Abstract
Young adults (aged 18 to 39 years) have the lowest hypertension control rates compared with older adults. Shorter follow‐up encounter intervals are associated with faster hypertension control rates in older adults; however, optimal intervals are unknown for young adults. The study objective was to evaluate the relationship between ambulatory blood pressure encounter intervals (average number of provider visits with blood pressures over time) and hypertension control rates among young adults with incident hypertension. A retrospective analysis was conducted of patients aged 18 to 39 years (n = 2990) with incident hypertension using Kaplan‐Meier survival and Cox proportional hazards analyses over 24 months. Shorter encounter intervals were associated with higher hypertension control: <1 month (91%), 1 to 2 months (76%), 2 to 3 months (65%), 3 to 6 months (40%), and >6 months (13%). Young adults with shorter encounter intervals also had lower medication initiation, supporting the effectiveness of lifestyle modifications. Sustainable interventions for timely young adult follow‐up are essential to improve hypertension control in this hard‐to‐reach population.
Keywords: clinical management of high blood pressure, hypertension‐general, primary care issues
1. INTRODUCTION
Hypertension is a potentially reversible contributor to more than 400 000 deaths annually in the United States.1 Historic blood pressures (BPs) predict the incidence of future cardiovascular events. Conversely, hypertension control can decrease rates of cardiovascular morbidity and mortality.2 Approximately 20% of young adult men and 15% of young adult women (aged 18 to 39 years)3 have hypertension.4 Unfortunately, hypertension is an underrecognized cardiovascular risk factor in young adults, contributing to premature heart failure, strokes, and chronic kidney disease.5, 6, 7 Overall, young adults have the lowest hypertension control rates when compared with middle‐aged and older adults,8 with <40% of young adults with hypertension achieving BP control.
Our previous studies demonstrated that young adults have lower rates of receiving an initial hypertension diagnosis and achieving hypertension control compared with older populations.8, 9 Despite quality measures for hypertension care,10 the optimal return encounter interval (average number of provider‐patient BP encounters over time) to achieve hypertension control among young adults remains unknown.11, 12 Encounter intervals also vary significantly between providers.13, 14, 15 Ambulatory encounters increase opportunities to reinforce hypertension lifestyle modifications, address patients' concerns, and initiate and/or titrate antihypertensive medication if necessary.16 Prior research demonstrated that shorter visit intervals were positively associated with hypertension control among middle‐aged adults (mean age 54.7±14.3 years). However, optimal BP encounter intervals or interventions have not been identified for young adults, a high‐risk population with longer exposure to high BPs.11 To address this critical gap in hypertension care among young adults, our objective was to evaluate the relationship of ambulatory encounter intervals and rates of hypertension control among young adults with incident hypertension.
2. METHODS
2.1. Sample
The University of Wisconsin‐Madison Health Sciences institutional review board approved this study with a waiver of written informed consent. This retrospective cohort analysis used electronic health record data of patients with uncontrolled hypertension from a large, Midwestern, multidisciplinary academic group practice. To construct the sample (Figure 1), we identified all patients 18 to 39 years who met criteria from the Wisconsin Collaborative for Healthcare Quality (WCHQ)17, 18 for being “currently managed” in the healthcare system between January 1, 2008, and December 31, 2011. WCHQ is a voluntary consortium of Wisconsin healthcare organizations committed to publicly reporting performance measures of quality and affordability of healthcare services.19 Per WCHQ criteria, eligible currently managed patients had to have two or more billable office encounters in an outpatient, nonurgent, primary care setting, or one primary care and one office encounter in an urgent care setting, in the 3 years prior to study enrollment, with at least one visit in the prior 2 years.20 Electronic health records were assessed for the date a patient met the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) criteria for a new diagnosis of hypertension1 (incident hypertension), meaning they had not received a previous diagnosis of or treatment for hypertension. JNC 7 criteria were used because they were the established US hypertension guidelines during the reporting period. A patient was determined as meeting hypertension eligibility criteria based on electronic health record data if there were: (1) three or more elevated outpatient BP measurements from three separate dates, ≥30 days apart, but within a 2‐year span (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg) or (2) two elevated BPs21, 22 (systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg), ≥30 days apart within a 2‐year period.8, 9, 23, 24, 25 If more than one BP was taken at a visit, the average was used.8 Hospital and emergency department BPs were excluded to avoid falsely elevated BPs. After meeting criteria for incident hypertension, patients were then excluded if they did not receive an electronic health record diagnosis of hypertension based on Tu criteria26 or if they had less than 6 months of follow‐up (Figure 1). The Tu algorithm for administrative data is used to define patients who have been diagnosed with hypertension using the following International Classification of Diseases, Ninth Revision (ICD‐9) codes:27 401.x (essential hypertension), 402.x (hypertensive heart disease), 403.x (hypertensive renal disease), 404.x (hypertensive heart and renal disease), and 405.x (secondary hypertension).
Figure 1.
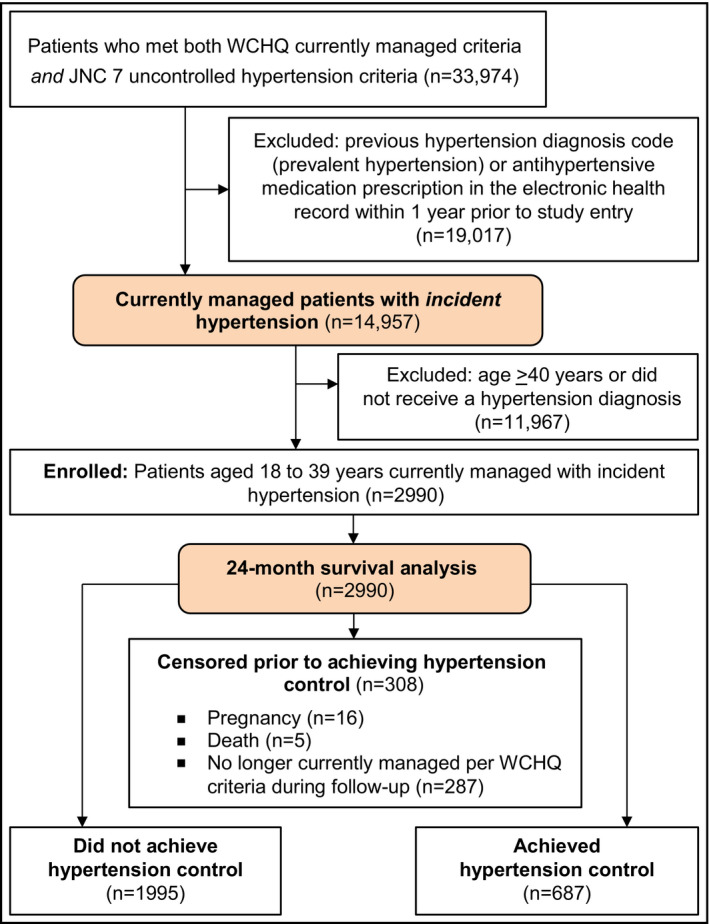
Study sample: enrollment and analysis. WCHQ indicates Wisconsin Collaborative for Healthcare Quality; JNC 7, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure
Each patient who met all eligibility criteria received an “index date” (the first date all criteria were met). A 365‐day period prior to this index date was the “baseline period” to assess patients' comorbidities and healthcare utilization. Patients were followed for 24 months to account for less frequent ambulatory visits among younger populations8 (eg, patients who may have returned solely for semiannual physicals). Patients continued to accrue time in the study from the index date until they achieved the primary outcome (hypertension control) or censoring occurred (death, end of primary care management, pregnancy, or end of study). Censoring for “end of primary care management” accounted for disruptions in healthcare access in this young population (eg, change in insurance, residence). Patients who were pregnant during the study were excluded 1 year before, during, and 1 year following pregnancy using a modified Manson approach28 (n = 16; 0.54%). The final sample included 2990 currently managed young adults with incident hypertension (Figure 1).
2.2. Primary explanatory variable
The return encounter intervals were calculated as visits over 24 months and categorized according to prior methodology:12 <1 month between visits, 1 to 2 months, 2 to 3 months, 3 to 6 months, and >6 months between visits. Ambulatory return visits required a BP entry into the electronic health record for that visit and included physicians (faculty, resident, fellow), nurse practitioners, and physician assistants in primary care clinics (defined in this academic center as Family Medicine/Family Practice, Internal Medicine, Obstetrics/Gynecology, and Pediatrics/Adolescent Medicine). Urgent care and emergency department BPs were not included for cohort development or for study follow‐up; the goal was to decrease the inclusion of BPs during acute illness/injury and to reflect routine ambulatory primary care.
2.3. Primary outcome variable
The primary outcome was time (days) from the index date to achieving hypertension control, defined as the first of three consecutive normal BPs (<140/90 mm Hg) on three separate dates.24 To account for BP variability, multiple clinic BPs were used to define hypertension control since 24‐hour ambulatory BP monitoring data were not available. Results are reported in months.
2.4. Other explanatory variables
Patient and provider variables to examine barriers to hypertension control were selected based on an established conceptual model for clinical inertia.29 Patient‐related factors included sociodemographics (age, sex, race/ethnicity, marital status, and Medicaid use during the baseline or study period), behavioral risk factors (baseline tobacco use and body mass index [BMI]), and comorbidities. Patients' race/ethnicity was included because of the increased prevalence of hypertension among young black patients.30 All of the patients self‐classified their race/ethnicity in the electronic health record (white, black, Asian, Hispanic/Latino, other [Native Hawaiian, Pacific Islander, multiracial], or unknown). Patient comorbidities were assessed at baseline using the following established algorithms: hyperlipidemia (ICD‐9 codes: 272.0‐272.4),31 diabetes mellitus with/without complications (ICD‐9 codes: 250.00–250.93, 357.2, 362.0–362.02, 366.41),32 chronic kidney disease (ICD‐9 codes: 016.0, 095.4,189.0, 189.9, 223.0, 236.91, 250.4, 271.4, 274.1, 283.11, 403.X1, 404.X2, 404.X3, 440.1, 442.1, 447.3, 572.4, 580‐588, 591, 642.1, 646.2, 753.12‐753.17, 753.19, 753.2, 794.4),33 and mental health conditions (depression [ICD‐9 codes: 296.2X, 296.3X, 300.4X]34 and anxiety [ICD‐9 codes: 300.0–300.02, 300.09, 300.21–300.23, 300.3, 309.24, 309.81]).34 Elixhauser and the Medicare Chronic Condition Data Warehouse Administrative algorithms were used to identify chronic pulmonary disease,35 stroke/transient ischemic attack,36 rheumatoid arthritis,37 inflammatory bowel diseases,37 thyroid diseases,35 and deficiency anemias.35 We created an indicator variable for the presence of any of these conditions because of their low prevalence.
Patients' morbidity burden can predict healthcare utilization, which may influence diagnosis and antihypertensive medication initiation rates.38, 39 Therefore, we used the Johns Hopkins Adjusted Clinical Group (ACG) case‐mix system (version 10.0), which assesses morbidity burden to predict future healthcare utilization.39, 40 The ACG risk score was selected because our study sample contains a diverse mix of government‐insured and privately insured ambulatory young adults. An ACG risk score of 1.0 represents expected healthcare utilization on an individual level according to the patient's age and sex.40 The number of primary care, specialty, and urgent care visits were measured in the baseline period. Primary care visits included physician, nurse practitioner, and physician assistant visits in Family Medicine/Family Practice, Internal Medicine, and lower prevalence primary care specialties (Obstetrics/Gynecology, Pediatrics/Adolescent Medicine) to reflect broader primary care options in this younger population.
Patients were assigned to the primary care provider they saw most frequently in outpatient face‐to‐face evaluation and management visits, as reported in professional service claims.20 Statistical models additionally controlled for providers' age, specialty (Internal Medicine, Family Medicine/Family Practice, Other), and sex, which were obtained from the provider group's human resource office and/or the American Medical Association (AMA) 2011 Masterfile data.
2.5. Statistical analysis
Analyses were conducted using SAS version 9.1.3 (SAS Institute, Inc.) and Stata version 13.1 (StataCorp). Baseline comparisons between individuals with different encounter intervals were performed using analysis of variance for continuous variables and chi‐square test for categorical descriptive statistics. Univariate Kaplan‐Meier survival curves41 were computed by encounter interval (<1 month, 1–2 months, 2–3 months, 3–6 months, and >6 months) to evaluate the probability of achieving hypertension control, as a function of time since meeting criteria for incident hypertension. Multivariate Cox proportional hazards regression analyses were conducted to obtain adjusted hazard ratios and 95% confidence intervals (CIs) for achieving hypertension control. Robust estimates of variance were used to account for within‐cluster correlation.42, 43 Explanatory variables used in the Cox regression models include patient sociodemographics (age, sex, race, Medicaid use), baseline comorbidities (dyslipidemia, diabetes mellitus, anxiety and/or depression, low prevalence conditions), behavioral risk factors (BMI, tobacco status), healthcare utilization (ACG risk score, baseline visit count), and provider characteristics (specialty). Statistical significance was defined as P < .002 after Bonferroni correction for multiple comparisons.44 The proportional hazards assumption for each model was tested using a generalized linear regression of the scaled Schoenfeld residuals on functions of time.45
2.6. Data availability
The data set generated and analyzed during the current study is not publicly available as a result of the data use agreement, but is available from the corresponding author upon reasonable request and necessary approvals.
3. RESULTS
3.1. Descriptive data
Overall, 2990 patients met inclusion criteria (Figure 1). Table 1 summarizes the study population by average encounter intervals of <1 month, 1 to 2 months, 2 to 3 months, 3 to 6 months, and >6 months. Among the study population of young adults with incident hypertension (mean age, 32 [5.4] years; 59% male), 77% had stage 1 (mild) hypertension, 64% were obese (BMI ≥30 kg/m2), and 58% were seen in a Family Medicine or Family Practice clinic. Overall, 13% of young adults had an encounter interval of <1 month, 19% had an encounter interval of 1 to 2 months, 16% an encounter interval of 2 to 3 months, 26% an encounter interval of 3 to 6 months, and 26% an encounter interval of >6 months. Young adults with the shortest encounter interval (<1 month) were more likely to be women (59%), have stage 1 (mild) hypertension, report Medicaid use, have comorbidities (hyperlipidemia, diabetes mellitus, mental health diagnoses), have higher ACG risk score, and a female provider. The mean number of annual primary care visits during a 12‐month calendar year, by encounter interval were: 3.0 (standard deviation [SD], 2.5) visits within the <1‐month interval, 3.4 (SD, 2.2) visits within the 1‐ to 2‐month interval, 3.0 (SD, 1.8) visits in the 2‐ to 3‐month interval, 2.4 (SD, 1.5) visits in the 3‐ to 6‐month interval, and 1.6 (SD, 1.1) visits in the >6‐month interval. There was a similar relationship between mean number of specialty visits and encounter intervals: 2.0 (SD, 1.9) specialty visits within the <1‐month interval, 1.7 (SD, 1.7) visits in the 1‐ to 2‐month interval, 1.3 (SD, 1.4) in the 2‐ to 3‐month interval, 0.93 (SD, 1.1) in the 3‐ to 6‐month interval, and 0.53 (SD, 0.74) in the >6‐month interval. During 24 months of follow‐up, patients were censored as a result of death (n = 5; 0.17%) and if they were no longer currently managed by the healthcare system (n = 287; 9.6%).
Table 1.
Baseline demographics of young adults (aged 18 to 39 years) with incident hypertension by encounter interval (N = 2990)
| Total population (N = 2990) | By encounter interval | ||||||
|---|---|---|---|---|---|---|---|
| <1 mo (n = 402) | 1–2 mo (n = 568) | 2–3 mo (n = 463) | 3–6 mo (n = 768) | >6 mo (n = 789) | P value | ||
| Patient characteristics | |||||||
| Age, mean (SD) | 32 (5.4) | 32 (5.1) | 32 (5.4) | 32 (5.2) | 32 (5.4) | 31 (5.6) | .06 |
| Men, No. (%) | 1763 (59) | 164 (41) | 254 (45) | 263 (57) | 489 (64) | 593 (75) | <.001 |
| Race/ethnicity, No. (%) | <.001 | ||||||
| White | 2474 (83) | 313 (78) | 460 (81) | 370 (80) | 650 (85) | 681 (86) | |
| Nonwhitea | 516 (17) | 89 (22) | 108 (19) | 93 (20) | 118 (15) | 108 (14) | |
| Marital status, No. (%) | .06 | ||||||
| Single/divorced/widowed | 1642 (55) | 227 (56) | 337 (59) | 260 (56) | 399 (52) | 419 (53) | |
| Married/partnered | 1348 (45) | 175 (44) | 231 (41) | 203 (44) | 369 (48) | 370 (47) | |
| Primary spoken language, No. (%) | <.001 | ||||||
| English | 2738 (92) | 370 (92) | 547 (96) | 441 (95) | 723 (94) | 657 (83) | |
| Other | 252 (8.4) | 32 (8.0) | 21 (3.7) | 22 (4.8) | 45 (5.9) | 132 (17) | |
| Tobacco use, No. (%) | .16 | ||||||
| Current tobacco use | 660 (22) | 94 (23) | 134 (24) | 114 (25) | 148 (19) | 170 (22) | |
| Never/former tobacco use | 2330 (78) | 308 (77) | 434 (76) | 349 (75) | 620 (81) | 619 (78) | |
| BMI, mean (SD), kg/m2 | 33 (8.6) | 33 (9.0) | 34 (9.1) | 34 (9.4) | 33 (8.2) | 33 (7.7) | .18 |
| BMI categories, No. (%) | .66 | ||||||
| BMI <25 kg/m2 | 361 (12) | 58 (14) | 75 (13) | 51 (11) | 85 (11) | 92 (12) | |
| BMI 25–29 kg/m2 | 724 (24) | 97 (24) | 141 (25) | 119 (26) | 187 (24) | 180 (23) | |
| BMI ≥30 kg/m2 | 1905 (64) | 247 (61) | 352 (62) | 293 (63) | 496 (65) | 517 (66) | |
| On Medicaid ever,b No. (%) | 524 (18) | 117 (29) | 134 (24) | 91 (20) | 101 (13) | 81 (10) | <.001 |
| JNC 7 hypertension stage,c No. (%) | <.001 | ||||||
| Stage 1: 140–159/90–99 mm Hg | 2317 (77) | 337 (84) | 456 (80) | 365 (79) | 597 (78) | 562 (71) | |
| Stage 2: ≥160–179/≥100 mm Hg | 673 (23) | 65 (16) | 112 (20) | 98 (21) | 171 (22) | 227 (29) | |
| Baseline SBP tertiles, No. (%) | <.001 | ||||||
| Lowest SBP tertile | 1051 (35) | 173 (43) | 217 (38) | 173 (37) | 242 (32) | 246 (31) | |
| Middle SBP tertile | 943 (32) | 126 (31) | 171 (30) | 152 (33) | 250 (33) | 244(31) | |
| Highest SBP tertile | 996 (33) | 103 (26) | 180 (32) | 138 (29) | 276 (36) | 299(38) | |
| Baseline DBP tertiles, No. (%) | .02 | ||||||
| Lowest DBP tertile | 1079 (36) | 157 (39) | 195 (34) | 171 (37) | 274 (36) | 282 (36) | |
| Middle DBP tertile | 993 (33) | 148 (37) | 202 (36) | 149 (32) | 262 (34) | 232 (29) | |
| Highest DBP tertile | 918 (31) | 97 (24) | 171 (30) | 143 (31) | 232 (30) | 275 (35) | |
| Baseline comorbid conditions, No. (%) | |||||||
| Hyperlipidemia | 240 (8.0) | 49 (12) | 38 (6.7) | 45 (9.7) | 64 (8.3) | 44 (5.6) | .001 |
| Diabetes mellitus | 93 (3.1) | 19 (4.7) | 20 (3.5) | 20 (4.3) | 25 (3.3) | 9 (1.1) | .003 |
| Anxiety and/or depression | 738 (25) | 166 (41) | 173 (30) | 112 (24) | 163 (21) | 124 (16) | <.001 |
| Low prevalence conditionsd | 335 (11) | 88 (22) | 89 (16) | 59 (13) | 64 (8.3) | 35 (4.4) | <.001 |
| ACGe score, young, mean (SD) | 1.1 (1.2) | 1.8 (1.9) | 1.3 (1.4) | 1.1 (1.2) | 0.8 (0.9) | 0.7 (0.6) | <.001 |
| ACGe score tertiles, young, No. (%) | <.001 | ||||||
| Lowest ACG tertile | 1035 (35) | 52 (13) | 119 (21) | 129 (28) | 323 (42) | 412 (52) | |
| Middle ACG tertile | 959 (32) | 124 (31) | 180 (32) | 160 (35) | 252 (33) | 243 (31) | |
| Highest ACG tertile | 996 (33) | 226 (56) | 269 (47) | 174 (38) | 193 (25) | 134 (17) | |
| Baseline annual ambulatory visit count, all visits, mean (SD) | |||||||
| Primary care visits | 2.5 (2.5) | 4.2 (3.6) | 2.9 (2.5) | 2.5 (2.3) | 2.0 (1.9) | 1.7 (1.7) | <.001 |
| Specialty care visits | 1.3 (1.8) | 2.6 (2.9) | 1.5 (1.9) | 1.2 (1.6) | 1.0 (1.3) | 0.7 (1.0) | <.001 |
| Urgent care visits | 0.9 (1.4) | 1.6 (2.1) | 1.0 (1.5) | 0.9 (1.4) | 0.7 (1.2) | 0.6 (0.9) | <.001 |
| Provider characteristics | |||||||
| Specialty providing majority of ambulatory care, No. (%) | .02 | ||||||
| Internal medicine | 828 (28) | 121 (30) | 186 (33) | 130 (28) | 203 (26) | 188 (24) | |
| Family medicine/family practice | 1742 (58) | 231 (57) | 317 (56) | 268 (58) | 444 (58) | 482 (61) | |
| Otherf | 420 (14) | 50 (12) | 65 (11) | 65 (14) | 121 (16) | 119 (15) | |
| Provider age,g mean (SD) | 43 (10) | 43 (10) | 43 (11) | 43 (11) | 43 (10) | 43 (10) | .42 |
| Female provider, No. (%) | 1361 (46) | 216 (54) | 300 (53) | 226 (49) | 322 (42) | 297 (38) | <.001 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; JNC 7, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; SBP, systolic blood pressure. Bolded P values are statistically significant.
Nonwhite: black (8.3%), Hispanic/Latino (3.2%), Asian (1.6%), Native Hawaiian/Pacific Islander (1.0%), American Indian/Alaska Native (0.54), and unknown (2.8%).
On Medicaid at any point during the baseline or study period.
Severity of blood pressure elevation at study entry.
Chronic kidney disease (0.6%), neurologic conditions (1.9%), anemia (0.6%), chronic pulmonary disease (5.2%), thyroid disorders (2.3%), rheumatologic disorders (0.5%), and inflammatory bowel disease (0.4%).
Adjusted Clinical Group (ACG) case‐mix assessment system.
Pediatrics/adolescent medicine and obstetrics/gynecology.
The American Medical Association (AMA) is the source for the raw physician data (provider ages only); statistics, tables, or tabulations were prepared by User‐Customer (M. Smith; PI: H. Johnson) using 2011 AMA Masterfile data.
3.2. Incident hypertension control rates by encounter interval
Among all patients aged 18 to 39 years, 52% (n = 1543) achieved hypertension control within 24 months after meeting criteria for incident hypertension. The Kaplan‐Meier curve (Figure 2) demonstrated that young adults with a <1‐month encounter frequency had the highest rates of hypertension control (91%), compared with young adults with longer encounter intervals: 1 to 2 months (76%), 2 to 3 months (65%), 3 to 6 months (40%), and >6 months (13%). The median (25th–75th percentile) time in months to hypertension control by encounter interval was: <1‐month encounter interval (2.8 [95% CI, 1.8–3.9] months to control), 1‐ to 2‐month interval (7.1 [95% CI, 5.1–11.3] months), 2‐ to 3‐month interval (10.5 [95% CI, 8.5–14.4] months), 3‐ to 6‐month interval (16.4 [95% CI 12.4–22.6] months), and >6‐month interval (23.9 [95% CI, 22.5–24.1] months).
Figure 2.
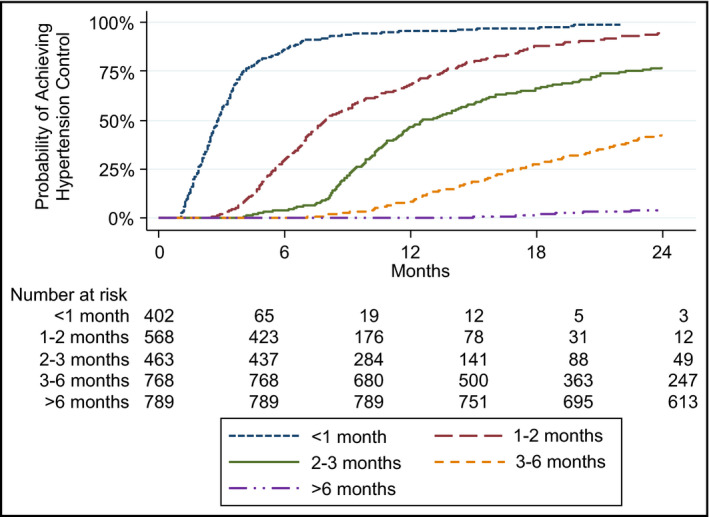
Probability of achieving hypertension control by encounter interval
3.3. Predictors of time to hypertension control by encounter interval
Unadjusted Cox proportional hazards models (Table 2) demonstrated that young adults with incident hypertension had a significantly lower rate of achieving hypertension control as the encounter interval increased. After adjusting for patient demographics, tobacco use, BMI, and comorbidities, young adults seen at the 1‐ to 2‐month interval had a 76% lower rate of achieving hypertension control (hazard ratio, 0.24; 95% CI, 0.18–0.31) and an even lower rate in the >6‐month interval (hazard ratio, 0.008; 95% CI, 0.005–0.011). Current tobacco use, obesity (BMI ≥30 kg/m2) and a high ACG risk score predicted a lower likelihood of achieving hypertension control at P < .05, but not after applying the conservative Bonferroni correction. Young adults with higher baseline systolic and diastolic BP had a significantly lower likelihood of achieving hypertension control. Provider factors (age, sex, and specialty) were not significant predictors for hypertension control.
Table 2.
HRs and 95% CIs of independent predictors for achieving hypertension control in patients aged 18 to 39 years (N = 2990)
| Variable | Unadjusted HR | Unadjusted 95% CI | P valuea | Adjusted HR | Adjusted 95% CI | P valuea |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Encounter interval | ||||||
| <1 mo (reference) | ‐ | ‐ | 1.00 | ‐ | ‐ | |
| 1–2 mo | 0.25 | (.22–.29) | <0.001 | .24 | (.18–.31) | <.001 |
| 2–3 mo | 0.12 | (.10–.13) | <0.001 | .11 | (.08–.14) | <.001 |
| 3–6 mo | 0.04 | (.03–.05) | <0.001 | .04 | (.03–.05) | <.001 |
| >6 mo | 0.008 | (.007–.010) | <0.001 | .008 | (.005–.011) | <.001 |
| Patient age | ||||||
| Lowest age tertile (reference) | 1.00 | ‐ | ‐ | |||
| Middle age tertile | 1.10 | (.94–1.29) | .25 | |||
| Highest age tertile | 0.88 | (.74–1.05) | .17 | |||
| Male sex | 0.97 | (.85–1.10) | .60 | |||
| Race/ethnicityb | ||||||
| White (reference) | 1.00 | ‐ | ‐ | |||
| Nonwhite | 0.84 | (.69‐1.03) | .10 | |||
| Tobacco use | ||||||
| Current tobacco use | 0.82 | (.68‐.98) | .03 | |||
| Never/former tobacco use (reference) | 1.00 | ‐ | ‐ | |||
| BMI, kg/m2 | ||||||
| BMI <25 kg/m2 (reference) | 1.00 | ‐ | ‐ | |||
| BMI 25–29 kg/m2 | 0.90 | (.73–1.12) | .35 | |||
| BMI ≥30 kg/m2 | 0.81 | (.68–.98) | .03 | |||
| On Medicaid everc | 0.96 | (.79–1.17) | .69 | |||
| Baseline SBP tertiles | ||||||
| Lowest SBP tertile (reference) | 1.00 | – | – | |||
| Middle SBP tertile | 0.71 | (.60–.84) | <.001 | |||
| Highest SBP tertile | 0.64 | (.53–.76) | <.001 | |||
| Baseline DBP tertiles | ||||||
| Lowest DBP tertile (reference) | 1.00 | – | – | |||
| Middle DBP tertile | 0.85 | (.72–.99) | .04 | |||
| Highest DBP tertile | 0.64 | (.53–.76) | <.001 | |||
| Baseline comorbid conditions | ||||||
| Hyperlipidemia | 1.09 | (.90–1.32) | .36 | |||
| Diabetes mellitus | 1.19 | (.88–1.59) | .26 | |||
| Anxiety and/or depression | 0.90 | (.77–1.06) | .20 | |||
| Low prevalence conditionsd | 1.06 | (.88–1.28) | .52 | |||
| ACGe risk score | ||||||
| Lowest ACG tertile (reference) | 1.00 | – | – | |||
| Middle ACG tertile | 1.10 | (.92–1.33) | .30 | |||
| Highest ACG tertile | 1.29 | (1.04–1.60) | .02 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; HR, hazard ratio; SBP, systolic blood pressure.
P value: Bonferroni's conservative correction was applied (Bolded P values are statistically significant at P < .002). Global P value for proportional hazards assumption: P = .1687.
Nonwhite: black (5.6%), Hispanic/Latino (2.1%), Asian (1.9%), Native Hawaiian/Pacific Islander (0.4%), American Indian/Alaska Native (0.3%), and unknown (2.7%).
On Medicaid at any point during the baseline or study period.
Low prevalence conditions include: chronic kidney disease, neurologic conditions, anemia, chronic pulmonary disease, thyroid disorders, rheumatologic disorders, and inflammatory bowel disease.
Adjusted Clinical Group (ACG) case‐mix assessment system.
Over 24 months, 26% (n = 772) of young adults with incident hypertension were started on one or more antihypertensive medication (the initial electronic health record entry of an antihypertensive medication prescription).9 Trends demonstrated that young adults with shorter encounter intervals had lower antihypertensive medication initiation rates: <1‐month encounter interval (n = 83, 21% prescribed medication), 1‐ to 2‐month interval (n = 150, 26%), 2‐ to 3‐month interval (n = 135, 29%), 3‐ to 6‐month interval (n = 209, 27%), and >6‐month interval (n = 195, 25%). Provider variables (age, sex, specialty) were not significant predictors for medication initiation.
4. DISCUSSION
Our findings demonstrate significant differences in rates of hypertension control among young adults with incident hypertension according to their follow‐up encounter interval. Young adults with a shorter encounter interval (<1 month) had higher rates of hypertension control compared with young adults with longer follow‐up intervals. The encounter interval remained a significant predictor of time to hypertension control even after adjusting for patient and provider factors. Shorter return visit intervals have been associated with higher rates of hypertension control among middle‐aged and older populations.12 However, this study highlights that the return encounter interval is an independent contributor to hypertension control specifically among young adults and beyond mean annual clinic visits.
Young adults have persistently low hypertension control rates30; however, there is a paucity of data on effective, sustainable approaches to address this concerning trend. Our findings demonstrate that shorter encounter intervals may be an effective tool for increasing rates of hypertension control among young adults. Interestingly, our data demonstrated that shorter encounter intervals were associated with lower antihypertensive medication initiation rates, likely underscoring the effectiveness of lifestyle modifications in young adults at the initial stages of BP elevation. An alternative explanation is that during subsequent visits, some individuals no longer met criteria for hypertension (ie, regression to the mean); however, this is less likely given the serially elevated baseline BPs prior to cohort entry.
It is also imperative to highlight the relationship between an encounter interval (the time between visits) and the total number of provider visits. Although the encounter interval was an independent predictor of time to hypertension control, we observed a similar absolute number of primary care visits between the <1‐month, 1‐ to 2‐month, and 2‐ to 3‐month intervals despite significant differences in hypertension control rates. Our findings that shorter encounter intervals lead to increased rates of hypertension control likely reflect visits with reinforcement of lifestyle modifications, and, if needed, timely initiation or titration of antihypertensive medication. In addition, early, more frequent follow‐up likely engages young adults with their primary care team, which supports ongoing ambulatory encounters. Patients with 3‐ to 6‐month and >6‐month intervals had lower absolute numbers of primary care visits; for these intervals, a lower number of visits may contribute to lower hypertension control rates. Interestingly, young adults with longer encounter intervals had higher baseline systolic and diastolic BPs. This group was also in the lower ACG tertile, which may be associated with fewer provider‐initiated return visits. Our data highlight that, given the high‐risk features of this population (eg, high prevalence of obesity and comorbid conditions), close hypertension follow‐up is indicated to achieve timely control.
Overall, our findings demonstrate that the optimal visit frequency is 1 month for young adults with uncontrolled hypertension, which supports prior recommendations in the JNC 7 guidelines.1 Unfortunately, young adults may have difficulty in adhering to the recommended frequency as a result of visit copayments, childcare needs, school, and/or work schedules. Prior studies have suggested using telephone follow‐up and team‐based care to bridge follow‐up visits46, 47, 48 and support hypertension care delivery in this hard‐to‐reach population.
5. STUDY STRENGTHS AND LIMITATIONS
The primary strength of this study was the ability to analyze a large sample of young adults with incident hypertension who received regular primary care in a large multispecialty group practice. However, the findings may not be generalizable to young adults without healthcare access attributable to lack of insurance or other transitions. Another limitation is the use of data from a single healthcare system, which limits the generalizability of the findings; treatment patterns may differ across systems and regions. However, this healthcare system is one of the 10 largest physician practices in the United States, including over 300 primary care physicians and 43 primary care clinics. Moreover, the inclusion of numerous covariates including patient demographics, comorbidities, and utilization data with provider data improves the validity and clinical applicability of our study. The use of retrospective administrative data raises the potential for misclassification of diagnoses, lack of documentation in the electronic health record, and inability to measure medication persistence. However, validated algorithms were used to identify hypertension and other comorbidities. Finally, we had a small sample size of young adults with diabetes mellitus, which prohibited stratified analyses with lower treatment thresholds.
6. CONCLUSIONS
Poor hypertension control rates among young adults underscores the critical need to develop effective interventions to improve hypertension control and reduce hypertension‐related morbidity and mortality. Understanding the impact of timely follow‐up and shorter encounter intervals on hypertension control rates among young adults provides healthcare providers, administrators, and policy makers an evidence‐based target to improve the delivery of hypertension care.
CONFLICT OF INTEREST
C. Bartels and H. Johnson have a clinical appointment with the academic group practice that has a financial interest in delivering care to the general population from which the study participants were drawn. Dr Bartels also receives grant funding from Independent Grants for Learning and Change (Pfizer). The remaining authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
H. Johnson had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. H. Johnson designed the study, performed the statistical analysis and interpretation of the data, and drafted the article. C. King, C. Bartels, E. Magnan, and J. Fink assisted in the interpretation of data, critical revision of the manuscript for important intellectual content, and provided final approval of the version to be published. M. Smith assisted with the design of the study, statistical analysis, interpretation of data, and critical article revisions, and provided final approval of the version to be published. All authors give the corresponding author (H. Johnson) permission to commit to all requirements for copyright transfer.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Katie Ronk, BS, and Patrick Ferguson, MPH, for data preparation, and Jamie LaMantia, BS, for article preparation.
King CC, Bartels CM, Magnan EM, Fink JT, Smith MA, Johnson HM. The importance of frequent return visits and hypertension control among US young adults: a multidisciplinary group practice observational study. J Clin Hypertens. 2017;19:1288–1297. 10.1111/jch.13096
Funding information
This original research was supported by the Clinical and Translational Science Award program, previously through the National Center for Research Resources (NCRR) UL1RR025011 and now by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR000427. Heather M. Johnson is supported by the National Heart, Lung, and Blood Institute of the NIH (K23HL112907). During this study, Christie M. Bartels was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH (K23AR062381). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders did not play any role in the study design; in the collection, analysis, or interpretation of the data; in the writing of the article; or in the decision to submit the article for publication. Additional funding for the project was provided by the UW Health Innovation Program and the University of Wisconsin (UW) School of Medicine and Public Health from The Wisconsin Partnership Program.
REFERENCES
- 1. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 2. Vasan RS, Massaro JM, Wilson PW, et al. Antecedent blood pressure and risk of cardiovascular disease: the Framingham heart study. Circulation. 2002;105:48‐53. [DOI] [PubMed] [Google Scholar]
- 3. Yoon SS, Carroll MD, Fryar CD. Hypertension prevalence and control among adults: United States, 2011‐2014. NCHS Data Brief. 2015;220:1‐8. [PubMed] [Google Scholar]
- 4. Tran CL, Ehrmann BJ, Messer KL, et al. Recent trends in healthcare utilization among children and adolescents with hypertension in the United States. Hypertension. 2012;60:296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . Vital signs: prevalence, treatment, and control of hypertension–United States, 1999‐2002 and 2005‐2008. MMWR Morb Mortal Wkly Rep. 2011;60:103‐108. [PubMed] [Google Scholar]
- 6. McCarron P, Smith GD, Okasha M, McEwen J. Blood pressure in young adulthood and mortality from cardiovascular disease. Lancet. 2000;355:1430‐1431. [DOI] [PubMed] [Google Scholar]
- 7. Vos LE, Oren A, Bots ML, Gorissen WH, Grobbee DE, Uiterwaal CS. Does a routinely measured blood pressure in young adolescence accurately predict hypertension and total cardiovascular risk in young adulthood? J Hypertens. 2003;21:2027‐2034. [DOI] [PubMed] [Google Scholar]
- 8. Johnson HM, Thorpe CT, Bartels CM, et al. Undiagnosed hypertension among young adults with regular primary care use. J Hypertens. 2014;32:65‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson HM, Thorpe CT, Bartels CM, et al. Antihypertensive medication initiation among young adults with regular primary care use. J Gen Intern Med. 2014;29:723‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Quality Measures Clearinghouse (NQMC) . Measure summary: Controlling high blood pressure: percentage of patients 18 to 85 years of age who had a diagnosis of hypertension (HTN) and whose BP was adequately controlled during the measurement year. www.qualitymeasures.ahrq.gov. Accessed April 4, 2017.
- 11. Guthmann R, Davis N, Brown M, Elizondo J. Visit frequency and hypertension. J Clin Hypertens (Greenwich). 2005;7:327‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turchin A, Goldberg SI, Shubina M, Einbinder JS, Conlin PR. Encounter frequency and blood pressure in hypertensive patients with diabetes mellitus. Hypertension. 2010;56:68‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeSalvo KB, Block JP, Muntner P, Merrill W. Predictors of variation in office visit interval assignment. Int J Qual Health Care. 2003;15:399‐405. [DOI] [PubMed] [Google Scholar]
- 14. Schwartz LM, Woloshin S, Wasson JH, Renfrew RA, Welch HG. Setting the revisit interval in primary care. J Gen Intern Med. 1999;14:230‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Welch HG, Chapko MK, James KE, Schwartz LM, Woloshin S. The role of patients and providers in the timing of follow‐up visits. Telephone care study group. J Gen Intern Med. 1999;14:223‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ornstein SM, Nietert PJ, Dickerson LM. Hypertension management and control in primary care: a study of 20 practices in 14 states. Pharmacotherapy. 2004;24:500‐507. [DOI] [PubMed] [Google Scholar]
- 17. Hatahet MA, Bowhan J, Clough EA. Wisconsin collaborative for healthcare quality (WCHQ): lessons learned. WMJ. 2004;103:45‐48. [PubMed] [Google Scholar]
- 18. Sheehy A, Pandhi N, Coursin DB, et al. Minority status and diabetes screening in an ambulatory population. Diabetes Care. 2011;34:1289‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wisconsin Collaborative for Healthcare Quality . WCHQ website. http://www.wchq.org/. Accessed April 4, 2017.
- 20. Thorpe CT, Flood GE, Kraft SA, Everett CM, Smith MA. Effect of patient selection method on provider group performance estimates. Med Care. 2011;49:780‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Myers MG, Tobe SW, McKay DW, Bolli P, Hemmelgarn BR, McAlister FA. New algorithm for the diagnosis of hypertension. Am J Hypertens. 2005;18:1369‐1374. [DOI] [PubMed] [Google Scholar]
- 22. Schmittdiel J, Selby JV, Swain B, et al. Missed opportunities in cardiovascular disease prevention?: low rates of hypertension recognition for women at medicine and obstetrics‐gynecology clinics. Hypertension. 2011;57:717‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ho AK, Bartels CM, Thorpe CT, Pandhi N, Smith MA, Johnson HM. Achieving weight loss and hypertension control among obese adults: a US multidisciplinary group practice observational study. Am J Hypertens. 2016;29:984‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ho AK, Thorpe CT, Pandhi N, Palta M, Smith MA, Johnson HM. Association of anxiety and depression with hypertension control: a US multidisciplinary group practice observational study. J Hypertens. 2015;33:2215‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallace ML, Magnan EM, Thorpe CT, Schumacher JR, Smith MA, Johnson HM. Diagnosis and treatment of incident hypertension among patients with diabetes: a U.S. multi‐disciplinary group practice observational study. J Gen Intern Med. 2015;30:768‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tu K, Chen Z, Lipscombe LL. Prevalence and incidence of hypertension from 1995 to 2005: a population‐based study. Can Med Assoc J. 2008;178:1429‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tu K, Campbell NR, Chen ZL, Cauch‐Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18‐e26. [PMC free article] [PubMed] [Google Scholar]
- 28. Manson JM, McFarland B, Weiss S. Use of an automated database to evaluate markers for early detection of pregnancy. Am J Epidemiol. 2001;154:180‐187. [DOI] [PubMed] [Google Scholar]
- 29. O'Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163:2677‐2678. [DOI] [PubMed] [Google Scholar]
- 30. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension‐related comorbidities from administrative data: what's the optimal approach? Am J Med Qual. 2004;19:201‐206. [DOI] [PubMed] [Google Scholar]
- 32. Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using medicare claims data. Am J Med Qual. 1999;14:270‐277. [DOI] [PubMed] [Google Scholar]
- 33. Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489‐495. [DOI] [PubMed] [Google Scholar]
- 34. Marciniak MD, Lage MJ, Dunayevich E, et al. The cost of treating anxiety: the medical and demographic correlates that impact total medical costs. Depress Anxiety. 2005;21:178‐184. [DOI] [PubMed] [Google Scholar]
- 35. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8‐27. [DOI] [PubMed] [Google Scholar]
- 36. Chronic Conditions Data Warehouse . 2011 Chronic Condition Reference List. http://www.ccwdata.org/cs/groups/public/documents/document/ccw_conditionreferencelist2011.pdf. Accessed April 4, 2017.
- 37. MacLean CH, Louie R, Leake B, et al. Quality of care for patients with rheumatoid arthritis. JAMA. 2000;284:984‐992. [DOI] [PubMed] [Google Scholar]
- 38. Campbell NR, So L, Amankwah E, Quan H, Maxwell C. Characteristics of hypertensive Canadians not receiving drug therapy. Can J Cardiol. 2008;24:485‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Starfield B, Weiner J, Mumford L, Steinwachs D. Ambulatory care groups: a categorization of diagnoses for research and management. Health Serv Res. 1991;26:53‐74. [PMC free article] [PubMed] [Google Scholar]
- 40. Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population‐oriented measure of ambulatory care case‐mix. Med Care. 1991;29:452‐472. [DOI] [PubMed] [Google Scholar]
- 41. StataCorp . STATA Survival Analysis and Epidemiological Tables Reference Manual: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 42. Rogers W. Regression standard errors in clustered samples. Stata J. 1993;3:319‐323. [Google Scholar]
- 43. Williams RL. A note on robust variance estimation for cluster‐correlated data. Biometrics. 2000;56:645‐646. [DOI] [PubMed] [Google Scholar]
- 44. Abdi H. The Bonferonni and Šidák corrections for multiple comparisons. In: Salking NJ, ed. Encyclopedia of Measurement and Statistics, vol. 2. Thousand Oaks, CA: SAGE Publications, Inc.; 2007. [Google Scholar]
- 45. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515‐526. [Google Scholar]
- 46. Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299:2857‐2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson HM, LaMantia JN, Warner RC, et al. MyHEART: a non randomized feasibility study of a young adult hypertension intervention. J Hypertens Manag. 2016;2:pii:016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set generated and analyzed during the current study is not publicly available as a result of the data use agreement, but is available from the corresponding author upon reasonable request and necessary approvals.


