Abstract
Rationale
Human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CM) are increasingly being used for modeling heart disease and are under development for regeneration of the injured heart. However, incomplete structural and functional maturation of hiPSC-CM including lack of t-tubules, immature excitation-contraction (EC) coupling, and inefficient Ca-induced Ca release (CICR) remain major limitations.
Objective
Thyroid and glucocorticoid hormones are critical for heart maturation. We hypothesized that their addition to standard protocols would promote t-tubule development and mature EC coupling of hiPSC-CM when cultured on extracellular matrix with physiological stiffness (Matrigel mattress).
Methods and Results
HiPSC-CM were generated using a standard chemical differentiation method supplemented with triiodo-L-thyronine (T3) and/or dexamethasone (Dex) during days 16–30 followed by single-cell culture for 5 days on Matrigel mattress. HiPSC-CM treated with T3+Dex, but not with either T3 or Dex alone, developed an extensive t-tubule network. Notably, Matrigel mattress was necessary for t-tubule formation. Compared to adult human ventricular CM, t-tubules in T3+Dex-treated hiPSC-CM were less organized and had more longitudinal elements. Confocal line scans demonstrated spatially and temporally uniform Ca release that is characteristic of EC coupling in the heart ventricle. T3+Dex enhanced elementary Ca release measured by Ca sparks as well as promoted ryanodine receptor (RyR2) structural organization. Simultaneous measurements of L-type Ca current and intracellular Ca release confirmed enhanced functional coupling between L-type Ca channels and RyR2 in T3+Dex cells.
Conclusions
Our results suggest a permissive role of combined thyroid and glucocorticoid hormones during the cardiac differentiation process which, when coupled with further maturation on Matrigel mattress, is sufficient for t-tubule development, enhanced CICR, and more ventricular-like EC coupling. This new hormone maturation method could advance the utility of hiPSC-CM for disease modeling and cell-based therapy.
Keywords: Induced pluripotent derived stem cell, t-tubules, calcium induced calcium release, Matrigel mattress, T3, Dexamethasone, electrophysiology, excitation-contraction coupling
Subject Terms: Calcium Cycling/Excitation-Contraction Coupling, Cellular Programing, Stem Cells, Electrophysiology
INTRODUCTION
Human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CM) are increasingly being used in biomedical research for cardiac disease modeling, cardiotoxicity screening, and regeneration of injured myocardium1, 2. Although considerable advances have been made in the maturation of hiPSC-CM2, the lack of t-tubule formation and respective functional consequences remain a major limitation3–6. T-tubules are organized invaginations in the cardiomyocyte sarcolemma and are critical for the synchronous Ca induced Ca release (CICR) that underlies efficient excitation-contraction (EC) coupling in human ventricular myocardium. Reduced t-tubule formation contributes to abnormal EC coupling in heart failure and may be responsible for the ventricular arrhythmias observed after cardiac transplantation of hiPSC-CM7. Early t-tubule development in PSC-CM may be possible using complex techniques such as nano-patterning or tissue engineering, however, the extent of t-tubule formation and its functional contribution to EC coupling has not been demonstrated8, 9.
Thyroid and glucocorticoid hormones are critical for cardiac maturation during development10, 11. While standard hiPSC-CM induction media contains both factors, supplementing the culture media with higher concentrations of thyroid and/or glucocorticoid was found to enhance CM maturation11–13. Independently, we and others have shown improved contractility and electrophysiological maturation when culturing hiPSC-CM on extracellular matrix substrates of physiological stiffness8, 14. We hypothesized that a combination of the thyroid hormone tri-iodo-l-thyronine (T3) and the glucocorticoid dexamethasone (Dex) with the flexible Matrigel substrate method14 would enhance the structural and functional maturation of hiPSC-CM. As such, at days 16–30 of the standard chemical differentiation protocol, the maintenance medium was supplemented with a combination T3 and Dex. Cells were dissociated after day 30 followed by single-cell culture on Matrigel mattress for an additional 5 days before experimental analyses.
METHODS
T3+Dex treated cells were generated as depicted in Figure 1A. In brief, media was supplemented with 100 nmol/L T3 and 1000 nmol/L Dex in 3 mL of media/well during days 16–30 of hiPSC-CM maintenance. Detailed materials and methods are included in the Online Data Supplement.
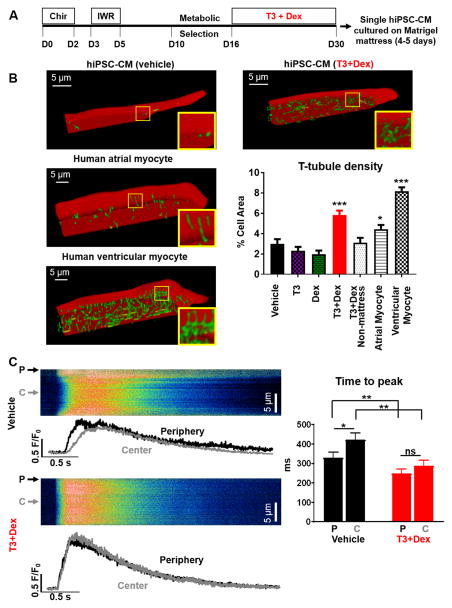
Figure 1. T3+Dex promotes t-tubule formation and synchronizes intracellular Ca release.
A) Hormone-based cardiac differentiation protocol (D=day, see online methods for details). B) Representative examples of t-tubule staining in hiPSC-CM (upper panels) and human adult myocardium (lower panels) with quantitation of t-tubule index (% cell area) (n=19–59 cells/group, *p<0.05 and ***p<0.001 vs vehicle). Images are planar projections of 3D reconstructions. C) Representative transverse line scans of Fluo-4AM loaded hiPSC-CM electrically-stimulated at 0.2 Hz (left panels) and summary data (right panel) comparing Ca transient time to peak between periphery (P) and center (C) of cell. Scan line was positioned across the middle of each cell. n=18–21 cells/group; *p<0.05, **p<0.01, ***p<0.001, vs vehicle, ns – non significant. All data are reported as mean±SEM.
RESULTS
T3+Dex promotes t-tubule formation and synchronizes intracellular Ca release
HiPSC-CM were stained using a lipophilic membrane dye and imaged using a confocal microscope. Images were deconvolved, and thresholded to the mean fluorescence intensity of the entire cell, and t-tubule density calculated by normalizing the supra-threshold signal within the cell interior to cross-sectional cell area15. Consistent with previous reports, vehicle treated cells essentially lacked t-tubules (Figure 1B). Treatment with either T3 or Dex alone did not significantly impact t-tubule density, whereas the combination of T3+Dex promoted t-tubule formation (Figure 1B and Online Video I and II). Notably, maturation on Matrigel mattress was required for t-tubule formation, as T3+Dex treated cells plated without thick Matrigel mattress were not different from control cells (Figure 1B). Cellular staining for bridging-integrator 1 (BIN1), junctophilin-2 (JP2), and caveolin-3 (Cav3), three regulators of t-tubule genesis16, 17, demonstrated striking changes in their subcellular localization. Whereas these t-tubule markers were located mostly perinuclear in vehicle-treated cells, after T3+Dex treatment staining was much more prominent throughout the whole cell (Online Figure IA). Importantly, co-immunostaining of sarcomeric α-actinin with JP2, which is required for anchoring and maturation of developing t-tubules17, demonstrated increased accumulation of JP2 along Z-lines (Online Figure IB,C).
We next compared t-tubule density of hiPSC-CM to that of adult human myocardium (Figure 1B and Online Video III). Ventricular tissue was acquired from organ donors deemed unsuitable for transplantation due to surgical reasons, and right atrial tissue from patients undergoing open heart surgery. T-tubule density was calculated from confocal images of cell-membrane stained cryosections (Online Figure IIA and Online Methods). T-tubule density of T3+Dex hiPSC-CM significantly exceeded that of healthy human atrial CM (p<0.05), but remained sparser and less organized than the t-tubule network of human ventricular CM (p<0.001) (Figure 1B and Online Video IV). Adult human ventricular CM exhibited a greater fraction of transversely-oriented tubules in comparison to longitudinal elements, while T3+Dex cells exhibited similar proportions of t-tubules in these two orientations (Online Figure IIA, B, C). This less organized appearance of t-tubules is analogous to that previously reported for rat CM during the postnatal period of development17.
In addition to promoting t-tubule development, T3+Dex also significantly increased the size of iPSC-CM, primarily cell width and cell volume (Online Figure IIIA, B). Results were confirmed in a second, independently-generated iPSC line (Online Figure IIIC). However, T3+Dex treated hiPSC-CM were still significantly smaller than adult human atrial or ventricular CM (Online Figure IIID).
Are the newly formed t-tubules functional and do they contribute to EC coupling? The juxtaposition of the L-type Ca channel (LTCC) located in the t-tubule membrane with the ryanodine receptor (RyR2) located in the junctional sarcoplasmic reticulum (SR) would be expected to synchronize SR Ca release throughout the cell. Consistent with their lack of t-tubules4, transverse confocal line-scans of Fluo-4 loaded vehicle treated hiPSC-CM demonstrated a U-shaped Ca release, with significantly faster Ca release at the cell periphery compared to the cell center (Figure 1C). In contrast, T3+Dex treated cells exhibited a uniform Ca release across the width of the cell, with a comparable amplitude and rate of Ca rise at both center and periphery of the cell (Figure 1C). Together, our findings indicate functional t-tubule development in hiPSC-CM as evidenced by increased t-tubule staining, synchronization of Ca release, and improved subcellular distribution of t-tubule related proteins.
T3+Dex treated hiPSC-CM demonstrate greater dependence on SR Ca release for EC coupling
We next examined the effect of the hormone treatment on cellular Ca handling using the ratiometric Ca indicator Fura-2 AM in electrically stimulated hiPSC-CMs (Figure 2A)14. Although diastolic and peak Fura-2 fluorescence were not significantly different between the groups, both time to peak and Ca decay rates were significantly accelerated by hormone treatment (Figure 2B, C, D and Online Table I). To determine what was responsible for the accelerated Ca removal, we performed a Ca flux balance analysis18 and calculated transport rate constants for SERCA, NCX, and non-NCX pathways (Online Table I). T3+Dex treated cells displayed faster Ca efflux via SERCA2 and NCX, although no differences in SR content were found (Online Table I). Consistent with the improved Ca handling, cell shortening and contractile kinetics measured by edge detection14 were also significantly enhanced by T3+Dex (Online Table II).
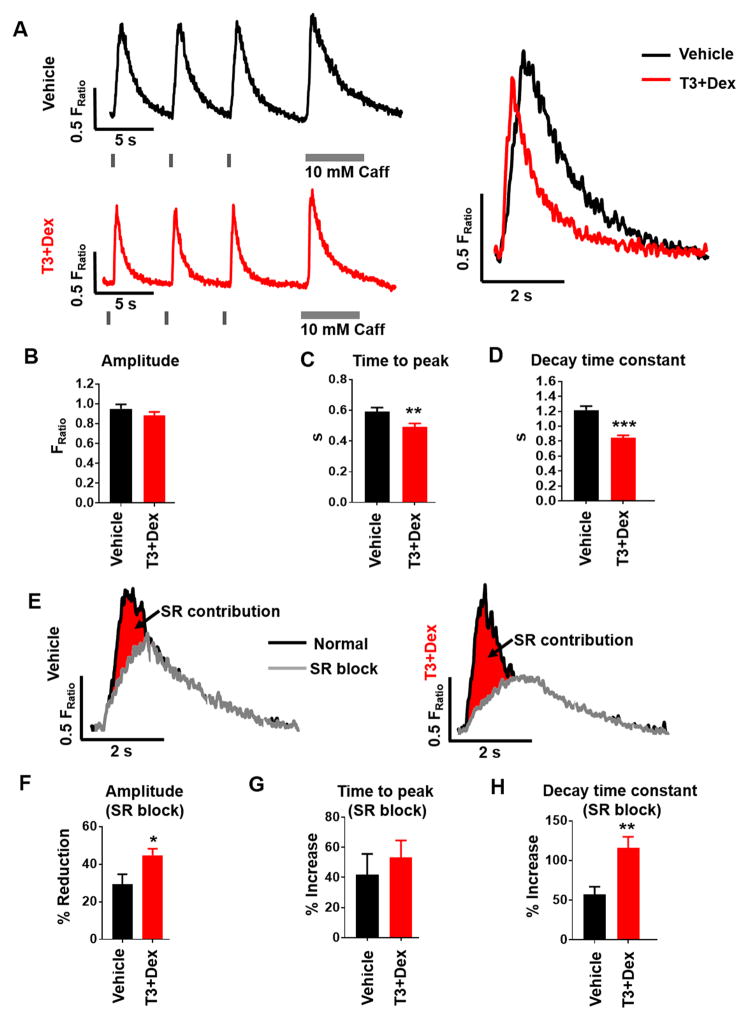
Figure 2. T3+Dex treated hiPSC-CM demonstrate greater dependence on SR Ca release for EC coupling.
A) Representative examples of intracellular Ca transients recorded from Fura-2AM loaded hiPSC-CM with an overlay of the Ca transient from a T3+Dex treated cell (red trace) and a vehicle-treated cell (black trace). Cells were paced at 0.2 Hz followed by rapid caffeine (Caff) application to measure SR Ca content. Note the significantly faster rate of Ca rise and rate of Ca decay in the T3+Dex treated cells. B-D) Summary data for Ca transient amplitude (B), time to peak (C), and transient decay tau (D); (n=68–73 cells/group). E) Representative paced Ca transients before (black) and after (grey) block of SR Ca release with thapsigargin and ryanodine. Note the larger contribution of SR Ca release to the Ca transient in T3+Dex treated cells. F–H) Summary data following SR blockade for Ca transient amplitude (F), time to peak (G), and decay tau (H). Data are expressed as percent change from baseline before SR block. Data reported as mean±SEM (n=16–21 cells/group); *p<0.05, **p<0.01, and ***p<0.001 vs vehicle.
To examine the contribution of SR Ca release to Ca handling, we repeated whole-cell Ca fluorescence recordings after blocking SR Ca release with thapsigargin and ryanodine (Figure 2E). T3+Dex treated cells displayed a greater reduction in Ca transient amplitude following SR blockade (Figure 2F). Both groups demonstrated a similar delay in time to peak following SR blockade (42% and 54% longer) (Figure 2G). Furthermore, Ca decay was significantly slower (i.e. increased τ) in the T3+Dex treated compared to vehicle-treated cells (Figure 2H). Taken together, T3+Dex produced cells with a greater contribution of SR Ca release to CICR and an overall improvement in EC coupling.
T3+Dex increases cell capacitance and EC-coupling gain
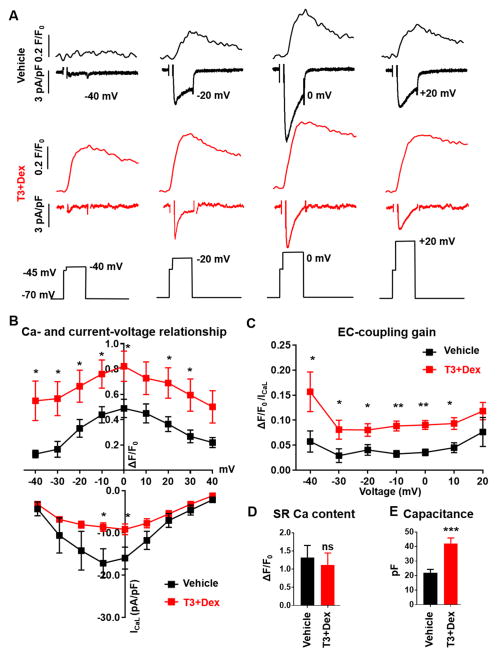
We next quantified the efficiency of coupling between LTCC current and SR-mediated Ca release by measuring the EC coupling gain (ECC gain) in voltage-clamped hiPSC-CM. ECC gain was calculated as the ratio of the amplitude of the cytoplasmic Ca release to the corresponding amplitude of the LTCC current at each membrane voltage. T3+Dex treated cells exhibited a greater rise in intracellular Ca for the respective membrane Ca current at each membrane potential (Figure 3A). On average, T3+Dex treatment significantly increased Ca transient amplitude despite reduced LTCC current density (Figure 3B). As a result, ECC gain was increased at most membrane potentials (Figure 3C). Equivalent SR Ca content was confirmed by rapid caffeine application following each voltage protocol (Figure 3D). Cell capacitance, a measure of cell surface membranes that includes t-tubules19, was significantly increased in the T3+Dex group (Figure 3E). The increase in cell capacitance (93%) was greater than the increase in cell surface area measured by 3D reconstruction, which excludes any membrane invaginations (54%, Online Figure IIIB). This result provides further evidence that the t-tubules formed after T3+Dex treatment were electrically connected to the sarcolemma.
Figure 3. T3+Dex increases cell capacitance and EC-coupling gain.
A) Representative examples of simultaneously-recorded intracellular Ca fluorescence traces (top) and L-type Ca currents (bottom) from vehicle-treated (black) and T3+Dex treated (red) hiPSC-CM. Records were obtained from voltage-clamped hiPSC-CM loaded with the fluorescent Ca indicator Fluo-4 and measured using the indicated voltage protocol. B) Average current-voltage relationship with associated intracellular Ca fluorescence at indicated membrane potentials in T3+Dex versus vehicle treated hiPSC-CM. C) EC coupling gain calculated as a ratio of normalized Ca fluorescence to normalized Ca current. D) SR Ca content. E) Cell capacitance. All data reported as mean±SEM (n=6–7); *p<0.05, **p<0.01, ***p<0.001, vs vehicle, ns – non significant.
A characteristic consequence of greater spatial proximity of LTCC, located primarily in t-tubules, and RyR2, located in the junctional SR, is faster LTCC inactivation. To assess this, a longer depolarization step was utilized to measure both voltage-dependent inactivation (τ1) and Ca-dependent inactivation (τ2) (Online Figure IVA). The significant acceleration of τ2 but not τ1 (Online Figure IVB, C) also supports closer spatial proximity of RyR2 and LTCC that occurs when t-tubules are present. Taken together, these results establish that T3+Dex enhances the efficiency of CICR in hiPSC-CM.
T3+Dex enhances elementary Ca release (Ca sparks) and RyR2 organization
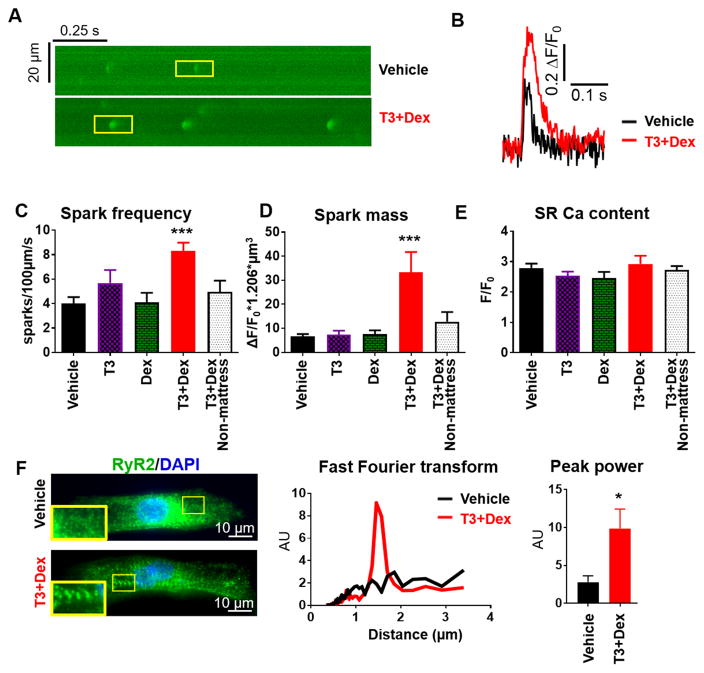
In adult ventricular myocardium, t-tubules form dyads where sarcolemmal LTCCs are closely juxtaposed to clusters of SR RyR2 Ca-release channels. As such, dyad formation promotes RyR2 clustering and hence RyR2-mediated Ca release. The activity of RyR2 clusters but not that of single isolated RyR2 can be assessed by measuring Ca sparks20. To date, only a few reports exist on the presence of Ca sparks in hiPSC-CM21–24. Representative longitudinal line scans of Ca sparks are shown in Figure 4A. T3+Dex treatment increased spark frequency, spark amplitude, and spark mass, with no significant differences in SR Ca content (Figure 4B, C, D, E and Online Table III). As RyR2 function was improved, we next assessed the structural organization of RyR2. Consistent with literature reports, vehicle treated cells showed diffuse RyR2 staining with predominant perinuclear localization25. In contrast, T3+Dex treated cells displayed reduced perinuclear RyR2 staining with striated organization and RyR2 clustering (Figure 4F). Taken together, these results provide evidence for enhanced organization and function of RyR2 in T3+Dex treated cells.
Figure 4. T3+Dex enhances elementary Ca release (Ca sparks) and RyR2 organization.
A) Representative line-scans of Ca sparks recorded from saponin permeabilized hiPSC-CM. B) Overlay of representative Ca spark. C–E) Summary data for spark parameters for the different treatment groups (n=15–37 cells/group). F) Representative cell images and fast Fourier transform of RyR2 immunostaining in T3+Dex and vehicle treated cells. Summary data of peak power of RyR2 immunostaining (n=8–10 cells/group). All data reported as mean±SEM; *p<0.05 and ***p<0.001 vs vehicle.
DISCUSSION
Here we report that a simple culture method – addition of T3+Dex during days 16–30 of cardiac induction followed by a 5 day culture on a Matrigel mattress – was sufficient to generate extensive t-tubule development in hiPSC-CM. Although still less organized than in adult human myocardium, the new t-tubules were electrically coupled to the cell surface, and drastically enhanced CICR and ECC gain. T3+Dex promoted a more ventricular-like RyR2 organization, which likely contributed to the robust appearance of Ca2+ sparks and the greater participation of SR Ca handling to the EC coupling process. This finding is also supported by our data showing a significant reduction (Figure 3) in LTCC current in T3+Dex treated cells, further evidence for increased coupling between the RyR2 and LTCC that allows for more efficient negative feedback of SR Ca release on LTCC (i.e., increased Ca-dependent inactivation of LTCC, Online Figure IV). The results reported here represent a significant advancement, because previous approaches that accelerate hiPSC-CM maturation (i.e., addition of growth factors, length of cultivation, culturing on various substrate/patterning, in vivo maturation) have not enhanced t-tubule formation or provided evidence of ventricular-like EC coupling to the extent shown here2.
The Matrigel mattress method (and others) has facilitated maturation of electrophysiological and Ca handling properties of hiPSC-CM, thereby demonstrating the importance of extracellular matrix for maturation2, 8, 14. Thyroid and glucocorticoid hormones are essential for optimal fetal and neonatal heart development and can also enhance hiPSC-CM maturation13. However, our results suggest that standard differentiation medium contains suboptimal concentrations of both hormones. Our findings highlight the importance of increasing fetal maturation factors during hiPSC-CM differentiation as well as the substrate dependence for promoting optimal CM development. Although mechanisms underlying this process are not yet elucidated, our data suggest that addition of T3+Dex primes the CM for functional and structural maturation and, when receiving proper substrate cues, is able to express these improvements.
In summary, our results suggest a permissive role of combined thyroid and glucocorticoid hormones during the cardiac differentiation process which, when coupled with further maturation on Matrigel mattress, is sufficient for robust t-tubule development, enhanced CICR, and more ventricular-like EC coupling in single hiPSC-CM. This provides proof of principle that functional t-tubule development can be achieved in single cell culture of hiPSC-CM. The hiPSC-CM structural and functional maturation appear to be mediated by two features: 1. Activation of pathways downstream of thyroid and glucocorticoid receptors and 2. Interaction with an extracellular matrix substrate of physiological stiffness. However, as our work does not provide an exhaustive list of molecular signatures of cardiomyocyte maturation, further assessment of the effects of T3+Dex mattress method on cellular metabolism and gene expression changes specific to EC coupling are warranted. Furthermore, t-tubule development in T3+Dex treated hiPSC-CM after 5 days is less organized than in adult human myocardium, and more reminiscent to that found in day 15–20 postnatal rat hearts17. Hence, future studies are required to establish optimal culture conditions to achieve further t-tubule maturation (e.g., longer culture time, tuning substrate tension, and optimizing hormone concentrations). Indeed, our discovery encourages investigation of gene expression pathways downstream of thyroid and glucocorticoid receptors and we anticipate this will yield novel targets for further maturation of hiPSC-CM. Nevertheless, the new maturation technique reported here overcomes a major barrier in the stem cell field, which will help improve the utility of hiPSC-CM for disease modeling and enable future research into the molecular pathways underlying t-tubule development.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CM) are utilized for modeling heart disease and under development for regenerating the injured myocardium.
Inefficient excitation-contraction (EC) coupling, fetal cardiomyocyte-like Ca handling and electrophysiological properties remain a major limitation of hiPSC-CM.
Central to efficient EC coupling in the human ventricle are functional t-tubules and ryanodine receptor 2 (RyR2) clustering, both of which are largely absent in hiPSC-CMs.
What New Information Does this Article Contribute?
The addition of thyroid and glucocorticoid hormones to standard protocols promotes robust t-tubule development and RyR2 clustering of hiPSC-CM when cultured on extracellular matrix with physiological stiffness (Matrigel mattress).
The new t-tubules are functional, resulting in spatially and temporally uniform Ca-induced Ca-release and efficient EC coupling that is characteristic of healthy adult ventricular myocardium.
The absence of t-tubules has remained a major concern for the utilization of hiPSC-CM. Although a number of methods have been reported that improve hiPSC-CM maturation (e.g. culture on soft, patterned substrates, increased length of culture time, hormone maturation), none of them produce functional t-tubules. We similarly observed that Matrigel mattress alone or treatment with hormone alone is not sufficient for development of t-tubules. Our work provides compelling evidence that combined activation of pathways downstream the thyroid and glucocorticoid receptors is sufficient for functional t-tubule development if hiPSC-CM are allowed to mature on a substrate of extracellular matrix with physiological stiffness. Furthermore, T3+Dex treated cells demonstrated more widespread cellular distribution of key t-tubule related proteins and displayed improved RyR2 organization. As such, our discovery not only helps to overcome a major barrier in the field, but also provides novel mechanistic insight into the genesis of t-tubules.
Acknowledgments
SOURCES OF FUNDING
The work was supported in part by grants from the US National Institutes of Health (R01HL71670, R01HL128044, R01HL124935, P50GM115305, U01HL131911 to BCK). S.P. was supported by NIGMS T32 GM07347, T32 GM07628 and NHLBI F30 HL131179; D.J.B. by T32 NS 007491; Imaging through VUMC Cell Imaging Shared Resource.
Nonstandard Abbreviations and Acronyms
- hiPSC-CM
human induced pluripotent stem cell-derived cardiomyocytes
- EC
excitation-contraction
- CICR
calcium induced calcium release
- T3
tri-iodo-l-thyronine
- Dex
dexamethasone
- RyR2
ryanodine Receptor 2
- τ
Tau (decay constant)
- Ca
Calcium
- SR
sarcoplasmic reticulum
- LTCC
L-type calcium channel
- PSC
iPSC or embryonic stem cell derived pluripotent stem cell
- BIN1
bridging integrator 1
- JP2
junctophilin-2
- Cav3
caveolin-3
- NCX
Na/Ca Exchanger
- SERCA
sarco/endoplasmic reticulum Ca ATPase
Footnotes
DISCLOSURES
None.
References
- 1.Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: At the heart of cardiovascular precision medicine. Nat Rev Cardiol. 2016;13:333–349. doi: 10.1038/nrcardio.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolanowski TJ, Antos CL, Guan K. Making human cardiomyocytes up to date: Derivation, maturation state and perspectives. Int J Cardiol. 2017 doi: 10.1016/j.ijcard.2017.03.099. [DOI] [PubMed] [Google Scholar]
- 3.Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–2363. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 4.Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, Huser T, Li RA. Absence of transverse tubules contributes to non-uniform ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev. 2009;18:1493–1500. doi: 10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, Yamanaka S, Kimura T. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J. 2013;77:1307–1314. doi: 10.1253/circj.cj-12-0987. [DOI] [PubMed] [Google Scholar]
- 6.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U. Allogeneic transplantation of ips cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A. 2015;112:12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pioner JM, Racca AW, Klaiman JM, Yang KC, Guan X, Pabon L, Muskheli V, Zaunbrecher R, Macadangdang J, Jeong MY, Mack DL, Childers MK, Kim DH, Tesi C, Poggesi C, Murry CE, Regnier M. Isolation and mechanical measurements of myofibrils from human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Reports. 2016;6:885–896. doi: 10.1016/j.stemcr.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Iismaa SE, Naqvi N, Nicks A, Husain A, Graham RM. Thyroid hormone action in postnatal heart development. Stem Cell Res. 2014;13:582–591. doi: 10.1016/j.scr.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Rog-Zielinska EA, Craig MA, Manning JR, Richardson RV, Gowans GJ, Dunbar DR, Gharbi K, Kenyon CJ, Holmes MC, Hardie DG, Smith GL, Chapman KE. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: A role for pgc-1alpha. Cell Death Differ. 2015;22:1106–1116. doi: 10.1038/cdd.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Rodriguez M, Pabon L, Fischer KA, Reinecke H, Regnier M, Sniadecki NJ, Ruohola-Baker H, Murry CE. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol. 2014;72:296–304. doi: 10.1016/j.yjmcc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birket MJ, Ribeiro MC, Kosmidis G, Ward D, Leitoguinho AR, van de Pol V, Dambrot C, Devalla HD, Davis RP, Mastroberardino PG, Atsma DE, Passier R, Mummery CL. Contractile defect caused by mutation in mybpc3 revealed under conditions optimized for human psc-cardiomyocyte function. Cell Rep. 2015;13:733–745. doi: 10.1016/j.celrep.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, Bloodworth N, Merryman WD, Lim CC, Wu JC, Knollmann BC, Hong CC. Matrigel mattress: A method for the generation of single contracting human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res. 2015;117:995–1000. doi: 10.1161/CIRCRESAHA.115.307580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisk M, Koivumaki JT, Norseng PA, Maleckar MM, Sejersted OM, Louch WE. Variable t-tubule organization and ca2+ homeostasis across the atria. Am J Physiol Heart Circ Physiol. 2014;307:H609–620. doi: 10.1152/ajpheart.00295.2014. [DOI] [PubMed] [Google Scholar]
- 16.Manfra O, Frisk M, Louch WE. Regulation of cardiomyocyte t-tubular structure: Opportunities for therapy. Curr Heart Fail Rep. 2017;14:167–178. doi: 10.1007/s11897-017-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziman AP, Gomez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol. 2010;48:379–386. doi: 10.1016/j.yjmcc.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang HS, Kryshtal DO, Feaster TK, Sanchez-Freire V, Zhang J, Kamp TJ, Hong CC, Wu JC, Knollmann BC. Comparable calcium handling of human ipsc-derived cardiomyocytes generated by multiple laboratories. J Mol Cell Cardiol. 2015;85:79–88. doi: 10.1016/j.yjmcc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brette F, Komukai K, Orchard CH. Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am J Physiol Heart Circ Physiol. 2002;283:H1720–1728. doi: 10.1152/ajpheart.00347.2002. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GQ, Wei H, Lu J, Wong P, Shim W. Identification and characterization of calcium sparks in cardiomyocytes derived from human induced pluripotent stem cells. PLoS One. 2013;8:e55266. doi: 10.1371/journal.pone.0055266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Cheng H, Tomaselli GF, Li RA. Mechanistic basis of excitation-contraction coupling in human pluripotent stem cell-derived ventricular cardiomyocytes revealed by ca2+ spark characteristics: Direct evidence of functional ca2+-induced ca2+ release. Heart Rhythm. 2014;11:133–140. doi: 10.1016/j.hrthm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhu WZ, Santana LF, Laflamme MA. Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLoS One. 2009;4:e5407. doi: 10.1371/journal.pone.0005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satin J, Itzhaki I, Rapoport S, Schroder EA, Izu L, Arbel G, Beyar R, Balke CW, Schiller J, Gepstein L. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:1961–1972. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 25.Fatima A, Xu G, Shao K, Papadopoulos S, Lehmann M, Arnaiz-Cot JJ, Rosa AO, Nguemo F, Matzkies M, Dittmann S, Stone SL, Linke M, Zechner U, Beyer V, Hennies HC, Rosenkranz S, Klauke B, Parwani AS, Haverkamp W, Pfitzer G, Farr M, Cleemann L, Morad M, Milting H, Hescheler J, Saric T. In vitro modeling of ryanodine receptor 2 dysfunction using human induced pluripotent stem cells. Cell Physiol Biochem. 2011;28:579–592. doi: 10.1159/000335753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.