Abstract
The increasing cost of prescription drugs is a burden for patients and threatens the financial stability of the US health care system. Rebates are a form of price concession paid by a pharmaceutical manufacturer to the health plan sponsor or the pharmacy benefit manager working on the plan’s behalf. Proponents argue that rebates result from vigorous negotiations that help lower overall drug costs. Critics argue that rebates have perversely increased the costs patients pay out of pocket, as well as the costs for Medicare as a whole. This special communication discusses how the availability of rebates for drugs covered by the Medicare Part D program may raise costs for patients and Medicare while increasing the profits of Part D plan sponsors and pharmaceutical manufacturers. Two policy alternatives are herein proposed that would reconfigure cost sharing to lower patient out-of-pocket costs and reduce cost shifting to Medicare.
When criticized by the US Congress in 2016 over the $600 cost of the epinephrine autoinjector (EpiPen; Mylan), the chief executive officer of Mylan pointed to the large and growing rebates the company provides to intermediaries such as pharmacy benefit managers. Rebates and other fees, she explained, resulted in the company netting only $274 from each sale. This is why, she explained, the drug’s price can vary between $600 and less than half that amount, depending on who pays.
In the United States, pharmaceutical pricing lacks transparency, leading to a system in which there is a range of prices for the same medication.1 At the center of this system are rebates, a form of price concession paid by the pharmaceutical manufacturer to the health plan sponsor or to the pharmacy benefit manager working on the plan’s behalf. Although the terms of rebates are generally confidential, rebates are typically offered in exchange for improved market access. For example, manufacturers might offer rebates in exchange for a favorable position on a plan’s drug formulary. Proponents argue that rebates are a result of vigorous negotiations that help lower overall drug costs.2 Critics argue that rebates have perversely increased the costs patients pay out of pocket and the costs for Medicare as a whole.3
We discuss how the availability of rebates for drugs covered by Medicare Part D may increase costs for beneficiaries and Medicare but may decrease costs for pharmaceutical manufacturers and the sponsors of Part D plans.
Role of Rebates in the Medicare Part D Benefit
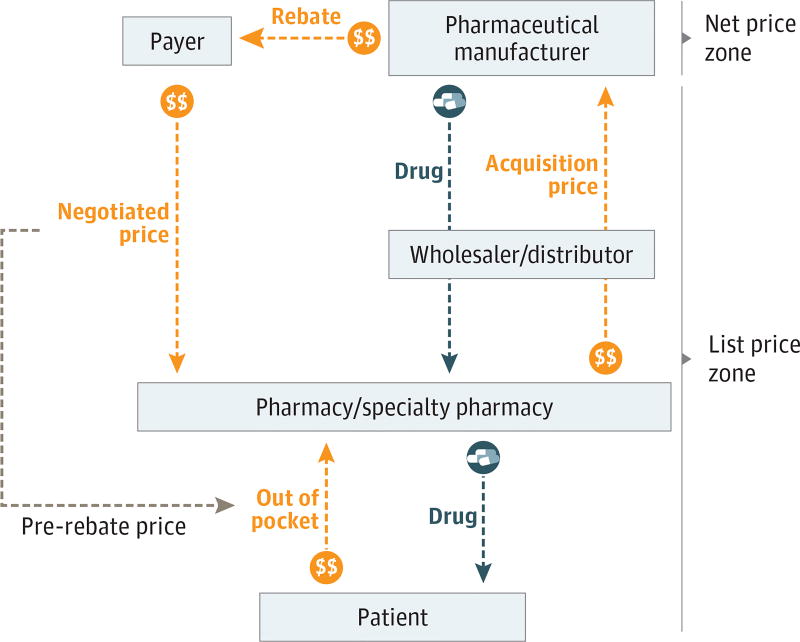
In the United States, the net price received by the drug manufacturer can differ substantially from the sale price at the pharmacy. Figure 1 provides an overview; at the top of the figure is the net price zone, where the price that the manufacturer receives is reduced when it gives a rebate to the payer. In Medicare, the payer is the Part D plan sponsor and the pharmacy benefit manager working on its behalf. The pharmacy sale occurs in the list price zone. The pharmacy pays an acquisition price when it purchases the drug and then receives a negotiated price from the payer (inclusive of payment from the patient) when it dispenses the drug. Although the negotiated price and the list price differ, they are usually reasonable approximations of each other. Regulations define the wholesale acquisition cost of a drug as the list price from a manufacturer to a wholesaler or another direct purchaser without discounts or rebates. For branded prescription medications with no direct competitors, acquisition prices for pharmacies are typically within a few percentage points of the wholesale acquisition cost.4
Figure 1. Drug Distribution and Payment System in the United States for Prescription Medications.
This schematic shows the differences between net price zone and list price zone.
Over time, the difference between net and list prices for drugs in the United States has widened, partly because of increases in list prices (higher prices at product launch and increases over time) and growing rebate percentages.5,6 For example, the average rebate percentage for drugs in the Part D program increased from 8.6% in 2006 to 14.3% in 2014 and was projected to increase further in 2015 and 2016.7 For branded drugs with competitors, rebates can be substantial, amounting to hundreds or thousands of dollars per prescription in some cases.
Impact of Rebates on Patient Out-of-Pocket Spending Under Medicare Part D
Patient out-of-pocket payments are determined by the Part D plan sponsor and are specific to the drug, benefit design, and phase.8 The standard 2017 benefit has a $400 deductible (where the patient pays 100%), and then drug costs are shared between the patient (who pays 25%) and the payer (which pays 75%) during the initial coverage phase until total drug spending reaches $3700. After this limit is reached, the Affordable Care Act mandates manufacturers discount by 50% the price of branded drugs in the coverage gap phase to ensure the patient’s payment is equivalent to 80% of the discounted price. Thus, the patient, payer, and manufacturer pay 40%, 10%, and 50%, respectively, of branded drug prices during the coverage gap phase (the “donut hole”) until total drug spending reaches $8071. In the coverage gap, the dollar value of the manufacturer’s payment (based on the list price of the brand name drug, not the discounted price in the coverage gap) counts toward patient out-of-pocket spending; the out-of-pocket spending to meet the catastrophic coverage threshold of $8071 is $4950 in 2017. (The rules are complicated in that manufacturer’s payments in the coverage gap count toward patient out-of-pocket spending, but the payments by plan sponsors or pharmacy benefit managers do not). When patients reach the catastrophic coverage threshold of $8071, the federal Medicare program bears 80% of costs, plans 15%, and patients 5%, with no upper limit on patient spending.
Although many patients pay copayments (eg, a flat fee of $10 per prescription) during these benefit phases, a growing proportion of prescriptions require patients to pay up to 100% of the drug’s price through deductibles or a fixed percentage through coinsurance.9 These percentage-based costs for drugs are based on the negotiated price (Figure 1). In Part D, the proportion of medications offered with coinsurance increased from 35% in 2014 to 58% in 2016.10 Coinsurance is particularly common for high-cost specialty medications, such as treatments for cancer, hepatitis C, and rheumatoid arthritis,10 mirroring trends in commercial plans.9
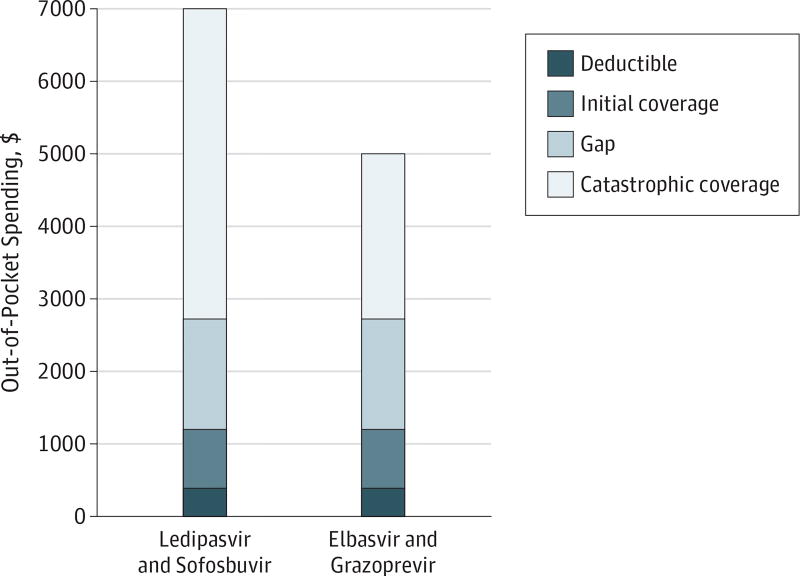
To understand how rebates can affect what patients pay for an expensive specialty drug, consider this illustration of patient out-of-pocket costs for 2 different 12-week (84 pills) courses of treatment for hepatitis C infection—ledipasvir and sofosbuvir (Harvoni; Gilead Sciences Inc) or elbasvir and grazoprevir (Zepatier; Merck Sharp & Dohme Corp). The Medicare Plan Finder is an online search tool that allows beneficiaries to estimate their out-of-pocket costs under Medicare Part D (see https://www.medicare.gov/find-a-plan/questions/home.aspx).11 Using data from the Medicare Plan Finder, we determined the patient costs for both regimens (Figure 2).12 The full cost for ledipasvir and sofosbuvir was $94 916, and the full cost for elbasvir and grazoprevir was $54 841. These list prices differ substantially, but because of rebates, the manufacturers may be receiving approximately the same net price per course of therapy.13 When Merck announced US Food and Drug Administration approval of elbasvir and grazoprevir, the company stated that it believed the list price “to be in the range of net prices for other commonly used HCV direct-acting antiviral regimens at 12 weeks of therapy.”13 Because a patient’s out-of-pocket cost is based on the negotiated price of a drug, not the net price, a patient who obtained ledipasvir and sofosbuvir in 2017 would pay $6995 out of pocket compared with $4991 for elbasvir and grazoprevir, a difference of $2004. This gap would be of the same magnitude if patients obtain their entire prescription under the catastrophic phase of the Part D benefit.
Figure 2. Estimated 2017 Patient Out-of-Pocket Spending for Either Ledipasvir and Sofosbuvir Regimen or Elbasvir and Grazoprevir Regimen.
Data derived from the Medicare Plan Finder for the AARP MedicareRX Saver Plus Part D plan using published methods.11,12 Prices were obtained in December 2016, excluded monthly premium amounts, and were applicable to patients not receiving extra help or low-income subsidy from Medicaid or Medicare (approximately 70% of patients enrolled in stand-alone Part D plans).
Impact of Rebates on Medicare Spending
Sponsors of Part D plans argue that rebates are used to support the funding of coverage for all Medicare beneficiaries: Patients who take high-cost specialty medications have out-of-pocket spending that is based on the list price of the drug, but the cost reductions from rebates are returned to all beneficiaries through lower premiums.14 According to this view, rebates would have no net effect on spending across beneficiaries and may even reduce overall spending. The current design of the Part D benefit does not guarantee this outcome, however, and incomplete information about the amounts of rebates precludes evaluations of their actual effect on premiums.
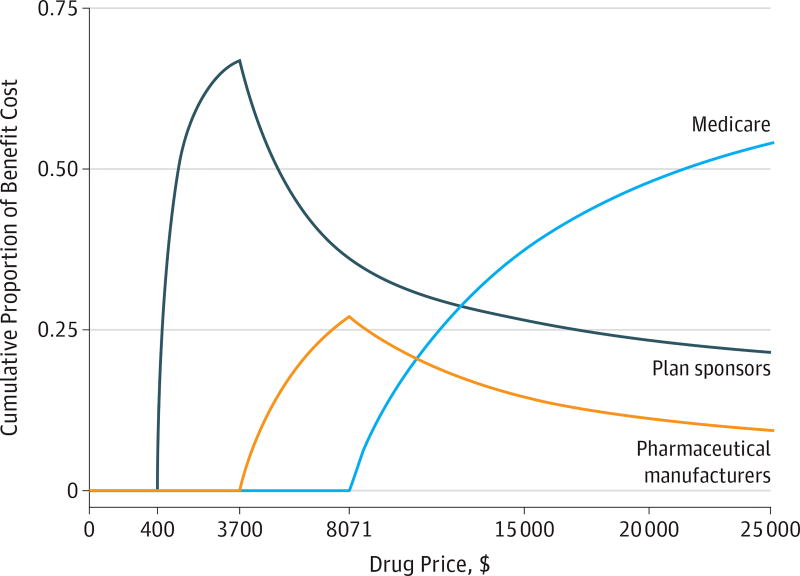
Progression through the phases of Part D coverage is determined by total drug spending and patient out-of-pocket spending, all of which are based on the list prices of drugs. Figure 3 illustrates that as a drug’s list price increases, the cumulative share of Part D spending shifts from plan sponsors and manufacturers to Medicare. Generally, plans bear most of the upfront costs, followed by pharmaceutical companies, for branded-drug prescriptions filled in the coverage gap. Once patients reach the catastrophic spending threshold, Medicare pays 80% of costs. Thus, when list prices for drugs are high, even when net prices are reduced by rebates, a patient progresses more quickly to the catastrophic phase of the Part D benefit under which Medicare bears most of the cost. Higher list prices also mean that the patient progresses more quickly through the coverage gap in which manufacturers discount the price of drugs by 50%.15
Figure 3. Cumulative Share of Drug Payments by Payer as Drug Prices Increase.
As a drug’s price increases under the 2017 Part D standard benefit, the cumulative share of Part D spending shifts from plan sponsors and pharmaceutical manufacturers to Medicare. The standard 2017 benefit has a $400 deductible (where the patient pays 100%), and then drug costs are shared between the patient (who pays 25%) and the payer (which pays 75%) during the initial coverage phase until total drug spending reaches $3700. After this limit, the patient, payer, and manufacturer pay 40%, 10%, and 50%, respectively, of branded drug prices during the coverage gap phase (the “donut hole”) until total drug spending reaches $8071. After this point, the patient enters the catastrophic phase in which Medicare bears 80% of costs, plans 15%, and patients 5%, with no upper limit on patient spending.
For specialty drugs with a net price of a few thousand dollars for a year’s prescription or more, an increase in the list price, as well as the rebate, lowers the share of the prescription that the plan is obligated to pay and that the pharmaceutical manufacturer is obligated to discount, saving money for both parties. Meanwhile, both the beneficiary’s out-of-pocket costs and Medicare spending increase. Although end-of-the-year reconciliation measures are included in the Part D program to compensate for such imbalances, they are asymmetric and incomplete. If a plan profits more than it projected when it bid for participation in Part D, the plan must return some of its excess profits to the government. Specifically, a plan keeps the first 5% of excess profit and then returns 50% of the next 5% and 80% beyond 10% of excess profit. If by the same mechanism Medicare pays more than was projected for catastrophic coverage, the federal government bears 100% of those excess costs.16
The details of rebates and net prices for individual drugs are proprietary and not publicly available. Nonetheless, trends in Medicare Part D spending suggest that the difference between list and net prices is widening. According to a 2017 report from the Office of Inspector General of the US Department of Health and Human Services, an increasing percentage of patients receiving high-priced drugs enter catastrophic coverage each year, and Medicare spending on catastrophic coverage has more than tripled, from $10.8 billion in 2010 to $33.2 billion in 2015.16 Although Part D plans have had to return some excess profits, they have simultaneously collected additional payments from Medicare for cost overruns in the catastrophic phase of coverage.16
Options for Medicare Part D Reform
This analysis of the effects of prescription drug rebates on patient and Medicare spending suggests that out-of-pocket spending and progression through the Part D benefit should no longer be linked to the list price of a drug. There are 2 general policy approaches: (1) reconfiguring cost sharing to lower patient out-of-pocket costs and (2) removing incentives for plans to progress quickly through the stages of the Part D benefit.
Under the first approach, patient cost sharing could be shifted from coinsurance to flat copayments. Plans could still use tiers with higher copayment requirements to discourage use of nonpreferred drugs. This approach could be made actuarially equivalent to the current structure of the plans if some of the current excess costs for patients needing specialty drugs were reincorporated into copayments for lower-tiered products.17 Alternatively, Part D plans could calculate the patient’s out-of- pocket costs on the basis of the payer’s expected net price for the drug after rebates, rather than on the basis of list price. Currently, these point-of-sale rebates are allowed for Part D plan sponsors but are not required; in 2008, only 4 of 258 sponsors offered plans that provided estimated rebates at the point of sale, and less than 1% of beneficiaries were enrolled in these plans.18 The Centers for Medicare & Medicaid Services could mandate point-of-sale rebates or highlight plans that offer these rebates during the annual enrollment period.19
Payers and pharmaceutical manufacturers may object to these approaches to lower patient out-of-pocket costs. Because actual rebate amounts are often determined after the fact, the amounts would have to be estimated. Payers and pharmaceutical manufacturers might also argue that providing point-of-sale rebates would unmask the size of the rebate, which is considered proprietary information. Despite these obstacles, point-of-sale rebates are used in commercial health insurance, and since 2011, pharmaceutical manufacturers have been providing this type of rebate to beneficiaries in the coverage gap, as required by the Affordable Care Act.15
Under the second approach, incentives could be realigned such that higher spending in the Part D program increases, rather than decreases, the share of costs borne by Part D plan sponsors. In 2016, the Medicare Payment Advisory Commission issued recommendations for restructuring the Part D benefit to provide incentives to sponsors to reduce catastrophic spending and offer better cost protections for patients.20 There commendations include requiring higher-cost sharing from Part D plan sponsors in the catastrophic phase of coverage. Higher-cost sharing would be accomplished by reducing Medicare’s reinsurance from 80% to 20% by incrementally increasing the proportion paid by the Part D plan sponsor and eliminating patient out-of- pocket contributions. By shifting spending back to plan sponsors in the catastrophic phase of coverage, this proposal would eliminate financial incentives for reaching the catastrophic spending level.
Choosing among these 2 options requires data on rebates at the individual drug level. At present, the Centers for Medicare & Medicaid Services does not obtain these data from Part D plans but should require their provision. Then, the Centers for Medicare & Medicaid Services should use this information to develop an alternative benefit design, such as point-of-sale rebates, which would lead to savings for Part D beneficiaries and Medicare.
Acknowledgments
Drs Dusetzina and Conti reported serving on the committee Ensuring Patient Access to Affordable Drug Therapies of the National Academy of Sciences, Engineering, and Medicine. Dr Conti reported receiving funding for her work on this study from The Commonwealth Fund, the American Cancer Society, and the Laura and John Arnold Foundation. Ms Yu reported owning Merck stock. Dr Bach reported receiving personal fees from the Association of Community Cancer Centers, America’s Health Insurance Plans, AIM Specialty Health, American College of Chest Physicians, American Society of Clinical Oncology, Barclays, Defined Health, Express Scripts, Genentech, Goldman Sachs, McKinsey and Company, MPM Capital, the National Comprehensive Cancer Network, the Biotechnology Industry Organization, The American Journal of Managed Care, The Boston Consulting Group, Foundation Medicine, Anthem Inc, Novartis, and Excellus Health Plan, as well as obtaining grants for work unrelated to this study from the National Institutes of Health (core grant P30 CA 008748), the Kaiser Foundation Health Plan, and the Laura and John Arnold Foundation.
Funding/Support: This study was funded in part by research scholar grant RSGI-14-030-01-CPHPS from the American Cancer Society (Dr Dusetzina).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Dusetzina and Bach had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dusetzina, Conti, Bach.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Dusetzina, Bach.
Administrative, technical, or material support: Bach.
Supervision: Bach.
Conflict of Interest Disclosures: No other disclosures were reported.
References
- 1.Testimony of Mylan CEO Heather Bresch before the United States House of Representatives Committee on Oversight and Government Reform [transcript] Washington, DC: House Oversight and Government Reform Committee; Sep 21, 2016. [Accessed December 13, 2016]. https://oversight.house.gov/wp-content/uploads/2016/09/2016-09-21-Mylan-CEO-Bresch-Testimony.pdf. [Google Scholar]
- 2.Drug price negotiations & rebates. Pharmaceutical Convention Management Association website. https://www.pcmanet.org/policy-issues/drug-price-negotiations-rebates/. Accessed Month day, year.
- 3.Langreth R. Secret rebates: why patients pay $600 for drugs that cost $300. [Accessed December 20, 2016];Bloomberg. 2016 Oct 5; https://www.bloomberg.com/news/articles/2016-10-05/patients-lose-out-on-big-pharma-s-secret-rebate-merry-go-round.
- 4.Berndt ER, Newhouse JP. Pricing and reimbursement in US pharmaceutical markets. In: Danzon P, Nicholson SN, et al., editors. The Oxford Handbook on the Economics of the Biopharmaceutical Industry. New York, NY: Oxford University Press; 2012. pp. 201–265. [Google Scholar]
- 5.Aitken M, Berndt ER, Cutler D, Kleinrock M, Maini L. Has the era of slow growth for prescription drug spending ended? Health Aff (Millwood) 2016;35(9):1595–1603. doi: 10.1377/hlthaff.2015.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dusetzina SB. Share of specialty drugs in commercial plans nearly quadrupled, 2003–14. Health Aff (Millwood) 2016;35(7):1241–1246. doi: 10.1377/hlthaff.2015.1657. [DOI] [PubMed] [Google Scholar]
- 7.Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. [Accessed December 14, 2016];2016 annual report of the boards of trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/ReportsTrustFunds/downloads/tr2016.pdf. Published June 22, 2016.
- 8.Hoadley J, Cubanski J, Hargrave E, Summer L. [Accessed December 21, 2016];Medicare Part D: a first look at plan offerings in 2014 [issue brief] https://www.pharmamedtechbi.com/~/media/Supporting%20Documents/The%20Pink%20Sheet%20DAILY/2013/October/medicarepartd2014firstlook.pdf. Published October 2013.
- 9.Cox C, Damico A, Levitt L. Examining high prescription drug spending for people with employer sponsored health insurance. [Accessed December 21, 2016]; http://www.healthsystemtracker.org/insight/examining-high-prescription-drug-spending-for-people-with-employer-sponsored-health-insurance/. Published October 27, 2016.
- 10.Pearson CF. [Accessed December 20, 2016];Majority of drugs now subject to coinsurance in Medicare Part D plans. http://avalere.com/expertise/managed-care/insights/majority-of-drugs-now-subject-to-coinsurance-in-medicare-part-d-plans. Published March 10, 2016.
- 11. [Accessed December 4, 2016];Medicare Part D Plan Finder website. https://www.medicare.gov/find-a-plan/questions/home.aspx.
- 12.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 13.Merck. [Accessed December 20, 2016];Merck receives FDA approval of ZEPATIER (elbasvir and grazoprevir) for the treatment of chronic hepatitis C virus genotype 1 or 4 infection in adults following priority review [press release] http://investors.merck.com/news/press-release-details/2016/Merck-Receives-FDA-Approval-of-ZEPATIER-elbasvir-and-grazoprevir-for-the-Treatment-of-Chronic-Hepatitis-C-Virus-Genotype-1-or-4-Infection-in-Adults-Following-Priority-Review/default.aspx. Published January 28, 2016.
- 14.America’s Health Insurance Plans. [Accessed December 20, 2016];The Medicare Part D program: a record of success. https://ahip.org/the-medicare-part-d-program/. Published September 28, 2016.
- 15.Center for Medicare & Medicaid Services. [Accessed February 21, 2017];Costs in the coverage gap. https://www.medicare.gov/part-d/costs/coverage-gap/part-d-coverage-gap.html.
- 16.Office of Inspector General, US Department of Health & Human Services. [Accessed December 27, 2016];High-price drugs are increasing federal payments for Medicare Part D catastrophic coverage. https://oig.hhs.gov/oei/reports/oei-02-16-00270.pdf. Published January 2017.
- 17.Pyenson BS, Dieguez G, Johnson RL. [Accessed December 20, 2016];Specialty tiers: benefit design considerations for Medicare Part D. http://us.milliman.com/uploadedFiles/insight/2013/specialty-tiers.pdf. Published June 26, 2013.
- 18.Office of Inspector General, US Department of Health & Human Services. [Accessed December 20, 2016];Concerns with rebates in the Medicare Part D program. https://oig.hhs.gov/oei/reports/oei-02-08-00050.pdf. Published March 2011.
- 19.Hargrave E, Hoadley J, Merrell K. [Accessed December 21, 2016];Toward meaningful quality and performance measures in Part D. http://67.59.137.244/documents/Oct10_PartDQualityandPerformanceMeasuresPartD_CONTRACTOR_RS.pdf. Published October 2010.
- 20.Medicare Payment Advisory Commission. [Accessed December 20, 2016];Improving Medicare Part D. http://www.medpac.gov/docs/default-source/reports/chapter-6-improving-medicare-part-d-june-2016-report-.pdf?sfvrsn=0. Published June 2016.