Abstract
Rationale
Pregnancy profoundly alters maternal physiology. The heart hypertrophies during pregnancy, but its metabolic adaptations are not well understood.
Objective
To determine the mechanisms underlying cardiac substrate use during pregnancy.
Methods and Results
We use here 13C-glucose, 13C-lactate, and 13C-fatty acid tracing analyses to show that hearts in late pregnant mice increase fatty acid uptake and oxidation into the tricarboxylic acid (TCA) cycle, while reducing glucose and lactate oxidation. Mitochondrial quantity, morphology, and function do not appear altered. Insulin signaling appears intact, and the abundance and localization of the major fatty acid and glucose transporters, CD36 and GLUT4, are also unchanged. Rather, we find that the pregnancy hormone progesterone induces pyruvate dehydrogenase kinase (PDK)-4 in cardiomyocytes, and that elevated PDK4 levels in late pregnancy lead to inhibition of pyruvate dehydrogenase (PDH) and pyruvate flux into the TCA cycle. Blocking PDK4 reverses the metabolic changes seen in hearts in late pregnancy.
Conclusions
Taken together, these data indicate that the hormonal environment of late pregnancy promotes metabolic remodeling in the heart at the level of PDH, rather than at the level of insulin signaling.
Keywords: Pregnancy, metabolism, PDH, PDK4, mitochondria, glucose, fatty acide
Subject Terms: Basic Science Research, Metabolism, Myocardial Biology, Pregnancy
INTRODUCTION
The maternal adaptations of pregnancy are complex, coordinated, and dynamic. Fetal demands take precedence over maternal needs. In early pregnancy, maternal metabolism is largely anabolic to promote the accumulation of substrates to prepare for catabolic late pregnancy, when fetal growth is at its highest.1 The developing fetus uses glucose, lactate, fatty acids, and amino acids for energy provision and biosynthetic activities. Maternal metabolism submits to those needs by deferring its own glucose usage. Insulin sensitivity is profoundly affected, so much so that the insulin resistance of late pregnancy is comparable to that found in type 2 diabetes.2 Consequent increased lipolysis in adipose tissue facilitates the use of lipids as the primary maternal energy source. Despite the insulin resistance, serum glucose levels drop, reflecting voracious consumption by the fetus. The mechanisms responsible for insulin resistance are unknown, although studies have shown that pregnancy hormones, such as tumor necrosis factor (TNF)-α, can affect insulin sensitivity.3
The maternal heart undergoes significant hemodynamic and structural changes during pregnancy. Cardiac output is augmented by 20–50% by late pregnancy as heart rate and stroke volume both increase.4 All four cardiac chambers and all valves enlarge, in response to preload increase and volume overload of pregnancy.5 Left ventricular mass increases by as much as 50%. These drastic alterations in cardiac morphology and function underscore the need for corresponding metabolic changes in the heart.
The metabolic adaptations of the heart during pregnancy are not well characterized.6 Previous studies in rats showed that the heart reduces its glucose consumption, by as much as 75% by late pregnancy, and fulfilling its needs instead with fatty acids.7–9 Recently, cardiac nitric oxide (NO) synthesis and endothelial NO synthase (eNOS) were shown to increase by late pregnancy, which was accompanied by a decrease in glucose uptake and use, and a corresponding increase in fatty acid uptake.10, 11 The mechanism for this substrate switch, however, has not been explored. It is often assumed that gestational insulin resistance affects the heart as it does other organs, but this has not been studied. In fact, if anything, phosphorylation of Akt is increased in late pregnancy, indicating that signaling through the insulin pathway is intact.12–14 Hormones secreted during pregnancy have been postulated to play a role, but studies in the context of pregnancy have not been reported.
Here, we sought to understand how cardiac metabolism is affected in pregnancy by exploring cardiac substrate utilization and metabolic regulation during late pregnancy in mice, using 13C-tracer analyses15 and ex vivo Langendorff perfused hearts.
METHODS
See supplemental material for complete Experimental Procedures.
Animal studies
All animal studies were performed according to procedures approved by the Institutional Animal Care and Use Committees (IACUC) at Beth Israel Deaconess Medical Center (BIDMC) and the University of Pennsylvania. Female mice undergoing estrus were mated with C57/bl6 breeder males. The presence of a copulatory plug was denoted as day 0 of pregnancy.
Statistical analysis
P-values were calculated using the two-tailed Student’s t-test. For statistical comparisons between study groups, two-way ANOVA was used. P < 0.05 was considered as statistically significant. Data are displayed as mean ± standard deviation or standard error (as indicated).
RESULTS
Cardiac fatty acid concentrations, uptake, and accumulation increase in late pregnancy
Cardiac metabolic changes during pregnancy in mice have not been reported to date. We used a late-pregnancy model in C57Bl/6 mice. Gestation typically lasts 19±0.5 days in this strain16, so we chose day 18 as representative of late pregnancy (LP). Morphometric analysis showed that at day 18, an approximately 13% increase in heart weight to tibia length ratios (HW:TL) is observed (Figure 1A), consistent with what has been found in previous studies and indicative of physiological hypertrophy. Heart weight to body weight (HW:BW) drops, reflecting gains in body weight during pregnancy. No significant change in plasma glucose was detected, while both triglyceride and free fatty acid concentrations were significantly higher in late gestation (Figure 1B), as seen in other species including humans, and consistent with the lipolytic environment of late pregnancy.
Figure 1. Cardiac fatty acid uptake and accumulation increases in late gestational mice.
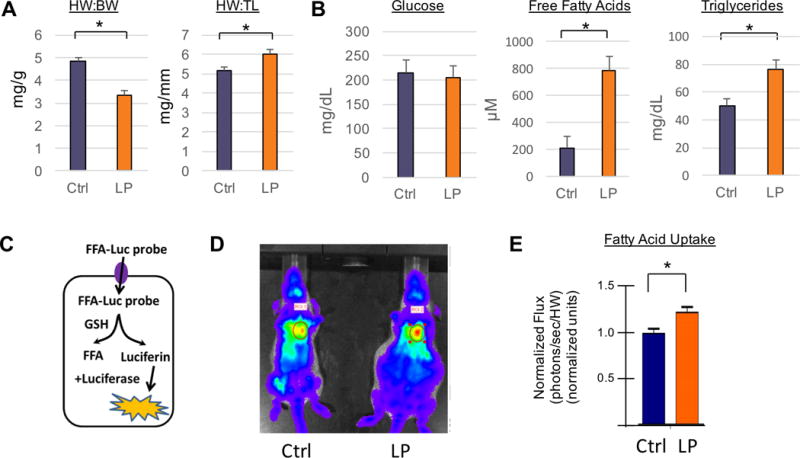
(A) Morphometric parameters including heart weight to body weight (HW:BW) and heart weight to tibia length (HW:TL) ratios in non-pregnant controls (n=5, blue) and late pregnant mice (n=5, orange). (B) Plasma glucose, free fatty acid, and triglyceride concentrations in late pregnant and non- pregnant mice (n≥5 for all groups). (C) Diagram of bioluminescence detection of free fatty acid probe conjugated to luciferin (FFA- Luc) upon uptake into mouse hearts. (D–E) In vivo bioluminescence image of non-pregnant (D, left) and late pregnant mouse hearts (D, right), and quantification (E) of photon flux at time of saturation (n≥6 for all groups). All data are represented as mean ±SEM; *, p < 0.05 compared with control.
We next quantified the extent of fatty acid uptake into the heart during late pregnancy. To do so, we used an assay recently described, in which a luciferin-conjugated long-chain fatty acid (FFA-LUC) is delivered intravenously to mice that ubiquitously express the luciferase enzyme.17 Uptake of FFA-LUC into tissues exposes the tracer to the reduced intracellular environment, which liberates the luciferin moiety, leading to bioluminescence in the presence of luciferase (Figure 1C). Quantification of FFA uptake can therefore be achieved noninvasively by in vivo bioluminescence imaging. As shown in Figure 1D–E, late pregnant mouse hearts take up 20% more fat than non-pregnant controls. These studies thus indicate that mice in late gestation increase the uptake and accumulation of cardiac fatty acids.
Substrate switch in late pregnant mouse hearts
The heart is generally omnivorous, consuming most substrates to which it is exposed.
Does the increase in cardiac uptake of FFA in late gestation thus reflect increased availability of FFA (Figure 1B), or is there inherent cardiac reprogramming of fuel preference? To address this question, we carried out 13C metabolic tracer experiments with explanted hearts perfused in Langendorff mode, in which substrate availability can be controlled. Hearts were perfused with a modified Krebs Henseleit Buffer that included physiological concentrations of glucose, palmitate, lactate, and pyruvate. Uniformly labeled glucose ([U-13C] glucose), lactate ([U-13C] lactate), or palmitate ([U-13C] palmitate) was then substituted for 30 minutes of perfusion, after which hearts were harvested for evaluation by mass spectrometry for incorporation of labeled isotopomer species into glycolytic and TCA metabolites (Figure 2A). As shown in Figure 2B and C, hearts from late gestational mice incorporate 30–50% less glucose and lactate into TCA cycle intermediates than hearts from non-pregnant controls. In contrast, there is a 15–20% increase in relative flux of fatty acids into citrate, α-ketoglutarate, glutamate, succinate, and malate (aspartate and succinate were not significant) (Figure 2D). Thus, during late pregnancy, a substrate switch occurs in the heart that decreases glycolytic flux into the TCA cycle and increases fatty acids usage as an energy source. No changes were detected in anapleurotic glycolytic flux into the TCA via pyruvate carboxylase (PC)-mediated carboxylation of pyruvate to oxaloacetate, as determined by step-increase in the M+3/M+2 ratio from succinate to aspartate, or comparison of M+3/M+2 ratio from glucose versus fatty acids (Online Figure IA–B). Both non-pregnant and late pregnant hearts were exposed to the same substrates but consumed them differently. Thus, differences in substrate utilization during pregnancy are due to adaptations that are intrinsic to the heart.
Figure 2. Isolated hearts from late gestational mice exhibit endogenous substrate switch of relative decreased oxidation of glucose and lacate, and increased oxidation of fatty acid.
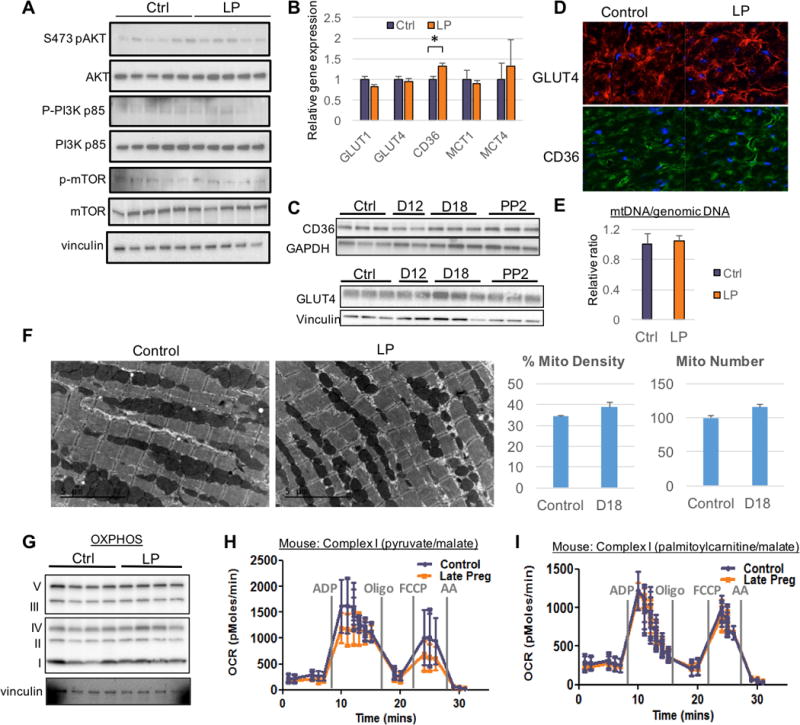
(A) Schematic of the formation of different labeled isotopomer species of TCA metabolites after utilization of 5mM uniformly labeled glucose ([U-13C]glucose), 1.5mM ([U-13C]lactate) or 0.4mM bovine serum albumin (BSA)-conjugated palmitate ([U-13C]palmitate). Red circles indicate labeled carbons and white circles are unlabeled carbons. (B–D) [U-13C]glucose (B), ([U-13C]lactate) (C), or [U-13C]palmitate (D) was perfused, along with unlabeled remaining fuels, via Langendorff on late pregnant and non-pregnant mouse hearts for 30 minutes before detection of TCA metabolites by mass spectrometry (n≥3 for all groups). Data are represented as mean ±SEM; *, p < 0.05 compared with control.
Insulin signaling and substrate transporter localization are unaltered
Maternal insulin resistance is a hallmark of pregnancy. Maternal tissues become less sensitive to insulin to promote fetal uptake of glucose. We tested if abnormal insulin signaling is responsible for the change in substrate utilization. We found, however, that the ratio of phosphorylated Akt to total Akt was unaltered in LP hearts (Figure 3A and ONLINE FIGURE IIA). In contrast, in skeletal muscle, a trend towards decreased Akt activity was observed in late pregnancy, suggesting insulin resistance in that tissue (Online Figure IIB). Also, there was no significant change in the ratio of phospho-PI3K/PI3K in the heart (Figure 3A and ONLINE FIGURE IIA). Addition of exogenous insulin led to robust induction of phosphorylated Akt in the heart, with no difference between nonpregnant and late pregnant mice (Online Figure IIC). In contrast, insulin-stimulated phosphorylation of Akt in the liver was blunted, consistent the with hepatic insulin resistance in late pregnancy. Thus cardiac tissue does not appear to develop blunted insulin signaling in late pregnancy, in contrast to other tissues. Interestingly, phospho-mTOR/mTOR was upregulated, consistent with the hypertrophic phenotype of late gestation (Figure 3A and ONLINE FIGURE IIA). Circulating insulin levels, as reported previously18, 19, were elevated in late pregnancy (Online Figure IID).
Figure 3. Insulin signaling, GLUT4 and CD36 expression and translocation, and mitochondrial function are unaltered in late gestational mouse hearts.
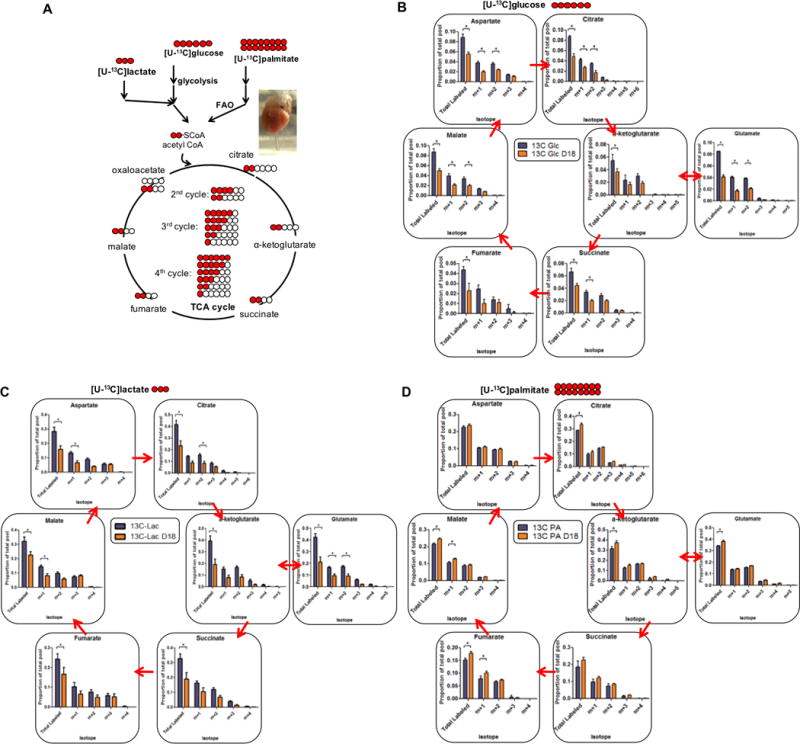
(A) Immunoblots of late pregnant and non-pregnant mouse hearts. Quantitation is presented in Online Figure IIA. (B) Quantitative, real-time PCR (qRT-PCR) analyses in hearts from mice during late gestation or non-pregnant controls (n=7 for all groups). (C) Immunoblots of CD36 and GLUT4 expression in late pregnant mouse hearts compared to control. Quantification is presented in Online Figure IIE. (D) Immunohistochemistry of GLUT4 and CD36 in mouse hearts from late gestational period vs. control. (E) Mitochondrial copy number determined by assessing the ratio of mitochondrial DNA to genomic DNA in control or late pregnant mouse hearts (n=3 for all groups). (F) Transmission electron micrographs demonstrating mitochondria among sarcomeres (left), and quantification of mitochondrial density and number (right). (G) Immunoblot of ETC component subunits. ATP5A (C V), UQCRC2 (C III), MT-CO1 (C IV), SDHB (C II) and NDUFB8 (C I). (H and I) Respiration of mitochondria isolated from indicated mice, assessed with pyruvate/malate (G) or palmitoylcarnitine/malate (H) as substrate (n=3 for all groups). Oligo: Oligomycin. FCCP: Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone. AA: Antimycin A. All data represented as mean ±SEM.
Insulin signaling increases glucose uptake via translocation of GLUT4 and other transporters to the plasma membrane. We found no differences in the cardiac expression of glucose transporters GLUT1 and GLUT4, or of lactate and ketone body membrane carriers MCT1 and MCT4, during late pregnancy (Figure 3B). Mild induction of gene expression of the fatty acid translocase CD36 was seen (Figure 3B), but no differences in protein levels of GLUT4 and CD36 were seen (Figure 3C and ONLINE FIGURE IIE). Importantly, there were also no alterations in the localizations of GLUT4 or CD36, as discerned by immunohistochemistry and subcellular fractionation (Figure 3D and ONLINE FIGURE IIF). Thus, it appears that the dramatic changes in substrate use seen in late pregnancy (Figure 2) cannot be explained by the suppression of insulin-mediated substrate transporter expression or localization, suggesting that mechanisms other than gestational resistance to insulin signaling are at play.
Late gestation does not affect mitochondria content or respiratory capacity
We examined the ratio of mitochondrial DNA to genomic DNA and observed no changes in mitochondrial copy number (Figure 3E). Transmission electron microscopy revealed no discernable change in the quantity, density, or morphology of mitochondria in the heart during late pregnancy (Figure 3F). In accord with this, expression of electron transport chain (ETC) subunits was also similar in hearts from late pregnant mice, compared with controls (Figure 3G), and neither nuclear nor mitochondrial encoded genes were altered (Online Figure IIIA–B). No differences were observed in mitochondrial fusion or fission genes, although PTEN-induced putative kinase 1 (PINK1) was significantly downregulated (Online Figure IIIC). To evaluate mitochondrial function directly, we used the Seahorse Extracellular Flux Analyzer to measure oxygen respiration in isolated mitochondria from hearts of late pregnant mice, versus nulliparous controls. We observed no significant difference in respiratory capacity, regardless of the choice of substrate of pyruvate/malate (Figure 3H) or palmitoylcarnitine/malate (Figure 3I), although we did note a trend for decreased consumption of pyruvate (Figure 3H). We conclude that the mitochondrial content, or respiratory capacity of mitochondria, in hearts from late pregnant mice is not distinctively different from control mouse hearts, and is thus unlikely to explain the observed strong changes in substrate use.
Progesterone induces PDK4 expression, leading to PDH phosphorylation in late pregnancy hearts
Pyruvate dehydrogenase kinases (PDKs) control the activity of pyruvate dehydrogenase (PDH) by inhibitory phosphorylation on multiple residues, thereby preventing entry of glycolytic products into the TCA cycle, in parallel leading to reciprocal increased fatty acid oxidation. While looking for insulin-independent changes that could explain the observed substrate switch in hearts in late gestation, we noted that PDK4 is upregulated in late pregnancy specifically in striated muscle, and more prominently in cardiac muscles than in skeletal muscles (Figure 4A and Online Figure IVA). Conversely, PDH phosphatase (PDP) 1 and 2, the prominent phosphatases that reverse the PDK-mediated phosphorylation of PDH, are downregulated in the heart and muscle in late pregnancy (Figure 4A and Online Figure IVA). The induction of PDK4 is exclusive to late pregnancy (Figure 4B). Protein expression of PDK4 mirrors the gene expression, with a 5-fold increase in late gestation (Online Figure IVB–D). Consistent with increased activity of PDK4, phosphorylation of PDH at the three primary inhibitory serine sites (232, 293, and 300) was strikingly increased in late pregnancy (Figure 4C), and PDH activity was dramatically reduced (Online Figure IVE).
Figure 4. The gestational hormone progesterone induces PDK4, and PDK4 inhibition reverses the endogenous substrate switch in hearts from late gestational mice.

(A) qRT-PCR analysis of the indicated genes in hearts from pregnant mice (n=7 for all groups). (B) qRT-PCR analysis of hearts from different time points during pregnancy (n=3 for all groups). (C) Representative immunoblots and respective quantification of phospho-PDH at serine 232, 293 and 300 in late pregnant mouse hearts versus control (n=8 for all groups). (D and E) qRT-PCR from NRVMs treated with different pregnancy hormones (D) or pre-treated with mifepristone for 1 hour before incubation with progesterone (E) for 8 hours (n=3 for all groups). Data are represented as mean ±SD. (F) [U-13C]glucose was perfused via Langendorff on late pregnant and non-pregnant mouse hearts, in the presence or absence of DCA, for 30 minutes before detection of TCA metabolites by mass spectrometry (n=4 for all groups). (G) Echocardiographic parameters in nulliparous (NP) versus D18 pregnant mice, in the presence or absence of DCA (n=4 for all groups). LVIDd: left ventricle internal diameter in diastole. FS: fractional shortening. LVMI: left ventricular mass index. RWT: rear wall thickness. All data are represented as mean ±SEM, unless otherwise noted; *, p < 0.05 compared with control; **, p < 0.05 compared with progesterone.
By what mechanism is PDK4 gene expression induced in late pregnancy? To investigate this question, isolated neonatal rat ventricular myocytes (NRVMs) were treated with various pregnancy hormones. While neither estrogen nor prolactin altered expression, progesterone increased PDK4 expression more than 8-fold (Figure 4D). Progesterone is induced during pregnancy, both in mice and humans12. To test if the progesterone receptor is required for this effect, we used a receptor partial agonist/antagonist, mifepristone (RU486) to pre-treat NRVMs for one hour prior to progesterone incubation. Mifepristone treatment alone led to a modest increase in PDK4 expression due to its partial agonist activity. The induction of PDK4 by progesterone, however, was fully blocked in the presence of mifepristone, indicating that the induction is progesterone receptor-mediated (Figure 4E). Data culled from chromatin immunoprecipitation sequencing (ChipSeq) experiments20 demonstrated that the progesterone receptor binds directly to the PDK4 promoter as well as a region approximately 10kb upstream, and receptor binding was markedly increased by the presence of progesterone (Online Figure IVF). ENCODE data21 demonstrates that both of these regions are hypersensitive to DNAseI digestion, and contain a high amount of histone acetylation at H3K27, indicating high transcriptional regulatory activity at both sites. Both regulatory sites contain strong candidate progesterone-binding sequences22 (Online Figure IVF), consistent with the observed progesterone receptor binding. The 15kb upstream region of PDK4 also contains three single nucleotide polymorphisms (SNPs) that act as expression quantitative trait loci (eQTLs) for PDK4 expression23, 24 (Online Figure IVF). Strikingly, two of these three eQTLs are found within the two progesterone receptor-bound regions. Even more strikingly, the eQTL identified in the proximal region directly modifies the progesterone receptor binding site (Online Figure IVF). These data thus unequivocally demonstrate that these sites regulate PDK4 expression, with significant contribution from progesterone receptor binding in response to the presence of progesterone. In vivo, treatment of nulliparous mice, or even male mice, with 14 days of estrogen plus progesterone, thereby mimicking pregnancy, was sufficient to drive cardiac PDK4 expression (Online Figure IVG). We conclude that progesterone drives the induction of cardiac PDK4 during pregnancy.
PDK4 inhibition reverses the substrate switch of late pregnancy
To assess if the increase in PDK4 in late pregnancy is responsible for the decrease in glycolytic input into the TCA cycle, hearts were again perfused in the Langendorff mode in the presence of 13C-labeled glucose, but this time treated with dichloroacetate (DCA), a well- established and widely used PDK inhibitor.25 Treatment of hearts from late pregnant mice with DCA led to a striking reversal of the inhibition of glucose oxidation, as manifested by recovered incorporation of 13C label into all TCA intermediates (Figure 4F). Treatment of nulliparous mouse heart did not produce a significant effect. DCA had no effect on PDK4 expression (Online Figure VA), consistent with its direct inhibition of PDK4 activity. DCA treatment also had no overt effect on cardiac contractile function, as measured by non-invasive echocardiography (Figure 4G and ONLINE FIGURE VB), indicating that the inhibition of PDH observed in late pregnancy is dispensable for normal contraction to occur. Interestingly, DCA treatment did reduce pregnancy-induced hypertrophy (Online Figure VC), suggesting that metabolic reprogramming may have a direct effect on growth parameters, for example by sparing glucose and lactate carbons for anabolic processes. We conclude that inhibition of PDH, most likely mediated by the induction of PDK4, is responsible for the substrate switch in cardiac fuel observed in late pregnancy.
DISCUSSION
Maternal insulin resistance occurs normally during pregnancy in order to ensure sufficient fetal consumption of glucose. Changes in insulin signaling and sensitivity during pregnancy have been well characterized in skeletal muscle and other tissues, but not in cardiac muscle.2 We show here that the insulin pathway, reflected in PI3K/Akt signaling and mTOR phosphorylation is in fact not repressed in the heart during late gestation, and likely if anything is induced. Others have reported similar observations.12–14 Insulin signaling and sensitivity thus do not decrease and instead may potentially increase in the heart during late pregnancy, unlike most other tissues in the body.
Despite insulin sensitivity, however, we find that cardiac glucose oxidation is decreased in late murine pregnancy. Previous studies demonstrated a similar substrate switch from glucose oxidation to increased fat import and consumption in rats and dogs7, 10, though this had not before been characterized in mice, an important consideration as numerous genetic models of pregnancy-related cardiac diseases, such as in peripartum cardiomyopathy, are murine. Moreover, we provide here direct 13C tracer analyses to demonstrate entry into the TCA cycle, in actively beating hearts, of the respective nutrient sources. Mechanistically, we find that the substrate switch is inherent to the heart, and does not merely reflect the dramatically altered availability of substrates during late pregnancy (e.g., 4-fold increase in circulating free fatty acids, Figure 1B). Instead, the substrate switch is caused in large part by the induction of PDK4, which leads to phosphorylation (and thus inhibition) of PDH. Conversely, expression of the phosphatases that reverse the inhibition of PDH (PDP1 and PDP2) are decreased in late gestation. Previous studies have reported conflicting results on the expression of PDK4, possibly reflecting species differences. Two groups found no change in PDH activity or PDK4 expression in day 18 pregnant rat hearts, while decreased PDH expression was observed in dogs.8, 10, 26 In mouse hearts, a significant induction was found, consistent with our observations.27 Thus, our data demonstrate not only clear induction of PDK4, but also that the induction is necessary for the substrate switch, since inhibition of PDK with DCA reverses the energetic changes of pregnancy (Figure 4F).
Substrate switching away from glucose thus occurs in the heart as in other tissues, but not via reduced sensitivity to insulin signaling. Why use an alternate mechanism? There are at least two plausible explanations. First, perhaps it is important to maintain cardiac Akt and mTOR signaling in late pregnancy. Indeed, late pregnancy is a catabolic state for most maternal tissues (to supply nutrients to the fetus), but catabolism of cardiac muscle would likely be detrimental. Maintaining Akt and mTOR activity likely spares this outcome. Cardiac insulin resistance does occur in type 2 diabetes, leading to a substrate switch very similar to that of late pregnancy. But in type 2 diabetes cardiac function declines, suggesting the need to maintain active insulin pathway signaling for cardiac health. Second, the heart in fact consumes lactate much more than glucose. Insulin resistance acts largely at the level of glucose entry into the cell, and thus would not spare lactate consumption. Inhibition of PDH, on the other hand, blunts consumption of both substrates, and thus spares lactate-derived gluconeogenic carbons for fetal use.
Our observations build on seminal work performed by Philip J Randle and others, who showed that fatty acid oxidation can suppress glucose oxidation in part by suppressing pyruvate flux through PDH28–30. These classic studies demonstrated that inhibition of PDH occurs by allosteric product-mediated inhibition, and partly via enzyme phosphorylation. We show here that this process is also hormonally activated during pregnancy in order to preserve glucose and gluconeogenic substrates for the fetus. Progesterone, which peaks at the end of pregnancy, induces PDK4 expression directly in cardiomyocytes, as has been noted in rat skeletal muscle.31 Other genomic and non-genomic effects of progesterone on cardiomyocytes have been reported previously, for example induction of hypertrophy of isolated cardiomyocytes12, inhibition of cardiomyocyte apoptosis32, and modulation of cardiac repolarization.33 But the effects of progesterone on cardiac metabolism have not been studied.34 Progesterone is widely used clinically, usually in combination with estrogen, as hormone-replacement therapy (HRT) in peri-menopausal women. We find that such a combination also induces PDK4 in cardiac cells both in cell culture and in vivo. It will thus be of interest to determine if progesterone inhibits glucose oxidation in this context, what are the functional consequences, and whether co-administration of estrogen impacts the outcome, especially in light of the disappointing effects of HRT on cardiac health.
Our data also have interesting implications for pregnancy-related cardiac diseases. For example, peripartum cardiomyopathy (PPCM), diagnosed by the onset of heart failure in late gestation or immediately postpartum, is a disease of unknown etiology35, 36. One model for PPCM is the cardiac PPARγ coactivator (PGC)-1α-null mouse, which develops heart failure during late pregnancy and has vascular insufficiency and metabolic deficiency.37 PGC-1α transcriptionally co-activates most nuclear receptors, regulates angiogenesis and mitochondrial biogenesis, and, in skeletal muscle, increases PDK4 expression during exercise or starvation.38, 39 Disruption of appropriate regulation of PDK4 in late pregnancy may thus compromise the ability of the heart to switch substrate preferences appropriately. There may also be implications for the gestational disease of pre- eclampsia, in which cardiac dysfunction and clinical heart failure is often seen40. Pre-eclampsia is accompanied by large excesses of circulating vaso-constrictive and anti-vascular agents, which likely limit oxygen and nutrient supply to the heart and elsewhere.41 Simultaneous inhibition of glucose oxidation by PDK4 may tip the balance to cardiac metabolic insufficiency. In other words, suppressing glucose oxidation to shunt glucose to the growing fetus may not be functionally maladaptive in a normal pregnancy (Figure 4), but it may become maladaptive in the context of additional stressors such as preeclampsia.
In conclusion, we use 13C tracer analyses to show that 1) late pregnancy promotes a metabolic switch in the heart, away from glucose oxidation and towards the use of fatty acids; 2) the choice of substrates reflects endogenous reprogramming in the heart, rather than exogenous fuel delivery; 3) the substrate switch occurs at the level of PDH, rather than at the level of insulin signaling or mitochondrial function; 4) and the process is driven by the hormonal environment of late pregnancy. These studies indicate that the late pregnancy hormonal milieu dictates the metabolic phenotype of the heart. A deeper understanding of these regulations could have significant impact on pregnancy-related cardiac diseases, such as PPCM or pre-eclamptic heart failure.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Diseases such as peripartum cardiomyopathy are triggered by pregnancy.
Pregnancy profoundly alters maternal cardiac physiology
What New Information Does This Article Contribute?
Metabolically, the heart dramatically decreases its consumption of glucose and lactate during pregnancy.
Low cardiac glucose consumption in pregnancy is not caused by insulin resistance.
Instead, it is caused by inhibition of pyruvate entry into the tricarboxylic acid, achieved by the late-gestational hormone progesterone
Pregnancy profoundly alters maternal cardiac physiology, but its metabolic adaptations are not well understood. Here the authors use state-of-the-art metabolomic heavy isotope tracing studies to show that hearts in late pregnant mice increase fatty acid uptake and oxidation into the tricarboxylic acid (TCA) cycle, while reducing glucose and lactate oxidation. Surprisingly, this is not achieved by suppression of insulin signaling. Instead, the gestational hormone progesterone induces in the heart the expression of pyruvate dehydrogenase kinase 4 (PDK4), which in turn phosphorylates and inhibits pyruvate dehydrogenase (PDH). As a consequence, pyruvate derived from glucose or lactate cannot enter the tricarboxylic acid (TCA) cycle, and glucose and lactate consumption are suppressed. Thus, the hormonal environment of late pregnancy directly promotes metabolic remodeling in the heart, possibly rendering it susceptible to diseases unique to pregnancy, such as peripartum cardiomyopathy.
Acknowledgments
We thank Ronglih Liao and Seoun Ngoy for providing invaluable technical advice and project insights.
SOURCES OF FUNDING
This work was supported by grants from the National Institutes of Health (NIA F31AG041598 to L.X.L. and HL094499 DK107667 to Z.A.), pilot funding from P30-DK19525, an Established Investigator Award the American Heart Association (Z.A.), and a grant by the Deutsche Forschungsgemeinschaft (DFG FL 267/7-2) to BKF.
Nonstandard Abbreviations and Acronyms
- TCA
tricarboxylic acid
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
- PDP
PDH phosphatase
- CD36
cluster of differentiation 36
- GLUT4
Glucose transporter type 4
- TNF
Tumor necrosis factor
- NO
nitric oxide
- eNOS
endothelial NO synthase
- HW
TL, heart weight to tibia length ratios
- HW
BW, Heart weight to body weight
- FFA
free fatty acid
- FFA-LUC
luciferin-conjugated long-chain FFA
- LP
late pregnancy
- ETC
electron transport chain
- PINK1
PTEN-induced putative kinase 1
- PGC-1α
PPARgamma coactivator 1 alpha
- HRT
hormone replacement therapy
- PPCM
peripartum cardiomyopathy
- DCA
dichloroacetate
Footnotes
DISCLOSURES
None.
References
- 1.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50:938–48. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 2.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes care. 2007;30(Suppl 2):S112–9. doi: 10.2337/dc07-s202. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, Kalhan SC, Catalano PM. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–13. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 4.Melchiorre K, Sharma R, Thilaganathan B. Cardiac structure and function in normal pregnancy. Curr Opin Obstet Gynecol. 2012;24:413–21. doi: 10.1097/GCO.0b013e328359826f. [DOI] [PubMed] [Google Scholar]
- 5.Chung E, Leinwand LA. Pregnancy as a cardiac stress model. Cardiovascular research. 2014;101:561–70. doi: 10.1093/cvr/cvu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu LX, Arany Z. Maternal Cardiac Metabolism in Pregnancy. Cardiovascular research. 2014;101:545–53. doi: 10.1093/cvr/cvu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugden MC, Changani KK, Bentley J, Holness MJ. Cardiac glucose metabolism during pregnancy. Biochemical Society transactions. 1992;20:195S. doi: 10.1042/bst020195s. [DOI] [PubMed] [Google Scholar]
- 8.Sugden MC, Holness MJ. Cardiac carbohydrate and lipid utilization during late pregnancy. Biochemical Society transactions. 1993;21(Pt 3):312S. doi: 10.1042/bst021312s. [DOI] [PubMed] [Google Scholar]
- 9.Sugden MC, Holness MJ. Control of muscle pyruvate oxidation during late pregnancy. FEBS letters. 1993;321:121–6. doi: 10.1016/0014-5793(93)80091-8. [DOI] [PubMed] [Google Scholar]
- 10.Williams JG, Ojaimi C, Qanud K, Zhang S, Xu X, Recchia FA, Hintze TH. Coronary nitric oxide production controls cardiac substrate metabolism during pregnancy in the dog. American journal of physiology Heart and circulatory physiology. 2008;294:H2516–23. doi: 10.1152/ajpheart.01196.2007. [DOI] [PubMed] [Google Scholar]
- 11.Williams JG, Rincon-Skinner T, Sun D, Wang Z, Zhang S, Zhang X, Hintze TH. Role of nitric oxide in the coupling of myocardial oxygen consumption and coronary vascular dynamics during pregnancy in the dog. American journal of physiology Heart and circulatory physiology. 2007;293:H2479–86. doi: 10.1152/ajpheart.00036.2006. [DOI] [PubMed] [Google Scholar]
- 12.Chung E, Yeung F, Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol. 2012;112:1564–75. doi: 10.1152/japplphysiol.00027.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 14.Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. American journal of physiology Heart and circulatory physiology. 2011;300:H931–42. doi: 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 15.Buescher JM, Antoniewicz MR, Boros LG, Burgess SC, Brunengraber H, Clish CB, DeBerardinis RJ, Feron O, Frezza C, Ghesquiere B, Gottlieb E, Hiller K, Jones RG, Kamphorst JJ, Kibbey RG, Kimmelman AC, Locasale JW, Lunt SY, Maddocks OD, Malloy C, Metallo CM, Meuillet EJ, Munger J, Noh K, Rabinowitz JD, Ralser M, Sauer U, Stephanopoulos G, St-Pierre J, Tennant DA, Wittmann C, Vander Heiden MG, Vazquez A, Vousden K, Young JD, Zamboni N, Fendt SM. A roadmap for interpreting (13)C metabolite labeling patterns from cells. Curr Opin Biotechnol. 2015;34:189–201. doi: 10.1016/j.copbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, Donahue LR. Mouse gestation length is genetically determined. PloS one. 2010;5:e12418. doi: 10.1371/journal.pone.0012418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henkin AH, Cohen AS, Dubikovskaya EA, Park HM, Nikitin GF, Auzias MG, Kazantzis M, Bertozzi CR, Stahl A. Real-time noninvasive imaging of fatty acid uptake in vivo. ACS chemical biology. 2012;7:1884–91. doi: 10.1021/cb300194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wharfe MD, Wyrwoll CS, Waddell BJ, Mark PJ. Pregnancy-induced changes in the circadian expression of hepatic clock genes: implications for maternal glucose homeostasis. American journal of physiology Endocrinology and metabolism. 2016;311:E575–86. doi: 10.1152/ajpendo.00060.2016. [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama C, Yamamoto M, Kotani T, Kikkawa F, Murata Y, Hayashi Y. Fertility and pregnancy-associated ss-cell proliferation in mice deficient in proglucagon-derived peptides. PloS one. 2012;7:e43745. doi: 10.1371/journal.pone.0043745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goddard LM, Murphy TJ, Org T, Enciso JM, Hashimoto-Partyka MK, Warren CM, Domigan CK, McDonald AI, He H, Sanchez LA, Allen NC, Orsenigo F, Chao LC, Dejana E, Tontonoz P, Mikkola HK, Iruela-Arispe ML. Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell. 2014;156:549–62. doi: 10.1016/j.cell.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyner C, Barber GP, Casper J, Clawson H, Diekhans M, Eisenhart C, Fischer CM, Gibson D, Gonzalez JN, Guruvadoo L, Haeussler M, Heitner S, Hinrichs AS, Karolchik D, Lee BT, Lee CM, Nejad P, Raney BJ, Rosenbloom KR, Speir ML, Villarreal C, Vivian J, Zweig AS, Haussler D, Kuhn RM, Kent WJ. The UCSC Genome Browser database: 2017 update. Nucleic acids research. 2017;45:D626–D634. doi: 10.1093/nar/gkw1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin P, Roqueiro D, Huang L, Owen JK, Xie A, Navarro A, Monsivais D, Coon JSt, Kim JJ, Dai Y, Bulun SE. Genome-wide progesterone receptor binding: cell type-specific and shared mechanisms in T47D breast cancer cells and primary leiomyoma cells. PloS one. 2012;7:e29021. doi: 10.1371/journal.pone.0029021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundberg E, Small KS, Hedman AK, Nica AC, Buil A, Keildson S, Bell JT, Yang TP, Meduri E, Barrett A, Nisbett J, Sekowska M, Wilk A, Shin SY, Glass D, Travers M, Min JL, Ring S, Ho K, Thorleifsson G, Kong A, Thorsteindottir U, Ainali C, Dimas AS, Hassanali N, Ingle C, Knowles D, Krestyaninova M, Lowe CE, Di Meglio P, Montgomery SB, Parts L, Potter S, Surdulescu G, Tsaprouni L, Tsoka S, Bataille V, Durbin R, Nestle FO, O’Rahilly S, Soranzo N, Lindgren CM, Zondervan KT, Ahmadi KR, Schadt EE, Stefansson K, Smith GD, McCarthy MI, Deloukas P, Dermitzakis ET, Spector TD, Multiple Tissue Human Expression Resource C Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nature genetics. 2012;44:1084–9. doi: 10.1038/ng.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu CH, Pal LR, Moult J. Consensus Genome-Wide Expression Quantitative Trait Loci and Their Relationship with Human Complex Trait Disease. OMICS. 2016;20:400–14. doi: 10.1089/omi.2016.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bersin RM, Stacpoole PW. Dichloroacetate as metabolic therapy for myocardial ischemia and failure. Am Heart J. 1997;134:841–55. doi: 10.1016/s0002-8703(97)80007-5. [DOI] [PubMed] [Google Scholar]
- 26.Rimbaud S, Sanchez H, Garnier A, Fortin D, Bigard X, Veksler V, Ventura-Clapier R. Stimulus specific changes of energy metabolism in hypertrophied heart. J Mol Cell Cardiol. 2009;46:952–9. doi: 10.1016/j.yjmcc.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Redondo-Angulo I, Mas-Stachurska A, Sitges M, Giralt M, Villarroya F, Planavila A. C/EBPbeta is required in pregnancy-induced cardiac hypertrophy. Int J Cardiol. 2016;202:819–28. doi: 10.1016/j.ijcard.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 29.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. American journal of physiology Endocrinology and metabolism. 2009;297:E578–91. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randle PJ, Sugden PH, Kerbey AL, Radcliffe PM, Hutson NJ. Regulation of pyruvate oxidation and the conservation of glucose. Biochem Soc Symp. 1978:47–67. [PubMed] [Google Scholar]
- 31.Campbell SE, Mehan KA, Tunstall RJ, Febbraio MA, Cameron-Smith D. 17beta-estradiol upregulates the expression of peroxisome proliferator-activated receptor alpha and lipid oxidative genes in skeletal muscle. Journal of molecular endocrinology. 2003;31:37–45. doi: 10.1677/jme.0.0310037. [DOI] [PubMed] [Google Scholar]
- 32.Morrissy S, Xu B, Aguilar D, Zhang J, Chen QM. Inhibition of apoptosis by progesterone in cardiomyocytes. Aging Cell. 2010;9:799–809. doi: 10.1111/j.1474-9726.2010.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura H, Kurokawa J, Bai CX, Asada K, Xu J, Oren RV, Zhu ZI, Clancy CE, Isobe M, Furukawa T. Progesterone regulates cardiac repolarization through a nongenomic pathway: an in vitro patch-clamp and computational modeling study. Circulation. 2007;116:2913–22. doi: 10.1161/CIRCULATIONAHA.107.702407. [DOI] [PubMed] [Google Scholar]
- 34.Wittnich C, Tan L, Wallen J, Belanger M. Sex differences in myocardial metabolism and cardiac function: an emerging concept. Pflugers Arch. 2013;465:719–29. doi: 10.1007/s00424-013-1232-1. [DOI] [PubMed] [Google Scholar]
- 35.Bello NA, Arany Z. Molecular mechanisms of peripartum cardiomyopathy: A vascular/hormonal hypothesis. Trends in cardiovascular medicine. 2015:499–504. doi: 10.1016/j.tcm.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arany Z, Elkayam U. Peripartum Cardiomyopathy. Circulation. 2016;133:1397–409. doi: 10.1161/CIRCULATIONAHA.115.020491. [DOI] [PubMed] [Google Scholar]
- 37.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del Monte F, Hilfiker-Kleiner D, Karumanchi SA, Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–8. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res. 2011;107:825–38. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol. 2005;25:10684–94. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bello N, Hurtado Rendon IS, Arany Z. The relationship between Preeclampsia and Peripartum Cardiomyopathy: a Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2013;62:1715–23. doi: 10.1016/j.jacc.2013.08.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–69. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


