Abstract
Some human behaviors, including aggression and activity level, differ on average for males and females. Here we report findings from two studies investigating possible relations between prenatal androgen and children’s aggression and activity level. For study 1, aggression and activity level scores for 43 girls and 38 boys, aged 4 to 11 years, with congenital adrenal hyperplasia (CAH, a genetic condition causing increased adrenal androgen production beginning prenatally) were compared to those of similarly-aged, unaffected relatives (41 girls, 31 boys). Girls with CAH scored higher on aggression than unaffected girls, d = 0.69, and unaffected boys scored higher on activity level than unaffected girls, d = 0.50. No other group differences were significant. For study 2, the relationship of amniotic fluid testosterone to aggression and activity level was investigated in typically-developing children (48 girls, 44 boys), aged 3 to 5 years. Boys scored higher than girls on aggression, d = 0.41, and activity level, d = 0.50. However, amniotic fluid testosterone was not a significant predictor of aggression or activity level for either sex. The results of the two studies provide some support for an influence of prenatal androgen exposure on children’s aggressive behavior, but not activity level. The within-sex variation in amniotic fluid testosterone may not be sufficient to allow reliable assessment of relations to aggression or activity level.
Keywords: Congenital adrenal hyperplasia, amniotic fluid testosterone, prenatal testosterone exposure, aggression, activity level
Introduction
Meta-analytic findings suggest that boys are more aggressive and more active than girls, with Cohen’s d values of 0.58 and 0.49, respectively (Eaton & Enns, 1986; Hyde, 1984). In humans, aggression is defined as behavior, typically directed toward another individual, that is carried out with the intent of causing harm (Bushman & Anderson, 2001) and activity level is defined as the typical level of energy that an individual expends through movement (Eaton & Enns, 1986). There is general agreement that gender differences in human behavior result from a combination of genetic, early hormonal and socio-cultural influences (Hines, 2015; Leaper, 2013). The current investigation examines the relation of the gonadal steroid, testosterone, prenatally to later aggression and activity level in children.
Prior research with nonhuman animals shows that exposure to the androgenic hormone, testosterone, during critical periods of prenatal or neonatal development exerts enduring influences on many behaviors that show sex differences (Arnold, 2009). These effects occur as part of general processes of sexual differentiation. The testes of male animals produce androgens, including testosterone, beginning prenatally, and these hormones act through steroid receptors to produce male-typical development of the external genitalia. Similar steroid receptors are present in certain brain regions, and these regions also are masculinized by androgen exposure during early life. These early effects of androgens on brain organization are thought to contribute to sex-related behaviors across the lifespan (Arnold, 2009; McCarthy, DeVries, & Forger, 2009), and experimental manipulations of testosterone during early development have been found to influence reproductive behaviors, as well as juvenile play behavior, aggression, and activity level in rodents (Hines, 2004, 2011a, 2011b; McCarthy et al., 2009).
Regarding aggression, male rodents typically show more aggression than female rodents, and treatment with testosterone early in life increases aggression in adulthood (Beatty, 1979; Hines, 2004; vom Saal, 1989). Similarly, early exposure to testosterone influences activity level in rodents (Beatty, 1979; Lightfoot, 2008; vom Saal, 1989). In humans, males are more active than females. In most other mammals, including rodents, however, females are more active than males. In keeping with the direction of this sex difference, early exposure to testosterone in female rodents reduces rather than increases activity level – that is, makes it more male typical (Broida & Svare, 1984; vom Saal, 1989).
The available evidence suggests that testosterone concentrations during early life also influence the development of some human behaviors that differ on average for males and females (Hines, 2011a). Male and female fetuses are exposed to androgens, including testosterone, that are produced primarily by the gonads, but also by the adrenal glands, the placenta and the maternal system (Braunstein, 2003; Fisher, 2003; Stewart, 2003). Testosterone levels are higher in human male than in human female fetuses from around week 8 to around week 24 of gestation (Smail, Reyes, Winter, & Faiman, 1981). Because the invasive experimental procedures used to study hormonal influences in nonhuman animals cannot be applied to humans, alternative methods have been used to assess prenatal hormone influences on human development. One such method is to study naturally occurring situations, such as endocrine disorders, in which hormones are altered. Using this approach, individuals who have been exposed to unusual levels of androgens prenatally, e.g., because of genetic disorders, are compared to individuals who have not been similarly exposed (e.g., unaffected relatives or matched controls). The most frequently studied endocrine disorder, in this context, is congenital adrenal hyperplasia (CAH), an autosomal recessive disorder that causes an enzymatic deficiency and results in overproduction of adrenal androgens beginning prenatally (White & Speiser, 2000). The most common form of the disorder, classic CAH, occurs in approximately 1 in 5,000 to 1 in 15,000 births in most populations (New, 1998) and typically involves deficiency in the enzyme 21-hydroxylase (21-OH). The deficiency causes reduced cortisol production and, as a consequence, overproduction of adrenal androgens beginning at around the seventh week of gestation. Because of this prenatal exposure to elevated adrenal androgens, girls with CAH – whose androgen levels at mid-pregnancy are similar to those seen in typically-developing boys – are often born with ambiguous (virilized) external genitalia, involving various degrees of labial fusion and clitoral enlargement. Typically, this genital ambiguity leads to diagnosis soon after birth and sex assignment as female, sometimes with surgical feminization of the external genitalia (Speiser et al., 2010). In contrast, androgen concentrations prenatally in boys with CAH appear to be largely within the normal male range and boys with CAH are born with male-typical external genitalia (Speiser et al., 2010).
Although most studies have found that gender-related behaviors in boys with CAH are not altered (for reviews see Cohen-Kettenis, 2010; Hines, 2015), females with CAH show increases in some male-typical behaviors from an early age. For example, girls with CAH are more likely than unaffected girls to prefer boys as playmates (Hines & Kaufman, 1994) and to prefer boys’ toys (e.g., vehicles versus dolls; Berenbaum & Hines, 1992) and activities (e.g., rough-and-tumble play; Pasterski et al., 2011). These outcomes of increased male-typical play have been reported both in comparison to matched controls and to unaffected female relatives of children with CAH, and in studies using questionnaires, interviews and behavioral observation (Hines, 2011b, 2015). Females with CAH also have been found to show reduced female gender identity (Pasterski et al., 2015; however, see Meyer-Bahlburg et al., 2004) and reduced heterosexual interests (Frisén et al., 2009; Hines, Brook, & Conway, 2004; Meyer-Bahlburg, Dolezal, Baker, & New, 2008).
Females with CAH also have been found to be more aggressive than unaffected female controls. For example, Berenbaum and Resnick (1997) studied aggression in 49 females and 41 males with CAH, ranging in age from approximately 3 to 35 years, and in their unaffected, similarly-aged relatives (28 females, 48 males). Data were analyzed separately for adult, adolescent and preadolescent participants. In all three samples, females with CAH scored higher on aggression than unaffected females, although the difference was not statistically significant in the preadolescent sample. Similarly, Pasterski et al. (2007) compared 3- to 11-year-old children with CAH (38 girls, 29 boys) to their unaffected siblings (25 girls, 21 boys), and found that girls with CAH were rated by their mothers as being more aggressive than unaffected girls. Finally, Mathews, Fane, Conway, Brook, and Hines (2009) studied recalled aggressive behavior at age 12 to 13 years in individuals with CAH (40 females, 29 males), aged 12 to 45 years, and their unaffected, similarly-aged relatives (29 females, 30 males), and found that females with CAH reported greater physical aggression than unaffected females.
Studies of activity level in children with CAH have generally found that girls with CAH show higher levels of activity than unaffected girls. For example, Ehrhardt, Epstein, and Money (1968) assessed physical energy expenditure in 15 girls with CAH, aged between 5 and 16 years, and 15 matched controls. Eleven of the girls with CAH were reported (by mothers or the children themselves) to engage in intense outdoor activities compared to only five of the matched controls. Similarly, Ehrhardt and Baker (1974) compared 17 girls and young women with CAH, aged 4 to 19 years, to 11 unaffected female siblings, aged 6 to 24 years. More females with CAH than unaffected females were described by their mothers as having a high level of intense physical energy expenditure. Finally, Pasterski et al. (2007) compared 3- to 11-year-old children with CAH (38 girls, 29 boys) to unaffected relatives of similar age (25 girls, 21 boys), and found that girls with CAH were rated by their mothers as being more active than unaffected girls.
Another approach to assessing influences of prenatal testosterone exposure on human behavior has involved measuring testosterone concentrations in typically-developing individuals. For example, testosterone concentrations have been measured in amniotic fluid obtained during clinical amniocentesis (Cohen-Bendahan, van de Beek, & Berenbaum, 2005; Constantinescu & Hines, 2012). Testosterone enters the amniotic fluid via diffusion through the fetal skin during early gestation and, from mid-gestation onwards, through fetal urination and lung fluid secretion (Brace, 1997; Robinson, Judd, Young, Jones, & Yen, 1977). The timing of amniocentesis, which is typically performed during the second trimester of pregnancy, coincides with the period during gestation when the sex difference in fetal testosterone exposure is large, between 8 and 24 weeks’ gestation (Smail et al., 1981). Thus, amniocentesis may provide a means for accessing a key developmental period during which testosterone influences human sexual differentiation.
Researchers have reported significant relations between concentrations of testosterone measured in amniotic fluid and some behaviors that differ on average for males and females, including empathy (Chapman et al., 2006) and traits related to autism (Auyeung, Baron-Cohen, Ashwin, Knickmeyer, Taylor, & Hackett, 2009; Auyeung et al., 2006; but, see Kung et al., 2016). There have been no studies to date, however, relating amniotic fluid testosterone to either aggression or activity level.
We conducted two studies to investigate the hypothesis that prenatal androgen exposure, in both the typical and atypical range, predicts increased aggression and activity. In the first study (the “CAH study”), we assessed aggression and activity level in children exposed to unusually high concentrations of adrenal androgens prenatally because of CAH and in their unaffected relatives, but using a larger sample than has been used in most prior studies. In the second study (the “amniotic testosterone study”), we assessed aggression and activity level in typically-developing children for whom testosterone had been measured in amniotic fluid. We evaluated three specific hypotheses: (1) typically-developing boys show higher levels of aggression and activity than typically-developing girls; (2) girls with CAH show higher levels of aggression and activity than girls without CAH; and (3) concentrations of amniotic fluid testosterone relate positively to aggression and activity level in boys and in girls.
Material and methods
Participants for the CAH study were 81 children with CAH (43 girls, 38 boys) and 72 unaffected relatives (41 girls, 31 boys) aged 4.00 to 11.93 years (M = 7.40 years, SD = 2.31). Participants for the amniotic testosterone study were 92 typically-developing children (48 girls, 44 boys) aged 3.81 to 5.22 years (M = 4.27 years, SD = 0.33). The research was undertaken with the understanding and written consent of parents; the written consent or assent of children, as age appropriate; the approval of the appropriate local research ethics committees; and in compliance with national legislation. Detailed information regarding the ethics procedures and sample recruitment and characteristics for the two studies has been published elsewhere (Browne et al., 2015; Hines et al., 2016; Kung et al., 2016; Pasterski et al., 2015). Assessment procedures for both studies were two-and-a-half to three hours in length and included a range of measures assessing children’s gender-related cognitive and motor abilities, characteristics and behaviors. In this paper we report on aggression and activity level. Other outcomes have been (Browne et al., 2015; Hines et al., 2016; Kung et al., 2016; Pasterski et al., 2015), or will be, reported separately.
Measures
Amniotic fluid testosterone
Participants in the amniotic testosterone study were recruited through Imperial College Healthcare NHS Trust in London, England. The sample was comprised of typically-developing children, born between June 2002 and June 2005, for whom measures of testosterone in amniotic fluid had been obtained. The reason for the amniocentesis was typically increased risk of trisomy 21 as indicated by maternal age or a serum screen result. All of the children were from healthy pregnancies and were identified as healthy at birth. Mothers were on average 37 years of age (ranging from 28 to 46 years) when their child was born. Amniotic fluid samples were obtained between gestational weeks 15 and 25 (M = 16.93 weeks, SD = 2.01). Boys and girls did not differ significantly in gestational age at amniocentesis, t(90) = 0.40, p = .69, or maternal age at birth, t(90) = 0.14, p =.99. All amniotic fluid samples were taken in the morning between 9:30am and 12:30pm. An aliquot of up to 4 ml of amniotic fluid surplus to clinical requirement was drawn for the study and stored at −80°C until assay. Total testosterone was measured in amniotic fluid by radioimmunoassay after prior extraction by diethylether to minimize cross-reactivity, using the ‘Coat-A-Count’ method (Coat-A-Count, DPC, Los Angeles, CA). As reported in Bergman, Glover, Sarkar, Abbott, and O’Connor (2010), the assay has high specificity for testosterone. Intra- and inter-assay coefficients of variation of our assay procedures were 7.5% and 8.9%, respectively.
Following others (e.g., Auyeung, Baron-Cohen, Ashwin, Knickmeyer, Taylor, & Hackett, 2009; Kung et al., 2016), we corrected for positive skewness in the distribution of values for amniotic fluid testosterone by adding one to each value and then calculating its log. This transformation removed the skewness both for the sample as a whole and separately for boys and girls. Unless otherwise stated, the analyses reported here are based on the transformed values.
Aggression and activity level
Aggression and activity level were measured using the Interests, Activities & Temperament Questionnaire – II (IATQ-II). The IATQ-II is a revision of Zucker & Bradley’s (1995) 17-item Activity Level/Extraversion Questionnaire, which was used in a prior study of aggression and activity level in children with CAH (Pasterski et al., 2007). For the IATQ-II, we increased the number of items, both to increase the potential variance in and the reliability of the measure and to reduce response bias by presenting the constructs in varied ways. The IATQ-II includes 50 items for which a caregiver indicates on a 4-point scale (1 = “not at all like my child” to 4 = “a lot like my child”) how similar their child’s behavior is to the behavior described in the item (see Appendix).
Because the IATQ-II was used for the first time in the two studies presented here, we used exploratory factor analysis (EFA) to identify underlying factors. Although EFA can be used to explore and describe a dataset, it is not appropriate to extrapolate the findings obtained from one sample to another sample or to the wider population (Costello & Osborne, 2005). Given this, separate EFAs were conducted for each of the two samples (see Appendix). For both samples, the items that clustered on the extracted factors suggested that the first factor represented aggression and the second represented activity level. Cronbach’s α values for the aggression items were α = .95 for the CAH study and α = .93 for the amniotic testosterone study, indicating good internal consistency. Cronbach’s α values for the activity level items were α = .84 for the CAH study and α = .82 for the amniotic testosterone study, again indicating good internal consistency.
For both studies, the IATQ-II was completed by a parent, usually the mother. Four fathers completed the IATQ-II for the children in the CAH study and six fathers completed the measure for the children in the amniotic testosterone study.
Control measures
The child’s age at testing was assessed as a control variable. It was expected that this variable would be most relevant in the CAH study, given the wider age range of the children, compared with the more uniformly-aged children in the amniotic testosterone study. The Vocabulary subtest of the Wechsler Intelligence Scale for Children (Wechsler, 2003) or the Wechsler Preschool and Primary Scale of Intelligence (Wechsler, 1967/2002) was used, as age appropriate, to provide estimates of general intelligence. Age-scaled Vocabulary subtest scores were included in the analyses, as appropriate, and were available for all of the children in the CAH study and all but three boys and one girl in the amniotic testosterone study.
Results for the CAH study
The four groups did not differ in age, F(3, 149) = 0.74, p = .53, η2 = .015, or vocabulary scores, F(3, 149) = 1.11, p = .35, η2 = .022. Aggression and activity level scores related to child age: r = −.23, p < .01 for aggression and r = −.36, p < .001 for activity level. In addition, vocabulary scores related to activity level scores, r = .22, p = .01, but not to aggression scores, r = −.05, p = .52. We therefore entered age as a covariate in all subsequent analyses and vocabulary as a covariate in subsequent analyses involving activity level scores. Two-way (sex x CAH status) ANCOVA was performed for each of the two dependent variables, aggression and activity level. Additional, one-way ANCOVAs were used to follow up these analyses and to test specific hypotheses. Effect sizes were calculated and used in conjunction with p values to assess the results. All analyses were two tailed, with α set at .05.
For aggression, a 2 (sex) × 2 (CAH status) ANCOVA, with age covaried, revealed a significant main effect of age, F(1, 148) = 6.81, p = .01, partial η2 = .044, and a significant main effect of CAH status, F(1, 148) = 11.13, p < .001, partial η2 = .070; children with CAH scored higher on aggression than unaffected children, d = 0.54. There were no other significant main or interaction effects. We used one-way ANCOVAs, with age covaried, to investigate our specific hypotheses (Table 1). Scores for unaffected boys did not differ from those of unaffected girls, F(1, 69) = 0.30, p = .58, partial η2 = .004, or from those of boys with CAH, F(1, 66) = 2.60, p = .11, partial η2 = .038. However, girls with CAH scored higher on aggression than unaffected girls, F(1, 81) = 10.15, p = .002, partial η2 = .111.
Table 1.
Gender differences and CAH-control comparisons for aggression and activity level for the children in the CAH study.
| Gender difference
|
Girls
|
Boys
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unaffected boys | Unaffected girls | p | d | CAH | Unaffected | p | d | CAH | Unaffected | p | d | |
| Aggression | ||||||||||||
|
| ||||||||||||
| Est. M | 1.73 | 1.66 | .58 | 0.13 | 2.11 | 1.67 | .002 | 0.69 | 1.97 | 1.74 | .11 | 0.38 |
| SE | .10 | .08 | .10 | .10 | .10 | .11 | ||||||
| n | 31 | 41 | 43 | 41 | 38 | 31 | ||||||
|
| ||||||||||||
| Activity level | ||||||||||||
|
| ||||||||||||
| Est. M | 3.41 | 3.13 | .05 | 0.50 | 3.33 | 3.13 | .17 | 0.31 | 3.31 | 3.44 | .25 | 0.27 |
| SE | .10 | .09 | .10 | .10 | .08 | .09 | ||||||
| n | 31 | 41 | 43 | 41 | 38 | 31 | ||||||
Note. Note that the adjusted mean (Est. M) and standard error (SE) values for unaffected girls and unaffected boys differ slightly depending on the group with whom they are being compared. This is because the calculation of these values takes into consideration the age, or age and vocabulary scores, of the children being compared. (Recall that we corrected for age in the analyses involving aggression, and we corrected for age and vocabulary in the analyses involving activity level.) So, for example, in the analysis comparing aggression scores for unaffected boys and unaffected girls, the calculations for the adjusted mean and standard error values take into consideration the ages of the 72 boys and girls included in the analysis. Likewise, in the analysis comparing aggression scores for unaffected girls and girls with CAH, the calculations for the adjusted mean and standard error values take into consideration the ages of the 84 girls included in this analysis. The effect size, d, was calculated using the adjusted means and standard errors (Lipsey & Wilson, 2000).
For activity level, a 2 (sex) x 2 (CAH status) ANCOVA, with age and vocabulary covaried, revealed a main effect of age, F(1, 147) = 15.81, p < 001, partial η2 = .097. There were no other significant main or interaction effects. We used one-way ANCOVAs, with age and vocabulary covaried, to investigate our specific hypotheses (Table 1). Unaffected boys scored higher on activity level than unaffected girls, F(1, 68) = 3.91, p = .05, partial η2 = .054. Scores for girls with and without CAH did not differ, F(1, 80) = 1.88, p = .17, partial η2 = .023, nor did scores for boys with and without CAH, F(1, 65) = 1.33, p = .25, partial η2 = .020.
Results for the amniotic testosterone study
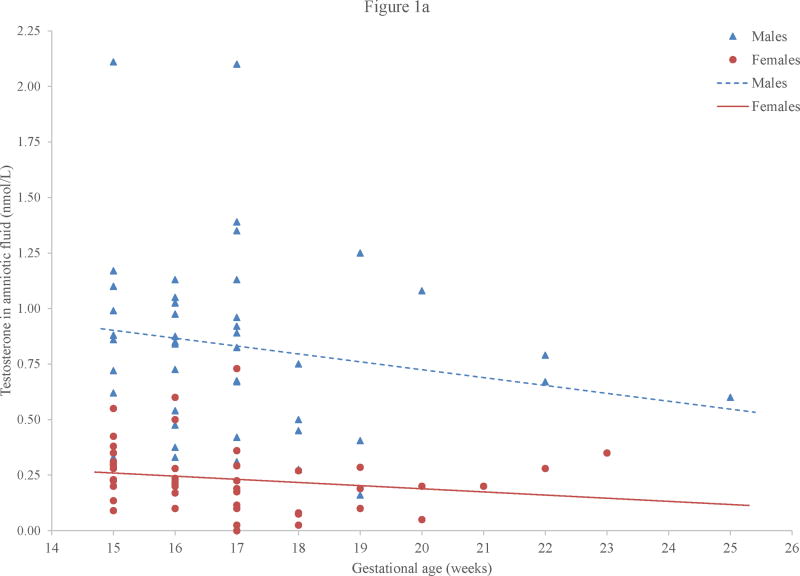
Because concentrations of fetal testosterone vary across gestation, preliminary analyses were conducted to determine if concentrations of amniotic fluid testosterone related to gestational age at amniocentesis, as calculated from medical records indicating the date of the mother’s last menstrual period. This relation was not statistically significant for boys, r = −.18, p = .24, or for girls, r = −.19, p = .19 (Figure 1a). The children in this sample were all relatively close in age (Table 2) and, perhaps as a consequence, aggression and activity level scores did not vary with age: r = .07, p = .51, for aggression and r = .00, p = .98, for activity level. Aggression and activity level scores also did not vary significantly with children’s vocabulary scores: r = .11, p = .33, for aggression and r = .10, p = .36, for activity level. Data were analyzed using Pearson correlations and independent-samples t tests. Effect sizes were calculated and used in conjunction with p values to assess the results. All analyses were two tailed, with α set at .05.
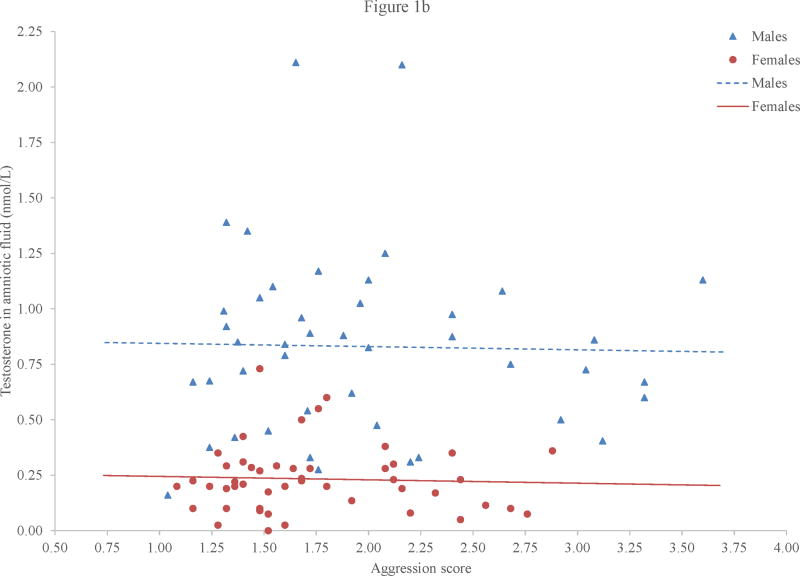
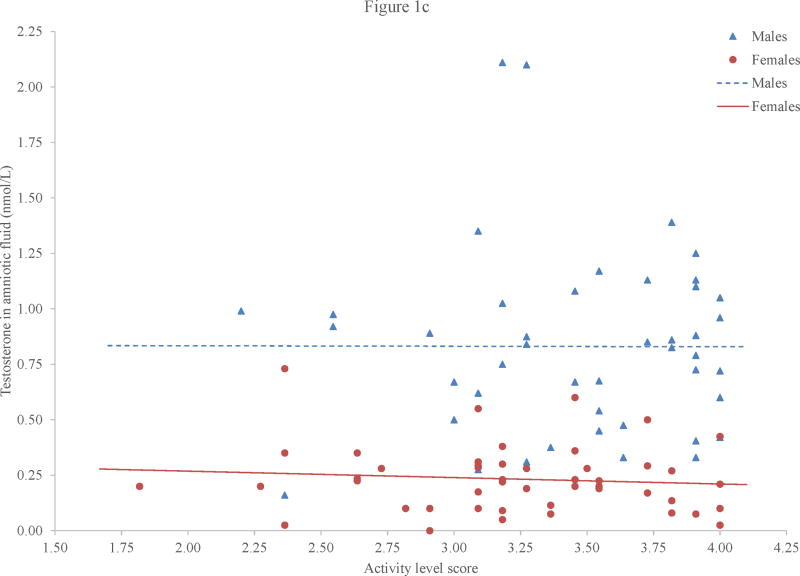
Figure 1.
Table 2.
Means (M), standard deviations (SD), p values from t tests and effect sizes (Cohen’s d) for assessing sex or gender differences in age, vocabulary, amniotic fluid testosterone concentrations, aggression and activity level for the children in the amniotic testosterone study.
| Boys n = 44 |
Girls n = 48 |
|||
|---|---|---|---|---|
| M (SD) | M (SD) | p | d | |
| Child’s age (years) | 4.31 (0.37) | 4.23 (0.29) | .28 | 0.23 |
| Vocabulary* | 10.46 (2.70) | 11.34 (2.27) | .10 | −0.35 |
| Amniotic fluid testosterone (nmol/L) | 0.83 (0.41) | 0.23 (0.15) | < .001 | 1.95 |
| Amniotic fluid testosterone (log transformed) | 0.25 (0.09) | 0.09 (0.05) | < .001 | 2.22 |
| Aggression | 1.98 (0.66) | 1.74 (0.46) | .05 | 0.41 |
| Activity level | 3.46 (0.47) | 3.21 (0.53) | .02 | 0.50 |
Note. Data missing for three boys and one girl. d = the mean difference divided by the weighted standard deviation (Cohen, 1988). Positive d values indicate higher values in boys.
Concentrations of amniotic fluid testosterone were higher for boys than for girls (Table 2). Furthermore, the mean amniotic fluid testosterone values that we obtained, for boys and for girls, were similar to those reported by other researchers who have measured testosterone in amniotic fluid during the second trimester (e.g., Auyeung et al., 2006). Aggression and activity level scores also were higher for boys than for girls (Table 2).
Although we saw sex or gender differences in amniotic fluid testosterone and in aggression and activity level, there were no significant relations between amniotic fluid testosterone and either behavioral construct (Figures 1b and 1c). The correlations between amniotic fluid testosterone and both aggression and activity level were negligible for boys: r = −.00, p = .99, for aggression and r = .03, p = .86, for activity level. For girls, the correlations were small to negligible: r = −.05, p = .75, for aggression and r = −.10, p = .51, for activity level.
Discussion
We conducted two studies to test the hypothesis that exposure to testosterone prenatally affects children’s aggressive behavior and activity level. The first study compared children with CAH to unaffected siblings and cousins. The second study related testosterone measured in amniotic fluid to aggression and activity level in typically-developing children. There was no gender difference in aggression in unaffected children in the CAH study, and ratings for boys with CAH did not differ from those of unaffected boys. However, girls with CAH were rated as more aggressive than unaffected girls. As expected, boys in the amniotic testosterone study were rated as more aggressive than girls, but testosterone measured in amniotic fluid did not relate to aggression in either girls or boys. Regarding activity level, we found a significant gender difference, favoring boys, among unaffected controls in the CAH study but no differences between children with and without CAH. For the children in the amniotic testosterone study, we found a gender difference – again, favoring boys – in activity level. There was no evidence, however, of a relation between activity level and amniotic fluid testosterone concentrations for boys or for girls. The results of the CAH study provide some support for the hypothesis that testosterone exposure prenatally influences children’s aggressive behavior, but we found no evidence, in either study, of an influence on activity level.
Aggression
As predicted, we found a gender difference in aggression, favoring boys, in the amniotic testosterone study. Contrary to prediction, however, we did not find a significant gender difference in aggression in the CAH study for unaffected boys and girls. This may have been because the unaffected boys in the CAH study scored at the low end of the range of scores for boys in general. Their scores resembled scores of unaffected girls more closely than they did boys or girls with CAH. They also scored lower than the typically-developing boys in the amniotic testosterone study. The unexpectedly low scores of the unaffected boys in the CAH study may have contributed to our finding that the difference in aggression scores between girls with and without CAH was larger than the gender difference in aggression in this study.
Although the gender difference in aggression scores in the CAH study was not significant, it was not outside the range of effect sizes suggested by meta-analyses. A meta-analysis by Card, Stucky, Sawalani, and Little (2008) found a moderate-to-large gender difference in direct aggression, d = 0.61, with boys showing more aggression than girls. Importantly, they also found that the size of this effect was moderated by informant. The gender difference was smaller (d = 0.30) when parents reported on children’s aggressive behavior, compared to when aggressive behavior was measured via behavioral observation, peer nomination, peer ratings or teacher reports (but not self reports). For the eight studies in the Card et al. (2008) meta-analysis that assessed direct aggression via parent report, the effect sizes ranged from d = 0.12 to d = 0.87. The sizes of the gender difference in aggression that we found in the amniotic testosterone study and the CAH study (d = 0.41 and d = 0.13, respectively) are within this range.
Our findings support the prediction that girls with CAH would be rated as more aggressive, on average, than their similarly-aged, unaffected female relatives – we found a large effect of CAH status on aggression in girls, d = 0.69. This finding falls within the range of effect sizes (from d = 0.41 to d = 1.02) found by others who have studied aggressive behavior in girls with and without CAH using multi-item questionnaires (Berenbaum & Resnick, 1997; Mathews et al., 2009; Pasterski et al., 2007) and provides further support for the hypothesis that androgen exposure prenatally influences this particular gender-related behavior. In line with prior research, which has generally not found increased masculine behavior in boys with CAH, aggression scores for boys with and without CAH did not differ.
In the amniotic testosterone study, testosterone concentrations were significantly higher for boys than for girls, and boys scored higher than girls on aggression. We found no significant within-sex relations between amniotic fluid testosterone and aggression, however, a point that is discussed in detail below.
Activity level
The typically-developing boys in both the CAH study and the amniotic testosterone study were rated as more active than the typically-developing girls. The size of the gender difference, for both studies, was moderate (d = 0.50 for the CAH study and d = 0.50 for the amniotic testosterone study) and similar to the average effect size for this gender difference (d = 0.49; Eaton & Enns, 1986).
Girls with and without CAH in the current study did not differ significantly in activity level and the size of the difference was small. In contrast, using a comparable parent report instrument to measure activity level in a sample of children similar in age to the children in our study, Pasterski et al. (2007) found a statistically significant difference of moderate size, d = 0.55, as well as a gender difference of moderate size, d = 0.59. Data from the current study suggest that any effect of prenatal androgen exposure on activity level may be smaller than suggested by prior research.
In the amniotic testosterone study, testosterone concentrations were significantly higher for boys than for girls, and boys scored higher than girls on activity level. We found no significant within-sex relations between amniotic fluid testosterone and activity level, however. These negative results, like those of no relation between amniotic fluid testosterone and aggression, may appear surprising, given other evidence that testosterone influences human behaviors that show gender differences.
Over the past three decades, several research groups have related measures of amniotic fluid testosterone to children’s later gender-typed behaviors. Studies that have used samples of fewer than around 60 participants per group generally have not found significant correlations between amniotic fluid testosterone and the gender-typed behavior(s) under investigation, have found that the direction of one or more correlations was opposite to that predicted or have found inconsistencies in the direction or strength of one or more correlations when comparing results for boys and girls (see e.g., Bergman et al., 2010; Grimshaw, Bryden, & Finegan, 1995; Grimshaw, Sitarenios, & Finegan, 1995; Knickmeyer, Baron-Cohen, Raggatt, & Taylor, 2005; Knickmeyer, Wheelwright, et al., 2005; Kung et al., 2016; van de Beek, van Goozen, Buitelaar, & Cohen-Kettenis, 2009). One research group has reported some significant within-sex relations between amniotic fluid testosterone and later gender-typed behaviors in samples similar in size to, or smaller than, ours, as well as in larger samples (around 100 participants per group). The studies using similarly-sized or smaller samples have found the predicted within-sex relations to amniotic fluid testosterone for autistic traits, attention to detail and empathy (Auyeung, Ahluwalia, et al., 2012; Auyeung, Knickmeyer, et al., 2012; Chapman et al., 2006). The studies of samples of over 100 participants of each sex have found the predicted within-sex relations for autistic traits and empathy, and for gender-typical childhood behavior (Auyeung, Baron-Cohen, Ashwin, Knickmeyer, Taylor, & Hackett, 2009; Auyeung, Baron-Cohen, Ashwin, Knickmeyer, Taylor, Hackett, et al., 2009; Chapman et al., 2006). Taken together, the evidence suggests that associations between amniotic fluid testosterone and human behavior, if they exist, may be small and may require larger samples than have typically been used, to provide adequate statistical power.
Limitations
As with previous research, the amniotic testosterone study is limited by using amniotic fluid testosterone concentrations as a measure of normal variability in prenatal androgen exposure. Mothers who undergo amniocentesis may not be representative of the general population – for example, the mothers of the children in our study were older and better educated than the average mother – and, consequently, our results may not generalize to the wider population. Nevertheless, the resemblance of our basic findings, e.g., regarding gender differences in the behavioral constructs we measured, to those reported by others provides some support for generalizability. The reliability of amniotic fluid testosterone as an index of prenatal androgen exposure may be limited, however, because typically only a single amniotic fluid sample is collected, usually uncontrolled for time of day or gestational age, although in our study time of day was controlled to some extent by taking all amniotic fluid samples in the morning. While it has been assumed that testosterone concentrations in amniotic fluid are a proxy for testosterone concentrations in fetal blood (Baron-Cohen, Lutchmaya, & Knickmeyer, 2004), the one study that we are aware of that has investigated the relation reported no correlation between the two (Rodeck, Gill, Rosenberg, & Collins, 1985). Therefore, testosterone in amniotic fluid may not be a sufficiently powerful measure of prenatal androgen exposure to detect relations to behaviors that show moderately-sized gender differences, such as aggression or activity level, in samples similar in size to ours.
There are also limitations related to studying children with CAH. Although CAH influences androgen concentrations in girls beginning prenatally, it has additional consequences that also could potentially influence behavior. Notably, although postnatal hormone treatment is intended to normalize the hormone environment, this is not always successful. Therefore, children with CAH may experience higher or lower levels of glucocorticoids and/or androgens at one or more periods during postnatal life. These possible postnatal differences in hormones are unlikely to explain our results, however, because there is no evidence that either glucocorticoids or androgens after early infancy and before puberty influence aggression or activity level in humans or in other species. In addition, if consequences of CAH other than unusual androgen concentrations prenatally explained the behavioral changes seen in girls with CAH, similar effects would be expected in boys with CAH as well, because only girls with CAH experience unusually high androgen concentrations prenatally, whereas both boys and girls with CAH experience postnatal treatment and possible higher or lower levels of glucocorticoids and androgens postnatally.
Finally, we conducted two studies on relatively small groups of children, and consequently cannot provide definitive evidence that a relation between testosterone and aggression or activity level does or does not exist. Nevertheless, we recruited a larger sample of children in our CAH study than has been recruited in most previous studies of aggression or activity level in children with CAH, and the sample in our amniotic testosterone study is larger than that in the previous amniotic fluid testosterone studies showing the biggest effects (Auyeung, Ahluwalia, et al., 2012; Auyeung, Knickmeyer, et al., 2012; Grimshaw, Sitarenios, et al., 1995). Importantly, both negative and positive results contribute to our understanding of the size, as well as the reliability, of relations between testosterone and gender-related behaviors, and our results are of interest not only on their own, but also in the context of prior findings (e.g., for meta-analytic studies).
Conclusions
Our primary goal was to examine the relation of prenatal androgen exposure to aggression and activity level. Consistent with prior research, we found that girls with CAH showed a statistically significant increase in aggression compared to unaffected girls. Although we found a small increase in activity level in girls with CAH, this increase was not statistically significant, suggesting that the contribution of prenatal androgen to activity level may be smaller than suggested by prior research. In addition, we found no significant within-sex correlations between amniotic fluid testosterone and either aggression or activity level, suggesting that amniotic fluid testosterone may be a relatively insensitive measure of prenatal androgen exposure.
Highlights.
Prenatal androgen exposure linked to aggression but not activity in girls with CAH.
Sex differences in aggression and activity found in healthy children.
Amniotic fluid testosterone unrelated to aggression or activity in healthy sample.
Amniotic fluid testosterone may not be an adequate measure for these outcomes.
Acknowledgments
My co-authors and I would like to acknowledge the contributions of the following groups and individuals for their contributions: Sue Elford and the Living with CAH support group; Kristin Bergman, Pampa Sarkar and Diana Adams; and all of the families whose participation made this study possible. This work was supported by USPHS National Institutes of Health grant numbers HD24542, MH073019 and MH073842, and by the March of Dimes.
Appendix: Factor analysis of the Interests, Activities and Temperament Questionnaire – Part II
As described in the main text, the Interests, Activities and Temperament Questionnaire - Part II (IATQ-II) includes 50 items for which a caregiver indicates on a 4-point scale (1 = “not at all like my child” to 4 = “a lot like my child”) how similar their child’s behavior is to the behavior described in the item. The items included in the measure were designed to tap into aggression (AG), activity level (AL) and being bullied by others (BBO). We used principal axis factoring (PAF; a form of exploratory factor analysis (EFA)) with oblique rotation, to analyze the data because the item scores were not normally distributed and because we had an a priori idea about how the items would relate to one another. A combination of scree plots and multiple test runs were used to determine the number of meaningful factors in each dataset. The cleanest factor structure – i.e., the one for which all item loadings > .30, there were no or few items that loaded at .32 or higher onto more than one factor and there were no factors with fewer than three items – consisted of two factors: one that represented aggression (AG) and one that represented activity level (AL) (see Table A1).
Table A1.
Results of the exploratory factor analyses for the IATQ-II.
| Item | Pattern matrix loadings | ||||
|---|---|---|---|---|---|
| Amniotic testosterone study | CAH study | ||||
| AG | AL | AG | AL | ||
| 1. | My child is curious and explores things.a | .57 | |||
| 2. | When my child moves about, s/he usually moves slowly. | .58 | .64 | ||
| 3. | My child starts fights with other children. | .80 | .72 | ||
| 4. | My child hits others when frustrated. | .78 | .75 | ||
| 5. | My child is off and running as soon as s/he wakes up in the morning. | .54 | .49 | ||
| 6. | My child rarely throws temper tantrums. | .40 | .56 | ||
| 7. | In the playground, my child runs, climbs, swings, and is constantly on the go. | .60 | .77 | ||
| 8. | My child rarely performs strenuous activity for long periods of time.b | .57 | |||
| 9. | My child is hardly ever provoked to fight. | .68 | .67 | ||
| 10. | My child rarely gets into arguments. | .59 | .70 | ||
| 11. | My child is noisy.b | .37 | .41 | ||
| 12. | My child is unlikely to take things (e.g. toys) from other children. | .45 | .51 | ||
| 13. | My child has a “quick temper.” | .41 | .64 | ||
| 14. | My child would never pick on children younger than himself/herself.a | .50 | |||
| 15. | My child bullies other children.a | .44 | |||
| 16. | My child is unlikely to be picked on by other children.ab | ||||
| 17. | My child is always on the go. | .79 | .71 | ||
| 18. | My child never gets into fights. | .68 | .68 | ||
| 19. | My child is timid and unadventurous.a | .63 | |||
| 20. | My child fights. | .82 | .69 | ||
| 21. | My child is physically aggressive. | .75 | .82 | ||
| 22. | My child hits (has been known to hit) others with various objects (e.g. toys). | .78 | .70 | ||
| 23. | My child rarely gets teased by other children at school.ab | ||||
| 24. | My child is likely to be disciplined at school for verbal misbehaviour (e.g. yelling, arguing). | .49 | .51 | ||
| 25. | My child would be likely to walk away from a fight. | .68 | .68 | ||
| 26. | My child avoids arguments with family members (e.g. siblings, cousins). | .39 | .48 | ||
| 27. | My child hits other children. | .65 | .76 | ||
| 28. | My child is low in energy. | .52 | .58 | ||
| 29. | My child almost never uses foul language (swears).ab | ||||
| 30. | When outdoors, in a playground or park, my child plays quietly with toys.b | .55 | |||
| 31. | My child would never throw an object at another child. | .54 | .67 | ||
| 32. | My child avoids rough-and-tumble play.b | .58 | .37 | ||
| 33. | My child is sometimes mean to animals.ab | ||||
| 34. | My child splashes hard in the bath and plays actively.b | .41 | .36 | ||
| 35. | My child hits me (and/or my partner/spouse). | .61 | .53 | ||
| 36. | My child rarely fights with other children. | .53 | .72 | ||
| 37. | My child is likely to be disciplined at school for physical misbehaviour (e.g. fighting).a | .57 | |||
| 38. | My child would never bite (has not bitten) other children.ab | .50 | |||
| 39. | When my child moves about in the house, s/he runs rather than walks. | .48 | .72 | ||
| 40. | My child is likely to be the victim of bullying.ab | ||||
| 41. | My child yells at me (and/or my partner/spouse). | .45 | .60 | ||
| 42. | My child likes aggressive video games.b | .43 | |||
| 43. | My child starts arguments with other children. | .69 | .68 | ||
| 44. | My child dislikes violent television programs.ab | ||||
| 45. | My child gets teased by family members (e.g. siblings, cousins).ab | ||||
| 46. | My child gets aggressive when s/he is angry (e.g. gets told “no”). | .67 | .68 | ||
| 47. | My child would only fight if provoked (i.e., does not start fights).b | .38 | |||
| 48. | My child avoids physical confrontation. | .64 | .74 | ||
| 49. | My child is known to others to be aggressive. | .73 | .71 | ||
| 50. | My child rarely yells at other children.a | .60 | |||
| Cronbach’s α | .93 | .82 | .95 | .84 | |
Note. N = 95 for the amniotic testosterone study; N = 169 for the CAH study. To improve readability, factor loadings < .32 are not displayed.
Item excluded from the amniotic testosterone study analyses due to low loading, cross loading or to maximize Cronbach’s α.
Item excluded from the CAH study analyses due to low loading, cross loading or to maximize Cronbach’s α.
Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy for the amniotic testosterone study = .81 and for the CAH study = .90. Bartlett’s test of sphericity for the amniotic testosterone study: χ2 (630) = 1744.27, p < .001; and for the CAH study: χ2 (741) = 3558.06, p < .001, indicating that, for both samples, correlations between items were sufficiently large for EFA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: The authors declare that there are no conflicts of interest.
References
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and behavior. 2009;55(5):570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Ahluwalia J, Thomson L, Taylor K, Hackett G, O’Donnell KJ, Baron-Cohen S. Prenatal versus postnatal sex steroid hormone effects on autistic traits in children at 18 to 24 months of age. Molecular Autism. 2012;3(1):1–5. doi: 10.1186/2040-2392-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G. Fetal testosterone and autistic traits. British Journal of Psychology. 2009;100(1):1–22. doi: 10.1348/000712608X311731. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, Hines M. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychological Science. 2009;20(2):144–148. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Chapman E, Knickmeyer R, Taylor K, Hackett G. Foetal testosterone and the child systemizing quotient. European Journal of Endocrinology. 2006;155(suppl 1):S123–S130. [Google Scholar]
- Auyeung B, Knickmeyer R, Ashwin E, Taylor K, Hackett G, Baron-Cohen S. Effects of Fetal Testosterone on Visuospatial Ability. Archives of Sexual Behavior. 2012;41(3):571–581. doi: 10.1007/s10508-011-9864-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Auyeung B, Norgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, Lombardo MV. Elevated fetal steroidogenic activity in autism. Molecular Psychiatry. 2014:1–8. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Lutchmaya S, Knickmeyer R. Prenatal testosterone in mind: Amniotic fluid studies. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- Beatty WW. Gonadal hormones and sex differences in nonreproductive behaviors in rodents: Organizational and activational influences. Hormones and behavior. 1979;12(2):112–163. doi: 10.1016/0018-506x(79)90017-5. doi: http://dx.doi.org/10.1016/0018-506X(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Hines M. Early androgens are related to childhood sex-typed toy preferences. Psychological Science. 1992;3(3):203–206. [Google Scholar]
- Berenbaum SA, Resnick SM. Early androgen effects on aggression in children and adults with congenital adrenal hyperplasia. Psychoneuroendocrinology. 1997;22(7):505–515. doi: 10.1016/s0306-4530(97)00049-8. [DOI] [PubMed] [Google Scholar]
- Bergman K, Glover V, Sarkar P, Abbott DH, O’Connor TG. In utero cortisol and testosterone exposure and fear reactivity in infancy. Hormones and behavior. 2010;57(3):306–312. doi: 10.1016/j.yhbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace RA. Physiology of amniotic fluid volume regulation. Clin Obstet Gynecol. 1997;40(2):280–289. doi: 10.1097/00003081-199706000-00005. [DOI] [PubMed] [Google Scholar]
- Braunstein GD. Endocrine changes in pregnancy. In: Larson PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. Tenth. Philadelphia, USA: Saunders; 2003. pp. 795–810. [Google Scholar]
- Broida J, Svare B. Sex differences in the activity of mice: Modulation by postnatal gonadal hormones. Hormones and behavior. 1984;18(1):65–78. doi: 10.1016/0018-506x(84)90051-5. doi: http://dx.doi.org/10.1016/0018-506X(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Browne WV, Hindmarsh PC, Pasterski V, Hughes IA, Acerini CL, Spencer D, Hines M. Working memory performance is reduced in children with congenital adrenal hyperplasia. Hormones and behavior. 2015;67:83–88. doi: 10.1016/j.yhbeh.2014.11.014. doi: http://dx.doi.org/10.1016/j.yhbeh.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman BJ, Anderson CA. Is it time to pull the plug on the hostile versus instrumental aggression dichotomy? Psychological Review. 2001;108(1):273–279. doi: 10.1037/0033-295x.108.1.273. [DOI] [PubMed] [Google Scholar]
- Card NA, Stucky BD, Sawalani GM, Little TD. Direct and Indirect Aggression During Childhood and Adolescence: A Meta-Analytic Review of Gender Differences, Intercorrelations, and Relations to Maladjustment. Child Development. 2008;79(5):1185–1229. doi: 10.1111/j.1467-8624.2008.01184.x. [DOI] [PubMed] [Google Scholar]
- Chapman E, Baron-Cohen S, Auyeung B, Knickmeyer R, Taylor K, Hackett G. Fetal testosterone and empathy: evidence from the empathy quotient (EQ) and the “reading the mind in the eyes” test. Social Neuroscience. 2006;1(2):135–148. doi: 10.1080/17470910600992239. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CCC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neuroscience & Biobehavioral Reviews. 2005;29(2):353–384. doi: 10.1016/j.neubiorev.2004.11.004. doi: http://dx.doi.org/10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cohen-Kettenis PT. Psychosocial and psychosexual aspects of disorders of sex development. Best Practice & Research Clinical Endocrinology & Metabolism. 2010;24(2):325–334. doi: 10.1016/j.beem.2009.11.005. doi: http://dx.doi.org/10.1016/j.beem.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- Constantinescu M, Hines M. Relating Prenatal Testosterone Exposure to Postnatal Behavior in Typically Developing Children: Methods and Findings. Child Development Perspectives. 2012;6(4):407–413. [Google Scholar]
- Costello AB, Osborne JW. Best practices in Exploratory Factor Analysis: Four recommendations for getting the most from your analysis. Practical Assessment, Research & Evaluation. 2005;10(7) [Google Scholar]
- Eaton WO, Enns LR. Sex differences in human motor activity level. Psychological Bulletin. 1986;100(1):19–28. [PubMed] [Google Scholar]
- Ehrhardt AA, Baker SW. Fetal androgens, human central nervous system differentiation, and behavior sex differences. In: Friedman RC, Richart RM, van de Wiele RL, Stern LO, editors. Sex differences in behavior. Oxford, England: John Wiley & Sons; 1974. p. xvi.p. 495. [Google Scholar]
- Ehrhardt AA, Epstein R, Money J. Fetal androgens and female gender identity in the early-treated adrenogenital syndrome. Johns Hopkins Medical Journal. 1968;122:165–167. [PubMed] [Google Scholar]
- Fisher DA. Endocrinology of fetal development. In: Larson PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. Tenth. Philadelphia, USA: Saunders; 2003. pp. 811–841. [Google Scholar]
- Frisén L, Nordenström A, Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. Journal of Clinical Endocrinology & Metabolism. 2009;94(9):3432–3439. doi: 10.1210/jc.2009-0636. [DOI] [PubMed] [Google Scholar]
- Grimshaw GM, Bryden MP, Finegan JAK. Relations between prenatal testosterone and cerebral lateralization in children. Neuropsychology. 1995;9(1):68–79. [Google Scholar]
- Grimshaw GM, Sitarenios G, Finegan JAK. Mental rotation at 7 years: Relations with prenatal testosterone levels and spatial play experiences. Brain and Cognition. 1995;29(1):85–100. doi: 10.1006/brcg.1995.1269. [DOI] [PubMed] [Google Scholar]
- Hines M. Brain Gender. New York: Oxford University Press; 2004. [Google Scholar]
- Hines M. Gender development and the human brain. Annual Review of Neuroscience. 2011a;34:69–88. doi: 10.1146/annurev-neuro-061010-113654. [DOI] [PubMed] [Google Scholar]
- Hines M. Prenatal endocrine influences on sexual orientation and on sexually differentiated childhood behavior. Frontiers in Neuroendocrinology. 2011b;32(2):170–182. doi: 10.1016/j.yfrne.2011.02.006. doi: http://dx.doi.org/10.1016/j.yfrne.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. Gendered Development. In: Lamb ME, Lerner RM, editors. Handbook of Child Psychology and Developmental Science. 7th. Vol. 3. Hoboken, NJ: John Wiley & Sons, Inc; 2015. pp. 1–46. [Google Scholar]
- Hines M, Brook C, Conway GS. Androgen and psychosexual development: Core gender identity, sexual orientation, and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH) Journal of sex research. 2004;41(1):75–81. doi: 10.1080/00224490409552215. [DOI] [PubMed] [Google Scholar]
- Hines M, Kaufman FR. Androgen and the development of human sex-typical behavior: rough-and-tumble play and sex of preferred playmates in children with congenital adrenal hyperplasia (CAH) Child Development. 1994;65(4):1042–1053. [PubMed] [Google Scholar]
- Hines M, Pasterski V, Spencer D, Neufeld S, Patalay P, Hindmarsh PC, Acerini CL. Prenatal androgen exposure alters girls’ responses to information indicating gender-appropriate behaviour. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2016;371(1688) doi: 10.1098/rstb.2015.0125. Retrieved from http://rstb.royalsocietypublishing.org/content/371/1688/20150125.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS. How large are gender differences in aggression? A developmental meta-analysis. Developmental Psychology. 1984;20(4):722–736. [Google Scholar]
- Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. Journal of Child Psychology and Psychiatry. 2005;46(2):198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R, Wheelwright S, Taylor K, Raggatt P, Hackett G, Baron-Cohen S. Gender-Typed Play and Amniotic Testosterone. Developmental Psychology. 2005;41(3):517–528. doi: 10.1037/0012-1649.41.3.517. [DOI] [PubMed] [Google Scholar]
- Kung KTF, Spencer D, Pasterski V, Neufeld S, Glover V, O’Connor TG, Hines M. No Relationship Between Prenatal Androgen Exposure and Autistic Traits: Convergent Evidence From Studies of Children With Congenital Adrenal Hyperplasia and of Amniotic Testosterone Concentrations in Typically-Developing Children. Journal of Child Psychology and Psychiatry. 2016 doi: 10.1111/jcpp.12602. doi: http://dx.doi.org/10.17863/CAM.344. [DOI] [PMC free article] [PubMed]
- Leaper C. Gender development during childhood. In: Zelazo PD, editor. The Oxford Handbook of Developmental Psychology. Vol. 2. 2013. pp. 326–377. Self and Other. [Google Scholar]
- Lightfoot JT. Sex Hormones’ Regulation of Rodent Physical Activity: A Review. International Journal of Biological Sciences. 2008;4(3):126–132. doi: 10.7150/ijbs.4.126. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2359866/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: SAGE Publications, Inc; 2000. [Google Scholar]
- Mathews GA, Fane BA, Conway GS, Brook CGD, Hines M. Personality and Congenital Adrenal Hyperplasia: Possible Effects of Prenatal Androgen Exposure. Hormones and behavior. 2009;55(2):285–291. doi: 10.1016/j.yhbeh.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, DeVries GJ, Forger NG. Sexual differentiation of the brain: Mode, mechanisms, and meaning. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, CA: Academic Press; 2009. pp. 1707–1744. [Google Scholar]
- Meyer-Bahlburg HFL, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI. Prenatal androgenization affects gender-related behavior but not gender identity in 5–12-year-old girls with congenital adrenal hyperplasia. Archives of Sexual Behavior. 2004;33(2):97–104. doi: 10.1023/b:aseb.0000014324.25718.51. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, Dolezal C, Baker SW, New MI. Sexual Orientation in Women with Classical or Non-classical Congenital Adrenal Hyperplasia as a Function of Degree of Prenatal Androgen Excess. Archives of Sexual Behavior. 2008;37(1):85–99. doi: 10.1007/s10508-007-9265-1. [DOI] [PubMed] [Google Scholar]
- New MI. Diagnosis and management of congenital adrenal hyperplasia. Annual Review of Medicine. 1998;49(1):311–328. doi: 10.1146/annurev.med.49.1.311. [DOI] [PubMed] [Google Scholar]
- Pasterski V, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and childhood sex segregation: Playmate and play style preferences in girls with congenital adrenal hyperplasia. Hormones and behavior. 2011;59(4):549–555. doi: 10.1016/j.yhbeh.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterski V, Hindmarsh P, Geffner ME, Brook C, Brain C, Hines M. Increased aggression and activity level in 3- to 11-year-old girls with congenital adrenal hyperplasia (CAH) Hormones and behavior. 2007;52:368–374. doi: 10.1016/j.yhbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterski V, Zucker KJ, Hindmarsh PC, Hughes IA, Acerini C, Spencer D, Hines M. Increased Cross-Gender Identification Independent of Gender Role Behavior in Girls with Congenital Adrenal Hyperplasia: Results from a Standardized Assessment of 4- to 11-Year-Old Children. Archives of Sexual Behavior. 2015:1–13. doi: 10.1007/s10508-014-0385-0. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Judd HL, Young PE, Jones OW, Yen SSC. Amniotic Fluid Androgens and Estrogens in Midgestation. The Journal of Clinical Endocrinology & Metabolism. 1977;45(4):755–761. doi: 10.1210/jcem-45-4-755. [DOI] [PubMed] [Google Scholar]
- Rodeck CH, Gill D, Rosenberg DA, Collins WP. Testosterone levels in midtrimester maternal and fetal plasma and amniotic fluid. Prenat Diagn. 1985;5(3) doi: 10.1002/pd.1970050303. [DOI] [PubMed] [Google Scholar]
- Smail PJ, Reyes FI, Winter JSD, Faiman C. The fetal hormonal environment and its effect on the morphogenesis of the genital system. In: Kogan SJ, Hafez ESE, editors. Pediatric andrology. The Hague: Martinus Nijhoff; 1981. pp. 9–19. [Google Scholar]
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, White PC. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology & Metabolism. 2010;95(9):4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PM. The adrenal cortex. In: Larson PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. Tenth. Philadelphia, USA: Saunders; 2003. pp. 491–551. [Google Scholar]
- van de Beek C, van Goozen SHM, Buitelaar JK, Cohen-Kettenis PT. Prenatal sex hormones (maternal and amniotic fluid) and gender-related play behavior in 13-month-old infants. Archives of Sexual Behavior. 2009;38(1):6–15. doi: 10.1007/s10508-007-9291-z. [DOI] [PubMed] [Google Scholar]
- vom Saal FS. Sexual Differentiation in Litter-Bearing Mammals: Influence of Sex of Adjacent Fetuses in Utero. Journal of Animal Science. 1989;67(7):1824–1840. doi: 10.2134/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Primary and Preschool Scale of Intelligence – Third edition (WPPSI-III) San Antonio, TX: Harcourt Assessment; 1967/2002. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children – 4th Edition (WISC-IV) San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- White PC, Speiser PW. Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency. Endocrine Reviews. 2000;21(3):245–291. doi: 10.1210/er.21.3.245. [DOI] [PubMed] [Google Scholar]
- Zucker KJ, Bradley SJ. Gender Identity Disorder and Psychosexual Problems in Children and Adolescents. London: Guilford Press; 1995. [DOI] [PubMed] [Google Scholar]