Abstract
With one of the highest mitochondrial densities in the body, the kidneys consume approximately 10% of total oxygen while constituting only 0.5% of body mass. Renal respiration is linearly correlated to solute extraction, linking mitochondrial oxidative metabolism directly to tubular function. This fundamental role of mitochondria in renal health may become an “Achilles heel” under duress. Acute kidney injury (AKI) related to each major class of stressor—inflammation, ischemia, and toxins—exhibits early and prominent injury to mitochondria. The classic mitochondrial biogenesis regulator, PGC1α (PPARγ-coactivator-1α) may confer protection to the tubules against these stressors. Recent work proposes that renal PGC1α directly increases levels of nicotinamide adenine dinucleotide (NAD+), an essential co-factor for energy metabolism that has recently also been proposed as an anti-aging factor. This mini-article presents a short summary of research on the topics of AKI, PGC1α, and NAD+ to focus on recent studies that propose a direct mechanism between the regulation of metabolic health and the ability to resist renal stressors.
Keywords: mitochondria, kidney, niacin, nicotinamide, AKI, PGC1α, NAD+, metabolism
Introduction
Renal purification of blood in higher organisms is powered by two tissues—the cardiac muscle provides the mechanical hydraulic force for passive filtration at the glomerulus whereas the renal tubule generates the electrochemical force for active and selective reabsorption of solutes across the nephron that is accompanied by water reclamation. In fact, oxygen consumption by the kidney is directly proportional to the degree of solute extraction, an observation that was reported over 80 years ago [1]. This latter force is generated primarily by abundant mitochondria found in the epithelium of the proximal tubule and thick ascending limb. Genetic and acquired disorders of the mitochondrion conclusively demonstrate that injury to this organelle is sufficient to impair tubular, and indeed, global renal function [2]. But is this organelle, and energy metabolism more broadly, important in the development of acute kidney injury (AKI)? And if so, can this process be measured and even targeted therapeutically?
In humans, electron microscopy studies in the 1970s from patients who died of shock with AKI showed evidence of ultrastructural mitochondrial injury in the tubular epithelium [3,4]. Even transient renal ischemia that is insufficient to provoke overt AKI still induces mitochondrial damage, suggesting that mitochondrial changes precede clinical impairments [5,6]. And experimental studies from laboratories around the world have linked injury-induced defects in oxidative metabolism to the pathogenesis of toxic, ischemic, and septic AKI (e.g., [7–21]).
PGC1α and Evidence of Extra-Renal Stress Resistance
Mitochondria exhibit a “life cycle” within cells that operates independently of the cell cycle and resembles the dynamics of unicellular organisms. Mitochondria “proliferate” by fission of existing organelles, and smaller organelles fuse together to grow and form intricate networks within a cell. Injurious stimuli trigger a pattern of fragmentation in this otherwise fused organellar network that resembles excessive fission. Injured mitochondria can undergo a permeability transition that allows water to enter the organelle and mediators of regulated cell death, such as calcium and caspases, to exit into the cytoplasm. Injured mitochondria can also be safely cleared by the cell by a disposal process called mitophagy. Finally, new mitochondrial mass can be generated by the transcription of genes encoding structural and enzymatic parts of the organelle. Termed mitochondrial biogenesis, this last part of the mitochondrial life cycle is regulated by a transcriptional co-activator called PGC1α (PPARγ-coactivator-1α) [22,23]. PGC1α binds to an array of transcription factors in the cell’s nucleus, driving the transcription of hundreds of genes that, collectively, increase the abundance of mitochondria within cells [24].
PGC1α is highly expressed in all metabolically active organs including brown fat, the heart, brain, skeletal muscle, liver, and kidney. Within the kidney, PGC1α expression is largely restricted to the cell types with abundant mitochondria that are found in the tubule [25]. By comparison, vascular and podocyte expression of PGC1α is very low. Germline deletion of PGC1α is not lethal, and animals have slightly reduced baseline mitochondrial abundance. But PGC1α appears to be important for the defense against stressors in these organs. Knockout mice develop an age-dependent neurodegenerative phenotype, suffer worse heart failure under pressure overload, and are less adept at tolerating nutrient stress [26–28].
PGC1α and the Kidney—Evidence of NAD+ as a Downstream Effector
During experimental and human AKI, renal PGC1α expression falls [17,25,29]. This change in expression does not appear to drive the mitochondrial pathology of AKI per se, but does appear to impair the cell’s ability to respond to mitochondrial injury. Global or tubule-specific knockout mice are more susceptible to models of septic and ischemic AKI [25,29]. On the other hand, artificial induction of PGC1α in the tubule (via a conditional nephron-specific transgenic mouse) protects against either stressor. Excess PGC1α restores the energy metabolism and ATP generation that is otherwise impaired by cytokines or oxidant stress [19,25]. Excess PGC1α in the tubule lowers the severity of AKI and accelerates functional resolution [29]. Inducing this one gene in this one renal cellular compartment is even sufficient to enhance overall survival following transient global renal ischemia [29].
Intriguingly, a similar pattern of results has been reported in models of tubulointerstitial fibrosis and diabetic kidney disease—PGC1α is suppressed by the injury event, and enhanced tubular PGC1α expression benefits renal function [30–33]. Following folate exposure, Kang et al., showed that fats, the preferred fuel of the proximal tubule, accumulates to perhaps noxious levels [30]. Fats have also been known to accumulate in tubular cells following AKI of diverse etiologies [10]. Since fats are oxidized in mitochondria—and to a lesser extent, peroxisomes—these results implicate defective mitochondrial energy metabolism resulting in less ATP, and in turn, impaired function in the tubule’s main task of solute and water reabsorption.
How does PGC1α impact metabolism in the kidney? This is a large question whose exploration has just begun. Tran, et al., applied the unbiased strategies of RNA sequencing and metabolomics to post-ischemic kidneys, PGC1α knockout kidneys, and PGC1α transgenic kidneys [29]. These studies led them to consider nicotinamide (Nam), a metabolite that is produced by the kidney and is the chief precursor for NAD+ (nicotinamide adenine dinucleotide), the universal electron carrier that is required for oxidation of glucose and fats [34,35]. Indeed, NAD+ levels have been shown to be rate-limiting for oxidative metabolism [36]. Renal Nam and NAD+ levels are strongly correlated to each other. Both fall during AKI, with NAD+ declining to a similar extent as ATP itself. Both are reduced at baseline in PGC1α knockout kidneys whereas both are elevated at baseline in transgenic kidneys. PGC1α coordinately regulates an eight-step enzymatic pathway for the de novo biosynthesis of NAD+. NAD+ reduction in AKI may reflect both an impairment of biosynthesis and excessive degradation by enzymes known to promote AKI [37].
Exogenous Nam boosts renal NAD+; normalizes the heightened post-ischemic response of PGC1α knockout mice; prevents toxic AKI induced by cisplatin; and rescues early post-ischemic AKI. These striking results propose that AKI constitutes a state of acute NAD+ deficiency that can be therapeutically targeted by methods to replete NAD+ [29]. Independent results with the immediate downstream intermediate between Nam and NAD+, nicotinamide mononucleotide (NMN), further affirm a potential therapeutic avenue for NAD+ augmentation in multiple etiologies of AKI [38]. There may be several effectors of renal tubular defense downstream of NAD+. Induction of fatty acid metabolism may resolve incipient lipotoxicity and promote the accumulation of beta-hydroxybutyrate, a ketone body that signals the production of the vasodilatory prostaglandin PGE2 [29,30]. NAD+ serves as a cofactor for deacetylase enzymes known as sirtuins that have been linked to renoprotection [38–40]. Maneuvers to increase NAD+ can boost mitochondrial function and enhance mitophagy [41]. While these pathways will require further study, the emerging body of work suggests that PGC1α-induced defense of NAD+ levels may be critical for the kidney to resist different classes of stressors.
Pursuing Clinical Translation
With the recognition of AKI as a rising global public health concern, the International Society of Nephrology set the ambitious goal of eliminating preventable deaths from renal failure through timely diagnosis and treatment of AKI [42]. However, the clinical translation of animal data into clinical trials faces significant challenges. Timely etiology-specific diagnosis and targeted treatment of AKI have remained elusive goals. A rise in serum creatinine, the standard clinical assay to diagnose kidney injury, lags the original insult behind by days and fails to distinguish broad categories of renal insult. Studies of myocardial infarction and stroke emphasize the importance of rapid recognition for optimization of clinical outcomes [43,44]. Related to this, Current state-of-the-art AKI biomarkers closely reflect severity of tissue injury, but do not necessarily provide dynamic surrogate measures of efficacy—for example, circulating LDL cholesterol levels report both a risk factor for cardiovascular disease and surrogate efficacy marker that can be measured easily and repeatedly to track the effectiveness of interventions to improve cardiovascular health.
If the PGC1α-NAD+ axis is indeed important for AKI pathogenesis across multiple etiologies, translational efforts could proceed in several directions. For the development of “diagnostic tools”—broadly referring to methods that would stratify risk, predict outcomes, and/or reflect therapeutic responses—a non-invasive measure would be desirable since pilot human results from renal biopsies suggest suppression of this axis in AKI and CKD [29,32]. In an early interventional study of NAD+ boosting compounds, leukocyte NAD+ levels were compared among recipients of three orally administered moieties [45]. While suitable for research studies, such a measure may not be sufficiently rapid in the context of early AKI to be actionable. Therefore, new ways to assess the PGC1α-NAD+ axis by simple blood or urine tests could be beneficial. Related to this, risk stratification for AKI currently accounts for factors such as age and CKD. Intriguingly, both aging and CKD have been related to reductions in PGC1α and NAD+, suggesting that new diagnostic tools could unveil underlying biological factors connecting these clinical contexts [46]. This kind of “metabolic risk stratification” could also help individualize management decisions by summating the underlying biological drivers of reduced stress resistance.
In terms of therapeutic development, the work from Tran et al., demonstrates that ischemic, septic, and toxic AKI share an modifiable reduction in renal PGC1α, mitochondrial health, and NAD+. Whereas upstream targeting of PGC1α has been challenging, several compounds exist to boost NAD+, including Nam itself and other NAD+ precursors commercially available as nutraceuticals. Compelling arguments could be made to pursue PGC1α or NAD+ augmentation in each of the related clinical scenarios, although each comes with challenges. For example, trials in cardiac surgery have been deployed to study candidate AKI interventions with the idea of capitalizing on a timed insult that enables both within-subject comparisons over time and preventative study designs. Yet, the event rate following cardiac surgery can be low, thus necessitating either a large sample size or specific enrollment criteria to enrich the AKI rate. In septic AKI, clinical heterogeneity is extremely wide, which increases the difficulty of equalizing baseline characteristics between placebo and drug arms even with randomization. Moreover, end-organ dysfunction in sepsis typically involves several organs, thus potentially transforming septic AKI trials into sepsis trials. Finally, toxic etiologies of AKI are not uncommon, but depending on the toxin, the evolution of AKI can be slower or stuttering. Implicating a single etiological factor can become challenging, particularly in cancer patients who are often receiving multiple toxic drugs simultaneously.
Conclusions
Since the discovery of the Krebs cycle [47], and over decades of biochemical experiments, mitochondria have been recognized as a major source of energy production for the highly active renal tubule. Recent studies have focused attention on impaired mitochondrial health as a contributor to AKI and CKD. Mitochondrial biogenesis induced by PGC1α may ameliorate a spectrum of pathological conditions. Downstream of PGC1α, NAD+ in the renal tubule may mediate the defense against acute and chronic stressors. As the metabolic underpinnings of renal health and disease become clearer, new opportunities come into focus for developing methods to stratify risk, individualize management, and treat patients in a targeted way. PGC1α and NAD+ may lie at the nexus of aging, CKD, and AKI.
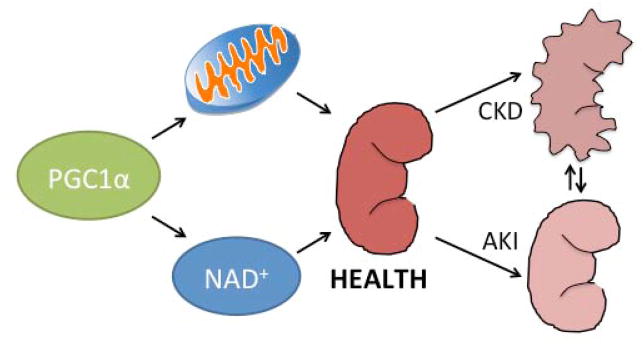
Figure 1. PGC1α and renal health.
PGC1α co-activates the transcription of hundreds of genes in the nucleus. Shortly after its discovery, PGC1α was characterized as a mitochondrial biogenesis regulator. Recent work suggests that PGC1α also induces the biosynthesis of the universal electron carrier, NAD+. PGC1α and these downstream effector pathways may inform the interplay of renal health with acute kidney injury (AKI) and chronic kidney disease (CKD).
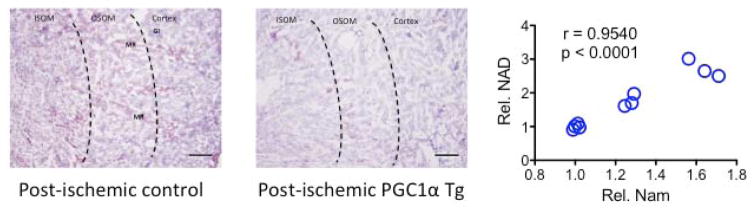
Figure 2. Tubular PGC1α and renal NAD+.
(left) Oil red O staining of control or tubular PGC1α transgenic kidney 24 hrs after transient bilateral renal ischemia reveals an accumulation of fat (reddish-purple) in tubular cells of the cortex, outer stripe of outer medulla (OSOM), medulla, and medullary rays (MR). This is attenuated in the transgenic mouse, which also resists functional injury more effectively following reperfusion. (right) Renal levels of nicotinamide (Nam) and NAD+ were assessed by mass spectrometry and enzyme activity assay, respectively. Nam is the chief precursor of NAD+ and its main breakdown product. Levels of Nam and NAD+ are highly correlated within the kidney.
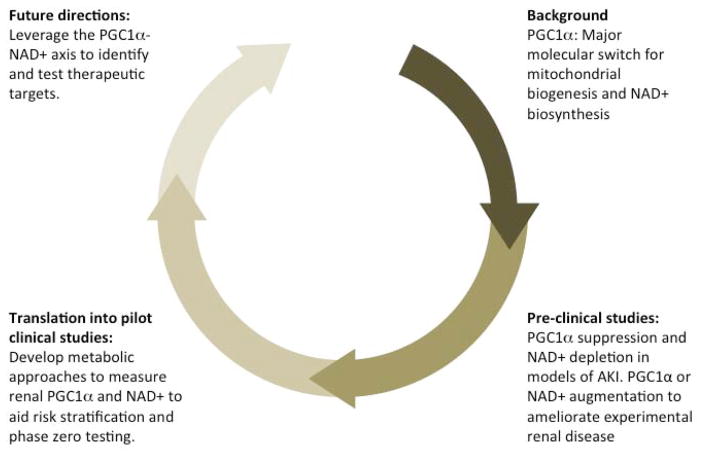
Figure 3. Moving toward clinical translation.
(clockwise from top right) i. Studies in cell culture models identified PGC1α as a mitochondrial biogenesis regulator. ii. Gain- and loss-of function genetic mouse models have facilitated the study of PGC1α in experimental AKI and CKD, proposing this protein as an inducer of resistance against acute and chronic stressors. Unbiased “-omics” strategies in these “genetics plus insult” contexts have implicated NAD+ biosynthesis as a downstream effector of PGC1α action. Strategies to boost NAD+ confer protection against experimental AKI arising after ischemia or from toxins. iii. Rapid and non-invasive surrogate markers of renal PGC1α-NAD+ status could catalyze the drug development process at multiple steps including risk stratification and early “phase zero” testing to assess whether candidate therapies are impacting renal PGC1α and/or NAD+ in humans. iv. Future interventional trials should capitalize on pre-clinical knowledge to optimize study design and implement surrogate markers.
Acknowledgments
For space considerations, the authors were unable to cite and discuss many important contributions to this topic. The authors would like to thank Drs. Zsuzsanna Zsengeller and Isaac Stillman at Beth Israel Deaconess Medical Center for histopathological images provided in this manuscript. Studies in SMP’s laboratory related to this topic are funded by NIH grant DK-R01-095072. APM is funded by the CAO’s Innovation grant at Beth Israel Deaconess Medical Center.
Footnotes
Disclosures: SMP is listed as an inventor on filings related to PGC1α and NAD+ submitted by Beth Israel Deaconess Medical Center. SMP has consulted for Merck on areas related to the present topic. Otherwise, there are no conflicts to disclose.
References
- 1.Van Slyke DD, Rhoads CP, Hiller A, Alving AS. Relationships between urea extraction, renal blood flow, renal oxygen consumption, and diuresis. The mechanism of urea excretion. Am J Physiol. 1934;109:336–374. [Google Scholar]
- 2.Emma F, Montini G, Parikh SM, Salviati L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol. 2016;12:267–280. doi: 10.1038/nrneph.2015.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tisher CC, Bulger RE, Trump BF. Human renal ultrastructure. I. Proximal tubule of healthy individuals. Laboratory investigation; a journal of technical methods and pathology. 1966;15:1357–1394. [PubMed] [Google Scholar]
- 4.Trump BF, Valigorsky JM, Jones RT, Mergner WJ, Garcia JH, Cowley RA. The application of electron microscopy and cellular biochemistry to the autopsy. Observations on cellular changes in human shock. Human pathology. 1975;6:499–516. doi: 10.1016/s0046-8177(75)80068-2. [DOI] [PubMed] [Google Scholar]
- 5.Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, Devarajan P, Venkatachalam MA. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24:506–517. doi: 10.1681/ASN.2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh SM, Yang Y, He L, Tang C, Zhan M, Dong Z. Mitochondrial function and disturbances in the septic kidney. Seminars in nephrology. 2015;35:108–119. doi: 10.1016/j.semnephrol.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberg JM, Davis JA, Venkatachalam MA. Cytosolic-free calcium increases to greater than 100 micromolar in ATP-depleted proximal tubules. J Clin Invest. 1997;100:713–722. doi: 10.1172/JCI119584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg JM, Venkatachalam MA. Preserving postischemic reperfusion in the kidney: a role for extracellular adenosine. J Clin Invest. 2012;122:493–496. doi: 10.1172/JCI60957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zager RA, Johnson AC, Hanson SY. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 2005;67:111–121. doi: 10.1111/j.1523-1755.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 11.Brooks C, Cho SG, Wang CY, Yang T, Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol. 2011;300:C447–455. doi: 10.1152/ajpcell.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Basnakian A, Bhatt R, Megyesi J, Gokden N, Shah SV, Portilla D. PPAR-alpha ligand ameliorates acute renal failure by reducing cisplatin-induced increased expression of renal endonuclease G. Am J Physiol Renal Physiol. 2004;287:F990–998. doi: 10.1152/ajprenal.00206.2004. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Gokden N, Okusa MD, Bhatt R, Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol. 2005;289:F469–480. doi: 10.1152/ajprenal.00038.2005. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int. 2009;76:1049–1062. doi: 10.1038/ki.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010;333:593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F853–864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007;355:734–739. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Rasbach KA, Schnellmann RG. Signaling of mitochondrial biogenesis following oxidant injury. J Biol Chem. 2007;282:2355–2362. doi: 10.1074/jbc.M608009200. [DOI] [PubMed] [Google Scholar]
- 20.Szeto HH, Liu S, Soong Y, Seshan SV, Cohen-Gould L, Manichev V, Feldman LC, Gustafsson T. Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1beta and IL-18 and Arrests CKD. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016070761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 24.Weinberg JM. Mitochondrial biogenesis in kidney disease. J Am Soc Nephrol. 2011;22:431–436. doi: 10.1681/ASN.2010060643. [DOI] [PubMed] [Google Scholar]
- 25.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 29.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, Parikh SM. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, Sharma K. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond-Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long J, Badal SS, Ye Z, Wang Y, Ayanga BA, Galvan DL, Green NH, Chang BH, Overbeek PA, Danesh FR. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016;126:4205–4218. doi: 10.1172/JCI87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins PB, Chaykin S. The management of nicotinamide and nicotinic acid in the mouse. J Biol Chem. 1972;247:778–783. [PubMed] [Google Scholar]
- 35.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A, Souza AL, Farrell L, Domos C, Yeh RW, Palacios I, Rosenfield K, Vasan RS, Florez JC, Wang TJ, Fox CS, Gerszten RE. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol. 2013;24:1330–1338. doi: 10.1681/ASN.2012101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebrahimkhani MR, Daneshmand A, Mazumder A, Allocca M, Calvo JA, Abolhassani N, Jhun I, Muthupalani S, Ayata C, Samson LD. Aag-initiated base excision repair promotes ischemia reperfusion injury in liver, brain, and kidney. Proc Natl Acad Sci U S A. 2014;111:E4878–4886. doi: 10.1073/pnas.1413582111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan Y, Wang SR, Huang XZ, Xie QH, Xu YY, Shang D, Hao CM. Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Sueyasu K, Washida N, Tokuyama H, Tzukerman M, Skorecki K, Hayashi K, Itoh H. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem. 2010;285:13045–13056. doi: 10.1074/jbc.M109.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, Novelli R, Remuzzi G, Benigni A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest. 2015;125:715–726. doi: 10.1172/JCI77632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang EF, Kassahun H, Croteau DL, Scheibye-Knudsen M, Marosi K, Lu H, Shamanna RA, Kalyanasundaram S, Bollineni RC, Wilson MA, Iser WB, Wollman BN, Morevati M, Li J, Kerr JS, Lu Q, Waltz TB, Tian J, Sinclair DA, Mattson MP, Nilsen H, Bohr VA. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta RL, Cerda J, Burdmann EA, Tonelli M, Garcia-Garcia G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, Cruz D, Jaber B, Lameire NH, Lombardi R, Lewington A, Feehally J, Finkelstein F, Levin N, Pannu N, Thomas B, Aronoff-Spencer E, Remuzzi G. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 43.Bagai A, Dangas GD, Stone GW, Granger CB. Reperfusion strategies in acute coronary syndromes. Circ Res. 2014;114:1918–1928. doi: 10.1161/CIRCRESAHA.114.302744. [DOI] [PubMed] [Google Scholar]
- 44.Saver JL. Time is brain--quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 45.Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nature communications. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krebs HA, Eggleston LV. The oxidation of pyruvate in pigeon breast muscle. Biochem J. 1940;34:442–459. doi: 10.1042/bj0340442. [DOI] [PMC free article] [PubMed] [Google Scholar]