Abstract
Introduction
The aim of this study was to assess alterations in median nerve biomechanics within the carpal tunnel resulting from ultrasound-guided hydrodissection in a cadaveric model.
Methods
Twelve fresh frozen human cadaver hands were used. Median nerve gliding resistance was measured at baseline and post-hydrodissection, by pulling the nerve proximally and then returning it to the origin. Six specimens were treated with hydrodissection, and 6 were used as controls.
Results
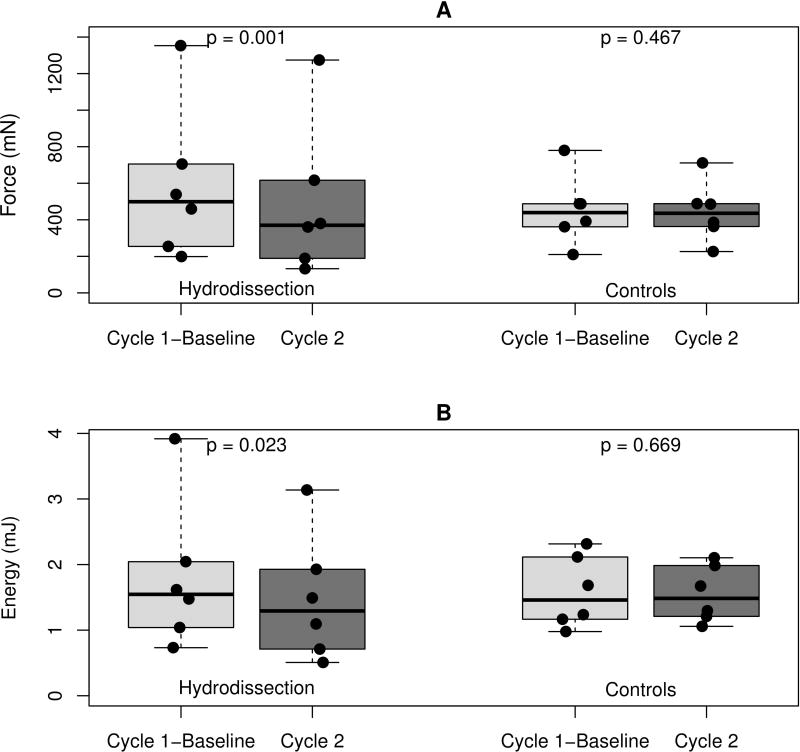
In the hydrodissection group there was a significant reduction in mean peak gliding resistance of 92.9 ± 34.8 mN between baseline and immediately post-hydrodissection (21.4% ± 10.5%, p= .001). No significant reduction between baseline and the second cycle occurred in the control group: 9.6 ± 29.8 mN (0.4% ± 5.3%, p= .467).
Discussion
Hydrodissection can decrease the gliding resistance of the median nerve within the carpal tunnel, at least in wrists unaffected by carpal tunnel syndrome. A clinical trial of hydrodissection seems justified.
Keywords: Hydrodissection, Ultrasound, Injection, Carpal tunnel, Nerve, Gliding resistance
Introduction
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy, with a reported annual incidence per 100,000 persons ranging from 324 to 524 among women and 135 to 303 among men1–4. Although the precise pathoetiology of idiopathic CTS remains undefined, the syndrome is characterized by increased pressure in the carpal tunnel, with consequent median nerve compression. The most common and effective treatment methods for CTS are corticosteroid injections and surgical decompression5,6. Corticosteroid injections often provide temporary relief, but approximately half of injected patients require further treatment within one year7,5,8.
Previous research has shown that the subsynovial connective tissue (SSCT) surrounding the tendons in the carpal tunnel is thickened and fibrotic in CTS patients compared to normal individuals9–12, and this thickening appears to result in reduced motion of the median nerve within the carpal tunnel13–16. While the relationship between fibrosis and neuropathy is still unknown, this reduction of mobility can prevent the nerve from moving aside as the tendons move anteriorly during strong grip or pinch17. This situation may compress the median nerve during gripping, thus contributing to development or progression of CTS. In addition, fixation can lead to traction neuropathy during hand activity as the tendons move along the nerve within the tunnel 18. Consequently, freeing the median nerve from any motion restriction might restore the dynamic balance in relative motion between the median nerve and the surrounding tissues, thus reducing CTS symptoms19,20.
Ultrasound-guided hydrodissection has recently been proposed to treat nerve entrapment21–23. The potential utility of hydrodissection in CTS is based on the theory that nerve entrapment is exacerbated by median nerve fixation to surrounding tissues such as the transverse carpal ligament (TCL). This fixation, which is often seen at the time of surgery, appears to correlate with the reduction in nerve motion visualized by ultrasound imaging14,15,13. Hydrodissection uses an ultrasound-guided injection of sterile saline to create a perineural fluid plane between the nerve and surrounding tissues and consequently improve the nerve mobility. Advantages of hydrodissection are that it can be performed using only sterile saline solution or a similar physiologically compatible fluid, and the injection can be done in the office. In addition, hydrodissection is performed under ultrasound-guidance, lessening chances of median nerve injury compared to a blind injection.
Several reports have anecdotally noted the use of hydrodissection to treat CTS21–23, but have not formally investigated whether the nerve is mobilized by this technique. Consequently, the aim of this study was to assess alterations in the biomechanics of the median nerve environment resulting from the hydrodissection procedure in an unembalmed cadaveric model. Our hypothesis was that gliding resistance of the median nerve would be decreased after ultrasound-guided hydrodissection, presumably due to disruption of fibrous connections between the median nerve and surrounding tissue. In addition, we performed cyclic testing to assess whether the potential effect of hydrodissection was permanent or changed over time, as SSCT fibers weakened or stretched by hydrodissection might be more likely to break with continuous cycling.
Methods
Experimental Set Up
Twelve fresh frozen human cadaveric hands were obtained from our institution’s anatomical bequest program for use in this experiment, with approval from our institutional biospecimen committee. Specimens were screened for a history of CTS, rheumatoid arthritis or traumatic injuries of the ipsilateral arm. We used cadaveric hands from donors unaffected by CTS, because the SSCT is expected to be less fibrous and therefore should be easier to dissect with the sterile saline injections. Hands were amputated approximately 13 cm proximal to the wrist joint. All soft tissue was removed 6 cm distal to the amputation site, in order to expose the proximal ends of the ulna and radius. The wrist was fixed in a neutral position with a customized external fixator, and the proximal ends of the ulna and the radius were locked into a clamp.
All digits were fixed in extension with 1.5-mm diameter K-wires. The median nerve and tendons in closest proximity to the median nerve in a neutral wrist position, namely the flexor pollicis longus (FPL), the flexor digitorum profundus of the index finger (FDP2) and the flexor digitorum superficialis of the index, middle and ring finger (FDS2 - FDS4) were exposed 2.5 cm proximal to the proximal wrist crease. Soft tissue in contact with the median nerve was removed to eliminate friction between the structures. The TCL was left intact (Figure 1A). Each tendon was connected proximally to a 50 gram weight suspended over a pulley to maintain tension. The three common palmar digital nerves, motor branch of the median nerve and the anastomotic branch to the ulnar nerve were divided distally and were sutured together. Before dividing the digital branches (Figure 1B), these and the underlying tendon were marked at the level of the proximal part of the metacarpal bones, using a 6.0 Prolene suture, in order to define the neutral position for the median nerve (Figure 1C). The sutured median nerve bundle was connected distally to a 50 gram weight suspended over a pulley to maintain tension. The proximal end of the median nerve was connected to a stepper-motor driven mechanical actuator controlled by a microcontroller (Arcus Technologies) and instrumented with a load cell (Transducer Techniques, Temecula, CA) (Figure 2). A similar experimental concept and apparatus has been used in previous studies from this laboratory to measure gliding resistance which indicated that when testing the same condition similar force-displacement curves were found repeatedly24–26.
Figure 1.
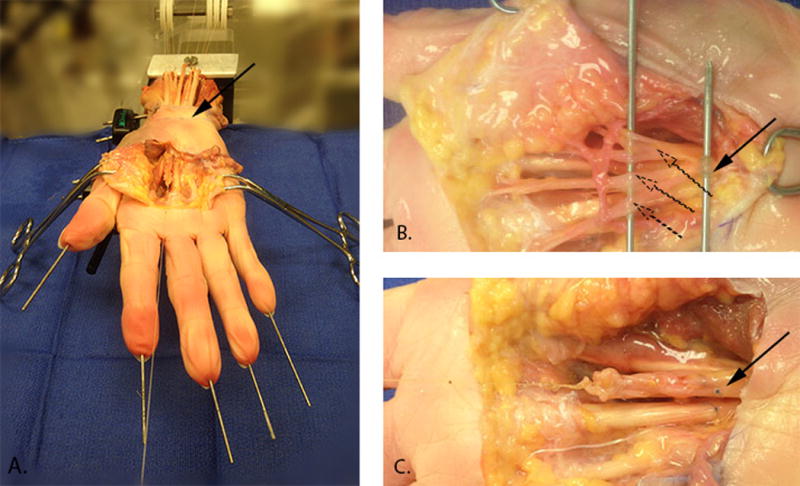
A) Median nerve and tendons in closest proximity to the median nerve exposed 2.5 cm proximal to the proximal wrist crease (arrow). Median nerve exposed distal to the carpal tunnel outlet, approximately 5cm distal to the proximal wrist crease depending on hand size. B) Three palmar digital branches (dotted arrows) and motor branch (solid arrow) of the median nerve identified. C) Branches of the median nerve sutured together: the neutral position of the nerve identified by markers at the same level of the nerve and underlying FDS3 tendon (arrow).
Figure 2.
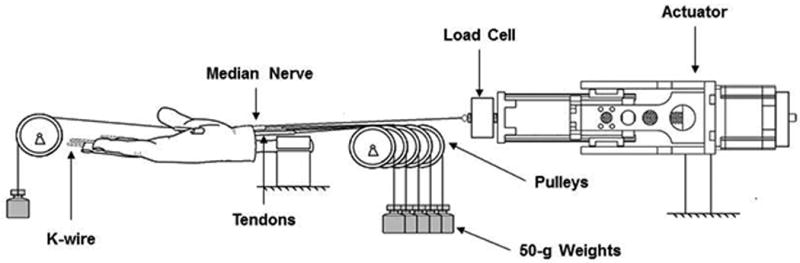
Experimental set up.
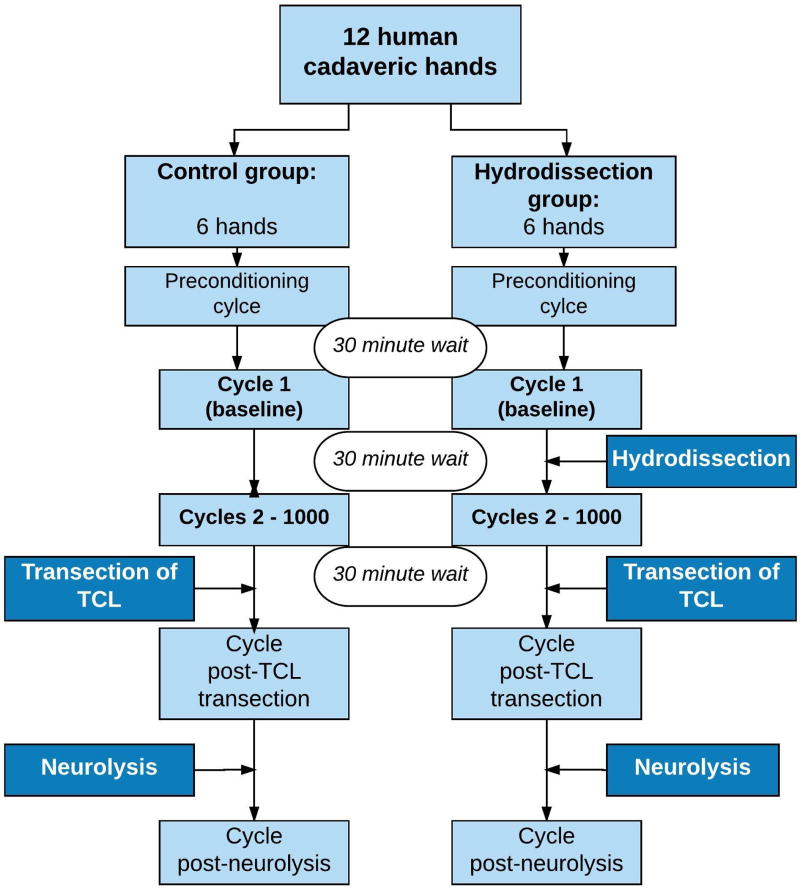
Prior to formal testing one preconditioning cycle was performed to ensure that any force reduction post treatment resulted from the hydrodissection, and not from the mechanical effect of the excursion/stretching. The median nerve was pulled proximally at a rate of 1 mm/s for a distance of 6 mm, which is within the physiological range of median nerve excursion with full finger motion27–29, and then returned to the neutral position. Following a 30 minute delay to allow viscoelastic recovery, the baseline median nerve gliding resistance was assessed by pulling the nerve using the same parameters. Subsequent to the hydrodissection procedure, the median nerve gliding resistance was measured again. To measure the potential effect of hydrodissection over time, the nerve was subsequently pulled for 1000 repetitive cycles post-hydrodissection at the same velocity and amplitude as described above. Six specimens were treated with ultrasound-guided hydrodissection using the ulnar approach and 6 specimens were used as a control group -testing the gliding resistance of the median nerve using the same experimental set up, without performing hydrodissection (Figure 3).
Figure 3.
Flow chart describing testing sequence.
Subsequently, in both groups the carpal tunnel was opened by transecting the TCL and the gliding resistance of the median nerve was measured. Finally, the gliding resistance was measured after neurolysis of the median nerve. With this final step, we aimed to investigate if fibrous connections that could not be dissected with hydrodissection can be dissected surgically, which then would result in an even lower gliding resistance. Force and displacement data were collected at a sample rate of 50 Hz during excursions.
Hydrodissection Technique
A Philips iE33 ultrasound machine (Philips Electronics, Best, The Netherlands) with an L15-7io MHz linear array transducer was used to guide the hydrodissection. We used the ulnar approach for performing hydrodissection as described previously by Smith et al.30. The ultrasound transducer was placed transversely along the proximal wrist crease (carpal tunnel inlet). First, the median nerve and the pisiform (on the ulnar side) were identified. Under ultrasound guidance, a 27 gauge needle was inserted into the skin on the ulnar side of the palmaris longus at the level of the wrist crease along a trajectory approximately parallel to the transducer footprint. Subsequently, the needle penetrated the TCL on the ulnar side of the carpal tunnel and under real time visualization the needle was directed to the superficial ulnar side of the median nerve. Then, the first injectate was delivered and the median nerve was hydrodissected from the undersurface of the TCL while advancing the needle between the superficial aspect of the median nerve and the overlying TCL (Figure 4A, supplemental video 1). After complete separation of the nerve and TCL was sonographically confirmed along the length of the carpal tunnel, the needle was withdrawn to the ulnar side of the median nerve and redirected to its deep surface. The remainder of the injectate was delivered, separating the nerve from the underlying SSCT and tendons (Figure 4B, supplemental video 2), once again confirming with ultrasound complete hydrodissection along the deep surface of the median nerve throughout the carpal tunnel within the TCL region (supplemental video 3). All hydrodissections were performed with a total volume of 5 mL of saline based on clinical experience and a pilot study of one cadaveric specimen demonstrating complete median nerve hydrodissection throughout the carpal tunnel with this volume. We then waited for 30 minutes to allow the saline to disperse within the wrist, before testing the median nerve gliding resistance again.
Figure 4.
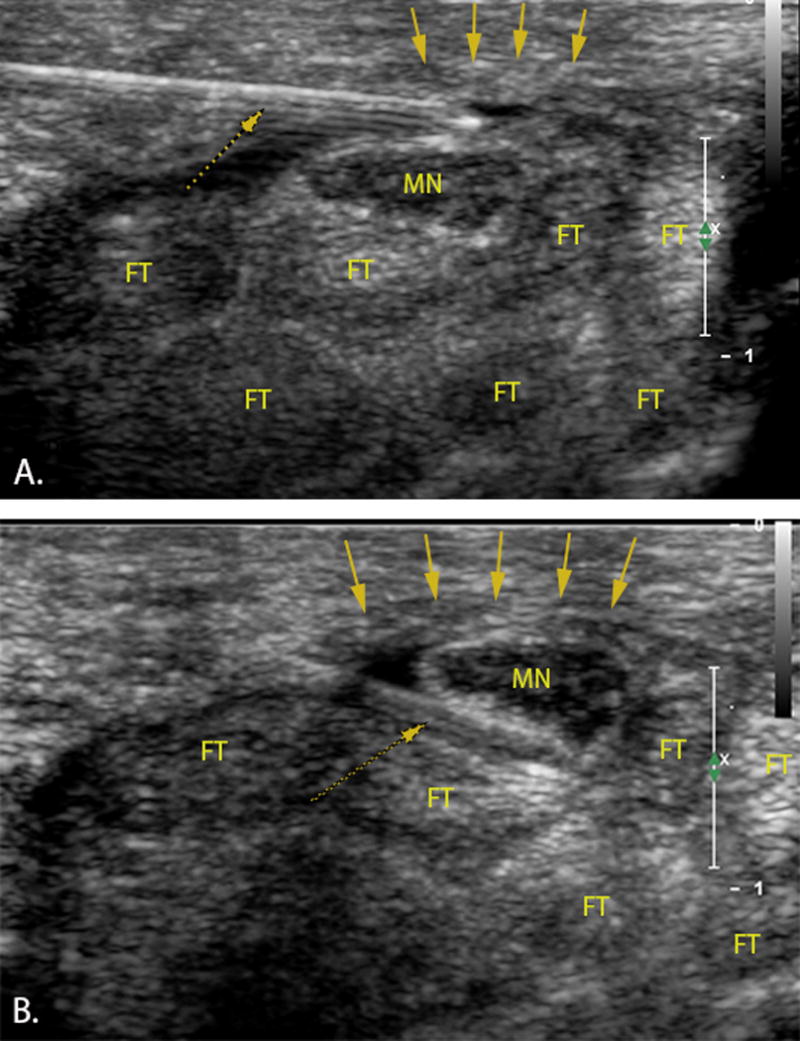
Transverse ultrasound image of the carpal tunnel. Left=ulnar. A 27-gauge needle (dotted arrow) penetrated the TCL (down pointing arrows) on the ulnar side of the carpal tunnel A) freeing the median nerve (MN) from the transverse carpal ligament B) separating the nerve from the underlying subsynovial connective tissue and flexor tendons (FT).
Data Analysis
The peak gliding resistance and total energy for each cycle were calculated as described in previous studies evaluating the characteristics of the SSCT31,32. Peak gliding resistance was the difference between the maximum force and the minimum force observed within each specific cycle and the energy absorption was the area under the curve of the resistance load-excursion curve, up to the maximum displacement. Peak gliding resistance of the median nerve and energy absorption were analyzed at baseline, at the second cycle and subsequently at intervals of 50 cycles up to cycle 1000, post-TCL transection and post-neurolysis. A custom MATLAB (version 2016a, MathWorks, Natick, MA) program was developed to analyze the repetitive force-excursion data, providing the peak gliding resistance and energy absorption for the selected cycles.
Statistical Analysis
Summary statistics are shown as mean ± standard deviation. Peak gliding resistance and energy absorption of the baseline conditions were compared to the same parameters obtained after hydrodissection using a paired t-test. The same test was applied to the control group in order to assess whether there was a significant difference between baseline measurement and the second cycle.
Independent t-test was used to compare the gliding resistance and the energy absorption of the selected cycles between the two groups. P-values less than 0.05 were considered significant. Statistical analyses were performed using the Statistical Package for Social Science (SPSS) software (version 22.0, Chicago, IL, USA) and R version 3.2.3.
Results
The hydrodissection and the control groups did not differ significantly in terms of age, gender or baseline peak gliding resistance and energy absorption. Mean age at death was 73 ± 12.3 years in the hydrodissection group and 73 ± 21.3 years in the control group (p = .961). There were 2 female and 4 male specimens in each group. Mean baseline peak gliding resistance was 584.9 ± 419.8 mN in the hydrodissection group and 452.9 ± 189.9 mN in the control group (p = .499). Mean baseline energy absorption was 1.8 ± 1.1 mJ in the hydrodissection group and 1.6 ± 0.5 mJ in the control group (p = .674).
In the hydrodissection group, we found a decrease in mean peak gliding resistance of 92.9 ± 34.8 mN between baseline and immediately post-hydrodissection and a decrease in mean energy absorption of 0.3 ± 0.2 mJ. In the control group a reduction in mean peak gliding resistance of 9.6 ± 29.8 mN occurred between baseline and cycle 2 and a reduction in mean energy absorption of 0.03 ± 0.15 mJ (Figure 5). The mean percentage difference in peak gliding resistance between baseline and post-hydrodissection was 21.4% ± 10.5%. The mean percentage difference between baseline and second cycle in the control group was 0.4% ± 5.3%.
Figure 5.
Difference in A) peak gliding resistance and B) energy absorption between baseline (cycle 1) and second cycle for both groups. The boxplots show the distribution of peak gliding resistance and energy absorption for the two groups. The boxes represent the 25th, 50th, and 75th percentiles. The whiskers show the range and points represent the data values.
There was no significant difference in mean peak gliding resistance between the preconditioning cycle and baseline cycle in the hydrodissection group (p = .161), indicating that a perturbation alone is not enough to disrupt the equilibrium.
Repetitive Motion Testing
The peak gliding resistance slightly decreased over 1000 repetitive cycles (difference between cycle 1 and cycle 1000) in both the hydrodissection and the control groups. However these changes were not statistically significantly different for either group (hydrodissection group p = .139, control group p = .276) or between groups (p = .615) (Figure 6A). In addition, there was no statistically significant difference in energy absorption over 1000 repetitive cycles in either group (hydrodissection group p = .056, control group p = .310). Greater reduction in energy absorption was found in the hydrodissection group during 1000 cycles compared to the control. However the difference between the two groups was not statistically significant (p = .308) (Figure 6B).
Figure 6.
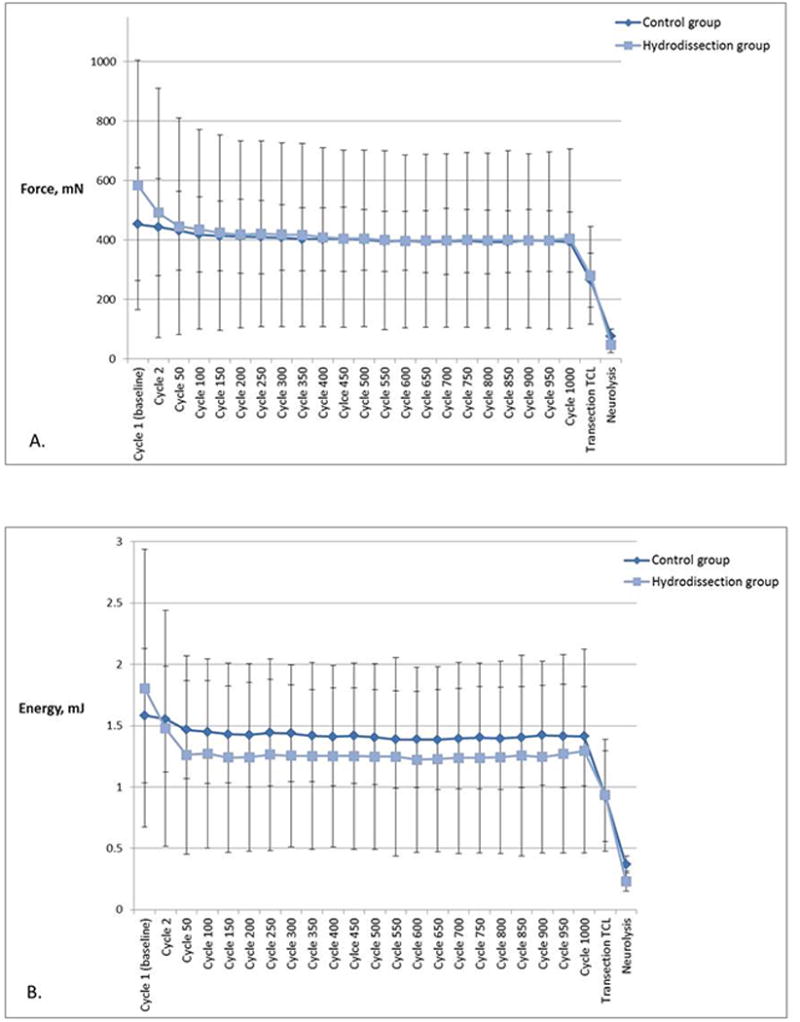
A) Mean peak gliding resistance at baseline, during repetitive motion testing, post-TCL transection and post-neurolysis of the median nerve. Error bars represent standard deviations. B) Energy absorption at baseline, during repetitive motion testing, post-TCL transection and post-neurolysis of the median nerve. Error bars represent one standard deviation.
Transection of the TCL resulted in a decrease of 26.3% ± 10.9% and 33.8% ± 8.5% in peak gliding resistance compared to the previous cycle, for the hydrodissection group and the control group respectively (hydrodissection group p = .100, control group p < .001, between groups p = .210). The greatest decrease in peak gliding resistance was found after neurolysis of the median nerve: a 69.1% ± 9.2% decrease in the hydrodissection group and a 81.0% ± 7.0% decrease in the control group compared to the previous cycle, resulting in a peak gliding resistance of 48.0 ± 26.9 mN and 77.0 ± 23.9 mN respectively (hydrodissection group p = .010, control group p = .002, between groups p = .523).
Discussion
Based on this cadaveric study we can conclude that ultrasound-guided hydrodissection can decrease the peak gliding resistance of the median nerve within the carpal tunnel, at least in wrists unaffected by CTS. Although the precise mechanism by which hydrodissection may reduce median nerve peak gliding resistance cannot be determined within the context of the current study, it is plausible that hydrodissection mechanically mobilizes the median nerve relative to the surrounding carpal tunnel structures. The most common histological finding in CTS is non-inflammatory fibrosis of SSCT surrounding the tendons in the carpal tunnel 12,9,33. Several studies suggest that fibrosis leads to decreased motion of the median nerve within the carpal tunnel 15,14,13. Freeing the median nerve from the TCL and surrounding tendons by dissecting the SSCT might result in restoration of the normal kinematics within the carpal tunnel, thereby reducing symptoms.
Our repetitive data indicate that the effects of hydrodissection may be long lasting and not entirely dependent on the persistence of a fluid bolus, which might have provided lubrication. Thus, the repetitive motion data suggest that there was no apparent additive effect of repetitive gliding and that there was no loss of hydrodissection effect over time, at least over 1000 repetitive cycles. TCL transection and neurolysis reduced the gliding resistance. Examining the effect of TCL transection and neurolysis validated our model, since we expected the gliding resistance to decrease.
Few studies have investigated the clinical effect of ultrasound-guided hydrodissection in patients with CTS. DeLea et al. treated 12 patients with scleroderma in the hand with ultrasound-guided hydrodissection (using 3 mL of 1% lidocaine) of entrapped structures within the carpal tunnel followed by a corticosteroid injection of 80 mg triamcinolone and included 14 patients with rheumatoid arthritis (RA) related CTS as a control group. They found that the treatment reduced the pain scores by 47% from baseline at two weeks in RA-related CTS and by 67% from baseline in scleroderma subjects 22. Malone et al. treated 44 wrists in 34 patients with CTS using hydrodissection of the median nerve and fenestration of the flexor retinaculum21. The median nerve was separated from the deep surface of the flexor retinaculum, using 9 cc of sterile saline, 1cc of 1% lidocaine and 1cc of 40mg/ml triamcinolone acetonide. Subsequently, a series of 150 fenestrations of the flexor retinaculum was made, using a 20 gauge needle tip. In 39 wrists the symptoms improved and in 5 wrists open carpal tunnel release was required after an average of 32 weeks. In addition, a prospective study on 75 cases of 44 patients with CTS receiving an injection of a 2 mL anesthetic-corticosteroid mixture using either one of two different ultrasound-guided approaches or a blind injection found a significantly greater improvement in symptoms using the in-plane ulnar approach with hydrodissection compared to the out-plane ulnar ultrasound-guided approach without hydrodissection or the blind injection group at twelve weeks23. In addition to the clinical improvement, a larger decrease in cross-sectional area of the median nerve and greater improvement in nerve conduction study were found with the in-plane ulnar approach compared to the out-plane approach and blind injection. A limitation of all of these clinical studies is that due to the study designs, the differential contributions of hydrodissection, injected steroid, lidocaine, or fenestration of the flexor retinaculum could not be determined.
Some limitations of our study should be considered. First, we have tested specimens unaffected by CTS only, which is the best case scenario since the SSCT will likely be fibrous and therefore more difficult to dissect in specimens with a history of CTS. In addition, the shape of the median nerve can be altered in patients with CTS and this might have an effect on the kinematics within the carpal tunnel 15. Conversely, it might be easier to mechanically see the effect of the treatment in specimens affected by CTS. This study could be repeated in specimens obtained from donors with clinical CTS. Second, a sham experiment, in which we just inserted a needle without injecting saline, was not performed. Although we do not expect a significant change in gliding resistance and energy absorption from just inserting the needle, this possibility has not been explored. Third, the groups had a slightly different gliding resistance at baseline and the standard deviations for all the measurements were relatively wide. This variation was probably due to normal inter-individual biological variability. Fourth, clinicians should exercise appropriate caution when extrapolating our cadaveric results to clinical populations. Although we have used fresh frozen specimens, it is possible the biomechanical and morphological properties of the median nerve and surrounding SSCT may be affected by the freezing and thawing34. In addition, the results of hydrodissection may be influenced in vivo by differing hydrostatic pressure conditions compared to those found in cadavers. Fifth, the experimental set up is artificial since we tested isolated median nerve motion relative to the immobilized structures around it in the longitudinal plane only. In vivo, these structures may all be moving relative to each other in both the longitudinal and transverse plane during functional activities. In our model, only the median nerve moved.
The strength of this study is that it provides information about the kinematic properties within the carpal tunnel. This information may contribute to a better understanding of the mechanism of action of hydrodissection. Clinical studies have already suggested that ultrasound-guided hydrodissection can reduce symptoms in patients with CTS as described above. However, this clinical improvement might be due to a placebo effect. With this study we have shown that the perineural fluid plane created by hydrodissection and visualized by ultrasound actually leads to altered forces within the carpal tunnel.
In conclusion, this study shows that ultrasound-guided hydrodissection can decrease the peak gliding resistance of the median nerve. The next step would be to assess whether the use of hydrodissection leads to improved nerve mobility, greater reduction in symptoms, or decreased recurrence rate in comparison with regular ultrasound-guided injections, with and without an anesthetic corticosteroid mixture. A clinical trial of ultrasound-guided hydrodissection seems justified.
Supplementary Material
Acknowledgments
This study was funded by a grant from NIH/NIAMS (RO1 AR62613).
We thank Dr. H. Liu and Dr. T.H. Yang for assistance with preparation of the specimens, and Dr. K.N. An for his valuable comments on data interpretation.
Abbreviations
- CTS
carpal tunnel syndrome
- FDP
flexor digitorum profundus
- FDS
flexor digitorum superficialis
- FPL
flexor pollicis longus
- FT
flexor tendon
- MN
median nerve
- RA
rheumatoid arthritis
- SSCT
subsynovial connective tissue
- SPSS
Statistical Package for Social Science
- TCL
transverse carpal ligament
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures: No conflicts of interest to report.1,2
Part of the material was contained within a poster presentation at the American Association for Hand surgery – Annual meeting 2017, Waikoloa Hawaii, January 11–14 and within a poster presentation at the Orthopedic Research Society 2017Annual meeting, San Diego, March 19–22.
References
- 1.Atroshi I, Englund M, Turkiewicz A, Tagil M, Petersson IF. Incidence of physician-diagnosed carpal tunnel syndrome in the general population. Archives of internal medicine. 2011;171(10):943–944. doi: 10.1001/archinternmed.2011.203. [DOI] [PubMed] [Google Scholar]
- 2.Roquelaure Y, Chazelle E, Gautier L, Plaine J, Descatha A, Evanoff B, et al. Time trends in incidence and prevalence of carpal tunnel syndrome over eight years according to multiple data sources: Pays de la Loire study. Scand J Work Environ Health. 2016 doi: 10.5271/sjweh.3594. [DOI] [PubMed] [Google Scholar]
- 3.Gelfman R, Melton LJ, 3rd, Yawn BP, Wollan PC, Amadio PC, Stevens JC. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72(1):33–41. doi: 10.1212/01.wnl.0000338533.88960.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bland JD, Rudolfer SM. Clinical surveillance of carpal tunnel syndrome in two areas of the United Kingdom, 1991–2001. J Neurol Neurosurg Psychiatry. 2003;74(12):1674–1679. doi: 10.1136/jnnp.74.12.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huisstede BM, Hoogvliet P, Randsdorp MS, Glerum S, van Middelkoop M, Koes BW. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments--a systematic review. Archives of physical medicine and rehabilitation. 2010;91(7):981–1004. doi: 10.1016/j.apmr.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. 2016 Feb 29; doi: 10.2106/JBJS.16.00719. www.aaos.org/ctsguideline. [DOI] [PubMed]
- 7.Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. The Cochrane database of systematic reviews. 2007;(2):CD001554. doi: 10.1002/14651858.CD001554.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Jarvik JG, Comstock BA, Kliot M, Turner JA, Chan L, Heagerty PJ, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074–1081. doi: 10.1016/S0140-6736(09)61517-8. [DOI] [PubMed] [Google Scholar]
- 9.Ettema AM, Amadio PC, Zhao C, Wold LE, O'Byrne MM, Moran SL, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg. 2006;118(6):1413–1422. doi: 10.1097/01.prs.0000239593.55293.c7. [DOI] [PubMed] [Google Scholar]
- 10.Ettema AM, Zhao C, Amadio PC, O'Byrne MM, An KN. Gliding characteristics of flexor tendon and tenosynovium in carpal tunnel syndrome: a pilot study. Clin Anat. 2007;20(3):292–299. doi: 10.1002/ca.20379. [DOI] [PubMed] [Google Scholar]
- 11.Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86-A(7):1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Tat J, Wilson KE, Keir PJ. Pathological changes in the subsynovial connective tissue increase with self-reported carpal tunnel syndrome symptoms. Clin Biomech (Bristol, Avon) 2015;30(4):360–365. doi: 10.1016/j.clinbiomech.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Filius A, Zhao C, Passe SM, Thoreson AR, An KN, et al. Altered median nerve deformation and transverse displacement during wrist movement in patients with carpal tunnel syndrome. Acad Radiol. 2014;21(4):472–480. doi: 10.1016/j.acra.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Doesburg MH, Henderson J, Mink van der Molen AB, An KN, Amadio PC. Transverse plane tendon and median nerve motion in the carpal tunnel: ultrasound comparison of carpal tunnel syndrome patients and healthy volunteers. PloS one. 2012;7(5):e37081. doi: 10.1371/journal.pone.0037081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filius A, Scheltens M, Bosch HG, van Doorn PA, Stam HJ, Hovius SE, et al. Multidimensional ultrasound imaging of the wrist: Changes of shape and displacement of the median nerve and tendons in carpal tunnel syndrome. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2015;33(9):1332–1340. doi: 10.1002/jor.22909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goetz JE, Thedens DR, Kunze NM, Lawler EA, Brown TD. Day-to-day variability of median nerve location within the carpal tunnel. Clin Biomech (Bristol, Avon) 2010;25(7):660–665. doi: 10.1016/j.clinbiomech.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sucher BM. Carpal tunnel syndrome: ultrasonographic imaging and pathologic mechanisms of median nerve compression. J Am Osteopath Assoc. 2009;109(12):641–647. doi: 10.7556/jaoa.2009.109.12.641. [DOI] [PubMed] [Google Scholar]
- 18.Hunter JM. Recurrent carpal tunnel syndrome, epineural fibrous fixation, and traction neuropathy. Hand Clin. 1991;7(3):491–504. [PubMed] [Google Scholar]
- 19.Kim SD. Efficacy of tendon and nerve gliding exercises for carpal tunnel syndrome: a systematic review of randomized controlled trials. J Phys Ther Sci. 2015;27(8):2645–2648. doi: 10.1589/jpts.27.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, O'Connor D, Pitt V, Massy-Westropp N. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;(6):CD009899. doi: 10.1002/14651858.CD009899. [DOI] [PubMed] [Google Scholar]
- 21.Malone DG, Clark TB, Wei N. Ultrasound-guided percutaneous injection, hydrodissection, and fenestration for carpal tunnel syndrome: description of a new technique. J Appl Res. 2010;10:116–123. [Google Scholar]
- 22.DeLea SL, Chavez-Chiang NR, Poole JL, Norton HE, Sibbitt WL, Jr, Bankhurst AD. Sonographically guided hydrodissection and corticosteroid injection for scleroderma hand. Clinical rheumatology. 2011;30(6):805–813. doi: 10.1007/s10067-010-1653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JY, Park Y, Park KD, Lee JK, Lim OK. Effectiveness of ultrasound-guided carpal tunnel injection using in-plane ulnar approach: a prospective, randomized, single-blinded study. Medicine. 2014;93(29):e350. doi: 10.1097/MD.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filius A, Thoreson AR, Ozasa Y, An KN, Zhao C, Amadio PC. Delineation of the mechanisms of tendon gliding resistance within the carpal tunnel. Clin Biomech (Bristol, Avon) 2017;41:48–53. doi: 10.1016/j.clinbiomech.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchiyama E, Kitaoka HB, Fujii T, Luo ZP, Momose T, Berglund LJ, et al. Gliding resistance of the posterior tibial tendon. Foot Ankle Int. 2006;27(9):723–727. doi: 10.1177/107110070602700912. [DOI] [PubMed] [Google Scholar]
- 26.Fujii T, Uchiyama E, Kitaoka HB, Luo ZP, Zhao KD, An KN. The influence of flatfoot deformity on the gliding resistance of tendons about the ankle. Foot Ankle Int. 2009;30(11):1107–1110. doi: 10.3113/FAI.2009.1107. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi T, Osamura N, Zhao C, An KN, Amadio PC. Relative longitudinal motion of the finger flexors, subsynovial connective tissue, and median nerve before and after carpal tunnel release in a human cadaver model. J Hand Surg Am. 2008;33(6):888–892. doi: 10.1016/j.jhsa.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshii Y, Zhao C, Zhao KD, Zobitz ME, An KN, Amadio PC. The effect of wrist position on the relative motion of tendon, nerve, and subsynovial connective tissue within the carpal tunnel in a human cadaver model. J Orthop Res. 2008;26(8):1153–1158. doi: 10.1002/jor.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuzuner S, Inceoglu S, Bilen FE. Median nerve excursion in response to wrist movement after endoscopic and open carpal tunnel release. J Hand Surg Am. 2008;33(7):1063–1068. doi: 10.1016/j.jhsa.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Smith J, Wisniewski SJ, Finnoff JT, Payne JM. Sonographically guided carpal tunnel injections: the ulnar approach. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2008;27(10):1485–1490. doi: 10.7863/jum.2008.27.10.1485. [DOI] [PubMed] [Google Scholar]
- 31.Filius A, Thoreson AR, Yang TH, Vanhees M, An KN, Zhao C, et al. The effect of low- and high-velocity tendon excursion on the mechanical properties of human cadaver subsynovial connective tissue. J Orthop Res. 2014;32(1):123–128. doi: 10.1002/jor.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanhees M, Morizaki Y, Thoreson AR, Larson D, Zhao C, An KN, et al. The effect of displacement on the mechanical properties of human cadaver subsynovial connective tissue. J Orthop Res. 2012;30(11):1732–1737. doi: 10.1002/jor.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. The Journal of bone and joint surgery American volume. 2004;86-A(7):1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Filius A, Dharan A, Kristin M, An KN, Zhao C, Amadio PC. The effect of freezing on biomechanical properties of the carpal tunnel subsynovial connective tissue. J Musculoskelet Res. 2015;18(04):8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.