens spilled over from managed bees to wild bees or the reverse. Thus, to demonstrate with more certainty the negative effects of pathogen transmission from managed bees to wild bees, future research should include experimental manipulative approaches to confirm transmission, and measure wild bee health, survival, or overall fitness with pathogens from managed bees. Nevertheless, the literature to date suggests that managed bees can transmit pathogens to wild bees [41], and that these pathogens may be contributing to wild bee population declines [50].
While our review found a substantial amount of research on the interactions between managed bees and wild bees, the relative effects of managed bees compared to factors such as habitat loss or pesticide exposure on wild bee populations are unknown and potentially confounding [12]. For example, it is difficult to examine the effects of managed bees in cropping systems independent of other aspects of agricultural management such as the use of pesticides or reduced plant diversity. Studies that control for these additional factors and compare wild bee responses in the presence/absence of managed bees, such as before-after-control-impact (BACI) analyses, or with varying densities of managed bees, are needed (e.g., [74–76]). Additionally, meta-analyses that compare the relative effects of different disturbances on wild bees would shed important insight on the role of managed bees in wild bee population declines. Currently, most meta-analyses have included factors related to habitat loss, habitat management, and fragmentation, but have not included the impact of managed bees [9–10, 77]. Understanding the relative magnitude of various disturbance factors is crucial for informing wild bee conservation priorities and the use of managed bees across both agricultural and natural habitats.
Finally, our review provides important insights on the relative risks of managed bees within and outside of their native ranges. While competition studies showed that managed bees outside of their native ranges are more likely to have negative effects on wild bees, studies on pathogen transmission suggest the opposite, with managed bees having greater negative effects on wild bees within their native ranges. Managed bees outside of their native ranges may have a competitive advantage over native wild bees due to reduced pressure from natural enemies [78–80]. Alternatively, managed bees within their native ranges may be more likely to transmit natural enemies to closely-related native wild species due to similarities in their foraging behaviors that could enhance transmission via flowers or direct contact [38, 40]. Additionally, wild populations may be more susceptible to pathogens or parasites transmitted by closely-related managed bees used within their native ranges in contrast to pathogens transmitted by distantly-related, exotic managed bees [45, 81–82]. Therefore, managed bees used both within and outside of their native ranges have the potential to affect wild bees, but the mechanisms responsible for such effects (i.e. competition versus pathogen transmission) may differ.
Conclusions
Our review found that the majority of studies reach the conclusion that managed bees negatively affect, or have the potential to negatively affect, wild bees through competition, changes in plant communities, or transmission of pathogens. However, there was significant variability in study results, particularly in the areas of competition and plant communities, with some studies finding no or even positive effects of managed bees. We also found that many studies to date do not show direct or causal relationships between managed bees and wild bees. That is, studies lack controls or experimental manipulations, or do not measure critical parameters such as wild bee fitness, population-level, or community-level responses to managed bees. While such studies can be logistically challenging, thereby limiting their number, recent studies provide examples of novel approaches, large-scale experiments, and/or the use of long-term data in order to better understand the effects of managed bees [41, 54, 58, 63, 74–76, 82–87]. The conclusions of these recent, more comprehensive studies largely mirror the conclusions of the literature as a whole: competition studies were highly variable (55% reporting negative effects, 33% no effects, and 11% mixed effects), studies on pathogens provide strong evidence for the transmission of pathogens between managed and wild bees, but the effects of these pathogens on wild bee health and fitness are variable and/or unknown, and the effects of managed bees on native plant populations can be positive in some contexts.
Managed bees provide benefits to humans, including crop pollination, and these benefits may outweigh the risks to native ecosystems in some cases. In order to limit the impact of managed bees, public land managers should consider site-specific attributes such as the species of managed bee and whether it is native to the region, the proposed densities of managed bees, relative resource availability (i.e. landscape diversity), whether managed bee colonies have been evaluated for pathogens and parasites, and whether there are declining wild bee species of conservation concern in the region before allowing managed bees on public lands. Commercial bee producers, including rearing centers, can furthermore limit the impact of managed bees by frequent screening for and treatment of pathogens. Industry guidelines that regulate the movement of managed bees across large regions will reduce the potential for pathogen introduction and spread. Finally, growers that use managed bees in greenhouse contexts could limit negative effects by ensuring that managed bees cannot escape to the wild, and growers that use managed bees in field settings may be able to reduce their impact by placing colonies in the center of agricultural fields or at maximum distances from natural habitats.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We thank Brian Spiesman, Dan Cariveau, Bryan Helm, Raphael Royaute, and three anonymous reviewers for feedback on early drafts. Additionally, we thank Savannah Bartel for help compiling and reviewing studies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The University of Wisconsin Vilas Associates Award and University of Wisconsin Hatch Funds (WIS201516) awarded to Claudio Gratton, and the United States Department of Agriculture Specialty Crop Block Grant (SCBG 15-02) awarded to Claudio Gratton and Hannah Gaines, provided funds to cover publication costs. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006; 313: 351–354. doi: 10.1126/science.1127863 [DOI] [PubMed] [Google Scholar]
- 2.Colla SR, Packer L. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodiversity & Conservation. 2008; 17: 1379–1391. doi: 10.1007/s10531-008-9340-5 [Google Scholar]
- 3.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: trends, impacts and drivers. Trends in Ecology & Evolution. 2010; 25: 345–353. doi: 10.1016/j.tree.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 4.Koh I, Lonsdorf EV, Williams NM, Brittain C, Isaacs R, Gibbs J, et al. Modeling the status, trends, and impacts of wild bee abundance in the United States. Proceedings of the National Academy of Sciences USA. 2015. doi: 10.1073/pnas.1517685113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey LM, Rebelo H, Rotheray E, Goulson D. Evidence for habitat and climatic specializations driving the long-term distribution trends of UK and Irish bumblebees. Diversity & Distributions. 2015; 21: 864–875. doi: 10.1111/ddi.12344 [Google Scholar]
- 6.Michener CD. The Bees of the World. Baltimore: JHU Press; 2000. [Google Scholar]
- 7.O’Toole C, Raw A. Bees of the World. London: Blandford Press; 1991. [Google Scholar]
- 8.Delaplane KS, Mayer DF. Crop Pollination by Bees. Wallingford, Oxfordshire: CABI; 2000. [Google Scholar]
- 9.Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology. 2009; 90: 2068–2076. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy CM, Lonsdorf E, Neel MC, Williams NM, Ricketts TH, Winfree R, et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecology Letters. 2013; 16: 584–599. doi: 10.1111/ele.12082 [DOI] [PubMed] [Google Scholar]
- 11.Goulson D. Effects of introduced bees on native ecosystems. Annual Review of Ecology, Evolution, and Systematics. 2003; 1–26. [Google Scholar]
- 12.Paini DR. Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees: a review. Austral Ecology. 2004; 29: 399–407. doi: 10.1111/j.1442-9993.2004.01376.x [Google Scholar]
- 13.Aizen MA, Harder LD. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Current Biology. 2009; 19: 915–918. doi: 10.1016/j.cub.2009.03.071 [DOI] [PubMed] [Google Scholar]
- 14.Breeze TD, Vaissière BE, Bommarco R, Petanidou T, Seraphides N, Kozák L, et al. Agricultural policies exacerbate honeybee pollination service supply-demand mismatches across Europe. PLoS ONE. 2014; 9: e82996 doi: 10.1371/journal.pone.0082996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winfree R, Williams NM, Gaines H, Ascher JS, Kremen C. Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. Journal of Applied Ecology. 2008; 45: 793–802. [Google Scholar]
- 16.Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013; 339: 1608–1611. doi: 10.1126/science.1230200 [DOI] [PubMed] [Google Scholar]
- 17.Klein AM, Steffan-Dewenter I, Tscharntke T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proceedings of the Royal Society B-Biological Sciences. 2003; 270: 955–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoehn P, Tscharntke T, Tylianakis JM, Steffan-Dewenter I. Functional group diversity of bee pollinators increases crop yield. Proceedings of the Royal Society B-Biological Sciences. 2008; 275: 2283–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallinger RE, Gratton C. Species richness of wild bees, but not the use of managed honeybees, increases fruit set of a pollinator-dependent crop. Journal of Applied Ecology. 2015; 52: 323–330. doi: 10.1111/1365-2664.12377 [Google Scholar]
- 20.Memmott J, Waser NM, Price MV. Tolerance of pollination networks to species extinctions. Proceedings of the Royal Society of London B- Biological Sciences. 2004; 271: 2605–2611. doi: 10.1098/rspb.2004.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontaine C, Dajoz I, Meriguet J, Loreau M. Functional diversity of plant—pollinator interaction webs enhances the persistence of plant communities. PLoS Biology. 2005; 4: e1 doi: 10.1371/journal.pbio.0040001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fruend J, Dormann CF, Holzschuh A, Tscharntke T. Bee diversity effects on pollination depend on functional complementarity and niche shifts. Ecology. 2013; 94: 2042–2054. doi: 10.1890/12-1620.1 [DOI] [PubMed] [Google Scholar]
- 23.Spiesman BJ, Gratton C. Flexible foraging shapes the topology of plant—pollinator interaction networks. Ecology. 2016; 97: 1431–1441. doi: 10.1890/15-1735.1 [DOI] [PubMed] [Google Scholar]
- 24.Di Pasquale GD, Salignon M, Conte YL, Belzunces LP, Decourtye A, Kretzschmar A, et al. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE. 2013; 8: e72016 doi: 10.1371/journal.pone.0072016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roulston TH, Cane JH. Pollen nutritional content and digestibility for animals. Plant Systematics and Evolution. 2000; 222: 187–209. doi: 10.1007/BF00984102 [Google Scholar]
- 26.Zurbuchen A, Cheesman S, Klaiber J, Müller A, Hein S, Dorn S. Long foraging distances impose high costs on offspring production in solitary bees. Journal of Animal Ecology. 2010; 79: 674–681. doi: 10.1111/j.1365-2656.2010.01675.x [DOI] [PubMed] [Google Scholar]
- 27.Dafni A, Kevan P, Gross CL, Goka K. Bombus terrestris, pollinator, invasive and pest: an assessment of problems associated with its widespread introductions for commercial purposes. Applied Entomology and Zoology. 2010; 45: 101–113. doi: 10.1303/aez.2010.101 [Google Scholar]
- 28.Goodell K. Invasive exotic plant-bee interactions In: Pitts-Singer JR, Pitts-Singer T, editors. Bee Pollination in Agricultural Ecosystems. Oxford University Press; 2008. pp. 166–183. [Google Scholar]
- 29.Abe T, Wada K, Kato Y, Makino S, Okochi I. Alien pollinator promotes invasive mutualism in an insular pollination system. Biological Invasions. 2011; 13: 957–967. doi: 10.1007/s10530-010-9882-9 [Google Scholar]
- 30.Barthell JF, Randall JM, Thorp RW, Wenner AM. Promotion of seed set in yellow star-thistle by honey bees: evidence of an invasive mutualism. Ecological Applications. 2001; 11: 1870–1883. doi: 10.2307/3061102 [Google Scholar]
- 31.Aizen MA, Morales CL, Morales JM. Invasive mutualists erode native pollination webs. PLoS Biology. 2008; 6: e31 doi: 10.1371/journal.pbio.0060031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kearns CA, Inouye DW, Waser NM. Endangered mutualisms: the conservation of plant-pollinator interactions. Annual Review of Ecology and Systematics. 1998; 29: 83–112. [Google Scholar]
- 33.Memmott J. The structure of a plant-pollinator food web. Ecology Letters. 1999; 2: 276–280. doi: 10.1046/j.1461-0248.1999.00087.x [DOI] [PubMed] [Google Scholar]
- 34.Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996; 77: 1043–1060. doi: 10.2307/2265575 [Google Scholar]
- 35.de Jong MCM, Diekmann O, Heesterbeek H. How does transmission of infection depend on population size? In Mollison D, editor. Epidemic Models: their Structure and Relation to Data. Cambridge: Cambridge University Press; 1995. pp. 84–94. [Google Scholar]
- 36.Chen Y, Evans J, Feldlaufer M. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. Journal of Invertebrate Pathology. 2006; 92: 152–159. doi: 10.1016/j.jip.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 37.Cameron SA, Lim HC, Lozier JD, Duennes MA, Thorp R. Test of the invasive pathogen hypothesis of bumble bee decline in North America. Proceedings of the National Academy of Sciences USA. 2016; 113: 4386–4391. doi: 10.1073/pnas.1525266113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh R, Levitt AL, Rajotte EG, Holmes EC, Ostiguy N, vanEngelsdorp D, et al. RNA viruses in Hymenopteran pollinators: evidence of inter-taxa virus transmission via pollen and potential impact on non-Apis Hymenopteran species. PLoS ONE. 2010; 5: e14357 doi: 10.1371/journal.pone.0014357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitehorn PR, Tinsley MC, Brown MJF, Goulson D. Investigating the impact of deploying commercial Bombus terrestris for crop pollination on pathogen dynamics in wild bumble bees. Journal of Apicultural Research. 2013; 52: 149–157. doi: 10.3896/IBRA.1.52.3.06 [Google Scholar]
- 40.Durrer S, Schmid Hempel P. Shared use of flowers leads to horizontal pathogen transmission. Proceedings of the Royal Society B-Biological Sciences. 1994; 258: 299–302. doi: 10.1098/rspb.1994.0176 [Google Scholar]
- 41.Fürst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature. 2014; 506: 364–366. doi: 10.1038/nature12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genersch E, Yue C, Fries I, de Miranda JR. Detection of Deformed Wing Virus, a honey bee viral pathogen, in bumble bees (Bombus terrestris and Bombus pascuorum) with wing deformities. Journal of Invertebrate Pathology. 2006; 91: 61–63. doi: 10.1016/j.jip.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 43.Kojima Y, Toki T, Morimoto T, Yoshiyama M, Kimura K, Kadowaki T. Infestation of Japanese native honey bees by tracheal mite and virus from non-native European honey bees in Japan. Microbial Ecology. 2011; 62: 895–906. doi: 10.1007/s00248-011-9947-z [DOI] [PubMed] [Google Scholar]
- 44.Levitt AL, Singh R, Cox-Foster DL, Rajotte E, Hoover K, Ostiguy N, et al. Cross-species transmission of honey bee viruses in associated arthropods. Virus Research. 2013; 176: 232–240. doi: 10.1016/j.virusres.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 45.Murray TE, Coffey MF, Kehoe E, Horgan FG. Pathogen prevalence in commercially reared bumble bees and evidence of spillover in conspecific populations. Biological Conservation. 2013; 159: 269–276. doi: 10.1016/j.biocon.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMahon DP, Fürst MA, Caspar J, Theodorou P, Brown MJF, Paxton RJ. A sting in the spit: widespread cross-infection of multiple RNA viruses across wild and managed bees. Journal of Animal Ecology. 2015; 84: 615–624. doi: 10.1111/1365-2656.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanbe Y, Okada I, Yoneda M, Goka K, Tsuchida K. Interspecific mating of the introduced bumblebee Bombus terrestris and the native Japanese bumblebee Bombus hypocrita sapporoensis results in inviable hybrids. Naturwissenschaften. 2008; 95: 1003–1008. doi: 10.1007/s00114-008-0415-7 [DOI] [PubMed] [Google Scholar]
- 48.Isaacs R, Tuell J, Fiedler A, Gardiner M, Landis D. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: the role of native plants. Frontiers in Ecology and the Environment. 2009; 7: 196–203. doi: 10.1890/080035 [Google Scholar]
- 49.Williams NM, Ward KL, Pope N, Isaacs R, Wilson J, May EA, et al. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecological Applications. 2015; 25: 2119–2131. doi: 10.1890/14-1748.1 [DOI] [PubMed] [Google Scholar]
- 50.Graystock P, Blane EJ, McFrederick QS, Goulson D, Hughes WOH. Do managed bees drive parasite spread and emergence in wild bees? International Journal for Parasitology: Parasites and Wildlife. 2016; 5: 64–75. doi: 10.1016/j.ijppaw.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson RA. Intraspecific resource partitioning in the bumble bees Bombus ternarius and B. pennsylvanicus. Ecology. 1986; 67: 133–138. doi: 10.2307/1938511 [Google Scholar]
- 52.Elbgami T, Kunin WE, Hughes WOH, Biesmeijer JC. The effect of proximity to a honeybee apiary on bumblebee colony fitness, development, and performance. Apidologie. 2014; 45; 504–513. doi: 10.1007/s13592-013-0265-y [Google Scholar]
- 53.Hudewenz A, Klein A-M. Competition between honey bees and wild bees and the role of nesting resources in a nature reserve. Journal of Insect Conservation. 2013; 17: 1275–1283. doi: 10.1007/s10841-013-9609-1 [Google Scholar]
- 54.Hudewenz A, Klein AM. Red mason bees cannot compete with honey bees for floral resources in a cage experiment. Ecology and Evolution. 2015; 5: 5049–5056. doi: 10.1002/ece3.1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paini DR, Roberts JD. Commercial honey bees (Apis mellifera) reduce the fecundity of an Australian native bee (Hylaeus alcyoneus). Biological Conservation. 2005; 123: 103–112. doi: 10.1016/j.biocon.2004.11.001 [Google Scholar]
- 56.Steffan-Dewenter I, Tscharntke T. Resource overlap and possible competition between honey bees and wild bees in central Europe. Oecologia. 2000; 122: 288–296. doi: 10.1007/s004420050034 [DOI] [PubMed] [Google Scholar]
- 57.Thomson DM. Detecting the effects of introduced species: a case study of competition between Apis and Bombus. Oikos. 2006; 114: 407–418. doi: 10.1111/j.2006.0030-1299.14604.x [Google Scholar]
- 58.Cane JH, Tepedino VJ. Gauging the effect of honey bee pollen collection on native bee communities. Conservation Letters. 2017; 10; 205–210. [Google Scholar]
- 59.Kuhn J, Hamm A, Schindler M, Wittmann D. Resource partitioning between the oligolectic leafcutter bee Megachile lapponica (Hymenoptera, Apiformes) and other visitors on flowers of Epilobium angustifolium (Onagracea). Mitteilungen der Deutschen Gesellschaft für allgemeine und angewandte Entomologie. 2006; 15; 389–392 [Google Scholar]
- 60.Neumayer J. Influence of honey-bees on nectar supply and native flower visitors. Entomologica Austriaca. 2006; 13; 7–14 [Google Scholar]
- 61.Thomson D. Competitive interactions between the invasive European honey bee and native bumble bees. Ecology. 2004; 85: 458–470. doi: 10.1890/02-0626 [Google Scholar]
- 62.Walther-Hellwig K, Fokul G, Frankl R, Buechler R, Ekschmitt K, Wolters V. Increased density of honeybee colonies affects foraging bumblebees. Apidologie. 2006; 37: 517–532. doi: 10.1051/apido:2006035 [Google Scholar]
- 63.Herbertsson L, Lindström SAM, Rundlöf M, Bommarco R, Smith HG. Competition between managed honeybees and wild bumblebees depends on landscape context. Basic and Applied Ecology. 2016. doi: 10.1016/j.baae.2016.05.001 [Google Scholar]
- 64.Cayuela L, Ruiz-Arriaga S, Ozers CP. Honeybees increase fruit set in native plant species important for wildlife conservation. Environmental Management. 2011; 48: 910–919. doi: 10.1007/s00267-011-9677-5 [DOI] [PubMed] [Google Scholar]
- 65.Gross CL, Mackay D. Honeybees reduce fitness in the pioneer shrub Melastoma affine (Melastomataceae). Biological Conservation. 1998; 86: 169–178. doi: 10.1016/S0006-3207(98)00010-X [Google Scholar]
- 66.Sun S-G, Huang S-Q, Guo Y-H. Pollinator shift to managed honeybees enhances reproductive output in a bumblebee-pollinated plant. Plant Systematics and Evolution. 2013; 299: 139–150. doi: 10.1007/s00606-012-0711-8 [Google Scholar]
- 67.Gross CL, Gorrell L, Macdonald MJ, Fatemi M. Honeybees facilitate the invasion of Phyla canescens (Verbenaceae) in Australia—no bees, no seed! Weed Research. 2010; 50: 364–372. doi: 10.1111/j.1365-3180.2010.00788.x [Google Scholar]
- 68.Goulson D, Rotheray EL. Population dynamics of the invasive weed Lupinus arboreus in Tasmania, and interactions with two non-native pollinators. Weed Research. 2012; 52: 535–541. doi: 10.1111/j.1365-3180.2012.00935.x [Google Scholar]
- 69.Schweiger O, Biesmeijer JC, Bommarco R, Hickler T, Hulme PE, Klotz S, et al. Multiple stressors on biotic interactions: how climate change and alien species interact to affect pollination. Biological Reviews. 2010; 85: 777–795. doi: 10.1111/j.1469-185X.2010.00125.x [DOI] [PubMed] [Google Scholar]
- 70.Graystock P, Yates K, Darvill B, Goulson D, Hughes WOH. Emerging dangers: deadly effects of an emergent parasite in a new pollinator host. Journal of Invertebrate Pathology. 2013; 114: 114–119. doi: 10.1016/j.jip.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 71.Graystock P, Yates K, Evison SEF, Darvill B, Goulson D, Hughes WOH. The Trojan hives: pollinator pathogens, imported and distributed in bumblebee colonies. Journal of Applied Ecology. 2013; 50: 1207–1215. doi: 10.1111/1365-2664.12134 [Google Scholar]
- 72.Koch JB, Strange JP. The status of Bombus occidentalis and B. moderatus in Alaska with special focus on Nosema bombi incidence. Northwest Science. 2012; 86: 212–220. [Google Scholar]
- 73.Szabo ND, Colla SR, Wagner DL, Gall LF, Kerr JT. Do pathogen spillover, pesticide use, or habitat loss explain recent North American bumblebee declines? Conservation Letters. 2012; 5: 232–239. doi: 10.1111/j.1755-263X.2012.00234.x [Google Scholar]
- 74.Paini DR, Williams MR, Roberts JD. No short-term impact of honey bees on the reproductive success of an Australian native bee. Apidologie. 2005; 36: 613–621. doi: 10.1051/apido:2005046 [Google Scholar]
- 75.Roubik DW, Wolda H. Do competing honey bees matter? Dynamics and abundance of native bees before and after honey bee invasion. Population Ecology. 2001; 43: 53–62. doi: 10.1007/PL00012016 [Google Scholar]
- 76.Lindstrom SAM, Herbertsson L, Rundlof M, Bommarco R, Smith HG. Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proceedings of the Royal Society B-Biological Sciences. 2016; 283 doi: 10.1098/rspb.2016.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scheper J, Holzschuh A, Kuussaari M, Potts SG, Rundlöf M, Smith HG, et al. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—a meta-analysis. Ecology Letters. 2013; 16: 912–920. doi: 10.1111/ele.12128 [DOI] [PubMed] [Google Scholar]
- 78.Connell JH. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees In: Den Boer PJ, Gradwell GR, editors. Dynamics of Populations. Wageningen, The Netherlands: Centre for Agricultural Publishing and Documentation; 1971. pp. 298–312. [Google Scholar]
- 79.Hanks LM, Denno RF. Natural enemies and plant water relations influence the distribution of an armored scale insect. Ecology. 1993; 74: 1081–1091. doi: 10.2307/1940478 [Google Scholar]
- 80.Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, et al. The population biology of invasive species. Annual Review of Ecology and Systematics. 2001; 32: 305–332. [Google Scholar]
- 81.Otterstatter MC, Thomson JD. Does pathogen spillover from commercially reared bumble bees threaten wild pollinators? PLoS ONE. 2008; 3: e2771 doi: 10.1371/journal.pone.0002771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dolezal AG, Hendrix SD, Scavo NA, Carrillo-Tripp J, Harris MA, Wheelock MJ, et al. Honey bee viruses in wild bees: viral prevalence, loads, and experimental inoculation. PLoS ONE. 2016; 11 doi: 10.1371/journal.pone.0166190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Graystock P, Goulson D, Hughes WOH. The relationship between managed bees and the prevalence of parasites in bumblebees. Peerj. 2014; 2 doi: 10.7717/peerj.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith-Ramírez C, Ramos-Jiliberto R, Valdovinos FS, Martínez P, Castillo JA, Armesto JJ. Decadal trends in the pollinator assemblage of Eucryphia cordifolia in Chilean rainforests. Oecologia. 2014; 176: 157–169. doi: 10.1007/s00442-014-3000-0 [DOI] [PubMed] [Google Scholar]
- 85.Hermansen TD, Britton DR, Ayre DJ, Minchinton TE. Identifying the real pollinators? Exotic honeybees are the dominant flower visitors and only effective pollinators of Avicennia marina in Australian temperate mangroves. Estuaries and Coasts. 2014; 37: 621–635. doi: 10.1007/s12237-013-9711-3 [Google Scholar]
- 86.Balfour NJ, Gandy S, Ratnieks FLW. Exploitative competition alters bee foraging and flower choice. Behavioral Ecology and Sociobiology. 2015; 69: 1731–1738. doi: 10.1007/s00265-015-1985-y [Google Scholar]
- 87.Thomson DM. Local bumble bee decline linked to recovery of honey bees, drought effects on floral resources. Ecology Letters. 2016; 19: 1247–1255. doi: 10.1111/ele.12659 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.