Abstract
BRCA genes are important for the integrity and stability of genetic material and play key roles in repairing DNA breaks via high fidelity homologous recombination. BRCA mutations are known to predispose carriers to gynecological malignancies, accounting for a majority of hereditary OC cases. Known to be lethal, OC is difficult to detect and control. Testing for BRCA mutations is a key step in the risk assessment, prognosis, treatment and prevention of OC and current clinical guidelines recommend BRCA mutation testing for all OCs of epithelial origin. Studies have established that ovarian tumors harboring BRCA mutations have distinct molecular and histo-pathological features that can be exploited for effective, targeted treatment. Deficiencies in DNA repair pathways that arise as a result of BRCA mutations make them hypersensitive to DNA-damaging treatments such as platinum chemotherapy and PARP inhibitors. Different combinations of treatment regimens which have the potential to greatly improve prognosis and disease outcomes are currently being evaluated. However, the issue of developing resistance to these treatments remains unresolved. This review emphasizes unique features of BRCA mutated OC and outlines the lay of the land in terms of diagnosis and treatment, while aiming to unravel the challenges that are part of its management.
Keywords: BRCA, ovarian cancer, pathogenesis, chemotherapy, targeted therapy
INTRODUCTION
Ovarian cancer (OC) is known to cause the largest number of deaths among cancers of gynecologic origin. Inheritable mutations increase the risk of development of OC in carriers [1, 2]. Dominant, autosomally transmitted hereditary OC arises from mutations in 2 key types of genes: the BRCA (breast cancer susceptibility) genes (called hereditary breast and OC [HBOC] syndrome) and the MMR (DNA mismatch repair) genes (called hereditary non-polyposis colorectal cancer [HNPCC] syndrome or Lynch II syndrome) [1]. Both sets of genes are involved in the repair of genetic lesions. However, of these two syndromes, mutations in the BRCA 1/2 (BRCA) genes responsible for HBOC syndrome are more common, accounting for 90% of all the hereditary OC cases [3]. The BRCA genes are tumor suppressors, and a mutation in either one is known to predispose carriers to several types of cancer [4, 5].
Although they have been associated with an increased risk of occurrence of various other types of cancer that are not of gynecologic origin, the majority of specific inherited mutations in BRCA1 and BRCA2 increase the risk of female breast and ovarian cancers. Together, inheritable mutations in BRCA1 and BRCA2 account for around 15% of OC cases. While approximately 1.3% of women in the general population are likely to develop OC, recent estimates suggest that about 40%-60% of women who inherit a BRCA1 mutation and between 11% and 27% of women who inherit a BRCA2 mutation will develop OC by 80 years of age, with the risk of development of OC being higher in BRCA1 carriers [6–10]. Thus, a mutation in either gene represents a significantly increased risk of OC in women [6]. The histopathology and molecular characteristics of OC that occur as a result of BRCA mutations are distinct from other types of ovarian malignancies, and several studies have also demonstrated that these features make such tumors more responsive to certain types of treatment regimens [11, 12]. Despite this, such tumors are hard to detect. The purpose of this review is to emphasize the recent advances in BRCA 1/2 linked OC prevention, detection, and treatment. In addition, it also highlights the possible areas of future investigation that have the potential to improve patient health and disease outcomes.
The BRCA genes
BRCA1 and BRCA2 are located on chromosomes 13 (13q12.3) and 17 (17q21), respectively. Both genes occupy very large regions of the human genome and span about 70 kb of DNA. BRCA1 contains 22 exons and BRCA2 contains 27 exons and both encode multi-domain proteins. Their primary sequences are rich in repetitive DNA elements [13–15]. They are broadly classified under the umbrella of tumor suppressors and are involved in cellular pathways for the repair of genetic lesions. In addition, a loss of function of either gene results in similar physiological consequences and also increases the risk of developing similar types of cancer, primarily breast and ovarian. However, both molecules are distinct in terms of genetic sequence and perform a non-redundant molecular function [13].
Many studies have focused on studying these genes in detail, in order to identify mutations that result in a loss of protein function and thus in an elevated risk of malignancy. Studies have also focused on classifying mutations based on the genetic location in which they occur, and mutations in specific regions of both genes are known to predispose carriers to certain types of malignancies. The ovarian cancer cluster region (OCCR) of BRCA2 has been well defined and studies have shown that mutations outside of the OCCR predispose carriers to malignancies that are not of ovarian origin. Conversely, mutations that lie within the OCCR of BRCA2 predispose carriers to a significantly elevated risk of ovarian cancer compared with other malignancies. Studies have also shown that mutations in the 3’ region of the BRCA1 gene are linked with a lower risk of OC, whereas mutations in regions further downstream are linked with an elevated risk of OC [16, 17]. Although the results of such studies are useful in estimating the risk of occurrence of certain kinds of malignancies in patients, they are not without exception and therefore must be interpreted with caution [18].
Epidemiology and prevalence of BRCA mutations in OC
Over the past 2 decades since their discovery and cloning, BRCA mutation studies have been conducted in various populations across the world. These studies show that the highest prevalence of BRCA mutations in OC is in women with the serous form of epithelial OC. Generally, mutations exist in either BRCA1 or BRCA2 and rarely does the same person have mutations in both the genes. Mutations in BRCA may be inherited within certain families and of germline origin or they may occur as tumor-only, non-inheritable somatic mutations in certain individuals [19]. Either germline or somatic mutations in BRCA account for 20% of all the OCs. The germline mutation rate in these genes is currently estimated at about 15% in OC cases, whereas somatic mutations in the BRCA genes account for about 5% [20–22].
The prevalence of inherited germline BRCA mutations in different populations is highly variable and dependent on ethnicity. The highest prevalence of germline BRCA mutations exists in women of Ashkenazi Jewish origin. Norwegian, Danish, Icelandic people, and people of French Canadian descent were also found to have a higher incidence of BRCA mutations [23–25]. In such closed populations, certain identifiable, specific mutations in the BRCA genes exist and these are known as founder mutations. For example, the 185delAG and 5382insC mutation in the BRCA1 gene and the 6174delT mutation in the BRCA2 gene are the known founder mutations that exist in Ashkenazi Jewish women [9, 26]. The prevalence of BRCA mutations in the Chinese population is similar to that in Western countries; however, a recent study found that the spectrum of mutations observed in Chinese populations differs from Western counterparts [27]. Another study also identified novel founder mutations present in populations from Eastern China [28]. In a multicenter BRCAm prevalence study conducted in 2016, Prof. Wu (Fudan University Shanghai Cancer Center) reported that the observed BRCAm prevalence was 28.5%, which is higher than what was found in other studies. This difference may be attributed to a higher percentage of Chinese ovarian cancer patients with high grade serous and late stage ovarian tumors observed in this group [29].
Pathogenesis, pathophysiology, and molecular characteristics of OC linked to BRCA mutations
Epithelial ovarian cancer (EOC) is classified as serous, mucinous, endometrioid, clear cell, or undifferentiated carcinoma etc., depending on the histological cell type. Of these, the first 4 are predominant in EOC [30]. The pathogenesis of EOC determines the type under which it is classified, with type I EOC developing through the low-grade pathway and type II EOC developing through the high-grade pathway. The main difference between the 2 types is that type I cancers show a spectrum of biogenesis from benign to malignant, whereas high-grade type II cancers arise de novo as aggressive neoplasms. Histological investigations of ovarian tissues obtained after preventive oophorectomy on BRCA mutation carriers were compared with those without BRCA mutations, and the only discernible difference was a higher frequency of surface micropapillae, the clinical significance of which has not been established [31]. As a consequence of these factors, there is no identifiable pre-cancerous lesion in type II carcinomas, and they are therefore detected at a later stage of progression [32, 33]. Although the claim needs to be supported by further investigation, there is preliminary evidence to show that fimbral dysplasia may be the pre-cancerous lesion for serous high-grade carcinomas [34, 35]. Fortunately, high-grade tumors have also been found to be largely chemo-sensitive. It is of particular interest that currently, type II disease is the predominantly occurring one in diverse populations.
Most OCs linked to BRCA mutations are high-grade, serous epithelial OCs (HGSOCs) and BRCA mutations are less likely to predispose carriers to other classes of EOC such as mucinous, endometrioid, or clear cell EOC [36–38]. BRCA mutations are present in more than one fifth of all high-grade serous OC cases [39]. However, BRCA mutations are purported to account for between 5%–15% of endometrioid and clear cell subtypes of OC [18, 40].
In addition, patients with BRCAm OC show a much higher incidence of HGSOC than patients having sporadic OC, and the majority of BRCAm OC-linked HGSOC tumors have been shown to arise in the fallopian tube [41–43]. Thus, as mentioned above, although the majority of BRCA-linked OC cases are of the high grade serous type, BRCAm OC also manifests as other histological sub-types, making BRCA testing an important tool for the effective treatment and resolution of various types of EOC [44]. BRCA mutation testing is also recommended by the NCCN guidelines as an important prerequisite for all patients presenting with EOC [45, 46]. At the tissue level, BRCA-linked cancers also generally show an increased tumor infiltration of immune cells. These factors are probably linked to the favorable prognostic outcome in BRCA mutated cancers compared with those cancers that are not a result of BRCA dysfunction [47]. Several studies have also shown that there is an increased frequency of p53 mutations and p53 overexpression in OCs arising from BRCA mutations compared with those that arise sporadically [37, 47, 48]. Analysis of EOC tissues has also shown that the homeobox gene HOX9 is up-regulated and this results in a more permissive environment for tumor growth and development through its effect on the differentiation of Cancer associated fibroblasts (CAF) [49]. In addition, other HOX genes have also been shown to be differentially expressed, with maximum genetic dysregulation occurring in case of HGSOC, the histological subtype that BRCA mutations are largely responsible for. Also, it has been shown that different HOX genes are dysregulated depending on whether the tumor is platinum sensitive or resistant and it is possible that this ties back to the BRCA mutation status of the tumor [50]. In contrast to breast cancers arising from BRCA mutations, HER2 expression in BRCAm OC have not been found to be significantly up-regulated compared with controls, although there are some studies that report an increase in HER2 expression in certain cases of HBOC.
Treatment and prognosis of OC caused by BRCA mutations
Although BRCA carriers are more likely to develop OC, they respond better to certain chemotherapy regimens and to some types of targeted treatment, notably platinum-based chemotherapy and poly-ADP ribose polymerase (PARP) inhibition. This augmentation in response rate can be attributed to the DNA-damaging effects of the treatment regimens that exploit the molecular and phenotypic characteristics of BRCA-linked tumors. Recently, it has been established that these molecular and phenotypic characteristics are not restricted to BRCA-linked tumors, but have also been found in other cancers that have a dysfunction in DNA repair genes. Thus, such characteristics are said to confer a BRCAness phenotype on the tumor and the benefits of DNA-damaging therapy can be extended and used to treat such tumors as well. This section of the review aims to characterize the responses of BRCA-linked tumors to different treatment regimens, since they can be extrapolated and adapted for the treatment of a larger population.
Several studies have reported that short-term prognosis and progression-free survival (PFS) in patients with BRCAm OC are better than in patients with OC because of non-BRCA–linked sporadic mutations in response to various treatment regimens [51, 52]. Moreover, because of improved prognosis in patients with BRCA dysfunction, VEGFR3 inhibition is being developed as a treatment to induce low levels of BRCA 1/2 in patients with sporadic OC or in patients who originally presented with BRCAm OC but experienced a BRCA gene reversion [53]. Despite these positive outcomes for patients with BRCAm OC, the issue of overall survival (OS) rates in patients with BRCAm OC versus control participants is controversial. A very early study that compared OS rates between control participants and patients with BRCAm OC by Ruben et al reported a significantly higher OS for patients with BRCAm OC [52]. However, since then, many studies that undertook similar investigations have reported no significant difference between OS rates in patients with BRCAm OC versus control participants [51].
A study in a cohort of Jewish women suggested that OC patients with germline BRCA mutations showed a better prognosis (survival period of 91 months and disease free interval of 49 months) than OC patients with somatic mutations (survival period of 54 months and disease free interval of 19 months) [54]. In addition, the results of a retrospective study suggested that OC patients with a mutation in BRCA2 (HR = 0.20) are likely to have higher PFS rates than OC patients with a mutation in BRCA1 (HR = 0.70) or no BRCA-related dysfunction [3, 55]. However, this conclusion was from an isolated study and conflicts with the results of a recent meta-analyses of 14 studies which suggested that both, BRCA1 and BRCA2 mutation status were equal predictors of a better OS rate (pooled HR of 0.65 in BRCA1 mutation carriers and a pooled HR of 0.61 in BRCA2 mutation carriers) in Ovarian Cancer patients [56]. Interestingly, this result was less convincing when mutations in the BRCA1 promoter region were analyzed, indicating that there might be differences in the course of treatment required for patient subgroups having mutations in different regions of the gene [57]. Presented below (and summarized in Table 1) is a discussion on the currently available options for the treatment of BRCAm OC.
Table 1. Summary of different treatments and outcomes in patients with OC based on BRCA mutation status.
| References | No. of patients | Type of OC | Treatment | BRCA mutation status | PFS | OS |
|---|---|---|---|---|---|---|
| Adams SF et al, 2011 [58] | 23 | EOC | (PLD) Doxil | BRCA 1/2 positive versus sporadic OC | 27.1 weeks versus 17 weeks | 89.1 weeks versus 48.3 weeks |
| Ledermann JA et al, 2012 [59]; Ledermann JA et al, 2014 [60] (Aka Study 19- basis for BRAC Analysis approval) |
254 | PSR HGSOC | Olaparib maintenance therapy versus placebo | BRCA 1/2 positive | 11.2 months versus 4.1 months | No difference reported |
| Oza AM et al, 2015 [61] | 107 | PSR HGSOC | Paclitaxel + carboplatin versus paclitaxel + carboplatin + olaparib maintenance | BRCA 1/2 positive versus BRCA 1/2 negative | 9.6 versus 12.2 months | Not reported |
| Louroso D et al, 2016 [11] | 100 | PSR OC versus PRR OC | Trabectedin |
BRCA 1/2 positive or BRCAness versus unreported |
No difference w.r.t. BRCA status | No difference w.r.t. BRCA status |
| Monk BJ et al, 2015 [62] | 41 | PRR OC | Trabectedin + PLD versus PLD | BRCA 1/2 positive | 13.5 versus 5.5 months | 23.8 versus 12.5 months |
| Liu JF et al, 2014 [63] | 90 | PSR HGSOC | Olaparib + cediranib versus olaparib | Mixed: BRCA positive + unknown | 17.7 versus 9 months | Not reported |
OC: Ovarian Cancer; BRCA: Breast cancer, early onset; PFS: Progression free survival; OS: Overall Survival; EOC: Epithelial OC; PSR HGSOC: Platinum Sensitive Recurrent High Grade Serous OC; PRR OC: Platinum resistant/refractory OC; PLD: Pegylated liposomal doxorubicin.
Surgical intervention
Surgical cyto-reduction and de-bulking of the tumor is largely considered the first line of treatment in patients who develop OC. Generally, bilateral salpingo-oophorectomy is recommended, and depending on the stage of the tumor and the age of the patient, a hysterectomy may also be recommended. In general, studies have reported an equal de-bulking rate in patients with BRCAm OC versus patients with sporadic OC, with no specific advantage for BRCA mutation carriers in terms of reduction in tumor size after surgery.
Chemotherapy
As in the case of other cancers, chemotherapy is a commonly used treatment in the resolution of BRCAm OC. However, systemic chemotherapy presents issues of high toxicity. It is well established that BRCAm OC tumors respond better to DNA-damaging agents, and therefore platinum-based DNA-damaging chemotherapy is a commonly recommended course of treatment for BRCAm OC.
Platinum-based chemotherapy
Platinum-based chemotherapy, either cisplatin or carboplatin, is commonly used in the treatment of OC. Those malignancies that have been treated with platinum-based therapies as a first line of treatment but worsen or recur after 12 months of initial treatment or do not worsen or recur at all are termed platinum sensitive. Those that progress within 6 months of initial treatment are termed platinum resistant and those progressing between 6-12 months of platinum therapy are called partially platinum sensitive. A platinum-paclitaxel combination is generally used as the first line of treatment for OC [64]. Ovarian tumors of BRCA origin are more sensitive to platinum-based chemotherapy than sporadic OC cases. Several studies have shown that patients with BRCA mutations of either germline or somatic origin respond better to platinum-based chemotherapeutic regimens and demonstrate better prognosis and improved survival rates over a median range [65, 66]. However, studies have also shown that in certain cases, previously platinum-sensitive BRCAm OC can become resistant to platinum treatment. Analysis has revealed that this switch is likely to occur due to a reversion of the BRCA mutation that restores protein function in tumor cells [67, 68].
Intra-peritoneal chemotherapy
The selected chemotherapy regimen may either be administered through an intravenous (IV) or intra-peritoneal (IP) route or by using a combination of the two depending on the treatment type and stage of presentation with OC. Currently, the standard of care for patients with EOC sometimes involves the administration of platinum-based chemotherapy and paclitaxel through a combination of routes, that is, either IV or IP. This combined model of treatment has been shown to be associated with a lower risk of death in patients for whom de-bulking surgery was effective [69]. However, such IP treatment causes very high toxicity within the peritoneal cavity.
A limited number of studies have assessed the use of IP chemotherapy in BRCA mutation positive versus BRCA mutation negative EOC. While studies have shown an improved PFS rate in BRCA mutation positive OC patients upon the use of platinum based IP chemotherapy (mainly cisplatin and paclitaxel) after surgical cyto-reduction, conclusive, direct evaluations of treatment efficacy between the IP and IV route based on BRCA mutation status are yet to be conducted [12, 70, 71]. Only one Phase III study, i.e., COG-172 has evaluated patient prognosis parameters after IP or IV chemotherapy based on BRCA mutation status. The results showed a better PFS in BRCA mutation positive patients treated with platinum based chemotherapy administered via the IP route, with a clinically insignificant effect on OS [72].
Pegylated liposomal doxorubicin
Pegylated liposomal doxorubicin (PLD) is a treatment that was initially approved for the recurrent form of epithelial OC in patients in whom platinum-based chemotherapy failed [73]. The formulation increases the concentration of doxorubicin specifically in tumor tissues, and thus shows a lower toxicity profile than platinum-based chemotherapy. Compared with only doxorubicin, a significantly lower number of severe adverse events (SAEs) occur, especially in terms of adverse cardiovascular events. In a recent study that investigated the use of PARPi versus PLD in BRCAm OC, there was an unexpectedly high PFS demonstrated in the PLD arm of the trial, implying that PLD may be prescribed in future as a treatment that is specifically efficacious in BRCA mutation carriers [58, 74].
Neo-adjuvant chemotherapy
In most OC cases, de-bulking surgery is recommended before the administration of chemotherapeutic or targeted treatment regimens to facilitate a reduction in the size of the primary tumor. However, in certain cases where the primary tumor is bulky, neo-adjuvant chemotherapy is recommended before surgical resection of the tumor. Neo-adjuvant chemotherapy can be used before de-bulking surgery to reduce the size of the primary tumor in patients presenting with stage III-IV EOC. In the CHORUS trial, the first line of treatment used was neo-adjuvant therapy, followed by surgery and standard chemotherapy. This was compared with the standard treatment regimen using first de-bulking surgery followed by chemotherapy. The investigators found that neo-adjuvant therapy was non-inferior and that there was no residual disease in the neo-adjuvant arm of treatment. However, this did not translate to an overall higher OS rate, and current research is focused on elucidating the biological mechanisms underlying this paradox [75].
Additionally, the efficacy of neo-adjuvant chemotherapy versus standard surgical de-bulking (prior to the administration of the chemotherapeutic regimen) based on BRCA mutation status remains to be evaluated. The study by Gorodnova et al., (conducted in Slovic patients) showed that the majority of BRCA mutation carriers had a complete clinical response to platinum-based neo-adjuvant chemotherapy, while only a small percentage of patients who were negative for BRCA mutations responded favorably [76]. However, this study did not provide a head to head evaluation between the standard mode of chemotherapy administration versus the use of neo-adjuvant therapy, and the enhanced response of BRCAm OC patients could also be attributed to the use of a platinum based regimen rather than to the mode of chemotherapy administration.
Targeted therapy
Aim: Improved efficacy and decreased toxicity treatment that exploits specific cellular and molecular characteristics linked to the origin of the cancer.
PARP inhibitors
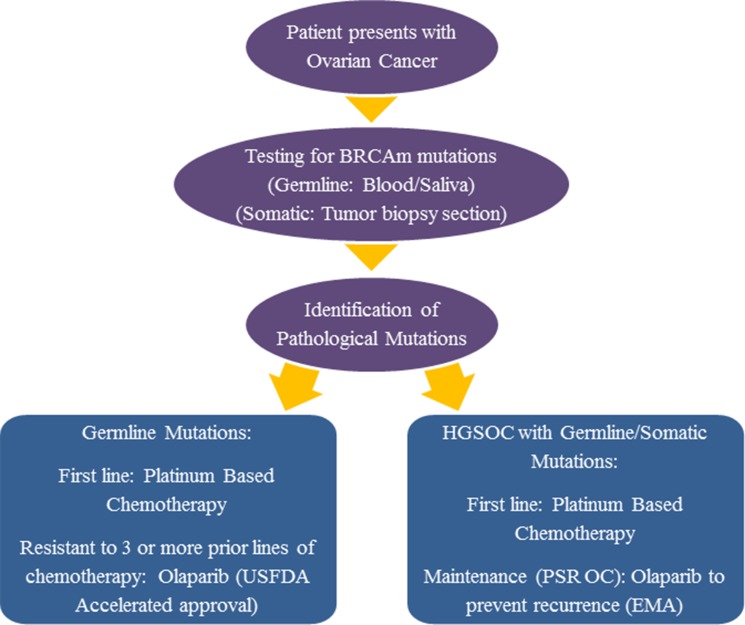
PARP inhibitors target the cellular enzymes PARP-1 and PARP-2 and thereby obstruct important molecular events necessary for effective DNA repair in the cell. Currently, there are numerous cellular pathways that they have been shown to interrupt. There are several hypotheses that attempt to explain the enhanced responsiveness of BRCA-deficient tumors to PARP inhibition. The most convincing of these is that they force the already BRCA-deficient cells to rely on HR as a repair mechanism, rendering them incapable of dealing with genetic lesions. This is known as synthetic lethality [77]. The current hypothesis for their improved effectiveness in treating OC resulting from BRCA mutations is linked to this fact. It was demonstrated in an open-label, non-randomized, phase II study that BRCAm OC is associated with a better response to apoptosis-inducing PARP inhibitors [78]. The proof of concept for the treatment of BRCAm OC with PARPi arose when laboratory tests showed that BRCA-deficient cells were extremely sensitive to PARP inhibition [77, 79]. In 2014, the FDA (Food and Drug Administration) and EMA (European Medicines Agency) approved the use of PARP inhibitor olaparib (AZD2281) for BRCAm OC. However, the setting for approval in both cases was different: the FDA approval was in the context of relapsing BRCAm OC and EMA approval was in the setting of maintenance treatment for BRCAm OC [80]. While the FDA approved the use of Olaparib for those gBRCA mutated Ovarian tumors resistant to at least three lines of prior chemotherapy, the EMA approved its use as maintenance therapy for HGSOC treated with first line chemotherapy. The 2017 NCCN guidelines for Ovarian Cancer are in line with the FDA approval for Olaparib. However, the NCCN panel does not recommend the use of Olaparib as maintenance therapy as the general consensus among experts was that a lack of evidence exists to support its use in this context (https://www.nccn.org). Olaparib was shown to be an effective course of monotherapy against advanced stage, relapsing BRCAm OCs with an ORR of 41%, leading to breakthrough approval that was granted by the FDA in 2014 [81]. Figure 1 provides a summary of the use of olaparib in the treatment of BRCAm OC in the context of FDA and EMA approval.
Figure 1. Incorporation of olaparib into the treatment regimen for BRCAm OC: USFDA versus EMA approval.
Outline of the testing and treatment procedure for patients presenting to the clinic with Ovarian Cancer. A comparison of the approval status for PARPi (Olaparib) in Europe versus America has been described. While the FDA has approved the use of Olaparib in patients with OC that failed to respond to three or more lines of chemotherapy, the EMA has approved Olaparib as maintenance treatment for PSR OC.
Anti-angiogenic treatment
Aggressive angiogenesis is one of the defining features of malignant tumors. Cancerous cells show an increased rate of blood vessel production compared with normal cells, and VEGF and VEGFR are molecules that are highly expressed during the process of angiogenesis. VEGF is secreted by cancer cells and stimulates endothelial cells by binding to the tyrosine kinase receptor VEGFR on their surface, activating an intracellular cascade that ultimately results in an increased production of blood vessels [82]. Many treatments have focused on targeting this molecular interaction and this can be achieved by using either antibodies that target VEGF (the ligand) or those that target VEGFR (the receptor). Cediranib (AZD2171) is one such molecule that is currently being studied to retard the rate of angiogenesis in BRCAm OC by inhibiting the activity of VEGFR. A phase III study evaluated its use in recurrent platinum-sensitive EOC in combination with chemotherapy and as maintenance therapy and found that although progression-free survival in patients improved, increased toxicity was still a concern [83]. Bevacizumab is a humanized monoclonal antibody that targets and neutralizes VEGF. It is currently being studied as an effective treatment in platinum-sensitive recurrent OC, although its efficacy in the treatment of BRCAm OC is unclear [84].
Combined treatment
Combined therapeutic regimens are common in the treatment of BRCAm OC and the rationale behind this is that the use of different agents enables the targeting of distinct, non-redundant molecular mechanisms that lead to malignancy and tumor growth. Several studies have already characterized the use of different combinatorial treatment regimens, and many such studies are ongoing. Combined treatment is also considered the current standard of care because it can enable the overall lowering of systemic toxicity of the treatment regimen. An open-label, phase II, randomized study on 173 patients from 12 countries that aimed to assess the efficacy of chemotherapy alone (paclitaxel + carboplatin) or chemotherapy along with olaparib maintenance in recurrent OC found that the latter group showed an enhanced progression-free outlook, with positive outlook further increased in patients with BRCAm OC [61]. Olaparib and cediranib were investigated in a combination treatment regimen for HGSOC, and improved PFS rates were observed. However, any specific advantage in BRCA-linked HGSOC was not studied or reported as part of this investigation [63]. Recently, it has been shown in a phase I trial of olaparib in combination with bevacizumab that PARP inhibitors and anti-angiogenic agents have a complementary method of action and that the efficacy of PARP inhibition could increase because of the hypoxic environment induced by the anti-angiogenic treatment [85].
Risk assessment and prevention of OC
Several studies have shown that cancers linked to BRCA mutations respond better to platinum-based chemotherapy and to PARP inhibitors and it is well established that BRCA mutation status can inform treatment. An observational study found that there were better survival rates in patients who tested for BRCA mutations and thus availed of therapies known to have better outcomes based on their genetic profiles [86]. Apart from improving treatment outcomes in patients who test for mutations after developing disease, hereditary screening for genetic BRCA mutations is a useful tool for the prevention of disease in patients with a known risk of developing certain malignancies. Various measures can be used to prevent the occurrence of OC in BRCA mutation carriers, including preventive surgery and the use of oral contraceptives [87, 88]. Preventive surgery, i.e., salpingo-oophorectomy (removal of the ovary and fallopian tubes), is used as a strategy for reducing the risk of OC occurrence in patients with known pathological BRCA mutations that predispose to OC. Several studies have shown that the risk of developing OC in patients with BRCA mutations can be significantly reduced, with one study reporting a decrease in the risk of OC occurrence of about 85% for patients who chose preventive surgery over surveillance programs as a strategy for risk reduction [89–91]. Currently, a phase II study is trying to evaluate both types of surgery, either RRSO (risk reducing salpingo-oophorectomy)or ISDO (interval salpingectomy with delayed oophorectomy), based on changes in sexual function, mental health, and quality of life (NCI-2016-00778, NCT02760849). Similar to the general population, studies have also shown that an increased number of anovulation periods during the lifetime of a woman are likely to reduce the risk of developing OC in BRCA mutation carriers, and oral contraceptives are used as a preventive strategy due to this fact [92–94]. In contrast, it was shown that stimulation of the ovaries by artificial methods such as IVF are likely to increase the risk of OC [95]. For those mutation carriers who do not accept risk reducing surgery, the use of oral contraceptives constitutes a viable alternative strategy which is supported by the results of extensive meta-analyses [87, 88]. One analysis of 3 studies reported that the risk of ovarian cancer decreased by almost 50% upon use of combined oral contraceptives. Interestingly, the results for risk reduction of breast cancer occurrence in BRCA mutation carriers were less convincing [87].
Homologous recombination deficiency in BRCAm OC and beyond
Several studies have established the role of BRCA1 and BRCA2 in the repair of genetic lesions via the homologous recombination repair (HRR) pathway and tissues that lack the function of these proteins show a stunted DNA damage repair mechanism that is solely reliant on other molecular cascades [13]. Deficiencies in the function of these proteins can lead to the deployment of other repair pathways in the cell-like non-homologous end joining (NHEJ), which are error prone and function at a lower fidelity. This can cause an accumulation of mutations that ultimately result in malignancy (two hit hypothesis). Such tissues that lack a functional HRR pathway are termed homologous repair deficient (HRD). Reliance on alternative mechanisms makes all tumors that lack the HRR pathway highly sensitive to therapies such as PARP inhibitors. PARPi specifically target the functioning of alternate pathways that these cells rely on in order to repair genetic lesions [77]. A deficiency in the function of molecules other than BRCA 1/2 can also confer the phenotype of HRD. Because HRD was first identified as a phenotypic characteristic of BRCA-linked tumors, it is one of the characteristics of BRCAness. Thus, the principle of synthetic lethality can be used to target such tissues. RAD50, RAD51, PALB2, and the components of the Fanconi anemia pathway (i.e., FANCA and FANCI) are examples of some such molecules [65, 96]. The Cancer Genome Atlas (TCGA) has reported that HRD could account for approximately 50% of all HGSOC cases [20]. Thus, current research is focused on establishing an HRD signature that can be used to test patient samples in future. This will help determine whether PARPi are likely to be an effective course of treatment depending on the specifics of the patient profile. It was recently shown that the myChoice companion diagnostics platform by Myriad Diagnostics can be used as an indicator of the inability of the tumor to repair DNA damage. This platform is capable of detecting loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions in tumor cells [97]. It was shown to be effective as a testing platform to predict patient responses to TESARO’s Niraparib, another oral PARP inhibitor which was shown to have positive effects on patient PFS independent of gBRCA mutation status when used as maintenance therapy [98]. Other PARP inhibitors are also under clinical evaluation. This includes Rucaparib, which showed encouraging results in a recent Phase II trial which evaluated dose dependent responses in patients with gBRCA mutated breast and ovarian cancer [99].
Ongoing investigations
Several studies that aim to assess the efficacy and safety of existing treatments in BRCAm OC are currently ongoing. New molecules are also being developed to overcome hurdles that exist currently in the treatment of BRCAm OC. Of note among these are the recent class of cell cycle checkpoint inhibitors that are being developed for the treatment of BRCAm OC [100]. Studies that aim to assess different combinatorial treatment regimens are also being conducted. A summary of all these is presented in Table 2.
Table 2. Summary of ongoing clinical trials being conducted for the treatment of BRCAm OC.
| Trial Number | Phase | Indication | Class of Drug | Treatment | Sponsor |
|---|---|---|---|---|---|
| NCT01874353 | III | PSR HGSOC | PARPi | Olaparib versus placebo | Astra Zeneca |
| NCT01844986 | III | Advanced stage (FIGO III-IV) ovarian cancer |
PARPi | Olaparib maintenance monotherapy versus placebo | Astra Zeneca |
| NCT01472783 | II | PSR EOC | PARPi | Veliparib | Vejle Hospital and Abbott |
| NCT01445418 | I | Recurrent OC | Chemotherapy + PARPi | Carboplatin + olaparib | National Cancer Institute |
| NCT00628251 | II | Platinum-resistant BRCAm OC advanced BRCAm OC |
PARPi | Dose titration of olaparib versus doxil | Astra Zeneca |
| NCT02282020 | III | PSR OC | PARPi | Single agent chemotherapy versus olaparib | Astra Zeneca |
| NCT02326844 | II | gBRCAm OC + disease progression post treatment with alternative PARPi | PARPi (second generation) |
Talazoparib | National Cancer Institute |
| NCT00679783 | II | BRCAm OC or recurrent high-grade OC | PARPi | Olaparib | Astra Zeneca |
| NCT01772979 | II | Recurrent BRCAm OC and BRCAness positive | DNA-binding agent (transcription blocking) | Trabectedin | Catholic University of the Sacred Heart |
| NCT00494442 | II | Advanced stage BRCA 1/2 positive OC Failed prior chemotherapy |
PARPi | Olaparib | Astra Zeneca |
| NCT02203513 | II | BRCAm OC/HGSOC | Chk 1/2 inhibitor (second generation, inhibits cell cycle progression) |
LY2606368 | National Cancer Institute |
| NCT01661868 | II |
BRCA 1/2 positive recurrent OC Treated with alternative PARPi/no PARPi exposure |
PARPi | Olaparib | Astra Zeneca |
| NCT01306032 | II | Refractory BRCA 1/2-positive OC/HGSOC | Chemotherapy + PARPi | Metronomic oral cyclophosphamide + veliparib | National Cancer Institute |
| NCT01482715 | II | gBRCA-positive OC | PARPi | Oral rucaparib | Clovis Oncology |
| NCT02855944 | III | BRCAm OC | PARPi versus chemotherapy | Rucaparib vs chemotherapy | Clovis Oncology |
| NCT02476968 | IV | PSR BRCAm OC | PARPi | Olaparib maintenance monotherapy | Astra Zeneca |
| NCT00892736 | I | Refractory BRCAm OC/platinum-resistant OC | PARPi | Veliparib | National Cancer Institute |
| NCT01853306 | I | BRCA 1/2 positive HGSOC | PARPi | Veliparib | AbbVie |
| NCT02286687 | II | Somatic BRCA linked OC | PARPi (second generation) |
Talazoparib | M.D. Anderson Cancer Center |
| NCT02345265 | II | Recurrent BRCAm OC or HGSOC | PARPi + VEGFRi | Olaparib + cediranib maleate | National Cancer Institute |
| NCT02354586 | II | BRCAm OC or HGSOC (received previous chemotherapy) | PARPi | Niraparib | Tesaro Inc. |
| NCT01237067 | I | Refractory/Recurrent BRCAm OC | Chemotherapy + PARPi | Carboplatin + olaparib | National Cancer Institute |
| NCT01989546 | I / II | Advanced stage BRCAm OC | PARPi | BMN 673 | National Cancer Institute |
| NCT01540565 | II | gBRCA-positive recurrent OC | PARPi | Veliparib | National Cancer Institute |
| NCT02482311 | Ib | BRCAm OC refractory to PARPi treatment | Wee1 kinase inhibitor (inhibits cell cycle progression) | AZD1775 | Astra Zeneca |
| NCT02470585 | III | BRCAm OC of epithelial origin | Chemotherapy + PARPi | Carboplatin + paclitaxel + veliparib maintenance versus carboplatin + paclitaxel + placebo maintenance |
AbbVie |
| NCT01286987 | I | Advanced stage/recurrent BRCAm OC | PARPi | Talazoparib | Medivation Inc. |
| NCT02489006 | II | BRCAm OC | PARPi | Neoadjuvant olaparib treatment (before surgery and chemotherapy) | University Health Network, Toronto |
OC: Ovarian Cancer; BRCAm: mutations in Breast cancer, early onset genes; FIGO: Federation of Gynecology and Obstetrics; PSR HGSOC: Platinum Sensitive Recurrent High Grade Serous OC; PARPi: poly ADP ribose polymerase inhibitor; VEGFRi: Vascular Endothelial Growth Factor Receptor inhibitor.
Conclusions and areas of future advancement
Ovarian cancer, primarily of epithelial origin, is one of the major tumor types that is associated with BRCA mutations. Among gynecological cancers, OC is the cause of significant morbidity in the female population. Testing for BRCA mutations is an important step in the risk assessment, treatment and prognosis of patients with OC, since BRCAm OC has been associated with distinct molecular and histopathological characteristics that result in differential responses to certain therapeutic regimens. However, the prevalence and type of BRCA mutations vary between countries/populations, ethnicity, and type of cancer. Despite the presence of several Consortiums and guidelines, screening for these mutations and annotating a specific clinical significance to them remain a challenge.
Although the presence of BRCA mutations represents a significant increase in the risk of occurrence of OC, BRCAm OC is known to be more responsive to certain types of treatments such as platinum-based chemotherapy and to targeted treatments which disrupt the DNA-damage response pathways of the cell, most notably PARP inhibitors such as Olaparib, Niraparib and Rucaparib. The recent approval of such targeted therapies for the treatment of BRCAm OCs is encouraging. However, an urgent need exists to solve the problem of increasing resistance to these compounds. Preliminary investigations have shown that combining PARPi with PI3K inhibitors constitutes an effective strategy which prevents resistance to treatments used for triple negative breast cancer. It has also been shown in mouse mammary tumors that the loss of 53BP1 is likely to cause resistance to PARP inhibitors [101, 102].
In addition, differences in response rates to treatments exist, depending on whether the OC is of BRCA1 or BRCA2 origin [41]. Such differences may also exist depending on which regions of these genes contain the mutation, and a limited number of studies have focused on characterizing these variations in response. A more detailed analysis of such differences can further improve the treatment course, quality of life, and overall survival rates in patients with BRCAm OC.
There is an increasing body of evidence to show that certain therapies such as PARPi, which were initially developed for the treatment of BRCAm OC, can be extended to treat a spectrum of malignancies that are not linked to BRCA mutations, but exhibit certain molecular characteristics in common with BRCA-associated disease, specifically HRD [97]. Such therapy has the potential to improve disease outcomes, not only in the restricted population of BRCA mutation carriers, but also for use in all tumors with HRD as a defining characteristic.
Acknowledgments
The authors acknowledge Dr Kripa Madnani (PhD) and Dr Amit Bhat (PhD) from Indegene Pvt. Ltd. for their medical writing assistance and critical evaluation of the supporting literature (funded by AstraZeneca) while drafting this review article.
Footnotes
CONFLICTS OF INTEREST
The authors have no Conflicts of Interest to declare.
REFERENCES
- 1.Floquet A, Stoeckle E, Croce S, Longy M, Mc Grogan G, Barouk E, Bubien V, Garbay D, Joly E, Guyon F. [Hereditary ovarian carcinomas: clinico-biological features and treatment] Bull Cancer. 2014;101:167–74. doi: 10.1684/bdc.2014.1888. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Prat J, Ribé A, Gallardo A. Hereditary ovarian cancer. Hum Pathol. 2005;36:861–70. doi: 10.1016/j.humpath.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Futreal PA, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, Tavtigian S, Bennett LM, Haugen-Strano A, Swensen J, Miki Y, Eddington K, McClure M, Frye C, et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994;266:120–22. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- 5.Lancaster JM, Wooster R, Mangion J, Phelan CM, Cochran C, Gumbs C, Seal S, Barfoot R, Collins N, Bignell G, Patel S, Hamoudi R, Larsson C, et al. BRCA2 mutations in primary breast and ovarian cancers. Nat Genet. 1996;13:238–40. doi: 10.1038/ng0696-238. [DOI] [PubMed] [Google Scholar]
- 6.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Easton DF, Ford D, Bishop DT, Breast Cancer Linkage Consortium Breast and ovarian cancer incidence in BRCA1-mutation carriers. Am J Hum Genet. 1995;56:265–71. [PMC free article] [PubMed] [Google Scholar]
- 8.Foulkes WD, Narod SA. Ovarian cancer risk and family history. Lancet. 1997;349:878. doi: 10.1016/S0140-6736(05)61782-5. [DOI] [PubMed] [Google Scholar]
- 9.King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–46. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 10.Søgaard M, Kjaer SK, Gayther S. Ovarian cancer and genetic susceptibility in relation to the BRCA1 and BRCA2 genes. Occurrence, clinical importance and intervention. Acta Obstet Gynecol Scand. 2006;85:93–105. doi: 10.1080/00016340500324621. [DOI] [PubMed] [Google Scholar]
- 11.Lorusso D, Perotto S. Ovarian cancer treatment in mutation carriers/BRCAness. Minerva Ginecol. 2016;68:566–78. [PubMed] [Google Scholar]
- 12.Tan DS, Kaye SB. Chemotherapy for Patients with BRCA1 and BRCA2-Mutated Ovarian Cancer: same or Different? Am Soc Clin Oncol Educ Book. 2015;35:114–21. doi: 10.14694/EdBook_AM.2015.35.114. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95:866–71. doi: 10.1111/j.1349-7006.2004.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, King MC. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250:1684–89. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 15.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G, Barfoot R, Hamoudi R, Patel S, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–92. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 16.Gayther SA, Warren W, Mazoyer S, Russell PA, Harrington PA, Chiano M, Seal S, Hamoudi R, van Rensburg EJ, Dunning AM, Love R, Evans G, Easton D, et al. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995;11:428–33. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- 17.Lubinski J, Phelan CM, Ghadirian P, Lynch HT, Garber J, Weber B, Tung N, Horsman D, Isaacs C, Monteiro AN, Sun P, Narod SA. Cancer variation associated with the position of the mutation in the BRCA2 gene. Fam Cancer. 2004;3:1–10. doi: 10.1023/B:FAME.0000026816.32400.45. [DOI] [PubMed] [Google Scholar]
- 18.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, LaPolla J, Hoffman M, Martino MA, Wakeley K, Wilbanks G, Nicosia S, Cantor A, Sutphen R. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 19.Geisler JP, Hatterman-Zogg MA, Rathe JA, Buller RE. Frequency of BRCA1 dysfunction in ovarian cancer. J Natl Cancer Inst. 2002;94:61–67. doi: 10.1093/jnci/94.1.61. [DOI] [PubMed] [Google Scholar]
- 20.Bell D, Berchuck A, Birrer M, Chien J, Cramer DW, Dao F, Dhir R, DiSaia P, Gabra H, Glenn P, Godwin AK, Gross J, Hartmann L, et al. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, Friedlander M, Fox S, Bowtell D, Mitchell G. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–28. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soegaard M, Kjaer SK, Cox M, Wozniak E, Høgdall E, Høgdall C, Blaakaer J, Jacobs IJ, Gayther SA, Ramus SJ. BRCA1 and BRCA2 mutation prevalence and clinical characteristics of a population-based series of ovarian cancer cases from Denmark. Clin Cancer Res. 2008;14:3761–67. doi: 10.1158/1078-0432.CCR-07-4806. [DOI] [PubMed] [Google Scholar]
- 24.Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–08. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 25.Belanger MH, Dolman L, Arcand SL, Shen Z, Chong G, Mes-Masson AM, Provencher D, Tonin PN. A targeted analysis identifies a high frequency of BRCA1 and BRCA2 mutation carriers in women with ovarian cancer from a founder population. J Ovarian Res. 2015;8:1. doi: 10.1186/s13048-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobias DH, Eng C, McCurdy LD, Kalir T, Mandelli J, Dottino PR, Cohen CJ. Founder BRCA 1 and 2 mutations among a consecutive series of Ashkenazi Jewish ovarian cancer patients. Gynecol Oncol. 2000;78:148–51. doi: 10.1006/gyno.2000.5848. [DOI] [PubMed] [Google Scholar]
- 27.Kim YC, Zhao L, Zhang H, Huang Y, Cui J, Xiao F, Downs B, Wang SM. Prevalence and spectrum of BRCA germline variants in mainland Chinese familial breast and ovarian cancer patients. Oncotarget. 2016;7:9600–12. doi: 10.18632/oncotarget.7144. https://doi.org/10.18632/oncotarget.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao WM, Gao Y, Yang HJ, Xie SN, Ding XW, Pan ZW, Ye WW, Wang XJ. Novel germline mutations and unclassified variants of BRCA1 and BRCA2 genes in Chinese women with familial breast/ovarian cancer. BMC Cancer. 2016;16:64. doi: 10.1186/s12885-016-2107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu XH, Wu L, Kong B, Liu J, Yin R, Wen H, Li N, Bu H, Feng Y, Li Q, Lu X, Wei J, Zhu X, et al. The first nationwide multicenter prevalence study of germline BRCA1 and BRCA2 mutations in Chinese ovarian cancer patients. Int J Gynecol Cancer. 2017;27:1650–1657. doi: 10.1097/IGC.0000000000001065. [DOI] [PubMed] [Google Scholar]
- 30.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casey MJ, Bewtra C, Hoehne LL, Tatpati AD, Lynch HT, Watson P. Histology of prophylactically removed ovaries from BRCA1 and BRCA2 mutation carriers compared with noncarriers in hereditary breast ovarian cancer syndrome kindreds. Gynecol Oncol. 2000;78:278–87. doi: 10.1006/gyno.2000.5861. [DOI] [PubMed] [Google Scholar]
- 32.Kurman RJ, Shih IM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151–60. doi: 10.1097/PGP.0b013e318161e4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih IM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–18. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 36.Bewtra C, Watson P, Conway T, Read-Hippee C, Lynch HT. Hereditary ovarian cancer: a clinicopathological study. Int J Gynecol Pathol. 1992;11:180–87. doi: 10.1097/00004347-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Lakhani SR, Manek S, Penault-Llorca F, Flanagan A, Arnout L, Merrett S, McGuffog L, Steele D, Devilee P, Klijn JG, Meijers-Heijboer H, Radice P, Pilotti S, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473–81. doi: 10.1158/1078-0432.ccr-1029-3. [DOI] [PubMed] [Google Scholar]
- 38.Maehle L, Apold J, Paulsen T, Hagen B, Løvslett K, Fiane B, Van Ghelue M, Clark N, Møller P. High risk for ovarian cancer in a prospective series is restricted to BRCA1/2 mutation carriers. Clin Cancer Res. 2008;14:7569–73. doi: 10.1158/1078-0432.CCR-08-0112. [DOI] [PubMed] [Google Scholar]
- 39.Vergote I, Bours V, Blaumeiser B, Baurain JF. New perspective on maintenance therapies for platinum- sensitive recurrent ovarian cancer in women with germline and somatic mutations in BRCA1 and BRCA2 genes. Facts Views Vis Obgyn. 2016;8:161–67. [PMC free article] [PubMed] [Google Scholar]
- 40.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, Jack E, Vesprini DJ, Kuperstein G, Abrahamson JL, Fan I, Wong B, Narod SA. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–10. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu G, Yang D, Sun Y, Shmulevich I, Xue F, Sood AK, Zhang W. Differing clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancer. Pharmacogenomics. 2012;13:1523–35. doi: 10.2217/pgs.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prat J, FIGO Committee on Gynecologic Oncology Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Schrader KA, Hurlburt J, Kalloger SE, Hansford S, Young S, Huntsman DG, Gilks CB, McAlpine JN. Germline BRCA1 and BRCA2 mutations in ovarian cancer: utility of a histology-based referral strategy. Obstet Gynecol. 2012;120:235–40. doi: 10.1097/AOG.0b013e31825f3576. [DOI] [PubMed] [Google Scholar]
- 44.Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A, Norquist B, Pennington KP, Garcia RL, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108:18032–37. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan RJ, Jr, Copeland L, Gershenson D, Locker G, McIntosh D, Ozols R, Teng N, The National Comprehensive Cancer Network NCCN Ovarian Cancer Practice Guidelines. Oncology (Williston Park) 1996;10(Suppl):293–310. [PubMed] [Google Scholar]
- 46.Morgan RJ, Jr, Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Behbakht K, Chen LM, Copeland L, Crispens MA, DeRosa M, Dorigo O, Gershenson DM, Gray HJ, Hakam A, et al. Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1134–63. doi: 10.6004/jnccn.2016.0122. [DOI] [PubMed] [Google Scholar]
- 47.McAlpine JN, Porter H, Köbel M, Nelson BH, Prentice LM, Kalloger SE, Senz J, Milne K, Ding J, Shah SP, Huntsman DG, Gilks CB. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol. 2012;25:740–50. doi: 10.1038/modpathol.2011.211. [DOI] [PubMed] [Google Scholar]
- 48.Ramus SJ, Bobrow LG, Pharoah PD, Finnigan DS, Fishman A, Altaras M, Harrington PA, Gayther SA, Ponder BA, Friedman LS. Increased frequency of TP53 mutations in BRCA1 and BRCA2 ovarian tumours. Genes Chromosomes Cancer. 1999;25:91–96. doi: 10.1002/(sici)1098-2264(199906)25:2<91::aid-gcc3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 49.Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E, Naora H. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122:3603–17. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly Z, Moller-Levet C, McGrath S, Butler-Manuel S, Kavitha Madhuri T, Kierzek AM, Pandha H, Morgan R, Michael A. The prognostic significance of specific HOX gene expression patterns in ovarian cancer. Int J Cancer. 2016;139:1608–17. doi: 10.1002/ijc.30204. [DOI] [PubMed] [Google Scholar]
- 51.Biglia N, Sgandurra P, Bounous VE, Maggiorotto F, Piva E, Pivetta E, Ponzone R, Pasini B. Ovarian cancer in BRCA1 and BRCA2 gene mutation carriers: analysis of prognostic factors and survival. Ecancermedicalscience. 2016;10:639. doi: 10.3332/ecancer.2016.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin SC, Benjamin I, Behbakht K, Takahashi H, Morgan MA, LiVolsi VA, Berchuck A, Muto MG, Garber JE, Weber BL, Lynch HT, Boyd J. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med. 1996;335:1413–16. doi: 10.1056/NEJM199611073351901. [DOI] [PubMed] [Google Scholar]
- 53.Lim JJ, Yang K, Taylor-Harding B, Wiedemeyer WR, Buckanovich RJ. VEGFR3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of BRCA1 and BRCA2. Neoplasia. 2014;16:343–53. doi: 10.1016/j.neo.2014.04.003. e1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–95. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 55.Hyman DM, Zhou Q, Iasonos A, Grisham RN, Arnold AG, Phillips MF, Bhatia J, Levine DA, Aghajanian C, Offit K, Barakat RR, Spriggs DR, Kauff ND. Improved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancer. Cancer. 2012;118:3703–09. doi: 10.1002/cncr.26655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res. 2015;21:211–20. doi: 10.1158/1078-0432.CCR-14-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun C, Li N, Ding D, Weng D, Meng L, Chen G, Ma D. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285. doi: 10.1371/journal.pone.0095285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams SF, Marsh EB, Elmasri W, Halberstadt S, Vandecker S, Sammel MD, Bradbury AR, Daly M, Karlan B, Rubin SC. A high response rate to liposomal doxorubicin is seen among women with BRCA mutations treated for recurrent epithelial ovarian cancer. Gynecol Oncol. 2011;123:486–91. doi: 10.1016/j.ygyno.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, Matei D, Macpherson E, Watkins C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–92. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 60.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CL, Meier W, Shapira-Frommer R, Safra T, Matei D, Fielding A, Spencer S, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 61.Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, Colombo N, Špaček J, Vuylsteke P, Hirte H, Mahner S, Plante M, Schmalfeldt B, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16:87–97. doi: 10.1016/S1470-2045(14)71135-0. [DOI] [PubMed] [Google Scholar]
- 62.Monk BJ, Ghatage P, Parekh T, Henitz E, Knoblauch R, Matos-Pita AS, Nieto A, Park YC, Cheng PS, Li W, Favis R, Ricci D, Poveda A. Effect of BRCA1 and XPG mutations on treatment response to trabectedin and pegylated liposomal doxorubicin in patients with advanced ovarian cancer: exploratory analysis of the phase 3 OVA-301 study. Ann Oncol. 2015;26:914–20. doi: 10.1093/annonc/mdv071. [DOI] [PubMed] [Google Scholar]
- 63.Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, Rimel B, Buss MK, Nattam S, Hurteau J, Luo W, Quy P, Whalen C, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–14. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.du Bois A, Neijt JP, Thigpen JT. First line chemotherapy with carboplatin plus paclitaxel in advanced ovarian cancer—a new standard of care? Ann Oncol. 1999;10(Suppl 1):35–41. doi: 10.1023/a:1008355317514. [DOI] [PubMed] [Google Scholar]
- 65.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, Agnew KJ, Pritchard CC, Scroggins S, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–75. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vencken PM, Kriege M, Hoogwerf D, Beugelink S, van der Burg ME, Hooning MJ, Berns EM, Jager A, Collée M, Burger CW, Seynaeve C. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011;22:1346–52. doi: 10.1093/annonc/mdq628. [DOI] [PubMed] [Google Scholar]
- 67.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, Boyd J, Reis-Filho JS, Ashworth A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–15. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 68.Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, Villegas E, Jacquemont C, Farrugia DJ, Couch FJ, Urban N, Taniguchi T. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–20. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA, Gynecologic Oncology Group Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 70.Kwa M, Edwards S, Downey A, Reich E, Wallach R, Curtin J, Muggia F. Ovarian cancer in BRCA mutation carriers: improved outcome after intraperitoneal (IP) cisplatin. Ann Surg Oncol. 2014;21:1468–73. doi: 10.1245/s10434-013-3277-y. [DOI] [PubMed] [Google Scholar]
- 71.Safra T, Grisaru D, Inbar M, Abu-Abeid S, Dayan D, Matceyevsky D, Weizman A, Klausner JM. Cytoreduction surgery with hyperthermic intraperitoneal chemotherapy in recurrent ovarian cancer improves progression-free survival, especially in BRCA-positive patients- a case-control study. J Surg Oncol. 2014;110:661–65. doi: 10.1002/jso.23688. [DOI] [PubMed] [Google Scholar]
- 72.Lesnock JL, Darcy KM, Tian C, Deloia JA, Thrall MM, Zahn C, Armstrong DK, Birrer MJ, Krivak TC. BRCA1 expression and improved survival in ovarian cancer patients treated with intraperitoneal cisplatin and paclitaxel: a Gynecologic Oncology Group Study. Br J Cancer. 2013;108:1231–37. doi: 10.1038/bjc.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oskay-Oezcelik G, Koensgen D, Hindenburg HJ, Klare P, Schmalfeldt B, Lichtenegger W, Chekerov R, Al-Batran SE, Neumann U, Sehouli J, Ovarian Cancer Study Group of the North-Eastern Germany Society of Gynecological Oncology (NOGGO) Biweekly pegylated liposomal doxorubicin as second-line treatment in patients with relapsed ovarian cancer after failure of platinum and paclitaxel: results from a multi-center phase II study of the NOGGO. Anticancer Res. 2008;28:1329–34. [PubMed] [Google Scholar]
- 74.Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, Amnon A, Bell-McGuinn KM, Chen LM, Friedlander M, Safra T, Vergote I, Wickens M, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30:372–79. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 75.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M, Dobbs S, Essapen S, Twigg J, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–57. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 76.Gorodnova TV, Sokolenko AP, Ivantsov AO, Iyevleva AG, Suspitsin EN, Aleksakhina SN, Yanus GA, Togo AV, Maximov SY, Imyanitov EN. High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation. Cancer Lett. 2015;369:363–67. doi: 10.1016/j.canlet.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 77.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 78.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, Yerushalmi R, Macpherson E, Carmichael J, Oza A. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–61. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 79.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–17. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 80.Deeks ED. Olaparib: first global approval. Drugs. 2015;75:231–40. doi: 10.1007/s40265-015-0345-6. [DOI] [PubMed] [Google Scholar]
- 81.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 82.Roskoski R., Jr Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol. 2007;62:179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Ledermann JA, Embleton AC, Raja F, Perren TJ, Jayson GC, Rustin GJ, Kaye SB, Hirte H, Eisenhauer E, Vaughan M, Friedlander M, González-Martín A, Stark D, et al. ICON6 collaborators Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387:1066–74. doi: 10.1016/S0140-6736(15)01167-8. [DOI] [PubMed] [Google Scholar]
- 84.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, Yi J, Nycum LR. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dean E, Middleton MR, Pwint T, Swaisland H, Carmichael J, Goodege-Kunwar P, Ranson M. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer. 2012;106:468–74. doi: 10.1038/bjc.2011.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Unni SK, Schauerhamer MB, Deka R, Tyczynski JE, Fernandes AW, Stevens V, Brixner DI, Stenehjem DD. BRCA testing, treatment patterns and survival in platinum-sensitive recurrent ovarian cancer - an observational cohort study. J Ovarian Res. 2016;9:18. doi: 10.1186/s13048-016-0227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cibula D, Zikan M, Dusek L, Majek O. Oral contraceptives and risk of ovarian and breast cancers in BRCA mutation carriers: a meta-analysis. Expert Rev Anticancer Ther. 2011;11:1197–207. doi: 10.1586/era.11.38. [DOI] [PubMed] [Google Scholar]
- 88.Iodice S, Barile M, Rotmensz N, Feroce I, Bonanni B, Radice P, Bernard L, Maisonneuve P, Gandini S. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275–84. doi: 10.1016/j.ejca.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 89.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, Ellis NA, Boyd J, Borgen PI, Barakat RR, Norton L, Castiel M, Nafa K, Offit K. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–15. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 90.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, Isaacs C, Evans DG, Lynch H, Eeles RA, Neuhausen SL, Daly MB, Matloff E, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26:1331–37. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, Garber JE, Evans G, Isaacs C, Daly MB, Matloff E, Olopade OI, Weber BL, Prevention and Observation of Surgical End Points Study Group Prophylactic oophorectomy in carriers of BRCA1 or BRCA2mutations. N Engl J Med. 2002;346:1616–22. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 92.McGuire V, Felberg A, Mills M, Ostrow KL, DiCioccio R, John EM, West DW, Whittemore AS. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol. 2004;160:613–18. doi: 10.1093/aje/kwh284. [DOI] [PubMed] [Google Scholar]
- 93.Tsilidis KK, Allen NE, Key TJ, Dossus L, Lukanova A, Bakken K, Lund E, Fournier A, Overvad K, Hansen L, Tjønneland A, Fedirko V, Rinaldi S, et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2011;105:1436–42. doi: 10.1038/bjc.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whittemore AS, Balise RR, Pharoah PD, Dicioccio RA, Oakley-Girvan I, Ramus SJ, Daly M, Usinowicz MB, Garlinghouse-Jones K, Ponder BA, Buys S, Senie R, Andrulis I, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911–15. doi: 10.1038/sj.bjc.6602239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stewart LM, Holman CD, Finn JC, Preen DB, Hart R. In vitro fertilization is associated with an increased risk of borderline ovarian tumours. Gynecol Oncol. 2013;129:372–76. doi: 10.1016/j.ygyno.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 96.Song H, Dicks E, Ramus SJ, Tyrer JP, Intermaggio MP, Hayward J, Edlund CK, Conti D, Harrington P, Fraser L, Philpott S, Anderson C, Rosenthal A, et al. Contribution of Germline Mutations in the RAD51B, RAD51C, and RAD51D Genes to Ovarian Cancer in the Population. J Clin Oncol. 2015;33:2901–07. doi: 10.1200/JCO.2015.61.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, Szallasi Z, Barry WT, Winer EP, Tung NM, Isakoff SJ, Ryan PD, Greene-Colozzi A, et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin Cancer Res. 2016;22:3764–73. doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Mądry R, et al. ENGOT-OV16/NOVA Investigators Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375:2154–64. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 99.Drew Y, Ledermann J, Hall G, Rea D, Glasspool R, Highley M, Jayson G, Sludden J, Murray J, Jamieson D, Halford S, Acton G, Backholer Z, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br J Cancer. 2016;114:723–30. doi: 10.1038/bjc.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Powell SN, Kachnic LA. Therapeutic exploitation of tumor cell defects in homologous recombination. Anticancer Agents Med Chem. 2008;8:448–60. doi: 10.2174/187152008784220267. [DOI] [PubMed] [Google Scholar]
- 101.Jaspers JE, Kersbergen A, Boon U, Sol W, van Deemter L, Zander SA, Drost R, Wientjens E, Ji J, Aly A, Doroshow JH, Cranston A, Martin NM, et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013;3:68–81. doi: 10.1158/2159-8290.CD-12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juvekar A, Burga LN, Hu H, Lunsford EP, Ibrahim YH, Balmañà J, Rajendran A, Papa A, Spencer K, Lyssiotis CA, Nardella C, Pandolfi PP, Baselga J, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2:1048–63. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]