Abstract
Objective
Newborn screening (NBS) has led to early diagnosis and early initiation of treatment for infantile onset Pompe Disease (IOPD). However, guidelines for management of late onset Pompe disease (LOPD) via NBS, especially with the IVS c.-32-13T>G are not clear. This IVS variant is noted in 68–90% cases with LOPD and has been presumed to result in “adult” disease in compound heterozygosity, with a few cases with earlier onset and a mild to no phenotype in homozygosity. Our study evaluates newborns with LOPD having IVS variant with a diligent multidisciplinary approach to determine if they have an early presentation.
Methods
Seven children with LOPD identified by NBS with IVS variant (3 compound heterozygous, and 4 homozygous) were evaluated with clinical, biochemical (CK, AST, ALT, and urinary Glc4), cardiac evaluation, physical therapy (PT), occupational, and speech/language therapy.
Results
All seven patients demonstrated motor involvement by age 6 months; the three patients with c.-32-13 T>G variant in compound heterozygosity had symptoms as neonates. Patients with c.-32-13 T>G variant in compound heterozygosity had more involvement with persistent hyperCKemia, elevated AST and ALT, swallowing difficulties, limb-girdle weakness, delayed motor milestones, and were initiated on ERT. The patients with c.-32-13T>G variant in homozygosity had normal laboratory parameters, and presented with very subtle yet LOPD specific signs, identified only by meticulous assessments.
Conclusion
This patient cohort represents the first carefully phenotyped cohort of infants with LOPD with the “late-onset” GAA variant c.-32-13T>G detected by NBS in the USA. It emphasizes not only the opportunity for early detection of skeletal and other muscle involvement in infants with c.-32-13T>G variant but also a high probability of overlooking or underestimating the significance of clinically present and detectable features. It can thus serve as a valuable contribution in the development of evaluation and treatment algorithms for infants with LOPD.
Keywords: Late onset Pompe disease, glycogen storage disease type II, newborn screening, enzyme replacement therapy, IVS variant, c.-32-13T>G
2. Introduction
Pompe disease is a progressive autosomal recessive neuromuscular disorder caused by deficiency of lysosomal acid α-glucosidase (GAA) [1]. It is broadly classified into classic infantile Pompe disease (IOPD), the most severe end of the spectrum with rapidly progressive hypertrophic cardiomyopathy at birth, generalized muscle weakness, and death within the first two years of life without treatment [2 3]; and late onset Pompe disease (LOPD), encompassing childhood, juvenile, and adult-onset disease, with variable severity of muscle involvement, presenting anywhere from infancy to the sixth decade of life [4–6]. Enzyme replacement therapy (ERT) with alglucosidase alfa remains the only FDA approved treatment for Pompe disease with evidence that early initiation of treatment results in best outcomes with dramatically improved survival [7–9].
Pompe disease was added to the recommended uniform screening panel (RUSP) for newborns by the U.S. Secretary of Health and Human Services in March, 2015. Currently Missouri, Illinois, New York, Kentucky, Mississippi, Ohio, Pennsylvania and Tennessee are screening for Pompe disease and many additional states are gearing towards this goal [10]. Newborn screening (NBS) has led to early diagnosis and early initiation of treatment for IOPD, as intended. However, NBS also identifies patients with “late-onset” GAA variants, which poses a clinical dilemma, as guidelines for management of LOPD in childhood are unclear. In the absence of NBS, early signs of LOPD such as subtle muscular weakness, swallowing difficulties, and respiratory compromise are often dismissed or overlooked as non-specific hypotonia or “developmental delay” in children, contributing to misdiagnoses and/or delayed diagnosis. NBS prevents the prolonged diagnostic odyssey for patients with LOPD, allowing for an understanding of early signs and symptoms and, thus, provides the opportunity to direct management and treatment considerations and decisions [11–14].
While immediate initiation of ERT is the standard of care for patients with variants consistent with IOPD identified by NBS, there is no consensus on if and when to initiate ERT for patients with “late-onset” GAA variants, especially with the leaky GAA splice site variant, c.-32-13T>G in intron 1 (IVS1-13T>G; IVS variant) [15]. This variant is found on at least one allele in 68–90% of Caucasian patients [16–18]. Data on the spectrum and severity of LOPD patients with c.-32-13T>G variant in homozygosity or compound heterozygosity are emerging. Patients with c.-32-13 T>G variant in compound heterozygosity and a second pathogenic variant were originally thought to have adult-onset LOPD, however the c.-32-13T>G variant has now been recognized across the disease continuum. Patients with the c.-32-13T>G variant in homozygosity have historically been thought to be asymptomatic or very mildly affected [16 19 20]. Contrary to that assumption, a recent report described six adult Pompe disease patients with c.-32-13T>G variant in homozygosity with myalgia, hyperCKaemia, and/or exercise induced fatigue, with symptom onset between 12–55 years [21]. The management and treatment of infants diagnosed with this “late-onset” GAA variant following NBS remains unclear, due to diagnostic delay and the paucity of published literature on this patient population [16 22–26]. Data from the Taiwan Pompe NBS program, which began in 2005 is a valuable resource [27–29]; however, absence of the IVS c.-32-13T>G splice site variant in Taiwan as compared to Caucasian populations, limits our ability to extrapolate conclusions from Taiwan’s LOPD program [30].
We present seven consecutive patients with “late-onset” GAA variants identified by NBS, consisting of three patients with c.-32-13T>G variant in compound heterozygosity and a second pathogenic variant and four patients with c.-32-13T>G variant in homozygosity. The purpose of this report is to summarize the clinical presentation of these seven patients as assessed utilizing a diligent multidisciplinary approach.
3. Methods
Written informed consent was obtained from a parent or guardian for all individuals as part of Duke Institutional Review Board approved Pompe long-term follow-up study (Pro00010830) and/or Determination of CRIM status in Pompe disease (Pro00001562). Data were extracted via retrospective chart review of seven consecutive patients identified via NBS with the c.-32-13T>G splice site variant in homozygosity or compound heterozygosity (Table 1). All patients had laboratory assessments (creatine kinase (CK), urinary glucose tetrasaccharide (Glc4), and complete metabolic profile), cardiac evaluation (electrocardiogram (ECG) and echocardiogram (ECHO)) (Table 1), genetics evaluation, physical therapy (PT) assessment, speech/language therapy (ST) and/or occupational therapy (OT) assessments as part of their evaluation at the Duke metabolic clinic (Table 2). PT assessments included qualitative assessment of posture, movement, musculoskeletal status, and standardized tests such as the Alberta Infant Motor Scale (AIMS) and Gross Motor Function Measure (GMFM). OT/ST evaluations assessed oropharyngeal muscle weakness and included video-fluoroscopic swallow study when indicated.
Table 1.
Patient demographics and baseline biochemical parameters
| Patient/Gender | Genotype | Age at ERT initiation (month s) | Current age* (years ) | Cardiac assessment | CK (N 70–320 U/L) | ALT (N 5–40 U/L) | AST (N 15–41 U/L) | ALP (N 24–170 U/L) | Urinary Glc4 (≤8.3 mmol/mol creatinine) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Allele 1 | Allele 2 | |||||||||
| 1/M | c.-32-13T>G | c.525delT | 1.0 | 4.0 | PFO | 378 | 26 | 45 | 211 | 3.7 |
| 2/M | c.-32-13T>G | c.525delT | 1.2 | 2.0 | PFO and LVH | 359 | 41 | 33 | 239 | 4.5 |
| 3/F | c.-32-13T>G; | c.2188G>T | 9.0 | 1.2 | WNL | 605 | 198 | 139 | 248 | 7.3 |
| 4/F | c.-32-13T>G | c.-32-13T>G | No ERT | 1.1 | WNL | 366 | NA | NA | NA | 4.8 |
| 5/F | c.-32-13T>G | c.-32-13T>G | No ERT | 0.4 | WNL | 174 | 27 | 36 | 352 | 4.1 |
| 6/M | c.-32-13T>G | c.-32-13T>G | No ERT | 0.7 | PFO | 176 | 30 | 90 | 249 | 2.6 |
| 7/F | c.-32-13T G | c.-32-13T>G | No ERT | 0.7 | PFO | 125 | 37 | 53 | 174 | NA |
Duke Metabolic Clinic; CK, Creatine Kinase; ALT, Alanine aminotransferase, AST, Aspartate aminotransferase; ALP, Alkaline phosphatase; Glc4, Glucose tetra saccharide; PFO, Patent Foramen Ovale; LVH, Left Ventricular Hypertrophy; WNL, Within Normal Limits; NA, Not Available
Age as of March 2017
Table 2.
Early symptoms and clinical parameters in neonates with Pompe disease with c.-32-13T>G GAA variant
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Presenting features | Within 1st week of life: Generalized hypotonia, delayed gross motor skills, bilateral scapular winging dysphagia, FTT | Since birth: Generalized hypotonia, delayed gross motor skills, dysphagia, positive family history | At age 5 months: Generalized hypotonia, delayed gross motor skills, regression in gross motor skills | Reported to be asymptomatic | Age 3 months: Regurgitation of feeds, especially when lying supine, self- limited gasping episodes | Reported to be asymptomatic | Reported to be asymptomatic |
| Standardized PT test (Age) | 7 months- AIMS: 10th to 25th percentile, GMFM: 23% | 3 months- AIMS: 10th to 25th percentile | 5 months- AIMS: 10th percentile; 6 months- AIMS: 3rd percentile, GMFM: 16% | 6 months - AIMS: 25th percentile, GMFM: 22% | 3 months chronological age & 2 months corrected age- AIMS: 10th percentile for chronological age & 25th to 50th percentile for corrected age | 6 months - AIMS: 1st percentile, GMFM: 12.3% | 6 months chronological age & 5 months corrected age- AIMS:1 0th to 25th percentile for chronological age &50th percentile for corrected age, GMFM: 16.55% |
| Hip extensor activity (balance between hip flexion & extension) | Decreased active hip extension in prone/sitting; sitting briefly (seconds) with/without support | Decreased active hip extension in prone | Decreased active hip extension; increased use of hip flexors | Decreased active hip extension in prone and sitting | Decreased active hip extension in prone (decreased posterior weight shift in prone to allow prone propping) | Decreased active hip extension. Muscle imbalance with greater hip flexion than extension in all positions | Decreased active hip extension in prone (decreased posterior weight shift in prone to allow prone propping) |
| LE positioning in: Flexion/Abduct ion/Extern al Rotation | Yes | Yes | Yes with plantar flexion tendency | Yes | Yes – slight | Yes – in all positions | Tendency for abduction and external rotation |
| LE muscle tightness/hypo-extensibility | IT band tightness bilaterally | Hip flexor and IT band tightness bilaterally | Hip flexor and IT band tightness bilaterally; plantar flexor hypo-extensibility bilaterally | IT band tightness bilaterally | IT band tightness – lateral thigh groove | Hip flexor hypo extensibility/IT band tightness bilaterally– lateral thigh groove | IT band tightness – lateral thigh groove |
| Abdominal & pelvic muscle s | No obvious involvement | No obvious involvement | Decreased use of abdominal obliques in rotation, decreased trunk rotation in transitions | Decreased use of abdominal obliques in active trunk rotation, increased use of spinal extension with momentum vs abdominals | Asymmetrical, as appropriate for corrected age of 2 months | Decreased use of abdominal oblique muscles-movement only in sagittal plane, inability to achieve posterior pelvic tilt in supine | No obvious involvement |
| LE weight bearing | Yes | Decreased | Yes – but with excessive hip and knee flexion and excessive plantarflexion | Yes | Yes | Yes but with tendency to shift forward and go up on toes | Yes – but with stiff extension and plantarflexion |
| Muscle tone | Decreased | Decreased | Decreased | Normal | Normal | Normal | Normal |
| Lower rib flaring | Not noted | Not noted | Present | Present | Not noted | Present | Not noted |
| Calf firmness | Not noted | Not noted | Present | Present | Not noted | Not noted | Not noted |
FTT, Failure to thrive; PT, Physiotherapy; AIMS, Alberta infant motor scale; GMFM, Gross motor function measure (of 5 dimensions); LE, Lower extremity: IT, Iliotibial
4. Patient reports
4.1 Patients with c.-32-13T>G variant in compound heterozygosity
Patient 1
Patient 1 is a Caucasian male with the c.-32-13T>G splice site variant and c.525delT (p.Glu176Argfs*45) variant, who presented with feeding difficulties, recurrent aspiration, poor weight gain, and hypotonia within a few days of life. ECHO noted a small patent foramen ovale at birth, which closed spontaneously, confirmed at age 4 months. ECG was normal. HyperCKemia was present since the early neonatal period (378 IU/L, day 8 of life; N: 60–305 IU/L), while AST and ALT were normal. Feeding assessment by Video-fluoroscopic swallow study (VFSS) performed locally at age 27 days showed moderate oropharyngeal dysphagia and poor muscle tone with a tendency for aspiration. Based on severity of his presentation, ERT at a dose of 20 mg/kg every 2 weeks was initiated at age 1 month, with joint consultation of the local physician and our team at Duke. CK levels were normalized on ERT by age 4 months (80 IU/L). Patient was first evaluated at Duke at age 7 months. PT evaluation at that time revealed generalized hypotonia with flexion/abduction/external rotation positioning of lower extremities, iliotibial (IT) band tightness, and delayed gross motor development with lack of independent sitting, AIMS score between 10th and 25th percentile, and GMFM at 23.16 %. VFSS evaluation at this time revealed significant improvement in feeding ability and oropharyngeal muscle tone. At age 23 months, he had achieved significant motor milestones; started sitting with support at 10 months and without support at 12 months, pulled to stand at 12 months, walked at 15 months and could feed himself with an immature pincer grasp. Swallow study was normal. He continues to receive ERT along with physical and occupational therapy.
Patient 2
Patient 2 is the younger brother of Patient 1 with an identical compound heterozygous genotype. Generalized hypotonia, weak oropharyngeal skills and poor suck were present since birth. The patient also had hyperCKemia (435 IU/L, day of life 4) and normal urinary Glc4 level. Similar to his brother, ECHO at birth revealed a patent foramen ovale, which resolved spontaneously. ECG and ECHO have been unremarkable since. The local physician consulted with our team on this patient. Given the early clinical symptoms and family history of Pompe disease, it was jointly decided to initiate ERT on day 35 of life at a dose of 20 mg/kg every 2 weeks. Medical evaluation at Duke at age 2 months noted generalized hypotonia with facial myopathy, and delayed gross motor milestones. PT evaluation at age 2 months revealed flexion/abduction/external rotation positioning of lower extremities with tightness bilaterally in hip flexors, hamstrings, and IT bands. He had a tendency to remain on toes when held in supported standing with additional support required for weight bearing through lower extremities. He had decreased head control for age, reflecting hypotonia/decreased neck strength. He also had difficulty lifting his head in prone, with asymmetric head lift to 45 degrees, and an inability to maintain head in midline when supine. AIMS score at age 2 months was between 10–25th percentile. Continued ERT and PT/OT were recommended.
Patient 3
Patient 3 is a Caucasian female, compound heterozygous for c.-32-13T>G and c.2188G>T variants, determined to be asymptomatic by two prior local genetics evaluations on day 8 of life and age 3 months, and was recommended to delay ERT until the development of overt symptoms. She had hyperCKemia since the second week of life (605 IU/L, day 8 of life; 513 IU/L, age 3 months). Local PT evaluation at age 5 months noted gross motor developmental delay, with inability to hold head up to 90 degrees in prone, ability to sit with slight support but unable to prop-sit, and with inability to bear weight on hands in prone. AIMS score was at the 10th percentile. PT evaluation at Duke at age 6 months revealed progression of delay in gross motor milestones, with AIMS score at the 3rd percentile (decreased from 10th percentile at 5 months). She was unable to sit upright or unsupported, and scored only 20% on Dimension B (Sitting) of the GMFM. She was unable to push up on arms in prone. CK was 602 IU/L. Imbalanced weakness was evident in trunk and pelvic musculature with predominant active use of, and positioning in, hip flexion/abduction/external rotation in supine, prone and sitting, decreased use of hip extensors and abdominals, and limitation in the use of abdominal obliques for active trunk rotation (Figures 1 and 2). Lower rib flaring was present with tightness in hip flexors, IT bands, and plantar flexors. Calf muscles felt firm on palpation and showed hypertrophy. ERT at 20 mg/kg every other week was initiated at age 9 months. After initiation of ERT and with continued PT, motor improvements were noted in the achievement of gross developmental milestones including independent sitting and crawling with improvement in AIMS score to 43rd percentile at age 11 months, along with normalized CK levels. Urinary Glc4 levels, ECG, and ECHO have been normal. PT evaluation at age 18 months showed continued improvement in gross motor skills, but with persistent residual subtle motor signs and slight delay in locomotor skills as measured by the Peabody Development Motor Scale (PDMS)-2. She began walking at age 16 months. Previous patterns of muscle involvement, positional tendencies and mild muscle tightness persisted, with weakness in abdominals, and in hip extension, ankle dorsiflexion, and ankle eversion; positioning and postural tendencies continuing to include excessive hip flexion, abduction and external rotation with an anterior pelvic tilt; and decreased flexibility in gastrocsoleus, hip flexors and iliotibial bands. She was standing and walking with excessive anterior pelvic tilt and lumbar lordosis. AIMS testing showed age appropriate function on all subscales, scoring in the 90th percentile for age on the AIMS. On the PDMS-2, with measurement of higher level gross motor skills, she was at the 50th percentile for age on the Stationary Subtest, which is considered in the average range for age, and the 16th percentile for age on the Locomotion Subtest, which is considered below average for age. On the GMFM, she showed 71.79% on Dimension D (Standing). She continues with PT 2x/week and ERT at 20 mg/kg every other week.
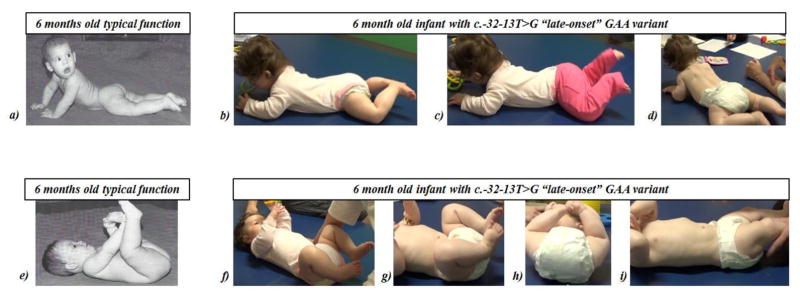
Figure 1. Function and Positioning in Prone and Supine.
a) Typical prone function at 6 months old: from Figure 6.9. In: Bly L. Motor skills acquisition in the first year: an illustrated guide to normal development. Tucson, Ariz.: Therapy Skill Builders; 1994. Used with permission. Typical function includes active hip extension & adduction, shoulder girdle stability & depression, humeral adduction, UE weight bearing with elbow extension, balanced neck flexion/extension, abdominal muscle activity. b, c, d) Prone function in a 6 month old with c.-32-13T>G “late-onset” GAA variant: excessive hip flexion, abduction, external rotation, excessive lumbar vs thoracic extension, increased hip and knee flexion with increased activity, decreased hip extension, adduction, internal rotation, decreased shoulder girdle depression, lack of upper extremity weight-bearing. With elbows placed under shoulders, can maintain propping but with scapular winging and lack of shoulder girdle stability. e) Typical supine function at 6 months old: from Figure 6.1. In: Bly L. Motor skills acquisition in the first year: an illustrated guide to normal development. Tucson, Ariz.: Therapy Skill Builders; 1994. Used with permission. Typical function includes use of abdominals for pelvic lifting, hip flexion with emerging knee extension and reaching with elbow extension. f, g, h) Supine function in 6 month old with c.-32-13T>G “late-onset” GAA variant: less use of abdominals, including abdominal obliques; lower rib flaring; greater hip abduction and external rotation; less pelvic lifting; less use of adductors; greater knee flexion; and less active knee extension. i) lower rib flaring and iliotibial band tightness.
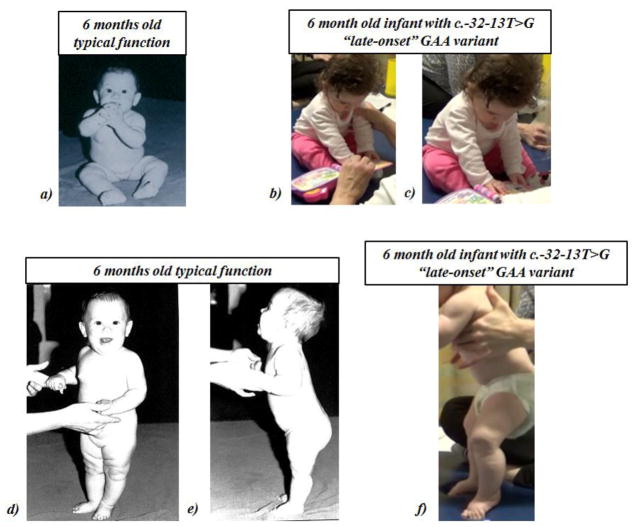
Figure 2. Function and Positioning in Sitting and Supported Standing.
a) Typical sitting function at 6 months old: From: Figure 6.29 in Bly L. Motor skills acquisition in the first year: an illustrated guide to normal development. Tucson, Ariz.: Therapy Skill Builders; 1994. Used with permission. Can typically sit with free hands b) and c): Sitting function in a 6 month old with c.-32-13T>G “late-onset” GAA variant: Facilitation of supported prop-sitting followed by brief independent prop-sitting; lower extremities in excessive flexion, abduction, and external rotation d) and e): Typical supported standing function at 6 months: From: Figure 6.33 in Bly L. Motor skills acquisition in the first year: an illustrated guide to normal development. Tucson, Ariz.: Therapy Skill Builders; 1994. Used with permission. Typically bear weight full weight through lower extremities with support at hands f) Supported standing in 6 month old with c.-32-13T>G “late-onset” GAA variant: excessive hip & knee flexion, excessive plantarflexion, firm calves.
4.2 Patients with c.-32-13T>G variant in homozygosity
Patient 4
Patient 4 is a Caucasian female, homozygous for the c.-32-13T>G splice site variant. HyperCKemia was detected in the first month (505 IU/L; 30–279 IU/L), this normalized spontaneously at 4 months (188 IU/L). Other laboratory assessments have been within normal limits along with normal ECG and ECHO. She was evaluated at Duke at age 6 months. She had no head lag when pulled to sit, had the ability to transfer objects and bring them to midline, and could bear weight through extremities. However, PT assessment revealed subtle motor signs, which included preferred position of ring sitting with hips in flexion/abduction/external rotation, and bilateral IT band tightness, which was more notable on left. She demonstrated imbalanced trunk flexion and extension with a tendency to use momentum for movement. Also notable was decreased use of abdominals and excessive use of spinal extension. She fatigued quickly with play. Asymmetry was noted in reaching objects in prone and weight shifting with lateral flexion and shortening on the left at the neck and trunk. AIMS score was at the 25th percentile and GMFM was 22.14 %. Given that she was not receiving any PT, and her general assessment was close to normal, the plan was to initiate PT locally and follow up closely for potential progression of symptoms. ERT was not initiated.
Patient 5
Patient 5 is a Caucasian female, homozygous for the c.-32-13T>G-splice site variant born at 36 weeks of gestation. CK levels, ECG, and ECHO have consistently been within normal limits. She was evaluated at Duke at age 3 months (2 months corrected for gestational age) for feeding difficulties. She had oral and nasal regurgitation despite adequate suck and swallow reflex and appropriate weight gain. Feeding and swallow evaluation confirmed regurgitation and possible fatigue during feeding. Difficulty in lying supine while eating, sleeping and self-limited gasping episodes was observed. Pulmonary evaluation noted a slightly reduced mean expiratory pressure. Good muscle tone was noted but PT evaluation showed mild consistent asymmetry with increased lateral head tilt to the right. Potential early signs of decreased use of pelvic girdle musculature, especially hip extensors, were suggested by a tendency towards flexion/abduction/external rotation positioning of lower extremities. Slight lateral thigh grooves reflected early IT band tightness, and she had decreased posterior weight shift and propping on forearms in prone. She had normal passive ranges of motion and appropriate weight bearing through lower extremities in supported standing. AIMS score was at 10th percentile for chronological age and 25th to 50th percentile for corrected age. Though the family history was positive for 15q26.3 duplication in an older brother and 15q deletion syndrome (Angelman syndrome) in a paternal first cousin, chromosomal microarray excluded similar alterations as the cause of feeding difficulties in this patient. Patient 5 started receiving PT at 3 months, with a planned follow up in 3 months. At age 6 months, 3 months post initiation of PT, there were remarkable improvements. She gained new motor skills and developmental milestones including the ability to roll over. Her PT assessment at age 6 months revealed good muscle tone and her AIMS score improved to 25th –50th percentile for chronologic age (50th –75th percentile for corrected age) while GMFM was reported as 22.72%. She had a slight tendency towards flexion/abduction/external rotation of hips and slight lateral grooves on thighs with passive adduction. In prone and supported sitting, active extension with adduction of hips and consistent preference for weight shift to left with head tilt towards right side was seen. She has been advised to continue PT. At this time ERT is not recommended, given her very subtle findings and very good improvement with PT intervention.
Patient 6
Patient 6 is a Caucasian male homozygous for the c.-32-13T>G-splice site variant. HyperCKemia was noticed in the first month (438 U/L; N 60–305) which normalized spontaneously by age 6 months. ECG and ECHO revealed patent foramen ovale without left ventricular hypertrophy. He was seen at Duke at age 6 months. PT evaluation revealed flexion/abduction/external rotation positioning of lower extremities and with bilateral IT band tightness. He had proximal muscle weakness including decreased active use of abdominal oblique muscles with a lack of active trunk rotation and with lower rib flaring. He had decreased active use of hip extensors; and decreased active use of lower abdominal muscles with inability to achieve a posterior pelvic tilt in supine. AIMS score was at 1st percentile for age and GMFM was at 12.3%. Other evaluations including cardiac, pulmonary, speech, and swallow were normal. Patient 6 is receiving PT and is being closely observed for potential progression of symptoms to determine if ERT needs to be initiated.
Patient 7
Patient 7 is a Caucasian female homozygous for the IVS c.-32-13T>G-splice site variant born at 36 weeks gestation. CK levels have been normal since birth. After a breech delivery, her hip ultrasound was off by 1/5th of a degree, which resolved spontaneously on follow up examination. Cardiac evaluation including ECHO and ECG showed a small patent ductus arteriosus at birth, premature atrial and ventricular contractions but without evidence of left ventricular hypertrophy. Duke evaluation at age 6 months, including PT evaluation, revealed subtle neck flexor weakness reflected by head lag during pull to sit. She had predominant overall flexion position of lower extremities with a tendency towards hip flexion/abduction/external rotation, with lateral thigh grooves suggestive of bilateral IT band tightness. She had lower extremity stiffness during supported standing with knees locked in extension and ankle plantarflexion positioning, remaining up on her toes in supported standing. AIMS score was between 10th and 25th percentile for chronological age and 50th percentile for her corrected age adjusted for prematurity while GMFM score was 16.55%. Other evaluations including pulmonary, speech, and swallow were normal. ERT has not been initiated. Patient 7 is receiving PT and is being closely observed for potential progression of symptoms.
5. Discussion
While NBS identifies individuals with “late-onset” GAA variants at birth, circumventing the diagnostic odyssey and offering a potential opportunity for better outcome, no evidence-based guidelines exist for assessment, management, and/or initiation of ERT for children with these “late-onset” GAA variants. Childhood management is further complicated in patients with the c.-32-13T>G variant. While originally thought to result in adult-onset disease, patients with c.-32-13 T>G variant in compound heterozygosity and a second pathogenic variant have been reported in retrospective case series as presenting unrecognized symptoms in early childhood [26 31 32]. However, these reports fall short as they do not include standardized developmental and functional motor testing, or evaluation of features in the early years, especially the first year of life. The patient cohort presented here represents a systematic characterization of clinical features in infants with the common IVS variant identified by NBS including feeding, respiratory, musculoskeletal, and functional motor involvement. Both compound heterozygous and homozygous infants with the common IVS variant are described, with the latter being a novel addition to our understanding.
All seven patients demonstrated some motor involvement by age 6 months; however patients (Patients 1, 2 and 3) with c.-32-13 T>G variant in compound heterozygosity were more severely affected and also presented with feeding difficulties, generalized hypotonia, and muscle weakness from the neonatal period. Patients with c.-32-13 T>G variant in compound heterozygosity also demonstrated persistent hyperCKemia, until the initiation of ERT. ERT with PT was initiated for all three patients (1, 2, and 3) with c.-32-13 T>G variant in compound heterozygosity as they demonstrated widespread musculoskeletal involvement. Patients 1 and 2 were initiated on ERT by their local physicians within 35 days as a joint decision with our team at Duke. Patient 3 had hyperCkemia, delayed development, and muscle weakness early in life, yet was presumed asymptomatic and denied treatment due to her “late-onset” c.-32-13T>G variant. Patient 3 was initiated on ERT at 9 months by our team at Duke based on findings of an in-depth evaluation and parental concern. She has shown remarkable improvement following 9 months on ERT. Thus, patients (Patients 1, 2 and 3) with c.-32-13 T>G variant in compound heterozygosity demonstrated significant and prominent developmental delays in gross motor milestones with evidence of muscular weakness requiring ERT initiation. It will be important to follow these patients carefully over time to gain a better understanding of response to ERT in infants identified via NBS. Patient 3 highlights the subtle motor signs of pelvic girdle muscle weakness consistent with Pompe disease that are often missed, resulting in this patient initially being considered asymptomatic. Subtle muscle deficits characteristic of infants with “late-onset” GAA variants as compared to a typically developing child 6 months of age are shown in Figures 1 and 2.
Subtle disease specific muscular signs characteristic of LOPD were observed in all four patients (Patient 4, 5, 6 and 7) with c.-32-13T>G variant in homozygosity. Patients homozygous for the c.-32-13T>G variant had more subtle motor signs than patients with c.-32-13 T>G variant in compound heterozygosity coupled with normal laboratory parameters (CK, AST, ALT, and urinary Glc4 levels) at the time of evaluation at Duke. HyperCKemia was present in two of four patients (Patient 4 and Patient 6) with c.-32-13T>G variant in homozygosity in the newborn period, which resolved spontaneously. Even though the skeletal muscle findings in the patients with c.-32-13T>G variant in homozygosity were subtle, they were characteristic of known features of Pompe disease and were missed before a vigilant disease-specific approach to evaluation. ERT has not been initiated in any of the patients with c.-32-13T>G variant in homozygosity at this time as they do not manifest the widespread motor involvement seen in the patients with c.-32-13 T>G variant in compound heterozygosity. Patients with c.-32-13T>G variant in homozygosity will be monitored and followed every 6 to 12 months, with initiation of ERT a future option should their disease course appears to be sufficiently progressive.
Based on carrier frequency of the common IVS variant as 1 in 154 in the general unaffected Dutch population, 16–40% of all adult Pompe patients should have c.-32-13T>G variant in homozygosity [33]. Clearly this has not been reported [20 21 34–36], suggesting that most c.-32-13T>G homozygotes are unaffected or have only a very subtle phenotype with the few symptomatic homozygotes described in the literature representing an extreme end of the phenotypic spectrum. Symptomatic patients with c.-32-13T>G variant in homozygosity could also be explained by other factors including modifier genes, other variants in the GAA gene, exercise, diet or other environmental factors influencing the clinical presentation. To date, only a few symptomatic patients have been reported. Musumeci et al describe only one patient, among six adult patients with c.-32-13T>G variant in homozygosity, with childhood motor disease onset at age 12 years who had a sole symptom of leg weakness at onset. The other 5 patients had a collective symptomatology of myalgia, hyperCKemia, and/or exercise induced fatigue at the age of onset (39–58 years) [21]. Another report by the same group describes two adult patients with c.-32-13T>G variant in homozygosity grouped with 27 adult patients with c.-32-13 T>G variant in compound heterozygosity with a phenotype of presymptomatic hyperCKemia (37 %), proximal/axial muscle weakness (53 %) and respiratory impairment [25]. Patients with c.-32-13T>G variant in homozygosity identified by NBS should be reassured that clinical symptoms are likely to be mild with treatment likely unnecessary, though continued follow-up is warranted to understand the disease course. The four patients with c.-32-13T>G variant in homozygosity reported here represent preliminary data as an emerging addition to the literature given the limited experience with this genotype.
Also notable in our cohort is the emerging early finding of feeding and swallowing difficulty in two of three patients with c.-32-13T>G variant in compound heterozygosity and one of four patients with c.-32-13T>G variant in homozygosity (Patients 1, 2 and 5), typically a feature of IOPD and also recognized in the Taiwan LOPD cohort (none have the IVS variant) [30]. Thus, this is a new finding associated with the IVS variant as a feature that may present in infancy.
The two cohorts of patients with c.-32-13T>G “late-onset” GAA variants diagnosed by NBS, compound heterozygotes and homozygotes, necessitate different levels of concern and management. A meticulous follow up is vital in both groups, yet treatment with ERT is likely not necessary for patients with c.-32-13T>G variant in homozygosity based on experience to date from patients identified in the clinical setting or this early experience in infants identified via NBS. Patients with c.-32-13T>G variant in compound heterozygosity and a second pathogenic variant are more likely to manifest symptoms warranting treatment with ERT. Patients 1 and 2 with c.-32-13T>G variant in compound heterozygosity initiated ERT at 1 month of age and displayed significant symptomatic improvement, yet with the continued presence of some gross motor developmental delays on follow up visits. It has been well documented that ERT results in significant improvement but not complete reversal and/or prevention of pre-existing damage and skeletal muscle involvement at the time of ERT in LOPD as well as in IOPD [5 37–41]. The outcome in LOPD can thus also be affected by early ERT to restore, preserve, and possibly reverse skeletal muscle pathology.
Initiation of treatment with ERT can be a difficult decision for infants with “late-onset” GAA variants especially the c.-32-13T>G variant. Treatment decisions should be multifactorial and differential, primarily based on a multidisciplinary evaluation along with parents and physicians’ views. Our report aims to increase awareness of the signs and symptoms of Pompe disease in infants with the c.-32-13T>G variant to establish an evolving phenotype, not to provide guidance on when to initiate ERT. Over time, as longitudinal data are gathered on patients identified by NBS, a better perspective and understanding of the natural history of patients with “late-onset” GAA variants from infancy through adulthood will be achieved. These long-term data could then serve as a basis for universal treatment decisions. At this time, the extent of disease involvement should guide individualized, patient specific decisions on if and when to initiate ERT.
We therefore recommend a thorough multi-dimensional approach to allow for early identification of typical and subtle features of patients with “late-onset” GAA variants [37 38 42]. Evaluation by a pediatric physical therapist with expertise in neuromuscular disorders is critical for an age appropriate assessment of muscular weakness, as it may not be appreciable on medical clinical examination alone. Postural and musculoskeletal features of Pompe disease may signal early involvement. These can limit or alter the motor development at a young age, and can contribute to compromised biomechanics for function and lead to contracture and secondary deformity if not treated [43]. Subtle findings as seen in our patients reflect early evidence of muscle compromise and underlying pathological damage within these muscles. Standardized functional gross motor assessments, especially in the 1st year of life, are of paramount importance in this population and could signal the need for initiation of intervention, including PT/OT and/or ERT before more severe symptoms with motor delays, regression, or secondary musculoskeletal involvement become evident by standardized assessments. AIMS scores on our patients were variable from the 1st percentile to the 50th percentile for age, at least 1 standard deviation (SD) below the mean for age, yet at or below the 50th percentile in all. It is imperative to look at the complete picture of qualitative musculoskeletal assessment in addition to seemingly borderline standardized PT tests to precisely determine the extent of involvement [44 45].
Musculoskeletal and developmental motor control features notable in this cohort resemble patterns characteristic of LOPD in older individuals, and appeared to reflect possible early signs of weakness. We have identified a pattern of involvement similar in all of our patients especially compared to the known components of normal motor development [46] for potential initial signs of LOPD pertaining typically to the trunk and limb girdle muscles (Figures 1 and 2). These features included a tendency towards positioning in lower extremity flexion/abduction/external rotation; decreased use of hip extensors and abdominals, especially abdominal obliques; and decreased flexibility in iliotibial bands and sometimes hip flexors and plantar flexors; all associated with functional difficulties in crucial aspects of developmental control of the pelvis, trunk, and shoulder girdle (Figures 1 and 2). It cannot be emphasized enough that clinicians should be aware of this early onset spectrum of LOPD which can occur in patients with IVS splice site variant to determine an individualized course of management.
In addition to ERT, regular physical therapy with age appropriate assessment, anticipatory, preventative intervention, and close monitoring based on specific and known signs and symptoms of Pompe disease should be an integral part of management, especially in the first year of life whether on ERT or not. Systematic follow-up with repeated assessment across systems and categories of the international classification of function (ICF) will be an important step in determining the early phenotype, progression, and outcome of patients with “late-onset” GAA variants identified by NBS. The Pompe Registry and the Newborn Screening Translation Research Network could serve as a platform for a comprehensive data collection and comparison. With a more comprehensive characterization of patients with “late-onset” GAA variants with early identification by NBS and detailed assessment across the entire lifespan, specific, individualized and more successful treatment of identified impairments will also be possible in tandem. As we know, the treatment of any neuromuscular disorder is optimized by early identification and ongoing, anticipatory, preventative treatment based on individual assessment [47].
Given this small sample of self-referred patients, also a likely selection bias, we cannot offer a comprehensive characterization of the spectrum of Pompe disease in infants with the c.-32-13T>G variant. Our patients here represent the first carefully phenotyped cohort in the USA of infants with Pompe disease with the c.-32-13T>G “late-onset” GAA variant detected by NBS. It can thus serve as a valuable contribution in the development of evaluation and treatment algorithms for infants with “late-onset” GAA variants, as more data becomes available. Though immediate symptomatic and motor improvement was seen by the parents of the patients initiated on ERT and/or PT, and confirmed by objective evaluations, the long term outcome, and an optimum timing of ERT initiation remains unknown. Long-term follow up studies on a larger cohort of patients with c.-32-13T>G variant in homozygosity and compound heterozygosity diagnosed via NBS is needed.
This patient cohort emphasizes not only the opportunity for early detection of skeletal muscle involvement in infants with “late-onset” GAA variants but also a high probability of overlooking or underestimating the significance of clinically present and detectable symptoms. With detailed evaluations, regardless of genotype or clinical disease classification, our current understanding and management of the early course of patients with “late-onset” GAA variants identified by newborn screening will continue to expand. It emphasizes the need for collective learning as this will allow for better management of cases identified by NBS, which is now a reality in USA. This is the first of a series of papers reporting on the expanded understanding and management, across the continuum and across the lifespan of patients with “late-onset” GAA variants identified by newborn screening.
6. Conclusion
This patient cohort represents the first carefully phenotyped cohort of infants with the c.-32-13T>G “late-onset” GAA variant detected by NBS in the USA. It emphasizes not only the opportunity for early detection of skeletal and other muscle involvement in infants with c.-32-13T>G variant but also a high probability of overlooking or underestimating the significance of clinically present and detectable features. It can thus serve as a valuable contribution in the development of evaluation and treatment algorithms for infants with “late-onset” GAA variants.
Acknowledgments
Funding: This study was partly funded by Genzyme Corporation and partly by the Lysosomal Disease Network, a part of the NIH Rare Diseases Clinical Research Network (U54NS065768). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This study was partly funded by the Lysosomal Disease Network, a part of the NIH Rare Diseases Clinical Research Network (RDCRN). The Lysosomal Disease Network (U54NS065768) is a part of the RDCRN, an initiative of the Office of Rare Diseases Research (ORDR), and the National Center for Advancing Translational Science (NCATS). This consortium is funded through collaboration between the NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The authors also acknowledge Lois Bly, PT, MA, C/NDT for graciously allowing us to republish the pictures of normal development in infancy from her textbook, Motor Skills Acquisition in the First Year of Life – an Illustrated Guide to Normal Development. In addition the authors are thankful to the Allen Boger family for their generous funding towards research in Pompe disease.
List of abbreviations
- NBS
Newborn screening
- IOPD
Infantile onset Pompe disease
- LOPD
Late onset Pompe disease
- GAA
Acid α-glucosidase
- ERT
Enzyme replacement therapy
- RUSP
Recommended uniform screening panel
- CK
Creatine kinase
- Glc4
Urinary glucose tetrasaccharide
- ECG
Electrocardiogram
- ECHO
Echocardiogram
- PT
Physical therapy
- ST
Speech therapy
- OT
Occupational therapy
- AIMS
Alberta infant motor scale
- GMFM
Gross motor function measure
- VFSS
video-fluoroscopic swallow study
- IT
Iliotibial
- PDMS
Peabody development motor scale
- ICF
International classification of function
- RDCRN
Rare disease clinical research network
- ORDR
Office of rare disease research
- NCATS
National center for advancing translational science
- NINDS
National institute of neurological disorders and stroke
- NIDDK
National institute of diabetes and digestive and kidney diseases
Footnotes
Ethics approval and consent to participate
Written informed consent was obtained from a parent or guardian for all individuals as part of Duke Institutional Review Board approved Pompe long-term follow-up study (Pro00010830) and/or Determination of CRIM status in Pompe disease (Pro00001562).
Authors’ Contributions
MVR, LB, ZBK, and PSK participated in the design of the study. PSK, MVR, LB, LEC, AKD, KB, and ZBK conceived of the study and participated in its design and coordination. LEC, LB, KB, JC, RG, RQ, and PSK were involved in the clinical care of the patients at Duke University. LEC, JC, RG, and RQ were involved with the PT assessments of the patients at Duke University. All authors helped to draft the manuscript, and approved the final manuscript.
Conflict of interest disclosures: MVR, LB, ZK, AKD, KB, RG, and RQ have no financial or proprietary interest in the materials presented herein. LEC has received honoraria from Genzyme Corporation of Sanofi; has participated in research supported by Genzyme Corporation of Sanofi and Roivant Sciences; has been awarded grant support from the National Skeletal Muscle Research Center; and is a member of the Pompe Registry North American Board of Advisors for Genzyme Corporation of Sanofi. JC has participated in research supported by Genzyme Corporation. PSK has received research/grant support and honoraria from Genzyme Corporation and Amicus Therapeutics. PSK is a member of the Pompe and Gaucher Disease Registry Advisory Board for Genzyme Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirschhorn R, Reuser AJJ. Glycogen storage disease type II: acid a-glucosidase (acid maltase) deficiency. In: Valle D, Scriver CR, editors. Scriver’s OMMBID the online metabolic & molecular bases of inherited disease. New York: McGraw-Hill; 2009. [Google Scholar]
- 2.van den Hout HMP, Hop W, van Diggelen OP, et al. The Natural Course of Infantile Pompe’s Disease: 20 Original Cases Compared With 133 Cases From the Literature. Pediatrics. 2003;112(2):332–40. doi: 10.1542/peds.112.2.332. [DOI] [PubMed] [Google Scholar]
- 3.Kishnani PS, Hwu W-L, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. The Journal of Pediatrics. 2006;148(5):671–76. e2. doi: 10.1016/j.jpeds.2005.11.033. doi: https://doi.org/10.1016/j.jpeds.2005.11.033 [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 4.Hagemans ML, Winkel LP, Van Doorn PA, et al. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain. 2005;128(Pt 3):671–7. doi: 10.1093/brain/awh384. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Felber W, Horvath R, Gempel K, et al. Late onset Pompe disease: clinical and neurophysiological spectrum of 38 patients including long-term follow-up in 18 patients. Neuromuscul Disord. 2007;17(9–10):698–706. doi: 10.1016/j.nmd.2007.06.002. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 6.Wokke JH, Escolar DM, Pestronk A, et al. Clinical features of late-onset Pompe disease: a prospective cohort study. Muscle Nerve. 2008;38(4):1236–45. doi: 10.1002/mus.21025. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Yang C-F, Yang CC, Liao H-C, et al. Very Early Treatment for Infantile-Onset Pompe Disease Contributes to Better Outcomes. The Journal of Pediatrics. 2016;169:174–80. e1. doi: 10.1016/j.jpeds.2015.10.078. doi: https://doi.org/10.1016/j.jpeds.2015.10.078 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 8.Gruhn KM, Heyer CM, Güttsches A-K, et al. Muscle imaging data in late-onset Pompe disease reveal a correlation between the pre-existing degree of lipomatous muscle alterations and the efficacy of long-term enzyme replacement therapy. Molecular Genetics and Metabolism Reports. 2015;3:58–64. doi: 10.1016/j.ymgmr.2015.03.010. doi: http://dx.doi.org/10.1016/j.ymgmr.2015.03.010 [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishnani PS, Corzo D, Leslie ND, et al. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pediatr Res. 2009;66(3):329–35. doi: 10.1203/PDR.0b013e3181b24e94. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matern D, Gavrilov D, Oglesbee D, Raymond K, Rinaldo P, Tortorelli S. Newborn screening for lysosomal storage disorders. Seminars in Perinatology. 2015;39(3):206–16. doi: 10.1053/j.semperi.2015.03.005. doi: http://dx.doi.org/10.1053/j.semperi.2015.03.005 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 11.Chien Y-H, Lee N-C, Thurberg BL, et al. Pompe Disease in Infants: Improving the Prognosis by Newborn Screening and Early Treatment. Pediatrics. 2009;124(6):e1116–e25. doi: 10.1542/peds.2008-3667. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 12.Chien Y-H, Hwu W-L, Lee N-C. Pompe Disease: Early Diagnosis and Early Treatment Make a Difference. Pediatrics & Neonatology. 2013;54(4):219–27. doi: 10.1016/j.pedneo.2013.03.009. doi: http://dx.doi.org/10.1016/j.pedneo.2013.03.009 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Byrne BJ, Kishnani PS, Case LE, et al. Pompe disease: Design, methodology, and early findings from the Pompe Registry. Molecular Genetics and Metabolism. 2011;103(1):1–11. doi: 10.1016/j.ymgme.2011.02.004. doi: http://dx.doi.org/10.1016/j.ymgme.2011.02.004 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 14.Kishnani PS, Amartino HM, Lindberg C, et al. Timing of diagnosis of patients with pompe disease: Data from the pompe registry. American Journal of Medical Genetics Part A. 2013;161(10):2431–43. doi: 10.1002/ajmg.a.36110. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 15.Kronn DF, Day-Salvatore D, Hwu W-L, et al. Management of Confirmed Newborn-Screened Patients With Pompe Disease Across the Disease Spectrum. Pediatrics. 2017;140(Supplement 1):S24–S45. doi: 10.1542/peds.2016-0280E. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.Kroos MA, Pomponio RJ, Hagemans ML, et al. Broad spectrum of Pompe disease in patients with the same c-32-13T->G haplotype. Neurology. 2007;68(2):110–5. doi: 10.1212/01.wnl.0000252798.25690.76. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 17.van Capelle CI, van der Meijden JC, van den Hout JM, et al. Childhood Pompe disease: clinical spectrum and genotype in 31 patients. Orphanet J Rare Dis. 2016;11(1):65. doi: 10.1186/s13023-016-0442-y. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laforet P, Laloui K, Granger B, et al. The French Pompe registry. Baseline characteristics of a cohort of 126 patients with adult Pompe disease. Rev Neurol (Paris) 2013;169(8–9):595–602. doi: 10.1016/j.neurol.2013.07.002. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 19.Laforet P, Nicolino M, Eymard PB, et al. Juvenile and adult-onset acid maltase deficiency in France: genotype-phenotype correlation. Neurology. 2000:55. doi: 10.1212/wnl.55.8.1122. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 20.Herzog A, Hartung R, Reuser AJJ, et al. A cross-sectional single-centre study on the spectrum of Pompe disease, German patients: molecular analysis of the GAA gene, manifestation and genotype-phenotype correlations. Orphanet Journal of Rare Diseases. 2012;7(1):1–14. doi: 10.1186/1750-1172-7-35. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musumeci O, Thieme A, Claeys KG, et al. Homozygosity for the common GAA gene splice site mutation c-32-13T>G in Pompe disease is associated with the classical adult phenotypical spectrum. Neuromuscular Disorders. 2015;25(9):719–24. doi: 10.1016/j.nmd.2015.07.002. doi: http://doi.org/10.1016/j.nmd.2015.07.002 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 22.Rafael Bretón Martínez J, Martínez AC. Long-term enzyme-replacement therapy (ERT) with alglucosidase alfa: Evolution of two siblings with juvenile late-onset Pompe disease. Journal of the Neurological Sciences. 2015;358(1–2):459–60. doi: 10.1016/j.jns.2015.08.007. doi: http://dx.doi.org/10.1016/j.jns.2015.08.007 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 23.Palmer RE, Amartino HM, Niizawa G, Blanco M, Pomponio RJ, Chamoles NA. Pompe disease (glycogen storage disease type II) in Argentineans: Clinical manifestations and identification of 9 novel mutations. Neuromuscular Disorders. 2007;17(1):16–22. doi: 10.1016/j.nmd.2006.09.004. doi: http://dx.doi.org/10.1016/j.nmd.2006.09.004 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 24.Turaça LT, de Faria DOS, Kyosen SO, et al. Novel GAA mutations in patients with Pompe disease. Gene. 2015;561(1):124–31. doi: 10.1016/j.gene.2015.02.023. doi: http://dx.doi.org/10.1016/j.gene.2015.02.023 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 25.Montagnese F, Barca E, Musumeci O, et al. Clinical and molecular aspects of 30 patients with late -onset Pompe disease (LOPD): unusual features and response to treatment. Journal of Neurology. 2015;262(4):968–78. doi: 10.1007/s00415-015-7664-0. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 26.van Capelle CI, van der Meijden JC, van den Hout JMP, et al. Childhood Pompe disease: clinical spectrum and genotype in 31 patients. Orphanet Journal of Rare Diseases. 2016;11:65. doi: 10.1186/s13023-016-0442-y. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chien Y-H, Goldstein JL, Hwu W-L, et al. Baseline Urinary Glucose Tetrasaccharide Concentrations in Patients with Infantile- and Late-Onset Pompe Disease Identified by Newborn Screening. JIMD Reports. 2015;19:67–73. doi: 10.1007/8904_2014_366. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang C-F, Liu H-C, Hsu T-R, et al. A large-scale nationwide newborn screening program for pompe disease in Taiwan: Towards effective diagnosis and treatment. American Journal of Medical Genetics Part A. 2014;164(1):54–61. doi: 10.1002/ajmg.a.36197. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 29.Chien YH, Lee NC, Huang PH, Lee WT, Thurberg BL, Hwu WL. Early pathologic changes and responses to treatment in patients with later-onset Pompe disease. Pediatr Neurol. 2012:46. doi: 10.1016/j.pediatrneurol.2011.12.010. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 30.Chien Y-H, Lee N-C, Huang H-J, Thurberg BL, Tsai F-J, Hwu W-L. Later-Onset Pompe Disease: Early Detection and Early Treatment Initiation Enabled by Newborn Screening. The Journal of Pediatrics. 2011;158(6):1023–27. e1. doi: 10.1016/j.jpeds.2010.11.053. doi: http://dx.doi.org/10.1016/j.jpeds.2010.11.053 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 31.Deroma L, Guerra M, Sechi A, et al. Enzyme replacement therapy in juvenile glycogenosis type II: a longitudinal study. European Journal of Pediatrics. 2014;173(6):805–13. doi: 10.1007/s00431-013-2258-2. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 32.Bembi B, Pisa FE, Confalonieri M, et al. Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. Journal of Inherited Metabolic Disease. 2010;33(6):727–35. doi: 10.1007/s10545-010-9201-8. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 33.Ausems MGEMJV, Hermans MMP, Kroos MA, Beemer FA, Wokke JHJ, Sandkuijl LA, Reuser AJJ, van der Ploeg AT. Frequency of glycogen storage disease type II in The Netherlands: implications for diagnosis and genetic counselling. European Journal of Human Genetics. 1999;7:713–16. doi: 10.1038/sj.ejhg.5200367. [DOI] [PubMed] [Google Scholar]
- 34.Kroos M, Pomponio RJ, van Vliet L, et al. Update of the Pompe disease mutation database with 107 sequence variants and a format for severity rating. Human Mutation. 2008;29(6):E13–E26. doi: 10.1002/humu.20745. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 35.Martiniuk F, Chen A, Mack A, et al. Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease. American Journal of Medical Genetics. 1998;79(1):69–72. doi: 10.1002/(SICI)1096-8628(19980827)79:1<69::AID-AJMG16>3.0.CO;2-K. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 36.Montalvo ALE, Bembi B, Donnarumma M, et al. Mutation profile of the GAA gene in 40 Italian patients with late onset glycogen storage disease type II. Human Mutation. 2006;27(10):999–1006. doi: 10.1002/humu.20374. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 37.Raben N, Wong A, Ralston E, Myerowitz R. Autophagy and mitochondria in Pompe disease: Nothing is so new as what has long been forgotten. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2012;160C(1):13–21. doi: 10.1002/ajmg.c.31317. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feeney EJ, Austin S, Chien Y-H, et al. The value of muscle biopsies in Pompe disease: identifying lipofuscin inclusions in juvenile- and adult-onset patients. Acta Neuropathologica Communications. 2014;2:2–2. doi: 10.1186/2051-5960-2-2. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoser BGH, Müller-Höcker J, Horvath R, et al. Adult-onset glycogen storage disease type 2: clinico-pathological phenotype revisited. Neuropathology and Applied Neurobiology. 2007;33(5):544–59. doi: 10.1111/j.1365-2990.2007.00839.x. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 40.Strothotte S, Strigl-Pill N, Grunert B, et al. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. Journal of Neurology. 2009;257(1):91. doi: 10.1007/s00415-009-5275-3. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 41.Bali DS, Goldstein JL, Banugaria S, et al. Predicting cross-reactive immunological material (CRIM) status in Pompe disease using GAA mutations: lessons learned from 10 years of clinical laboratory testing experience. Am J Med Genet C Semin Med Genet. 2012;160C(1):40–9. doi: 10.1002/ajmg.c.31319. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim J-A, Li L, Kakhlon O, Myerowitz R, Raben N. Defects in calcium homeostasis and mitochondria can be reversed in Pompe disease. Autophagy. 2015;11(2):385–402. doi: 10.1080/15548627.2015.1009779. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Case LE, Beckemeyer AA, Kishnani PS. Infantile Pompe disease on ERT: update on clinical presentation, musculoskeletal management, and exercise considerations. Am J Med Genet C Semin Med Genet. 2012;160C(1):69–79. doi: 10.1002/ajmg.c.31321. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 44.Suh-Fang J, Kuo-Inn Tsou Y, Li-Chiou C, Shu-Fang H. Alberta infant motor scale: Reliability and validity when used on preterm infants in Taiwan. Physical Therapy. 2000;80(2):168–78. [PubMed] [Google Scholar]
- 45.Darrah J, Piper M, Watt M-J. Assessment of gross motor skills of at-risk infants: predictive validity of the Alberta Infant Motor Scale. Developmental Medicine & Child Neurology. 1998;40(7):485–91. doi: 10.1111/j.1469-8749.1998.tb15399.x. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 46.Bly L. Motor Skills Acquisition in the First Year - An Illustrated Guide to Normal Development. Tucson, Arizona: Therapy Skill Builders; 1994. [Google Scholar]
- 47.Case LE, Kishnani PS. Physical therapy management of Pompe disease. Genet Med. 2006;8(5):318–27. doi: 10.1097/01.gim.0000217789.14470.c5. [DOI] [PubMed] [Google Scholar]