Abstract
Multiple sclerosis (MS) is a disabling, chronic disease that imposes a significant economic burden on patients and the US healthcare system. The largest cost component for individuals with MS are prescription drugs, specifically disease-modifying therapies (DMTs). Despite an increase in the number and diversity of DMTs over the past 10 years, acquisition costs for all DMTs have escalated dramatically at rates substantially higher than medical inflation. Currently, costs for most DMTs exceed $70,000 a year. Recent cost-effectiveness studies suggest the cost for nearly all DMTs exceeds generally accepted thresholds for what is considered a good value in the USA, even after factoring expected rebates. The high cost of DMTs is symptomatic of systemic dysfunction in the pharmaceutical market. Strategies aimed at reigning in high-cost medications include proposals ranging from increasing pricing transparency to allowing Medicare to negotiate directly with manufacturers. Because the economics of pharmaceuticals are inherently complex, a diversity of approaches will be required.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-017-0566-3) contains supplementary material, which is available to authorized users.
Keywords: Healthcare economics, pharmaceuticals, multiple sclerosis, disease modifying therapy
Introduction
Multiple sclerosis (MS) is a chronic autoimmune condition affecting the central nervous system and associated with significant premature disability [1]. The global prevalence of MS is estimated to be > 2.3 million; > 400,000 individuals in the USA are affected [2]. The total economic burden of MS in the USA has been estimated to be approximately $2.5 billion and lifetime costs for individual patients exceed $4 million [3, 4].
The peak incidence of MS occurs around 30 years of age, making it a leading cause of nontraumatic disability in young to moderately aged individuals [5, 6]. Nearly 30% of individuals with MS in the USA are reliant on public disability insurance (i.e., Social Security Disability Insurance) [7]. MS is a disabling condition that primarily affects younger working-age individuals. Consequently, the indirect costs of lost productivity are substantial. Workers with MS have approximately 4-fold higher disability and absenteeism-related costs than employees without MS [8]. Costs due to lost productivity are over one-third of total costs for MS [9]. However, the single largest driver of MS-related healthcare expenditures are prescription drugs, which account for more than half of all direct medical costs [9]. In particular, the costs for disease-modifying therapies (DMTs) have increased dramatically over the last 10 years [10, 11]. Acquisition costs for nearly all DMTs presently exceed $70,000 annually. The high costs for DMTs has a cascade of negative consequences on patients, ranging from excessive cost-sharing or deductible amounts [12] to restrictive insurance barriers, which can negatively affect care [13]. Additionally, high-cost DMTs place considerable burden on the healthcare system. The objective of this narrative review is to summarize current economic issues related to MS DMTs. Specifically, this review will highlight current pricing and reimbursement trends, describe the economics of the pharmaceutical marketplace, review recent cost-effectiveness studies, and outline potential strategies that respond to these issues.
Drug Pricing
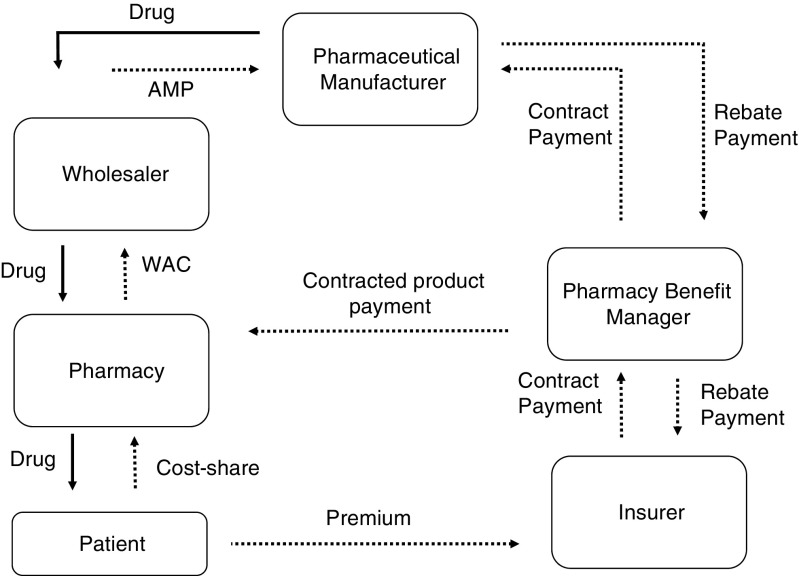
The high cost of medical care for individuals with MS is fueled primarily by spending and price inflation among MS DMTs. The soaring costs of prescription drugs is largely attributable to market dysfunction that is characterized by lack of price transparency, regulatory structures that foster monopolistic conditions, uncertainty in the clinical evidence-base, and payer and supply-chain issues [14]. A comprehensive discussion of trends in pricing cannot be had without considering the broader context of the pharmaceutical market. Pharmaceutical purchasing, which involves multiple stakeholders, is one of the least transparent and convoluted transactions in healthcare [15, 16]. Figure 1 describes the complex flow of money and drug products through pharmaceutical distribution channels. Within this framework, drug products are purchased from manufacturers by distributors who, in turn, sell and deliver product to individual pharmacies. The framework and payment arrangements are somewhat different for pharmaceuticals that are administered in a physician’s office. Currently, 85% of all pharmaceutical sales are consolidated through 3 large disturbers (AmerisourceBergen, Cardinal Health, and McKesson) [16]. The flow of money is more complex. While payments for outpatient pharmaceuticals (nonclinic or hospital administered) are anchored to publically available benchmark list prices, the ultimate cost for drugs is determined by a complex web of proprietary contracts and agreements between payers, distributors, pharmacies, and other intermediaries. The most widely used pricing benchmark for branded drugs is Wholesale Acquisition Cost (WAC), which is set by the manufacturer. WAC is defined by regulators as the list price paid to distributors without any rebates or discounts [15]. From this benchmark, discounts and rebates are applied by a variety of different stakeholders in the drug distribution channel. These relationships can vary dramatically between public [Medicaid, Medicare, Department of Veterans Affairs (VA)] and private payers, with profound consequences. For instance, the VA is commonly cited as a model to emulate because they are able to extract substantial discounts from industry. However, the VA’s ability to negotiate these discounts is predicated on the organization’s vertical integration and strict control of utilization. More typically, drug purchasing is mediated through pharmacy benefit managers, who negotiate discounts (reductions in price) and rebates (funds returned to payer) for most commercial and public payers (Medicare, Medicaid). Medicaid, which is financed jointly by federal and state governments, is statutorily required to receive the lowest price available to most payers (Medicaid Best Price). At a minimum, manufacturers are required to pay a 23.1% rebate off the Average Manufacture Price for branded drugs and 13.1% for generics [15]. This rebate is generally the floor and can be larger if other payers have negotiated larger concessions. Moreover, states can negotiate individual supplemental rebates. Consequently, the Medicaid program acquires considerably better prices than Medicare, which is administered through hundreds of individual Part D plans [17]. Regardless, contracted discounts and rebates are confidential and the true net costs to payers is rarely known.
Fig. 1.
Schematic of the flow of money and drug product in the drug distribution system. Sold lines represent drug product movement. Dashed lines represent payments. Figure adapted from Academy of Managed Care Pharmacy Guide to Pharmaceutical Payment Methods (2013 Update).
AMP = Average Manufacturer Price; WAC = Wholesale Acquisition Cost
DMT Pricing Trends and Spending
Figure 2 summarizes the rapid escalation in annual acquisition costs for DMTs from 1993 to 2017. As previously reported, prices for interferon (IFN) and glatiramer acetate-based platform therapies have accelerated dramatically over the past 15 years [10]. Despite the approval of a variety of novel DMTs, including the first generic—glatiramer acetate (GlatopaTM) in June 2015—there is little evidence that pricing trends have abated. In Table 1, WAC-based annual prices, estimates of net cost to specific payers, and median annual pricing changes are reported. Prior to the approval of subcutaneous IFN-β1a (Rebif™) in 2002, annual price increases for available DMT platform therapies (IFNs or glatiramer acetate) remained < 3% per year. However, between 2002 and the approval of fingolimod, the first oral DMT, in 2010, the annual cost of IFN-β1b (Betaseron™), IFN-β1a (Avonex™), and glatiramer acetate (Copaxone™) increased rapidly from 16% to 18% per year. Annual price inflation for these drugs has only marginally declined following the approval of fingolimod. Median annual price inflation for the 3 oral DMTs (fingolimod, teriflunomide, dimethyl fumerate) has varied between 5% and 18% a year. Also summarized in Table 1 are estimated annual costs for state Medicaid programs and the VA. As previously mentioned, the VA is able to extract large discounts from manufacturers because of their size and ability to purchase drugs directly from manufacturers. Consequently, VA prices for DMTs are often 20% to 50% lower than list price.
Fig. 2.
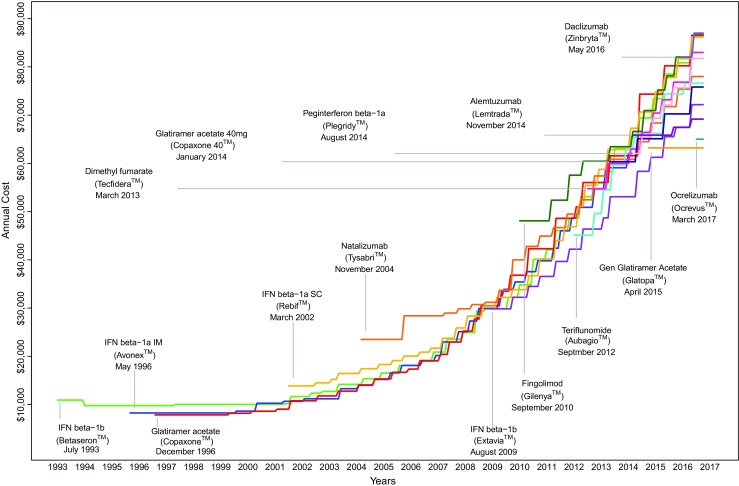
Trends in disease modifying therapy (DMT) pricing. Pricing estimated from WAC for year of therapy. Alemtuzumab estimate is based on average of first- (60 mg) and second-year dose (36 mg)
IFN = interferon; SC = subcutaneous
Table 1.
Disease modifying therapy (DMT) annual costs from market introduction to present, estimated Medicaid costs, and VA costs
| DMT (approval date) | Annual cost at market introduction ($) | Median annual cost increases (%) | 2017 annual cost ($)* | 2017 cost net standard medicaid rebate ($)† | 2017 big-4 pricing ($) | ||
|---|---|---|---|---|---|---|---|
| 1998– 2001 | 2002–09 | 2010– 16 | |||||
| IFN-β1b; Betaseron™ (July 1993) | 10,920 | 0.0 | 15.9 | 14.1 | 86,421 | 66,458 | 38,261 |
| IFN-β1a IM; Avonex™ (May 1996) | 8261 | 2.3 | 17.6 | 16.1 | 81,731 | 62,851 | 46,452 |
| Glatiramer acetate; Copaxone™ 20 mg (December 1996) | 7852 | 2.5 | 18.1 | 16.5 | 86,554 | 66,560 | 48,821 |
| IFN-β1a SC; Rebif™ (March 2002) | 13,875 | NA | 13.7 | 12.5 | 86,179 | 66,272 | 31,409 |
| Natalizumab; Tysabri™ (November 2004‡) | 23,500 | NA | 6.1 | 11.3 | 78,000 | 59,982 | 41,299 |
| IFN-β1b; Extavia™ (August 2009) | 29,842 | NA | NA | 13.4 | 72,160 | 55,491 | 35,006 |
| Fingolimod; Gilenya™ (September 2010) | 48,083 | NA | NA | 9.5 | 86,966 | 66,877 | 48,610 |
| Teriflunomide; Aubagio™ (September 2012) | 45,124 | NA | NA | 18.3 | 76,612 | 58,915 | 48,987 |
| Dimethyl fumerate; Tecfidera™ (March 2013) | 54,750 | NA | NA | 5.0 | 82,977 | 63,809 | 46,745 |
| Glatiramer acetate; Copaxone 40 mg™ (January 2014) | 60,336 | NA | NA | 7.9 | 75,816 | 58,303 | 47,661 |
| Pegylated IFN-β1a; Plegridy™ (August 2014) | 62,036 | NA | NA | 10.6 | 81,731 | 62,851 | 46,591 |
| Alemtuzumab; Lemtrada™* (November 2014) | 65,833 | NA | NA | 1.2 | 69,166 | 53,189 | 60,466 |
| Glatiramer acetate; Glatopa™ (April 2015) | 63,193 | NA | NA | 0 | 63,193 | 48,595 | 32,552 |
| Daclizumab; Zinbryta™* (May 2016) | 82,000 | NA | NA | NA | 86,838 | 66,778 | 61,251 |
| OcrevusTM* (March 2017) | 65,000 | NA | NA | NA | 65,000 | 49,985 | 49,400 |
Wholesale Acquisition Cost (WAC) data accessed through FirstDataBank. Big Four Pricing data accessed through (https://www1.va.gov/nac/Pharma/List). IFN = interferon; SC = subcutaneous; NA = not available
*June 2017
†Acquisition costs estimating using wholesale acquisition costs. Medicaid rebate estimated to be 23.1% of WAC
‡Natalizumab withdrawn from market in February 2005; reintroduced in June 2006
There have been several important developments in the last several years. First, in January 2015, Glatopa, the first generic of glatiramer acetate 20 mg, was approved. In contrast to prevailing trends, pricing of Glatopa has remained unchanged for nearly 3 years. The much anticipated ocrelizumab (Ocrevus™; Roche) was approved in March of 2017 with a WAC of $16,250 per 10 ml or $65,000 per year, significantly lower than most other DMTs. Whether or not the pricing trajectory of ocrelizumab remains significantly below other DMT remains to be seen.
It is important to consider the net costs to payers, because most WAC-based price estimates do not include any potential rebates or discounts. Although this is challenging to estimate, a recent report produced through the Massachusetts Office of the Attorney General finds that rebates have not risen at rates large enough to compensate for price increase overall and within the specialty drug class [18]. Importantly, analysis of specific DMTs revealed that net rebate costs for 10 DMTs increased substantially from approximately $36,000 in 2001 to $60,000 in 2015.
The single largest payer of MS-related costs in the USA is the Medicare program. Table 2 summarizes Medicare Part D expenditures for 12 DMTs from 2011 to 2015. Between 2011 and 2015, expenditures for DMTs more than doubled from $1.5 billion to $4.0 billion. In 2015, Medicare spent over $1.3 billion on glatiramer acetate alone. In comparison, Skolarus et al. [19] reported that Medicare paid neurologists $1.15 billion for clinical services in 2012. Rising expenditures have been driven largely by expansion in the DMT market and price increases. Among IFN and glatiramer-based platform therapies, there was very little change in the number of prescriptions or patients treated but the average cost per patient increased 68% from $28,975 in 2011 to $48,987 in 2015.
Table 2.
Medicare Part D utilization and spending on disease-modifying therapies 2011 to 2015
| 2011 | 2015 | |||||||
|---|---|---|---|---|---|---|---|---|
| Spending ($) | Claims (n) | Patients (n) | Spending/patient ($) | Spending ($) | Claims (n) | Patients (n) | Spending/ patient ($) | |
| IFN-β1b; Betaseron™ | 155,981,338 | 47,873 | 5830 | 26,755 | 241,573,749 | 40,255 | 4859 | 49,717 |
| IFN-β1a IM; Avonex™ | 349,108,226 | 106,302 | 12,314 | 28,351 | 348,107,873 | 61,330 | 7490 | 46,476 |
| Glatiramer acetate; Copaxone™* | 707,777,690 | 188,828 | 22,731 | 31,137 | 1,382,386,515 | 237,976 | 27,621 | 50,048 |
| IFN-β1a SC; Rebif™ | 194,050,544 | 63,470 | 7399 | 26,227 | 378,253,134 | 64,671 | 7752 | 48,794 |
| Natalizumab; Tysabri™ | 7,770,739 | 2204 | 313 | 24,827 | 30,548,895 | 5828 | 775 | 39,418 |
| Interferon-β1b; Extavia™ | 10,462,795 | 4215 | 644 | 16,247 | 24,035,265 | 5324 | 747 | 32,176 |
| Fingolimod; Gilenya™ | 62,818,219 | 14,402 | 2889 | 21,744 | 387,672,781 | 57,470 | 7213 | 53,746 |
| Teriflunomide; Aubagio™ | NA | NA | NA | NA | 292,038,853 | 46,263 | 6910 | 42,263 |
| Dimethyl fumerate; Tecfidera™ | NA | NA | NA | NA | 875,583,352 | 136,426 | 18,798 | 46,579 |
| Pegylated IFN-β1a; Plegridy™ | NA | NA | NA | NA | 8,050,336 | 1310 | 318 | 25,316 |
| Glatiramer acetate; Glatopa™ | NA | NA | NA | NA | 29,181,271 | 5854 | 1802 | 16,194 |
| Alemtuzumab; Lemtrada™ | NA | NA | NA | NA | 4,349,084 | 48 | 41 | 106,075 |
| Total | 1,487,969,550 | 427,294 | 52,120 | 28,549 | 4,001,781,108 | 662,755 | 84,326 | 47,456 |
Source data derived from Medicare Part D drug spending public use files (https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Information-on-Prescription-Drugs/2015MedicareData.html). Spending does not include negotiated rebates or discounts
IFN = interferon; SC = subcutaneous; NA = not available
*20- and 40-mg doses are not differentiated in data
The Effect of High Drug Prices on Patient Care
The ultimate consequence of high drug prices for persons with MS is reduced patient access to DMTs, which can manifest in a number of ways. First, the growing use of high deductible/high-cost-share health plans can expose individuals to excessive out-of-pocket costs [12]. While copay cards and other patient-assistance programs can mitigate exorbitant out-of-pocket expenses for patients, they do nothing to reduce total healthcare payer expenditures [20, 21]. Additionally, individuals who receive government-funded healthcare, such as Medicare, are prohibited from using these programs because of federal antikickback laws [21]. Second, rising DMT expenditures have resulted in increasing levels of restriction by insurance and pharmacy benefit management companies. DMT selection, which should involve shared decision-making that takes into account clinical evidence and patient preferences, is often driven by rigid policies that require “failure” of one or more preferred DMT before using a nonpreferred agent [13].
The impact of higher DMT costs or insurance company restrictions on clinical outcomes is uncertain. Several studies have examined the relationship between out-of-pocket expenses and DMT adherence using administrative medical and pharmacy claims data. Two studies have examined the impact of co-insurance level on DMT adherence in commercially insured individuals with MS [22, 23]. In a study of nearly 3500 patients with MS, those who had the highest cost-sharing level (i.e., DMT out-of-pocket expenditures as a proportion of total expenditures) were significantly less likely to initiate a DMT relative to those with lower cost-sharing levels [22]. A similar study further examined the relationship between out-of-pocket expenditures and DMT adherence by comparing patients with coinsurance to those with copayments [23]. This study found that DMT adherence was only adversely affected among those with coinsurance (i.e., out-of-pocket expense is a proportion of total expense), and not those with copayment-type pharmacy benefits. Specifically, patients with the highest monthly out-of-pocket spending (average of $1162 per month) only filled their DMT 25% of the time, whereas those with the lowest out-of-pocket expenditures were 100% adherent.
Two other studies in Medicare populations show a consistent relationship between cost exposure and adherence [24, 25]. In one analysis of 4180 Medicare beneficiaries with MS, investigators compared DMT adherence and persistence (how long patients continued to take their DMT) between those with full copayments, those with partially subsidized copayments, and those with no copayment [24]. Similar to the commercial populations, copayment amount was significantly related to an erosion in DMT adherence over time. Additionally, patients with the highest copayment levels were significantly more likely to discontinue their therapy after entering the Medicare Part D coverage gap (i.e., the “donut hole”). Although another study that examined discontinuation of intramuscular IFN-β1a and glatiramer acetate after beneficiaries entered the coverage cap found no effect, this study had a small sample and large confidence intervals around its estimates of discontinuation [25].
The evidence of how restrictive drug policies or increasing out-of-pocket expenditures affect clinical outcomes is less clear. Several studies have been conducted to examine the relationship between DMT adherence and clinical and economic outcomes using pharmacy and medical claims data in commercially insured populations.26–29 These studies consistently demonstrate an inverse relationship between adherence, typically measured using the medication possession ratio, MS relapse, emergency department visits or hospitalizations, and medical (nonpharmacy) spending [26–28]. Another study found that patients with MS who switched or discontinued their DMT have significantly higher medical utilization and costs than those who remained on therapy [29], suggesting disruptions in therapy may have serious unintended consequences.
Assessing the Economic Value of MS DMTs
Although DMT costs are central to issues of access and affordability, they also need to be considered in the context of value. Value in healthcare, defined as the efficiency with which interventions deliver outcomes with respect to their costs [30], is typically measured using cost-effectiveness metrics. Available DMTs have been shown to reduce relapses, decrease disability, and improve health-related quality of life to varying degrees. The extent to which DMTs can prolong life, prevent premature disability, and improve individual quality of life impacts the ultimate value of these therapies. Cost-effectiveness analyses facilitate comparisons of different healthcare interventions by estimating costs and benefits over a defined period of time. It is important to note that while most DMT clinical trials are 1 to 2 years in length, MS disability develops over decades. Therefore, the long-term economic and health consequences of interventions are often projected over an extended time horizon using mathematical models.
In a comprehensive cost-effectiveness study, costs should include not only the direct costs of the intervention (e.g., DMT acquisition costs), but also all other associated downstream costs of the therapy and the condition. These can include costs associated with DMT harms or harms-prevention strategies (monitoring for side effects), costs attributed to relapses and hospitalizations, and costs accrued as a result of increased disability (home care, mobility devices). Additionally, experts recommend that indirect costs associated with lost productivity of the individual be factored in. This is particularly notable for individuals with MS, who often must leave the workforce as a result of disability. To facilitate comparisons across different conditions where outcomes are heterogeneous, cost-effectiveness analyses commonly use the quality-adjusted life year (QALY) to capture both the quantity and quality of health expected from different interventions. QALYs are estimated by essentially discounting each additional year of life by a utility weight that approximates an individual’s preference for being in a particular health state. Utilities can range from 0 to 1, where 1 indicates a state of perfect health and 0 is death. For example, an intervention that, on average, prolonged life by 10 years with a utility of 0.65 would represented by 6.5 QALYs.
Following estimation of both costs and benefits, value is expressed using an incremental cost-effectiveness ratio (ICER). The ICER is calculated by dividing the incremental total costs of an intervention by the incremental total benefits. Inherent in the ICER concept is that applying no active treatment (e.g., supportive care) has both costs and health consequences. Therefore, it is important to emphasize that ICERs represent the incremental cost of generating an additional unit of benefit (e.g., cost per additional QALY). Although there is no uniformly agreed upon cost-effectiveness threshold in the USA, interventions with ICERs < $150,000 per QALY are broadly considered to be a good value. This threshold is based on assumptions espoused by economists and some authorities that a ICER threshold 3 times a nation’s per capita income is reasonable, although the empiric basis for this not well established [31].
Numerous cost-effectiveness studies of DMTs have been published over the years with considerable methodologic, analytic, and reporting heterogeneity [32, 33]. Further, many of these studies examine the relative value individual therapies, often failing to capture the real-world intricacies such as imperfect adherence and DMT switching. In a recent systematic review of cost-effectiveness studies of DMTs for MS, US-based studies consistently reported ICERs in excess of $150,000 per QALY [32]. One study, which reported ICERs in excess of $900,000/QALY for the IFNs and glatiramer, presented sensitivity analyses suggesting that a reduction in DMT prices to levels similar to that of the UK would bring ICERs in line with traditionally accepted US thresholds [34]. Choice of comparison is an important consideration in the design and interpretation of cost-effectiveness analyses. A standard active comparator is challenging to recommend uniformly because there is no consensus on the optimal therapeutic approach for MS. In a recent review of 37 economic studies, Hawton et al. [33] noted that nearly three-quarters reported comparisons to supportive care. Because the incremental differences in efficacy between DMTs are not as pronounced relative to supportive care, studies examining the comparative value of DMTs typically result in much higher ICERs. A preferred approach would be to present cost and effectiveness estimates for all comparators, including supportive care, so all possible incremental assessments can be explored.
The Institute for Clinical and Economic Review, a US-based nonprofit organization that conducts clinical and economic health technology appraisals for treatments and diagnostics, recently completed a comprehensive systematic review and cost-effectiveness analysis of all DMTs for both relapsing-remitting MS and primary-progressive MS [35]. Their relapsing-remitting MS economic model simulated patient progression through 20 health states based on the Expanded Disability Status Scale. Disability progression for each DMT relative to supportive care (placebo) was informed by a network meta-analysis showing ocrelizumab, alemtuzmab, daclizumab, and natalizumb [relative risk (RR) 0.42–0.56] were the most effective, followed by dimethtyl fumerate, pegylated IFN-β1a, IFN-β1b, and fingolimod (RR 0.62–0.68), and finally teriflunomide, glatiramer acetate, and the other IFNs (RR 0.72–0.86). Using WAC-based drug pricing net-estimated rebates, ICERs for all but one DMT (excluding ocrelizumab, which was without pricing data at the time of analysis) were well above accepted cost-effectiveness thresholds and ranged from $183,240 for subcutaneous IFN-β1b (Extavia™) to $355,115 for subcutaneous IFN-β1a (Rebif™, 22 μg). At $38,277 per QALY, alemtuzumab was the only DMT to have an ICER < $150,000 per QALY. While the primary analysis used a healthcare system perspective (only direct medical costs), a secondary analysis factoring in lost productivity (i.e., societal perspective) was quantitatively similar. The relatively low ICER for alemtuzumab is likely attributable to its unique dosing strategy (5 infusions in first year and 3 in the second year) and modeling assumptions where a diminishing proportion of patients (< 20%) require an annual dose after year 2.
Relative to generic glatiramer acetate, ICERs were economically unattractive (> $150,000 per QALY) for all DMTs except IFN-β1b 250 μg (Extavia™; $144,873 per QALY) and alemtuzumab, which was more effective and less costly.
The ICER for ocrelizumab was not explicitly estimated because of a lack of pricing data at the time. However, using the $150,000 per QALY threshold, the authors report ocrelizumab would require an annual net price of $58,608 to be considered cost-effective. At its current WAC-based list price ($65,000 annually), rebates of approximately 10% could achieve this net cost. For the remainder of DMTs, discounts from WAC of between 50% and 90% would be required in order to achieve accepted costs per QALY thresholds. For primary-progressive MS, where ocrelizumab is the only Food and Drug Administration-approved DMT, the annual cost required to achieve the $150,000 per QALY thresholds was $14,367.
Potential Solutions
Excessive pricing of prescription drugs has been under intense scrutiny by the public, media, and legislators. Recent public polls report that a large majority of Americans believe prescription drug affordability should be a top healthcare legislative priority [36]. A variety of policy proposals have been put forth intended to address dysfunction in the pharmaceutical market place. Efforts are underway in many states to pass legislation that would require pharmaceutical companies to publically disclose and justify high-priced drugs [37]. Included in most of these proposals are requirements that industry disclose product specific costs for research, manufacturing, and marketing under certain circumstances (e.g., excessive price increases or high initial acquisition costs). Proponents of these efforts argue that enhanced transparency of drug prices would allow decision-makers to counter the prevailing notion that high prices and profits are required to offset the costs and risk involved in bringing drugs to market. Additionally, increased public scrutiny could incentivize manufactures to moderate their pricing strategy. Indeed, faced with mounting public pressure, several manufacturers have made public pledges to keep drug price increases below a certain level [38, 39]. A separate transparency issue concerns the disconnect between list prices and net prices after rebates. As previously discussed, transactions in the pharmaceutical marketplace differ markedly by payer, are notoriously complex, and typically involve proprietary rebate and discount negotiations. Savings extracted from manufacturers through contracted rebates are not directly shared with patients. Cost-sharing by patients with high deductible or co-insurance (vs copayment) types of pharmacy benefit plans is based on list price and not net price, which is often substantially lower [40]. Consequently, patients are often faced with very large out-of-pocket costs. Over the last 5 years, large increases in list prices and growing rebates have driven an increase in the gap between list and net prices [41].
Another popular proposal concerns how Medicare purchases prescription drugs. Medicare is the largest single purchaser or prescription drugs in the USA, yet its establishing legislation prohibits the government from directly negotiating drug costs with manufacturers. Instead, the program relies on imbedded incentives for contracted Part D plans that negotiate individually with manufacturers and compete for beneficiaries. There is overwhelming public support and among many professional organizations, including the American Academy of Neurology, that Medicare should be permitted to use its purchasing power to leverage greater savings for patients and the government, similar to how it sets prices for other services [14, 42–44]. Because Medicare is also constrained in its ability to implement coverage restrictions, the ultimate effect of these potential negotiations on expenditures is not clear [45].
Finally, generic drugs provide significant economic relief in most therapeutic spaces. With the exception of the orally available agents, most DMTs are considered biologics and therefore generic manufacturers cannot bring competitors through traditional channels. In April of 2015, Sandoz received approval to market a generic version of glatiramer acetate (Glatopa™), making it the first generically available DMT [46]. Glatopa was approved through the traditional Abbreviated New Drug Application pathway and therefore is considered clinically substitutable with Copaxone 20 mg (Teva™). While Glatopa is the only product currently available, generic competitors of the 40-mg version of glatiramer acetate should appear in the near future [47]. Novartis will lose patent protection and marketing exclusivity for fingolimod, setting the stage for more generic competition in 2019 [48].
In the absence of systematic changes to the current pharmaceutical market, Kister and Corboy [49] have suggested several clinical approaches aimed at reducing the burden of the high cost of MS DMTs. First, they advocate research that supports alternative DMT dosing schedules. Specifically, pharmacologic mechanism of action and some preliminary clinical data support less frequent administration of fingolimod, glatiramer acetate (20 mg), and natalizumab. Additionally, they note evidence supporting the off-label use of rituximab, which is substantially less expensive than available Food and Drug Administration-approved DMTs. Evidence from randomized trials and observational studies suggest that infusions of 1 to 2 g annually (WAC $16,704 per 1000 mg) are effective [50–52]. Finally, leflunomide, which is available as a lower-cost generic (WAC $150 for 30 × 20-mg tablets), is almost completely converted to teriflunomide in vivo [53], and may be a reasonable option for some individuals, although there are no clinical data to support this practice.
Conclusions
In summary, prescription drugs, and specifically DMTs, are the single largest medical expenditure for individuals with MS. Over the last decade, the acquisition costs for DMTs have risen at annual rates substantially above medical inflation and now exceed $70,000 for many agents. In addition to stress on the healthcare system, the rapid rise in DMT costs also has negative effects on patient care and access to DMTs. Cost-effectiveness analyses suggest most DMTs are excessively priced given established value benchmarks. While the reasons for high and escalating drug costs are multifactorial, many point to fundamental dysfunction in the pharmaceutical marketplace. As such, proposals to confront the high cost of prescription drugs have included a diversity of strategies that, applied in isolation, will likely not resolve this issue. Although the current presidential administration has publically telegraphed awareness and concern about the high cost of prescription drugs, specific policy solutions have yet to be advanced at the federal level.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Electronic supplementary material
(PDF 1224 kb)
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Dilokthornsakul P, Valuck RJ, Nair KV, Corboy JR, Allen RR, Campbell JD. Multiple sclerosis prevalence in the United States commercially insured population. Neurology. 2016;86(11):1014–1021. doi: 10.1212/WNL.0000000000002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens GM. Economic burden of multiple sclerosis and the role of managed sare organizations in multiple sclerosis management. Am J Manag Care. 2016;22(6 Suppl):s151–s158. [PubMed] [Google Scholar]
- 4.O'Brien JA, Ward AJ, Patrick AR, Caro J. Cost of managing an episode of relapse in multiple sclerosis in the United States. BMC Health Serv Res. 2003;3(1):17. doi: 10.1186/1472-6963-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9(5):520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 6.Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2 Suppl):S15–S20. [PubMed] [Google Scholar]
- 7.Iezzoni LI, Ngo L. Health, disability, and life insurance experiences of working-age persons with multiple sclerosis. Mult Scler. 2007;13(4):534–546. doi: 10.1177/1352458506071356. [DOI] [PubMed] [Google Scholar]
- 8.Ivanova JI, Birnbaum HG, Samuels S, Davis M, Phillips AL, Meletiche D. The cost of disability and medically related absenteeism among employees with multiple sclerosis in the US. Pharmacoeconomics. 2009;27(8):681–691. doi: 10.2165/11314700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Adelman G, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United States: a systematic review of the literature. J Med Econ. 2013;16(5):639–647. doi: 10.3111/13696998.2013.778268. [DOI] [PubMed] [Google Scholar]
- 10.Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? Neurology. 2015;84(21):2185–2192. doi: 10.1212/WNL.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bin Sawad A, Seoane-Vazquez E, Rodriguez-Monguio R, Turkistani F. Price analysis of multiple sclerosis disease-modifying therapies marketed in the United States. Curr Med Res Opin. 2016;32(11):1783–1788. doi: 10.1080/03007995.2016.1208644. [DOI] [PubMed] [Google Scholar]
- 12.Esper GJ, Hartung D, Avitzur O. The patient protection and affordable care act and chronic neurological illnesses: benefits and challenges. JAMA Neurol. 2015;72(7):739–740. doi: 10.1001/jamaneurol.2015.0273. [DOI] [PubMed] [Google Scholar]
- 13.Bourdette DN, Hartung DM, Whitham RH. Practices of US health insurance companies concerning MS therapies interfere with shared decision-making and harm patients. Neurol Clin Pract. 2016;6(2):177–182. doi: 10.1212/CPJ.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel H. Stemming the escalating cost of prescription drugs: a position paper of the american college of physicians. Ann Intern Med 2016 Mar 29. [DOI] [PubMed]
- 15.Tag T, Rubinstein E. AMCP Guide to Pharmaceutical Payment Methods, 2013 Update (Version 3.0). Academy of Managed Care Pharmacy Aug 2013. 1083-4087.
- 16.Dabora MC, Turaga N, Schulman KA. Financing and distribution of pharmaceuticals in the United States. JAMA. 2017;381:21–22. doi: 10.1001/jama.2017.5607. [DOI] [PubMed] [Google Scholar]
- 17.Medicaid Rebates for Brand-Name Drugs Exceeded Part D Rebates by a Substantial Margin. In: General OoI, ed2015.
- 18.Examination of Health Care Cost Trends and Cost Drivers. Office of the Attorney General Commonwealth of Massachusetts; October 7, 2016.
- 19.Skolarus LE, Burke JF, Callaghan BC, Becker A, Kerber KA. Medicare payments to the neurology workforce in 2012. Neurology. 2015;84(17):1796–1802. doi: 10.1212/WNL.0000000000001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard DH. Drug companies' patient-assistance programs—helping patients or profits? N Engl J Med. 2014;371(2):97–99. doi: 10.1056/NEJMp1401658. [DOI] [PubMed] [Google Scholar]
- 21.Ross JS, Kesselheim AS. Prescription-drug coupons—no such thing as a free lunch. N Engl J Med. 2013;369(13):1188–1189. doi: 10.1056/NEJMp1301993. [DOI] [PubMed] [Google Scholar]
- 22.Romley J, Goldman D, Eber M, Dastani H, Kim E, Raparla S. Cost-sharing and initiation of disease-modifying therapy for multiple sclerosis. Am J Manag Care. 2012;18(8):460–464. [PubMed] [Google Scholar]
- 23.Dor A, Lage MJ, Tarrants ML, Castelli-Haley J. Cost sharing, benefit design, and adherence: the case of multiple sclerosis. Adv Health Econ Health Serv Res. 2010;22:175–193. doi: 10.1108/S0731-2199(2010)0000022011. [DOI] [PubMed] [Google Scholar]
- 24.Banahan BF, Datar M, Mendonca CM, Bentley JP, Phillips AL, Stewart M. Effect of medicare part d coverage on adherence with disease modifying drug therapy for multiple sclerosis. Annual Meeting of the Academy of Managed Care Pharmacy; April 18–20, 2012; San Francisco.
- 25.Tamariz L, Uribe CL, Luo J, et al. Persistence with biologic therapies in the Medicare coverage gap. Am J Manag Care. 2011;17(11):753–759. [PubMed] [Google Scholar]
- 26.Ivanova JI, Bergman RE, Birnbaum HG, Phillips AL, Stewart M, Meletiche DM. Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis in the US. J Med Econ. 2012;15(3):601–609. doi: 10.3111/13696998.2012.667027. [DOI] [PubMed] [Google Scholar]
- 27.Oleen-Burkey M, Cyhaniuk A, Swallow E, Retrospective US database analysis of persistence with glatiramer acetate vs. available disease-modifying therapies for multiple sclerosis: 2001-2010. BMC Neurol. 2014;14:11. doi: 10.1186/1471-2377-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010;30(2):89–100. doi: 10.2165/11533330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds MW, Stephen R, Seaman C, Rajagopalan K. Healthcare resource utilization following switch or discontinuation in multiple sclerosis patients on disease modifying drugs. J Med Econ. 2010;13(1):90–98. doi: 10.3111/13696990903579501. [DOI] [PubMed] [Google Scholar]
- 30.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 31.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto D, Campbell JD. Cost-effectiveness of multiple sclerosis disease-modifying therapies: a systematic review of the literature. Autoimmun Dis. 2012;2012:784364. doi: 10.1155/2012/784364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawton A, Shearer J, Goodwin E, Green C. Squinting through layers of fog: assessing the cost effectiveness of treatments for multiple sclerosis. Appl Health Econ Health Policy. 2013;11(4):331–341. doi: 10.1007/s40258-013-0034-0. [DOI] [PubMed] [Google Scholar]
- 34.Noyes K, Bajorska A, Chappel A, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based study. Neurology. 2011;77(4):355–363. doi: 10.1212/WNL.0b013e3182270402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Disease-Modifying Therapies for Relapsing-Remitting and Primary-Progressive Multiple Sclerosis: Effectiveness and Value. Institute for Clinical and Economic Review; 2017.
- 36.Kirzinger A, Wu B, Brodie M. Kaiser Health Tracking Poll: Health Care Priorities for 2017. January 6, 2017.
- 37.Sarpatwari A, Avorn J, Kesselheim AS. State initiatives to control medication costs—can transparency legislation help? N Engl J Med. 2016;374(24):2301–2304. doi: 10.1056/NEJMp1605100. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee S. This drug company just made a huge pledge on its prices. Fortune. May 9, 2017.
- 39.Johnson A. Drugmakers pledge restraint, but prices will still soar. Associated Press. Februrary 27, 2017.
- 40.Dusetzina SB, Conti RM, Yu NL, Bach PB. Association of Prescription Drug Price Rebates in Medicare Part D With Patient Out-of-Pocket and Federal Spending. JAMA Intern Med. 2017;177:1185–1188. doi: 10.1001/jamainternmed.2017.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medicines use and spending in the U.S: A Review of 2016 and Outlook to 2021. IMS Institute for Healthcare Informatics; 2017.
- 42.Singh R, Palosky C. Majorities of Democrats, Republicans and Independents Support Actions to Lower Drug Costs, Including Allowing Americans to Buy Drugs from Canada. The Henry J. Kaiser Family Foundation; 2017.
- 43.Drug Coverage & Spending Reports From Council on Medical Service. AMA; 2015.
- 44.AAN Position Statement: Prescription Drug Prices.Minneapolis, MN; 2017.
- 45.Shih C, Schwartz J, Coukell A. How would government negotiation of medicare part d drug prices work? HealthAffairs Blog 2016.
- 46.Bourdette D, Hartung D. Equivalence of glatiramer acetate generics with branded glatiramer acetate in efficacy and cost for the treatment of multiple sclerosis. JAMA Neurol. 2015;72(12):1411–1413. doi: 10.1001/jamaneurol.2015.2605. [DOI] [PubMed] [Google Scholar]
- 47.Helfand C. Teva faces billions in lost sales as court tosses four long-acting Copaxone patents. FiercePharma 2017.
- 48.Sagonowsky E. Novartis’ Gilenya patent loss sets MS market up for battle with early generics. FiercePharma http://www.fiercepharma.com/pharma/novartis-loses-gilenya-patent-appeal-knocking-off-years-protection Publication date: Apr 13, 2017.
- 49.Kister I, Corboy JR. Reducing costs while enhancing quality of care in MS. Neurology. 2016;87(15):1617–1622. doi: 10.1212/WNL.0000000000003113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958. doi: 10.1002/ana.24651. [DOI] [PubMed] [Google Scholar]
- 51.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 52.Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 53.Claussen MC, Korn T. Immune mechanisms of new therapeutic strategies in MS: teriflunomide. Clin Immunol. 2012;142(1):49–56. doi: 10.1016/j.clim.2011.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)