Abstract
Burn is associated with a considerable burden of morbidity worldwide. Early excision of burned tissue and skin grafting of the resultant wound has been established as a mainstay of modern burn therapy. However, in large burns, donor sites for autologous skin may be limited. Numerous alternatives, from cadaver skin to synthetic substitutes have been described, each with varying benefits and limitations. We previously proposed the use of genetically modified (alpha-1,3-galactosyl transferase knockout, GalT-KO) porcine skin as a viable skin alternative. In contrast to wild type porcine skin, which has been used as a biologic dressing following glutaraldehyde fixation, GalT-KO porcine skin is a viable graft, which is not susceptible to loss by hyperacute rejection, and undergoes graft take and healing, prior to eventual rejection, comparable to cadaver allogeneic skin. In the current study we aimed to perform a detailed functional analysis of GalT-KO skin grafts in comparison to allogeneic grafts for temporary closure of full thickness wounds using our baboon dorsum wound model. Grafts were assessed by measurement of fluid loss, wound infection rate, and take, and healed appearance, of secondary autologous grafts following xenograft rejection. Comparison was also made between fresh and cryopreserved grafts. No statistically significant difference was identified between GalT-KO and allogeneic skin grafts in any of the assessed parameters, and graft take and function was not adversely effected by the freeze–thaw process. These data demonstrate that GalT-KO porcine grafts are functionally comparable to allogeneic skin grafts for temporary closure of full thickness wounds, and support their consideration as an alternative to cadaver allogeneic skin in the emergency management of large burns.
Keywords: Temporary burn wound closure, Split-thickness skin graft, Xenogeneic skin, Allogeneic skin, GalT-KO
1. Introduction
Burns are responsible for a considerable burden of injury, with the World Health Organization estimating that each year burns lead 11 million individuals worldwide to require medical attention, and are responsible for 265,000 deaths [1]. Mortality rates have declined in recent years, and the size of a “survivable” burn has increased, attributable to targeted resuscitation and early surgical intervention [2]. Tangential excision of burned tissue and wound closure with autologous skin grafts is a mainstay of modern burn treatment. However, in burns affecting large percentages of total body surface area (TBSA), the availability of un-burned autologous donor sites suitable for skin graft harvest may be limited, necessitating the use of alternative approaches to temporarily achieve wound coverage and restore barrier function.
The current gold standard therapy is allogeneic cadaver skin. Allogeneic cadaver grafts routinely undergo vascularization over a matter of days in a manner comparable to autologous skin grafts and provide a viable, functional skin barrier offering protection against pathogen entry and homeostatic dysregulation. Although anecdotal reports of prolonged survival of allogeneic skin can be found, in general these grafts are lost to rejection in between 7 and 12days as a result of immunologic incompatibility between patient and skin donor [3]. However, allogeneic cadaver skin grafts require extensive and costly screening to mitigate the risk of pathogen transmission, and supply is often limited [4,5].
Numerous alternatives have been suggested, including cultured autologous keratinocytes and synthetic dermal constructs [6,7]. Cultured keratinocytes offer the benefit of autologous wound coverage which will not undergo immunologic rejection and require later definitive closure, however several weeks can be required to culture adequate quantities and the grafts are delicate and prone to injury [8,9]. Synthetic dermal constructs, such as Biobrane™ and Integra™, offer a more durable and more easily handled coverage solution, but require vessel ingrowth for optimal function and ultimately coverage with autologous skin grafts to achieve definitive wound closure [10,11].
Porcine skin has been suggested as an alternative to allogeneic cadaver skin grafts in this capacity, citing the anatomic and physiologic similarities between porcine and human skin [12]. Porcine skin preserved by treatment with glycerol has been used as a temporary covering for burn wounds, acting as a biologic dressing but lacking some of the benefits of viable, vascularized tissue [13]. However, the presence of the α-1,3-galactose (gal) epitope on the surface of porcine cells results in hyperacute rejection of viable wild-type porcine skin due to naturally occurring anti-gal antibodies when grafted onto humans, or indeed, old world primates [14]. Overcoming hyperacute rejection mediated by anti-gal antibodies has been a major focus of research in the field of xenotransplantation, which has resulted in production of genetically modified α-1,3-galactosyltransferase knockout swine, which lack the gal epitope [15,16].
We previously reported that skin grafts from GalT-KO swine enjoy comparable survival to allogeneic skin grafts on both partial- and full-thickness wounds in a pig-to-baboon xenotransplantation model [17]. We also demonstrated a lack of cross-sensitization between GalT-KO and allogeneic skin grafts, which suggests that serial grafting with both GalT-KO and allogeneic skin could be used to prolong the period of temporary wound coverage in severe burns with very limited availability of autologous donor sites [18]. In the current study, we utilized our pig-to-baboon model system to perform a detailed study of GalT-KO porcine skin graft function, in terms of barrier function as determined by fluid loss and infection rates, graft survival following cryopreservation, and cosmetic outcome.
2. Materials and methods
2.1. Animals
All studies were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (IACUC) and performed in accordance with The Guide for the Care and Use of Laboratory Animals [19]. GalT-KO swine skin donors were obtained from the MGH miniature swine herd. Baboons (Papio hamadryas), aged between 2–5 years and weighing 6–10kg, were obtained from the Mannheimer Foundation, Inc, Homestead, Florida. All animals underwent routine pathogen screening and quarantine prior to commencement of studies.
2.2. Skin graft harvest
Baboon donors were pre-medicated with 0.1mg/kg Atropine IM and 20mg/kg Ketamine IM and transferred to the operating room. The dorsum was shaved using clippers. Endotracheal intubation was performed and anesthesia maintained with 2% isoflurane and oxygen. Swine donors were anesthetized with 2mg/kg Telazol intramuscular (IM) injection, intubated, and anesthesia maintained using 2% isoflurane and oxygen. Baboons were placed under general anesthesia in prone position, swine donors in lateral recumbency. Pre-operative skin preparation was achieved with 2% (w/v) chlorhexidine acetate (NolvasanR Surgical Scrub, Fort Dodge Animal Health, Fort Dodge, IA), 70% isopropyl rubbing alcohol (NolvasanR Surgical Scrub, Owens & Minor, Mechanicsville, VA), and povidone-iodine, 10% (Betadine Solution, Purdue Products, L. P., Stamford, CT) scrubs, and animals draped. Split-thickness skin grafts were harvested from the dorsum (baboons) or flank (swine) with an air-driven dermatome (Zimmer U.K. Ltd, Wiltshire, U.K.). Grafts for fresh use were stored at 4°C pending preparation of the recipient site, which was performed immediately following graft harvest without undue delay beyond that necessary for anesthesia and preparation of the recipient. Grafts for cryopreservation were immediately prepared and frozen according to the protocol below.
2.3. Skin graft cryopreservation and thawing
Freeze media was prepared by adding equal parts cryoprotective media (Lonza BioWhittaker, Basel, Switzerland) and fetal porcine serum to a 0.45 µM filter unit and cooled to 4°C. Working in a tissue culture hood, freshly harvested skin was placed in culture dishes and cut into approximately 4cm × 5cm pieces. Skin samples were then sandwiched in sterile mesh to prevent self-adherence of dermis during the freeze, rolled and placed into 8mm diameter reagent vials (Sarstedt, Germany). Freeze media was then added to the vials until the skin grafts were immersed. The samples were then placed in a phase freezer, which had been pre-cooled to 4°C and brought down to −80°C prior to storage at this temperature until required.
To thaw skin, samples were removed from the freezer and placed in 37°C water bath until freeze media was just thawed enough to be able to remove samples from vials. Thawing was completed in three successive washes in tissue culture media augmented with fetal pig serum.
2.4. Skin graft transplant
Baboon recipients were anesthetized and prepared for surgery as described for donors. 4 × 5cm full-thickness defects were then prepared by excision of skin, subcutaneous tissue and fascia until dorsal muscles were revealed. Hemostasis was accomplished with electro-cautery. Skin was tacked down to muscle bed with 2–0 Vicryl suture (Ethicon Inc.). Split-thickness skin grafts were sutured to full-thickness wound with 3–0 Ethilon (Ethicon Inc.). Non-adherent dressings were applied and secured with veterinary wrapping under protective jackets. For fluid loss studies, skin grafts were dressed with 4 × 4cm non-adhesive absorbent pad (Allevyn, Smith & Nephew Medical Ltd., Hull, England) and adhesive occlusive dressings applied.
2.5. Skin graft assessment and measurement of fluid loss
Skin grafts have previously been described as rejected when less than 10% of the original graft remains viable, this definition was applied throughout the current study. Baboons were sedated for graft checks daily for the first three post-operative days, and then on alternate days until grafts were determined to be rejected. To quantify wound exudates from allo and GalT-KO porcine skin grafts, Allevyn™ absorbent dressings were used. Dressings were collected and replaced daily for the first three days post-transplant. Pre- and post-weights of the dressing (in grams) were recorded. Exudates were also collected by centrifugation. Cell strainers (70 µm, BD Falcon, Franklin Lakes, New Jersey) were placed in the top of 50mL polypropylene conical tubes (BD Falcon, Franklin Lakes, New Jersey), Allevyn pads were then placed in the filters, conicals were capped and centrifuged at 3000rpm for 10min at room temperature. The resulting collections of exudate were then measured in a graduated cylinder.
The gross appearance of skin grafts was documented photographically at each dressing change. For assessment of cosmetic outcome following rejection and re-grafting with autologous skin, de-identified graft photographs were shown to a panel of blinded observers who were asked to score the appearance of each image on a visual analog scale.
2.6. Data analysis
For fluid loss studies (Fig. 1A and B) data are presented as mean volume or mass of fluid lost, with error bars of one standard deviation from the mean. Means were compared using the Mann–Whitney U test. Mean appearance scores for healed wounds following primary GalT-KO or allogeneic skin grafting were compared using the Mann–Whitney U test.
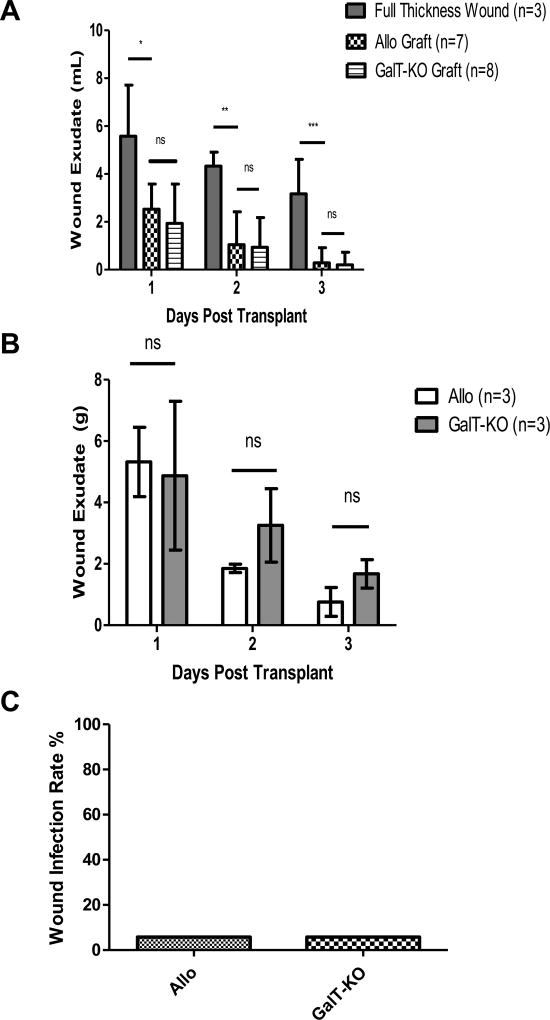
Fig. 1.
Control of fluid loss from full thickness wounds by GalT-KO porcine split thickness grafts is equivalent to allogeneic skin grafts. (A) Wound exudates measured by volume (mL) eluted from absorbent dressings. GalT-KO skin and allogeneic skin grafts both significantly reduced fluid loss in comparison to untreated full-thickness wounds over first three days following grafting (*, Day 1 Full thickness wound vs. allo graft p=0.0132; vs. GalT-KO p=0.0137: **, Day 2 Full thickness wound vs. allo graft p=0.0046; vs. GalT-KO p=0.0016: ***, Day 3 Full thickness wound vs. allo graft p=0.0017; vs. GalT-KO p=0.0005). (B) Wound exudates assessed daily as delta wet-dry weight (grams) of absorbent dressing. Exudates fell to undetectable levels after day 3. No statistical significance was observed between allo and GalT-KO grafts at any point (Day 1: p=0.7865; Day 2: p=0.1144; Day 3: p=0.0746). (C) Percentage wound infection rates for allogeneic and GalT-KO xenogeneic skin grafts. Clinical signs of wound infection were observed in 5.8% (1/17) grafts in each group. Common skin commensal organisms were cultured, and neither animal developed signs of systemic illness.
3. Results
3.1. Barrier function of GalT-KO skin is equivalent to allogeneic skin
Following preparation of full thickness wounds, fluid loss from wounds grafted with GalT-KO porcine skin (n=8), and allogeneic skin (n=7) was measured by volume of fluid, in milliliters, eluted from absorbant dressings daily over the first three post-operative days. Fluid loss from ungrafted wounds (n=3) was measured for comparison (Fig. 1A). Mean fluid loss for GalT-KO porcine skin for days 1, 2, and 3 was 1.93mL, 0.938mL and 0.202mL respectively. The mean fluid loss for allogeneic skin was 2.53mL, 1.04mL, and 0.287mL respectively. Mean fluid loss for full-thickness wound beds for days 1, 2, and 3 were 5.58mL, 4.33mL, and 3.17mL respectively. GalT-KO porcine skin most effectively prevented fluid loss on all three days but did not differ significantly from allogeneic skin grafts. The full-thickness wound bed allowed significantly more fluid loss than both experimental skin grafts (Day 1 Full thickness wound vs. allo graft p=0.0132; vs. GalT-KO p=0.0137: Day 2 Full thickness wound vs. allo graft p=0.0046; vs. GalT-KO p=0.0016: Day 3 Full thickness wound vs. allo graft p=0.0017; vs. GalT-KO p=0.0005).
We sought to confirm these findings by daily evaluation of fluid loss by comparison of wet and dry dressing weights for wounds grafted with GalT-KO and allogeneic skin on three baboons; as before, no significant difference was observed between GalT-KO and allogeneic skin (Fig. 1B). GalT-KO skin grafts allowed for a mean of 4.87g of fluid collected on day 1, 3.25g on day 2, and 1.67g on day 3. Allo skin grafts allowed for a mean of 5.31g on day 1, 1.85g on day 2, and 0.76g on day 3. There was no significant difference in fluid loss between GalT-KO porcine skin and allogeneic skin over the three-day period (Day 1: p=0.7865; Day 2: p=0.1144; Day 3: p=0.0746).
In addition to fluid homeostasis, control of infection is an important component of the barrier function of skin. All experimental grafts and wounds were routinely observed for clinical signs of infection, and if detected, wound swabs and blood samples submitted for bacteriological culture and sensitivity. A total of 17 grafts were performed in both GalT-KO and allogeneic groups in this study, of which one graft (5.8%) in each group demonstrated clinical signs of infection (Fig. 1C). In both cases, only skin commensals were cultured from wound swabs and neither animal developed systemic signs of infection such as fever, leukocytosis or positive blood culture.
3.2. Cryopreservation has no significant impact on GalT-KO split-thickness skin graft survival
Frozen and fresh GalT-KO split-thickness skin grafts were placed on naïve baboons. Fresh and cryopreserved grafts were initially placed on groups of four baboons each. Analysis of mean graft survival time for these equal groups demonstrated no significant difference between fresh and cryopreserved grafts (data not shown), therefore, cryopreserved grafts were utilized for all experiments in the barrier function and secondary autologous graft take groups. In the interests of completeness, survival data for frozen grafts is therefore reported for all recipients in this paper, a total of n=13.
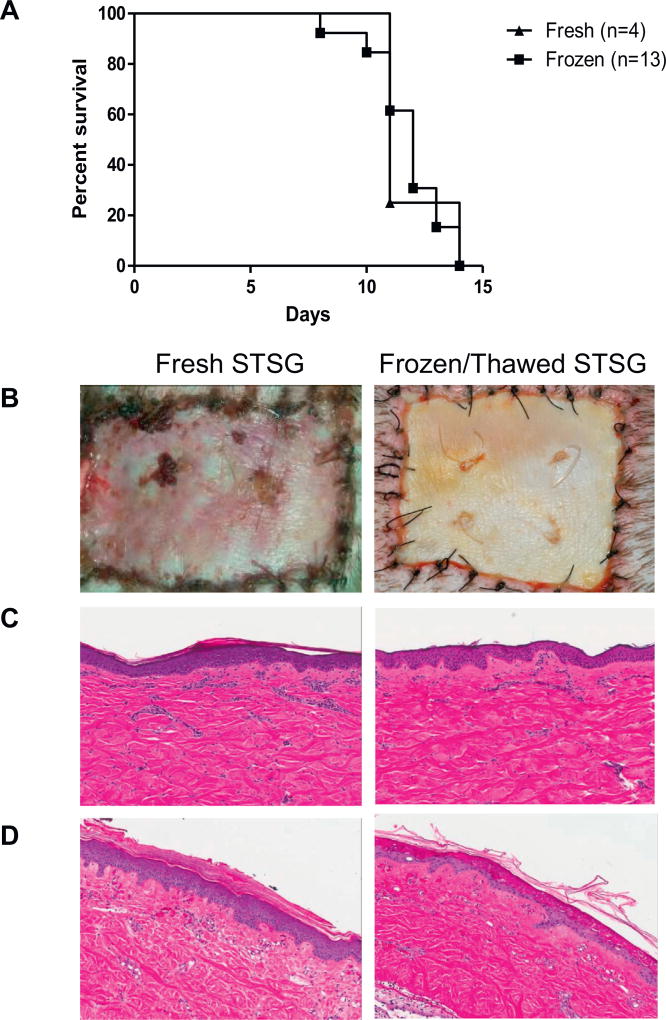
In previous studies in this series, skin graft rejection was determined as having occurred when less than 10% viable graft tissue remained: the same standard has been applied throughout this study [20]. The day of rejection for each graft was recorded and presented in a Kaplan–Meier survival curve (Fig. 2A). Three fresh grafts were deemed rejected by day 11 with the fourth rejecting on day 14, thus indicating a mean rejection time for fresh grafts of 11.75 days. Similarly, frozen GalT-KO skin graft rejection occurred between days 8 and 14 with a mean rejection of 11.8 days.
Fig. 2.
Cryopreservation does not significantly effect survival of GalT-KO split-thickness skin grafts. (A) Kaplan–Meier survival curve demonstrating equivalent survival of GalT-KO split-thickness skin grafts whether placed immediately following harvest, or following period of cryopreservation at −80°C. (B) Representative images demonstrating visible appearance of fresh (left) and thawed cryopreserved (right) GalT-KO grafts four days after grafting; obvious pink coloration and fixed staining is event in fresh grafts. (C) Representative hematoxylin and eosin stained images of pre-implantation specimens of fresh (left) and thawed cryopreserved (right) GalT-KO grafts and (D) corresponding images from biopsies obtained 4days after grafting.
Despite comparable survival, differences in appearance of the grafts were observed between fresh and thawed cryopreserved grafts, with the latter, while clearly adherent and vascularized, demonstrating less marked coloration and vascular staining (Fig. 2B). To further investigate this difference, GalT-KO skin grafts were subjected to biopsy and histological analysis at the time of grafting and at various post-operative times prior to loss of the graft to rejection. Thawed grafts appeared histologically indistinguishable from fresh skin at the time of grafting (Fig. 2C). At day 4, the overall histological appearance of the grafts remained similar with comparable evidence of mononuclear cell infiltration, and erythrocytes were visible throughout the dermal vascular plexi. However, diffuse separation of the dermo-epidermal junction was noted in grafts which had been cryopreserved and thawed. The epidermis generally remained in approximation to the dermal portion of the biopsy samples, and evidence of proliferation and spreading of basal keratinocytes was observed (Fig. 2D).
3.3. Autologous skin grafts take and heal following rejection of GalT-KO skin grafts comparably to autologous skin following allogeneic skin grafts
Upon rejection of either GalT-KO (n=5) or allogeneic skin grafts (n=3), wound beds were debrided and autologous split-thickness skin grafts were placed to determine if rejection of temporary skin grafts might induce wound-bed changes precluding take of autologous skin grafts and definitive wound closure. Regardless of primary skin graft, secondary autologous skin grafts that took and were vascularized post forty-eight hours of operation survived indefinitely (Fig. 3A). In the initial two cases, following GalT-KO skin graft rejection, failure to adequately fenestrate and secure the autologous skin graft contributed to formation of seroma, preventing vascularization and resulting in loss of at least 50% of graft. However, subsequent re-grafting of affected area resulted in 100% take and vascularization of graft, surviving indefinitely.
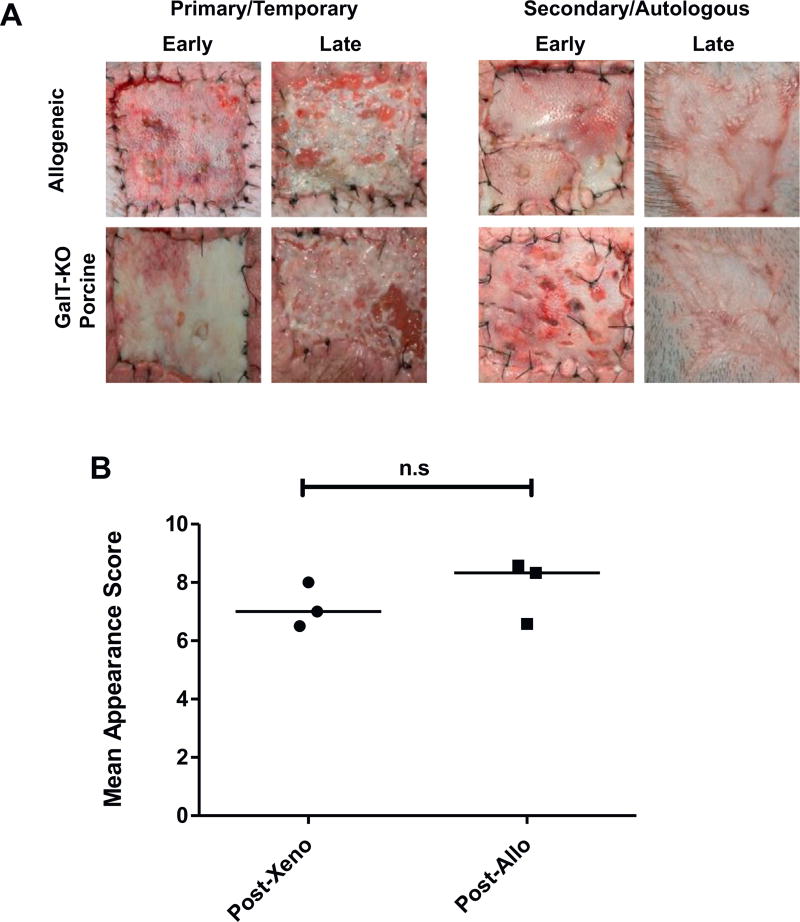
Fig. 3.
Definitive closer of wounds with autologous split-thickness skin grafts is not affected by rejection of preceding GalT-KO grafts, nor allografts. (A) Time course of split-thickness skin graft appearance. Primary (left panel) GalT-KO porcine (upper row) and allografts (lower row) vascularized (early, POD 6) then rejected (late, POD 13) as expected within 14 days. Secondary autologous grafts (right panel) showed evidence of take and vascularization early (POD 2) and all healed to provide definitive wound closure (late, POD 48). Representative images are shown. (B) Mean cosmetic outcome scores for three autologous skin grafts post-GalT-KO and three autologous grafts post-allografts. A panel of blinded observers scored images of autologous grafts from 1 to 10 based upon cosmetic appearance. Statistical significance was determined using the student’s t-test (p=0.40). Two additional grafts are excluded from cosmetic outcome analysis due to technical failure and delayed re-grafting.
Cosmetic outcome of these autologous skin grafts were compared. A panel of blinded observers scored images of the autologous skin grafts at a consistent time point on a scale of 1–10. Mean ratings for three auto-post-GalT-KO and three auto-post-allo skin are represented in Fig. 3B. The two initial grafts in the post-GalT-KO group, which required reoperation due to seroma formation were excluded from this analysis. Mean score for auto-post-allo skin grafts was 7.83. Mean score for auto-post-GalT-KO skin grafts was 7.17 (p=0.40). No statistical significance was seen for cosmetic outcome of autologous skin grafts.
4. Discussion
Cadaveric allograft skin has long been a mainstay in achieving temporary closure of burn wounds, revascularizing in a manner comparable to autologous grafts and mimicking a healed wound environment until rejection intervenes. However, limitations are imposed by cost, availability, and stringent regulations governing testing for transmissible pathogens. Xenogeneic skin grafts have been considered as an alternative, but their efficacy has generally been limited by rapid loss due to hyperacute rejection. We previously reported that skin grafts from swine genetically modified to lack the chief antigenic target resulting in this hyperacute rejection, 1,3-α-gal, demonstrate take and survival comparable to allogeneic grafts in a pig-to-baboon model [17]. Furthermore, these GalT-KO xenogeneic skin grafts fail to elicit cross-sensitization with allografts, facilitating their application in series to achieve an extended period of temporary coverage should it be required [18]. In the current study we have undertaken a detailed analysis of the function of xenogeneic skin grafts from GalT-KO swine in comparison to allogeneic skin. Our findings demonstrate that these xenogeneic grafts provide equivalent acute phase barrier function to allograft; that their eventual rejection results in a wound mileu amenable to autologous graft take, facilitating long-term outcomes comparable to use of allograft; and, importantly for practical application, that cryopreservation and thawing has no significant impact on graft survival duration.
The chief aim, following excision to remove necrotic tissue from the burn wound, is to restore integrity of the cutaneous barrier. In the absence of sufficient autologous skin donor sites to achieve definitive closure with the patient’s own skin any alternative used should provide comparable barrier and protective function [6,11]. Cadaveric allograft skin is well established in this regard, undergoing rapid graft take and providing a healed would environment comparable to autologous skin graft, until loss of the donor skin to rejection, usually within 2–3 weeks. In the current study, GalT-KO xenogeneic grafts demonstrated survival for a mean of approximately 11 days, with no statistically significant difference between fresh and cryopreserved grafts. This is somewhat shorter than the 14–21days which would be ideal for clinical application, but does not differ significantly from the survival of allogeneic grafts observed in previous experiments using this model [20]. Direct comparison of graft survival in this model with clinical data may be misleading however, as the experience of the authors with a variety of non-human primate models of transplantation indicates that in general these animals mount a comparably more aggressive immune response than do humans, requiring higher doses of immunosuppressive agents to achieve comparable clinical effect. In addition, this model does not take into account the potential down regulation of immune function resulting from a large burn. Taken together, these factors indicate that the most valid comparison is to allogenic skin survival in same model system, and that determination of clinically useful survival time would most appropriately be an outcome measure for an early phase clinical trial of GalT-KO xenografts.
Our findings demonstrate that GalT-KO xenograft offers comparable protection against fluid balance and wound infection to allograft. Other approaches to a lack of autologous skin include application of dermal substitutes or cultured autologous keratinocytes. Dermal substitutes are readily available, however optimal protective function typically requires ingrowth of blood vessels from the wound bed, a process which can take several weeks, and autologous skin grafts are still required for definitive closure [10,21,22]. Keratinocyte cultures have the advantage of achieving autologous wound closure, but handling properties remain suboptimal and the resulting skin quality often remains fragile [7,9]. Production aslo requires several weeks, during which time temporary wound closure GalT-KO xenogeneic skin and subsequent autologous donor site re-harvest could have been undertaken.
Clearly it is essential that any material used for temporary closure of burn wounds results in, following its loss or debridement, a mileu conducive to take and healing of autologous grafts. In our model, rejection of both GalT-KO xenograft and allograft resulted in a healthy wound bed which readily accepted autografts. We investigated this further by following secondary autografts until maturation was well advanced and assessing scar appearance at this point; again finding no significant difference between wounds treated with xenograft and allograft.
From a practical perspective, non-autologous graft materials must be readily available and conveniently stored and transported. Synthetic and decellularized skin substitutes have advantages in this domain, needing no special storage conditions. GalT-KO skin is a viable product, akin to allogeneic skin, and therefore requires some method of preservation to facilitate storage. Cryopreservation is an established means of skin storage and our studies have confirmed that GalT-KO skin is tolerant of the freeze–thaw process with no significant degredation in survival time.
This study is limited by the use of relatively small wounds to model a clinical scenario of large, contiguous burn wounds, and by the use of healthy recipient animals free from the systemic insult associated with a large burn. Clearly however, neither preparation of very large surgical skin wounds, nor intentional burning of non-human primates would be morally acceptable and we believe that our model, which has close immunologic and physiologic analogy to humans, and which is a well established system for xenotransplantation research, represents a useful proxy for the debrided full thickness burn wound.
Clearly introduction of any new therapy requires demonstration of not only efficacy, but safety. The potential safety of xenogeneic tissue and products has been a subject of considerable debate, but evidence suggests that swine and humans share only a limited number of pathogens, reducing the risk of disease transmission [23,24]. Furthermore, swine for production of xenogeneic tissue can be maintained in climate controlled, pathogen-free environment, further reducing the risk of contaminated grafts in a manner not possible with allograft. While not a specific aim of the current study, we observed no adverse reactions to xenograft in any recipient.
Taken together with our previous studies demonstrating equivalent survival of GalT-KO xenogeneic, and allogeneic skin grafts in this system [20], and the lack of cross-sensitization between these grafts when applied in series [18], we find the results reported herein encouraging, and believe that GalT-KO porcine skin grafts warrant further investigation in a phase 1 clinical trial to confirm safety, as this technology has the potential to offer an attractive alternative or addition to allogeneic skin in the management of severe burns.
Acknowledgments
This study was supported by funding from the U.S. Department of Defense W81XWH-09-1-0419 and from the U.S. National Institutes of Health Grant No. 1C 06 RR 20135-01 for construction of the swine production facility.
References
- 1.World Health Organisation: Burns. [Accessed March 2016];2015 Apr; http://www.who.int/mediacentre/facsheets/fs365/en/
- 2.Palmieri T, Przkira R, Meyer WJ, III, Carrougher GJ. Measuring burn injury outcomes. Surg Clin North Am. 2014;94:909–16. doi: 10.1016/j.suc.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Ninnemann JL, Fisher JC, Frank HA. Prolonged survival of human skin allografts following thermal injury. Transplantation. 1978;25:69–72. doi: 10.1097/00007890-197802000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kealey GP, Aguiar J, Lewis RW, Rosenquist MD, Strauss RG, Bale JF. Cadaver skin allografts and transmission of human cytomegalovirus to burn patients. J Am Coll Surg. 1996;182:201–5. [PubMed] [Google Scholar]
- 5.Herndon DN, Rose JK. Cadaver skin allograft and the transmission of human cytomegalovirus in burn patients: benefits clearly outweigh risks. J Am Coll Surg. 1996;182:263–4. [PubMed] [Google Scholar]
- 6.Nyame TT, Chiang HA, Orgill DP. Clinical applications of skin substitutes. Surg Clin North Am. 2014;94:839–50. doi: 10.1016/j.suc.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Atiyeh BS, Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns. 2007;33:405–13. doi: 10.1016/j.burns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns. 2007;33:946–57. doi: 10.1016/j.burns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Lootens L, Brusselaers N, Beele H, Monstrey S. Keratinocytes in the treatment of severe burn injury: an update. Int Wound J. 2013;10:6–12. doi: 10.1111/j.1742-481X.2012.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee LF, Porch JV, Spenler W, Garner WL. Integra in lower extremity reconstruction after burn injury. Plast Reconstr Surg. 2008;121:1256–62. doi: 10.1097/01.prs.0000304237.54236.66. [DOI] [PubMed] [Google Scholar]
- 11.Sheridan R. Closure of the excised burn wound: autografts, semipermanent skin substitutes, and permanent skin substitutes. Clin Plast Surg. 2009;36:643–51. doi: 10.1016/j.cps.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Debeer S, Le Luduec J-B, Kaiserlian D, Laurent P, Nicolas J-F, Dubois B, et al. Comparative histology and immunohistochemistry of porcine versus human skin. Eur J Dermatol. 2013;23:456–66. doi: 10.1684/ejd.2013.2060. [DOI] [PubMed] [Google Scholar]
- 13.Basile AR. A comparative study of glycerinized and lyophilized porcine skin in dressings for third-degree burns. Plast Reconstr Surg. 1982;69:969–74. doi: 10.1097/00006534-198206000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–62. [PubMed] [Google Scholar]
- 15.Dor FJMF, Tseng Y-L, Cheng J, Moran K, Sanderson TM, Lancos CJ, et al. alpha1,3-Galactosyltransferase gene-knockout miniature swine produce natural cytotoxic anti-Gal antibodies. Transplantation. 2004;78:15–20. doi: 10.1097/01.tp.0000130487.68051.eb. [DOI] [PubMed] [Google Scholar]
- 16.Zhong R. Gal knockout and beyond. Am J Transplant. 2007;7:5–11. doi: 10.1111/j.1600-6143.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- 17.Barone AAL, Mastroianni M, Farkash EA, Mallard C, Albritton A, Torabi R, et al. Genetically modified porcine split-thickness skin grafts as an alternative to allograft for provision of temporary wound coverage: preliminary characterization. Burns. 2015;41:565–74. doi: 10.1016/j.burns.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Albritton A, Leonard DA, Barone AL, Keegan J, Mallard C, Sachs DH, et al. Lack of cross-sensitization between α-1,3-galactosyltransferase knockout porcine and allogeneic skin grafts permits serial grafting. Transplantation. 2014;97:1209–15. doi: 10.1097/TP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Touitou Y, Portaluppi F, Service UPH, Smolensky MH, Rensing L. Ethical principles and standards for the conduct of human and animal biological rhythm research. Chronobiol Int. 2009;21:161–70. doi: 10.1081/cbi-120030045. [DOI] [PubMed] [Google Scholar]
- 20.Barone AAL, Mastroianni M, Farkash EA, Mallard C, Albritton A, Torabi R, et al. Genetically modified porcine split-thickness skin grafts as an alternative to allograft for provision of temporary wound coverage: preliminary characterization. Burns. 2015;41:565–74. doi: 10.1016/j.burns.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Brusselaers N, Pirayesh A, Hoeksema H, Richters CD, Verbelen J, Beele H, et al. Skin replacement in burn wounds. J Trauma. 2010;68:490–501. doi: 10.1097/TA.0b013e3181c9c074. [DOI] [PubMed] [Google Scholar]
- 22.Lineen E, Namias N. Biologic dressing in burns. J Craniofac Surg. 2008;19:923–8. doi: 10.1097/SCS.0b013e318175b5ab. [DOI] [PubMed] [Google Scholar]
- 23.Fishman JA, Scobie L, Takeuchi Y. Xenotransplantation-associated infectious risk: a WHO consultation. Xenotransplantation. 2012;19:72–81. doi: 10.1111/j.1399-3089.2012.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur M, De A, Gore M. Microbiological assessment of cadaver skin grafts received in a Skin Bank. Burns. 2009;35:104–6. doi: 10.1016/j.burns.2008.04.001. [DOI] [PubMed] [Google Scholar]