Abstract
Thiazolidinediones are sulfur containing pentacyclic compounds that are widely found throughout nature in various forms. Thiazolidinedione nucleus is present in numerous biological compounds, e.g., anti-malarial, antimicrobial, anti-mycobacterium, anticonvulsant, antiviral, anticancer, anti-inflammatory, antioxidant, anti-HIV (human immunodeficiency virus) and antitubercular agent. However, owing to the swift development of new molecules containing this nucleus, many research reports have been generated in a brief span of time. Therefore seems to be a requirement to collect recent information in order to understand the current status of the thiazolidinedione nucleus in medicinal chemistry research, focusing in particular on the numerous attempts to synthesize and investigate new structural prototypes with more effective antidiabetic, antimicrobial, antioxidant, anti-inflammatory, anticancer and antitubercular activity.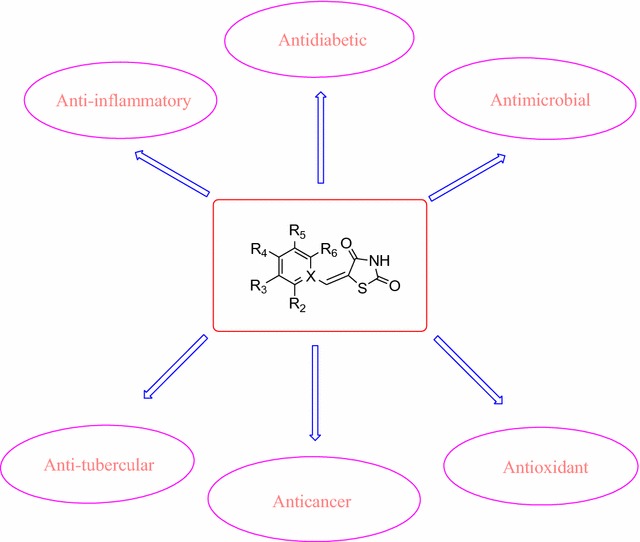
Keywords: Thiazolidinedione derivatives, Antidiabetic, Antimicrobial, Anti-inflammatory
Introduction
The number of antimicrobial drugs available in the market is vast, but there is a need to discover novel antimicrobial agents with better pharmacodynamic and pharmacokinetic properties with lesser or no side effects. Most of thiazolidinediones exhibit good bactericidal activity against various Gram-positive and Gram-negative bacteria. The bactericidal activity of thiazolidinediones derivatives depends on the substitution on the heterocyclic thiazolidine ring rather than the aromatic moiety.
Thiazolidinedione (Scheme 1) along with their derivatives draw attention as they have diverse biological as well as clinical use. Researchers focus on this moiety because it is involved in the control of various physiological activities. Heterocyclic moieties having Nitrogen and Sulfur are involved in a broad range of pharmacological processes. This created interest among researchers who have synthesized variety of thiazolidinediones derivatives and screened them for their various biological activities. In the present study, we have made an attempt to collect biological properties of thiazolidinediones and its derivatives of synthetic origin.
Scheme 1.
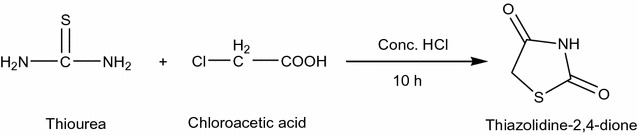
Synthesis of Substituted thiazolidine-2,4-dione
Biological activities of thiazolidinediones derivatives in the new millennium
Thiazolidinedione derivatives as antidiabetic agents
Diabetes mellitus (DM), also known as diabetes, is represented by the high blood sugar level over a period of prolonged time. There are three types of diabetes: (i) type 1 DM in which pancreas fails to produce insulin. Previously, it was referred as “insulin-dependent diabetes mellitus” or “juvenile diabetes”, (ii) type-2 DM a condition in which cells does not respond to insulin. Previously, it was referred as “non insulin-dependent diabetes mellitus”, (iii) gestational diabetes is the third main type and arises in pregnant women with no prior record of diabetes with high blood sugar levels [1].
The fundamental reasons of diabetes are a low production of insulin, the inability of the body to use it, or a combination of both (hormone which regulate carbohydrate, fat and protein metabolism). Normally it is a long-standing syndrome having different clinical revelation, with a number of problems such as cardiovascular, hypertension, renal, neurological. It is a disease in which pancreas does not secrete sufficient insulin or cells prevent reacting toward secreted insulin, that’s why cells cannot absorb blood glucose. Its symptoms are recurrent urination, tiredness, too much dehydration and hunger. It is cured by change in food habits, by regulation of proper diet; oral prescription and few situations include insulin injection [2, 3]. The thiazole moiety is a significant heterocyclic unit in drug invention. Literature survey shows that the wide-spread studies have been carried out on the production of thiazolidinediones. Thiazolidiones compounds shows a number of pharmacological activities such as antimicrobial, antitubercular, anti-tumor, anti-viral, anti-HIV, anti-inflammatory and anti-diabetic effects [4–6].
Datar et al. [7] synthesized a new series of thiazolidinediones by the reaction of thiazolidenedione with several benzaldehyde derivatives using Scheme 2. In vitro anti-diabetic activity of synthesized compound was performed by SLM model. In this series compounds 1 and 2 found to be most active [5-(3,4-dimethoxy)benzylidine-2,4-thiazolidinedione,5-(3,4,5 trimethoxy)benzylidine-2,4-thiazolidenedione] due to presence of methoxy group and comparable to standard drug pioglitazone studies. The results of the most active compound are indicated Tables 1 and 2 (Datar et al. [7]).
Scheme 2.
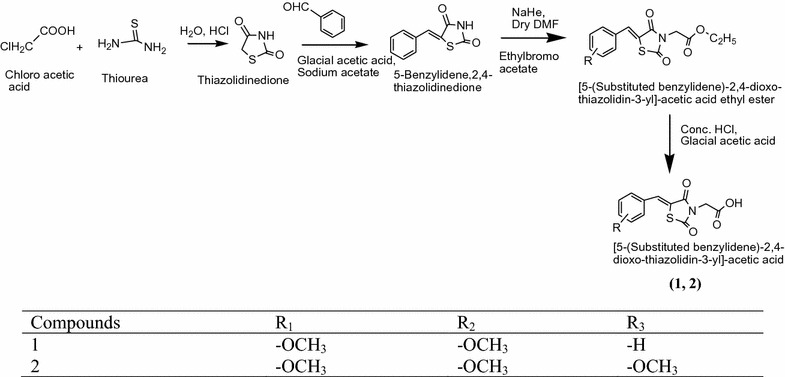
Synthesis of [5-(Substituted benzylidene)-2,4-dioxo-thiazolidin-3-yl]-acetic acid
Table 1.
Blood glucose level in experimental animals (mg/dl)
| Compounds | Time (min) | ||||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | |
| DMSO | 145 | 150 | 150 | 147 | 141 |
| Pioglitazone | 139 | 105 | 110 | 112 | 115 |
| 1 | 141 | 112 | 117 | 118 | 112 |
| 2 | 147 | 110 | 112 | 107 | 104 |
Table 2.
Decrease in blood glucose levels by AUC method
| Compounds | Time (min) | ||||
|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | % reduction in blood glucose level | |
| DMSO | + 11 | + 05 | + 02 | − 04 | + 31 |
| Pioglitazone | − 34 | − 39 | − 29 | − 26 | − 23.07 |
| 1 | − 29 | − 25 | − 24 | − 27 | − 21.71 |
| 2 | − 37 | − 35 | − 28 | − 24 | − 22.84 |
Swapna et al. [8] synthesized novel thiazolidinediones by using Scheme 3. In vitro antidiabetic activity performed by alloxan induced tail tipping method. From this series compound 3, 4, 5 showed highest activity as comparable to standard drug metformin because of presence of electron donating group. The results of most active derivatives showed in Table 3 (Swapna et al. [8]).
Scheme 3.
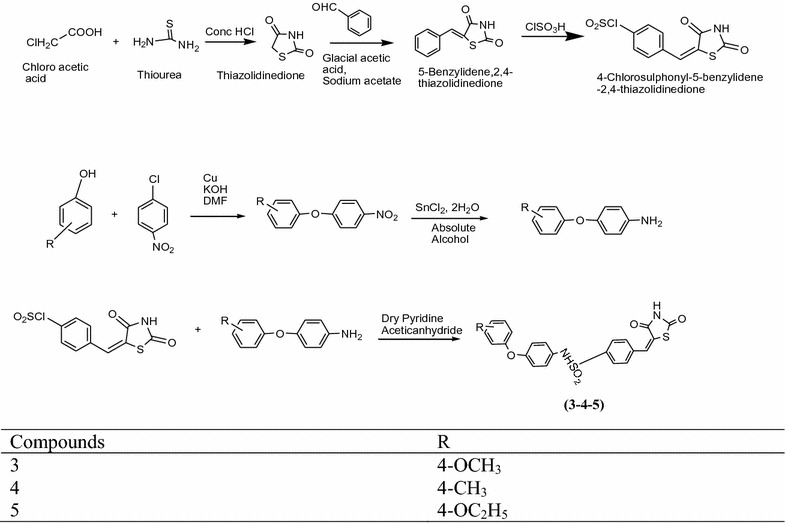
Synthesis of 5-[4-Substituted) sulphonyl benzylidene]-2,4-thiazolidinedione
Table 3.
Blood glucose level (mg/dl) of synthesized thiazolidinediones derivatives
| Compounds | Blood glucose level (mean ± SE) | ||
|---|---|---|---|
| 0 h | 3 h | 6 h | |
| 3 | 343 ± 5.797 | 313.8 ± 9.411 | 303.2 ± 9.827 |
| 4 | 341.5 ± 6.158 | 320.5 ± 6.737 | 313 ± 9.500 |
| 5 | 353.7 ± 6.026 | 315.8 ± 8.109 | 311.2 ± 9.297 |
| Positive control | 335.7 ± 5.168 | 345.5 ± 5.488 | 354 ± 8.135 |
| Normal control | 125.0 ± 4.497 | 126.3 ± 4.047 | 127.7 ± 3.703 |
| Metformin | 343.3 ± 6.206 | 322.8 ± 4.989 | 292.0 ± 7.767 |
Pattan et al. [2] synthesized a new series of thiazolidinediones derivatives [5-(4-substitutedsulfonylbenzylidene)-2,4-thiazolidinedione] using Scheme 4. The In vitro antidiabetic activity performed by ANOVA and Dunnet’s ‘t’ test. From this series 6, 7 and 8 compound showed moderates activity and comparable to the standard drug glibenclamide. The results of active compound are given in Table 4 (Pattan et al. [2]).
Scheme 4.
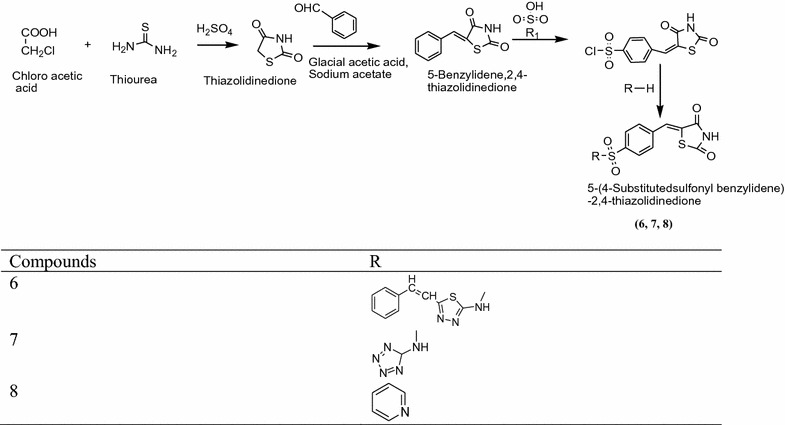
Synthesis of 5-(4-Substituted sulfonyl benzylidene)-2,4-thiazolidenedione
Table 4.
Blood glucose level (mg/dl) in synthesized compounds
| Compounds | Blood glucose level (mean ± SE) | |||
|---|---|---|---|---|
| 0 h | 1 h | 3 h | 6 h | |
| 6 | 320.5 ± 15.81 | 145.5 ± 2.26 | 137.0 ± 3.80 | 123.5 ± 1.10 |
| 7 | 213.5 ± 8.78 | 140.7 ± 3.30 | 106.3 ± 6.91 | 95.75 ± 6.06 |
| 8 | 283.5 ± 43.76 | 205.75 ± 49.7 | 166.3 ± 38.92 | 124.5 ± 13.16 |
| Standard | 385.8 ± 21.37 | 230.8 ± 12.35 | 156.8 ± 10.87 | 93.4 ± 4.98 |
Badiger et al. [9] synthesized novel thiazolidinediones derived from 4-fluorophenylacetic acid and thiosemicarbazide in phosphorous oxychloride using Scheme 5. The in vitro antidiabetic activity of synthesized compound [5-{[2-(4-alkyl/aryl)-6-arylimidazo[1,2][1,3,4]thiadiazol-5-yl]metylene}-1,3-thiazolidine-2,4-dione] were performed by alloxan induced tail tipping method. Among them, compounds 9 and 10 found to be most active due to presence of napthyl and coumarinyl groups at C5 position as compared to standard drug pioglitazone. The results of synthesized compounds presented in Table 5 (Badiger et al. [9]).
Scheme 5.
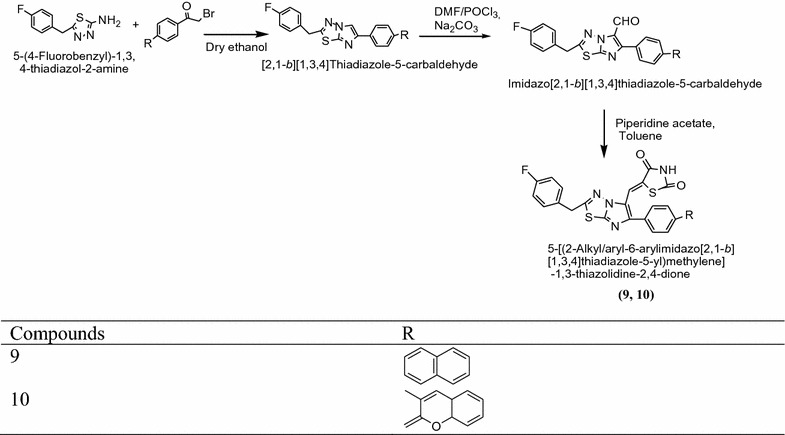
Synthesis of 5-{[2-(4-Fluorobenzyl)-6-arylimidazo[2,1-b] [1, 3, 4] thiadiazol-5-yl]methylene}thiazolidine-2,4-diones
Table 5.
Plasma glucose level of 3–4 at various drug doses
| Compounds | % decrease in plasma glucose level (PG) at various drug doses (mg/kg bodyweight) | ||
|---|---|---|---|
| 10 mg | 30 mg | 60 mg | |
| 9 | 42.48 + 3.25 | 62.24 + 3.42 | 70.35 + 3.14 |
| 10 | 45.42 + 1.25 | 58.36 + 2.36 | 68.42 + 2.16 |
| Pioglitazone | 47.25 + 5.50 | 64.59 + 5.42 | 75.43 + 3.40 |
Patil et al. [10] synthesized a new series of thiazolidinedione derivatives derived from thiourea and chloroacetic acid in ethanol/DMF as presented in Scheme 6. The In vitro antidiabetic activity of synthesized compounds was performed by alloxan induced tail tipping method. From these series compounds 11, 12 and 13 showed better activities compared to pioglitazone and metformin as standard drug. The results of most active derivatives showed in Table 6 (Patil et al. [10]).
Scheme 6.
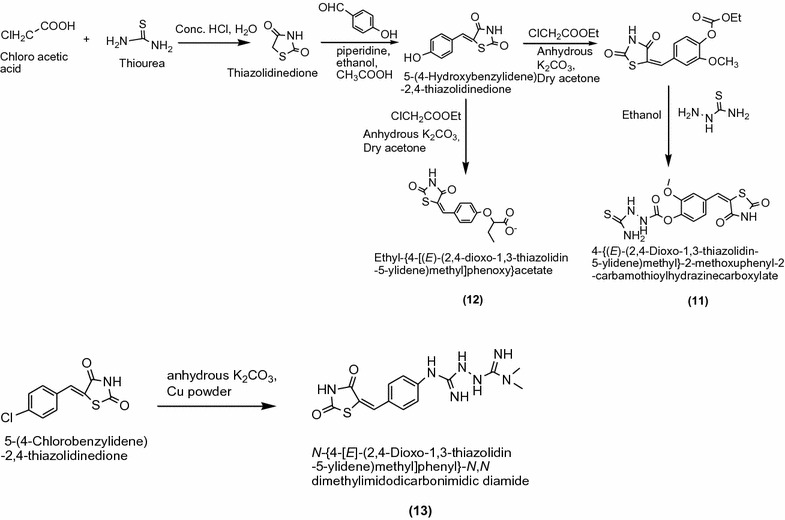
Synthesis of 5-(Substituted benzylidene)-2,4-thiazolidinedione
Table 6.
Hypoglycemic effect of synthesized compounds
| Compounds | Blood glucose level mg/dl (mean ± SE) | |||
|---|---|---|---|---|
| 0 h | 3 h | 6 h | 24 h | |
| 11 | 376.4 ± 21.00 | 342.8 ± 21.58 | 315.2 ± 21.66 | 276 ± 21.79 |
| 12 | 326.2 ± 25.32 | 300 ± 25.03 | 278.2 ± 25.76 | 245.2 ± 25.91 |
| 13 | 355 ± 24.59 | 322.8 ± 24.10 | 253.8 ± 23.45 | 231.4 ± 23.48 |
| Pioglitazone | 402.2 ± 28.7 | 363.4 ± 26.08 | 302.4 ± 26.87 | 232.2 ± 20.53 |
| Metformin | 441.8 ± 18.71 | 399.4 ± 17.72 | 289.4 ± 18.46 | 219.6 ± 18.40 |
| Vehicle control | 304.2 ± 36.81 | 308.2 ± 36.85 | 309 ± 37.92 | 310.4 ± 39.57 |
| Diabetic control | 322.2 ± 22.96 | 337 ± 23.59 | 347 ± 24.01 | 363.4 ± 24.0 |
| Normal control | 120.33 ± 7.76 | 125.66 ± 2.08 | 126.66 ± 3.05 | 129.33 ± 1.52 |
Srikanth et al. [11] synthesized an innovative sequence of thiazolidinediones using 4-fluoroaniline, methyl acrylate and thiourea using proper solvent as showed in Scheme 7. The In vitro antidiabetic activities of synthesized compounds were confirmed by tail vein method and ANOVA method. In this series compounds 14, 15, 16 and 17 showed significant activity as compared to standard drug rosiglitazone. The results of synthesized compounds presented in Table 7 (Srikanth et al. [11]).
Scheme 7.
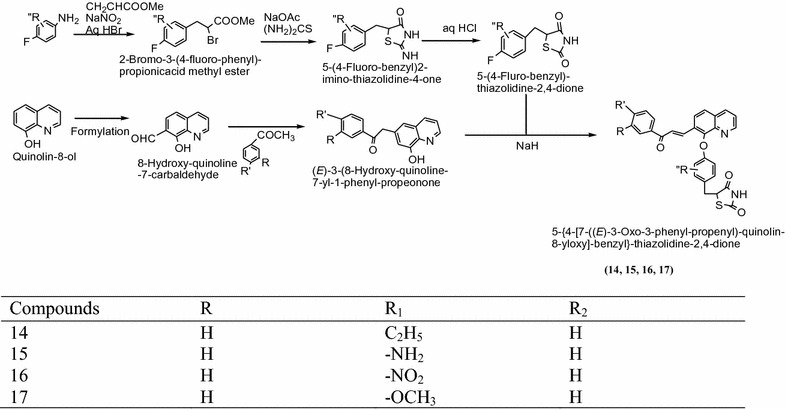
Synthesis of 5-{4-[7-((E)-3-Oxo-3-phenyl-propenyl)-quinolin-8-yloxy]-benzyl}-thiazolidine-2,4-dione
Table 7.
Antidiabetic activities of synthesized compounds (mg/dl)
| Compounds | Blood glucose level (mean ± SE) |
|---|---|
| 14 | 82.81 ± 1.115 |
| 15 | 86.31 ± 0.993 |
| 16 | 87.21 ± 1.233 |
| 17 | 97.91 ± 1.870 |
| Rosiglitazone | 65.58 ± 1.013 |
Nikalje et al. [12] designed few thiazolidinediones derivatives from thiazolidindione via 4-hydroxy, 3-ethoxy benzaldehyde in ethanol, benzoic acid and piperidine using Scheme 8. The In vitro antidiabetic activity of synthesized compounds was confirmed by ANOVA, alloxan induced diabetic rat model and dunnet’ t test. From this series compounds 18, 19, 20, 21, and 22 showed better activity as compared to standard drug rosiglitazone. The results of synthesized compounds presented in Table 8 (Nikalje et al. [12]).
Scheme 8.
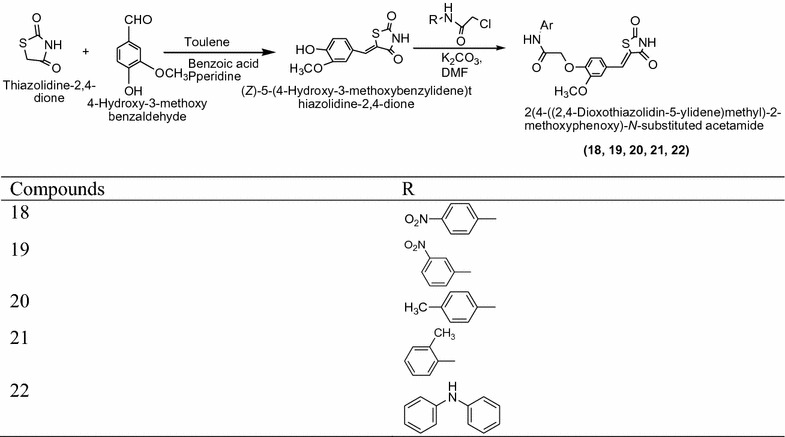
Synthesis of 2-(4-((2,4-Dioxothiazolidin-5-ylidene) methyl)-2-methoxyphenoxy)-N-substituted acetamide derivatives
Table 8.
Evaluation of hypoglycemic activity: effect of compound on % decrease in blood glucose in diabetic mice
| Compounds | 0 h | 2 h | 4 h | 6 h | 24 h |
|---|---|---|---|---|---|
| Control | 252.53 ± 4.254 | 4.74 ± 0.68 | 7.9 ± 4.32 | 13.43 ± 2.68 | 3.18 ± 4.35 |
| Piogiltazone | 250.75 ± 5.21 | 31.07 ± 6.74 | 37.48 ± 5.37 | 45.41 ± 3.67 | 10.3 ± 6.53 |
| 18 | 252.79 ± 2.85 | 29.34 ± 4.53 | 36.52 ± 5.43 | 46.64 ± 4.52 | 6.70 ± 6.51 |
| 19 | 252.19 ± 4.35 | 24.7 ± 3.97 | 34.76 ± 6.51 | 37.89 ± 5.43 | 5.19 ± 7.74 |
| 20 | 254.38 ± 4.53 | 26.64 ± 5.28 | 34.26 ± 5.67 | 37.05 ± 4.62 | 4.19 ± 5.43 |
| 21 | 253.60 ± 5.64 | 22.9 ± 4.72 | 35.6 ± 5.53 | 40.41 ± 5.97 | 3.87 ± 6.53 |
| 22 | 252.73 ± 5.23 | 29.01 ± 6.54 | 36.47 ± 4.65 | 39.21 ± 5.74 | 3.0 ± 3.75 |
Jiwane et al. [13] synthesized a new series of thiazolidine-2,4-dione derivatives from 5-(benzylidene)thiazolidine-2,4-dione with N N 1-dimethylformamide in diethyl amino as presented in Scheme 9. The In vitro anitdiabetic activity of synthesized compound [3-((diethyl amino)methyl)-5-(4-methoxybenzylidine)thiazolidine-2,4-dione] were confirmed by alloxan induced diabetic rat model. From this series, compounds 23 and 24 showed remarkable activity as that of the standard rosiglitazine, which indicates that the substitution of α-amino methyl group at position-3 show different hypoglycemic activity. The results of most active derivatives showed in Table 9 (Jiwane et al. [13]).
Scheme 9.
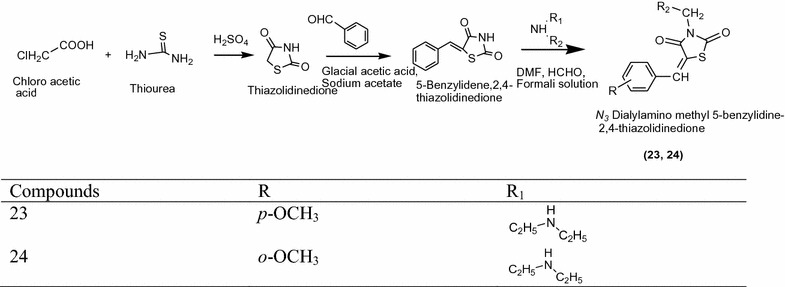
Synthesis of N 3-dialylamino methyl 5-benzylidine 2,4-thiazolidinedione derivatives
Table 9.
Hypoglycemic activity of synthesized derivatives
| Compounds | Dose (mg/kg) | Mean blood glucose level (mg/dl) | % reduction in blood glucose level | |||
|---|---|---|---|---|---|---|
| Before 1st dose | After 2 h | After 4 h | After 2 h | After 4 h | ||
| 23 | 50 | 400 | 56 | 48 | 86 | 88 |
| 24 | 50 | 275 | 63 | 79 | 72 | 65 |
| Rosiglitazone | 50 | 400 | 56 | 48 | 86 | 88 |
Grag et al. [14] designed novel thiazolidinediones derivative from 3-benzylthiazolidine-2,4-dione with selected various substituted aromatic aldehydes in ethanol, benzoic acid and piperidine using Scheme 10. In vitro antidiabetic activity of synthesized compound [5-arylidene-3-benzyl-thiazolidine-2,4-diones] was confirmed by ANOVA, alloxan induced diabetic rat model and dunnet’ t test. From this series compounds 25, 26 and 27 showed highest activity because of methoxy group as compared to standard rosiglitazone. The results of synthesized compounds presented in Table 10 (Grag et al. [14]).
Scheme 10.
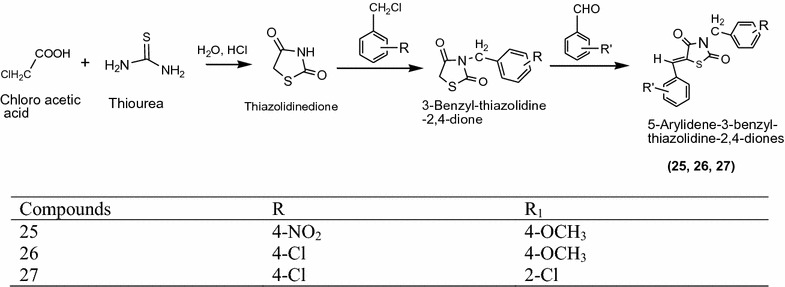
Synthesis of 5-Substituted-arylidene-3-substituted-benzyl-thiazolidine-2,4-dione derivatives
Table 10.
Hypoglycemic activity of synthesized derivatives
| Treatment (mg/kg) | Blood glucose level (mg/dl) | |||
|---|---|---|---|---|
| 0 day | 3rd day | 5th day | 7th day | |
| 25 | 86.11 ± 0.98 | 85.67 ± 0.58 | 84.68 ± 0.54 | 86.23 ± 0.48 |
| 26 | 188.23 ± 1.14 | 189.56 ± 0.98 | 185 ± 0.86 | 182.36 ± 1.25* |
| 27 | 189.35 ± 1.18 | 206.38 ± 0.86 | 192.30 ± 1.2 | 188.36 ± 1.23 |
| Rosiglitazone | 194.99 ± 1.70 | 207.45 ± 0.69 | 189.64 ± 1.33 | 172.38 ± 2.24 |
* indicates high reduction in glucose level after seven days
Bhat et al. [15] synthesized a new series of thiazolidinediones derivatives derived from 5-arylidene-2,4-thiazolidinedione using Scheme 11. The In vitro antidiabetic activity of synthesized compound [5-(4-methoxy-benzylidene)-2,4-dioxo-thiazolidin-3-yl]-acetic acid] and [5-(substituted)-2,4-dioxo-thiazolidin-3-yl]-acetic acid substituted ester were performed by alloxan induced tail tipping method and SLM. Among them compounds 28, 29, 30, 31, 32, 33, 34, 35 and 36 found to be most active or higher than rosiglitazone and metformin using as standard drug. The results of most active derivatives showed in Table 11 (Bhat et al. [15]).
Scheme 11.
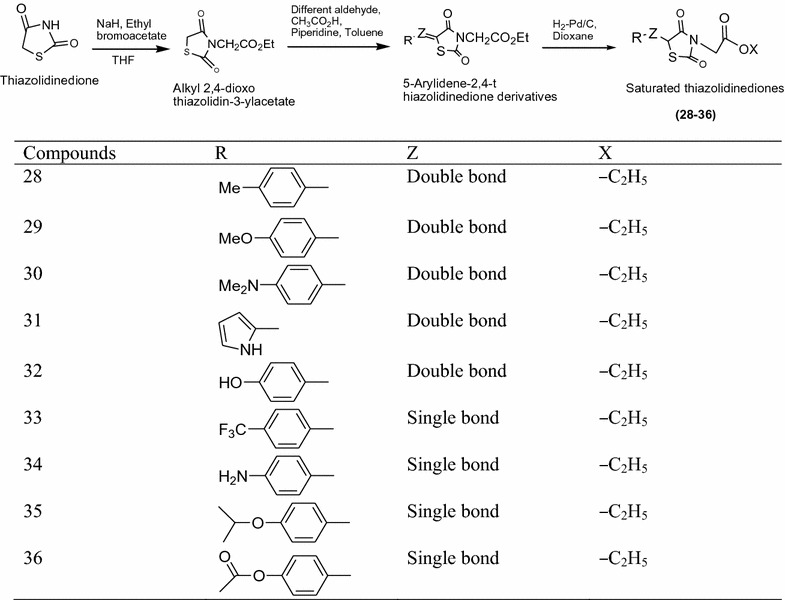
Synthesis of [5-(4-Methoxy-benzylidene)-2,4-dioxo-thiazolidin-3-yl]-acetic acid
Table 11.
Antihyperglycemic activity profile of title compounds thiazolidine-2,4-dione derivatives
| Compounds | Antihyperglycemic activity, SLM | PPARc | |
|---|---|---|---|
| 10 nmol | 1000 nmol | ||
| 28 | − 22.1 | 9 | 9 |
| 29 | − 22.2 | 7 | 8 |
| 30 | − 15.8 | – | – |
| 31 | + 9.00 | – | – |
| 32 | − 26.7 | 10 | 12 |
| 33 | − 12.3 | 9 | 11 |
| 34 | − 12.7 | 8 | 10 |
| 35 | − 4.1 | – | – |
| 36 | − 26.8 | – | – |
| Rosiglitazone | 11.6 | 92 | 248 |
| Metformin | 34.1 | – | – |
PPAR c proxisome proliferator activated receptor
Jawale et al. [16] synthesized innovative chain of thiazolidinediones derived from maleic anhydride and thiourea was treated with water using Scheme 12. The In vitro antidiabetic activity of synthesized compounds was performed by alloxan induced tail tipping method using wister rat, dunnet’ t test and SLM model. Among them compounds 37, 38, 39 and 40 found to be significant activity metformin using as standard drug. The results of most active derivatives showed in Table 12 (Jawale et al. [16]).
Scheme 12.
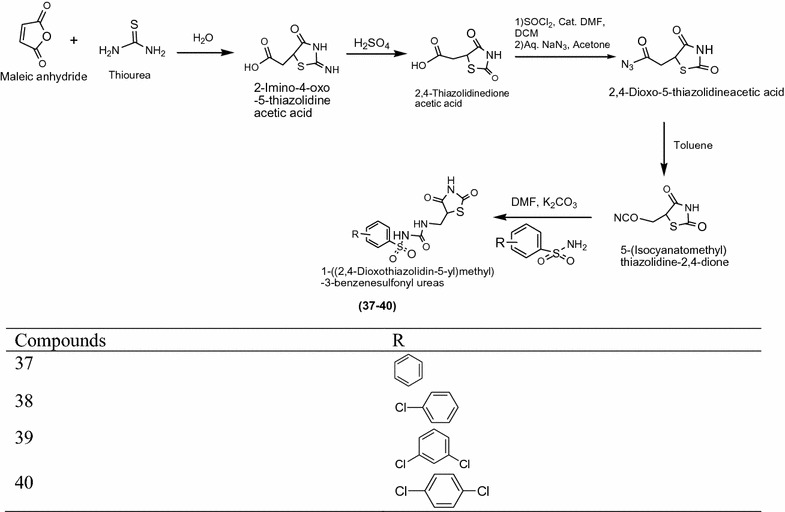
Synthesis of 1-((2,4-Dioxothiazolidin-5-yl)methyl)-3-substitued benzene sulphonyl ureas
Table 12.
Antidiabetic activity of synthesized compounds
| Compounds | Dose (mg/dl) | % activity | Significance |
|---|---|---|---|
| 37 | 100 | 15.8 | p < 0.01 |
| 38 | 100 | 17.2 | p < 0.01 |
| 39 | 100 | 14.3 | p < 0.05 |
| 40 | 100 | 16.5 | p < 0.01 |
| Metformin | 100 | 27.0 | p < 0.001 |
Thiazolidinedione derivatives as antimicrobial agents
Long-ago, contagious diseases caused by multidrug-resistant microorganisms have become a serious issue, representing a growing threat to human health and being a major problem in many countries worldwide. There has been a significant increase in clinical drug resistance over the past few decades, owing to exploitation of antimicrobial agents, thus many infectious disease can no longer be treated successfully with general anti-infective agents [17]. Modern therapies and management technique such as bone marrow or solid-organ transplants, and newer much aggressive chemotherapy have resulted in a rapidly inflating number of immune-suppressed patient. So, in order to meet above mentioned challenges, there is an urgent need for the development of novel antimicrobial agents [18].
In this study, Nawale et al. [19] synthesized a new series of 5-Substituted 2,4-thiazolidinedione derivatives (Scheme 13) and evaluated for in vitro antimicrobial activity against two species of Gram-positive bacteria, Bacillus subtilis, Staphylococcus aureus and Gram-negative bacteria, Pseudomonas aeruginosa using broth dilution method. Among the synthesized derivatives, compounds 41, 42, 43 and 44 exhibited highest activity on all tested microorganisms. The results of synthesized compounds presented in Table 13 (Nawale et al. [19]).
Scheme 13.
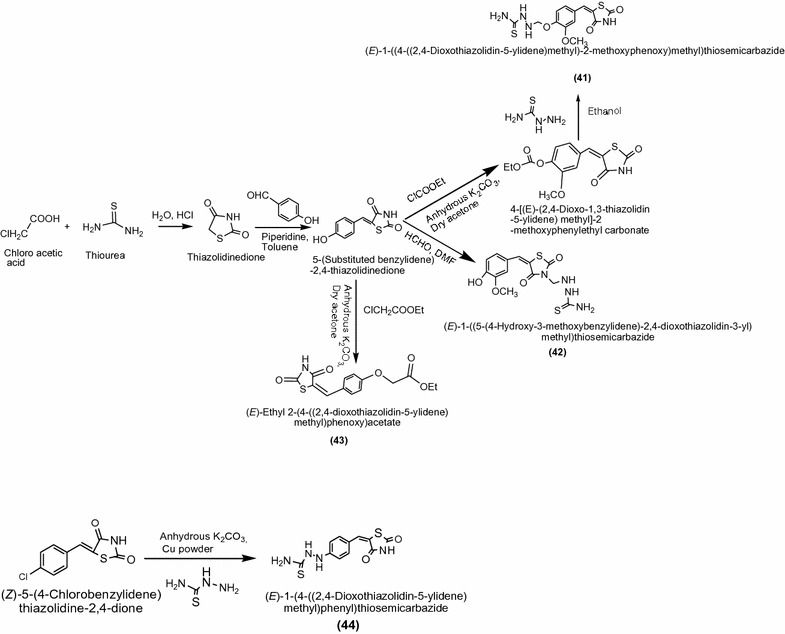
Synthesis of 5-Substituted benzylidenethiazolidine-2,4-dione
Table 13.
MIC (μg/ml) values for the screened thiazolidinediones compounds
| Compounds | Microorganisms | ||
|---|---|---|---|
| Bacillus subtilis | Staphylococcus aureus | Pseudomonas aeruginosa | |
| 41 | 31.25 | 31.25 | 31.25 |
| 42 | 31.25 | 31.25 | 31.25 |
| 43 | 62.5 | 125 | 62.5 |
| 44 | 31.25 | 62.5 | 125 |
| Streptomycin | 3.90 | 3.90 | 3.90 |
Nastas et al. [20] synthesized a series of novel 5-(Chromene-3-yl)methylene-2,4-thiazolidinedione derivatives as presented in Scheme 14 and tested for its in vitro antimicrobial potency towards Gram-positive bacteria (Listeria monocytogenes, Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli, Salmonella typhi) pathogenic bacteria and fungi (Candida albicans) using broth dilution method and the disk diffusion method. Among the synthesized derivatives, compounds 45, 46 and 47 antimicrobial activity against all tested bacteria and fungi. The results of most active derivatives showed in Table 14 (Nastas et al. [20]).
Scheme 14.
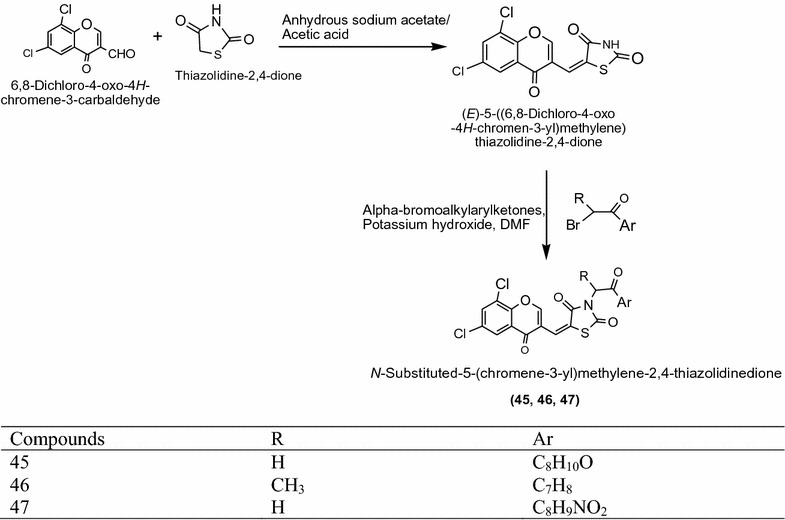
Synthesis of 5-(Chromene-3-yl)methylene-2,4-thiazolidinediones
Table 14.
Antimicrobial activity of 5-(chromene-3-yl)methylene-2,4-thiazolidinediones
| CP 10/5/1(mg/ml) | Gram-positive | Gram-negative | Fungi | ||
|---|---|---|---|---|---|
| L. monocytogenes | S. aureus | E. coli | S. typhi | C. albicans | |
| 45 | 18/22/18 | 22/12/12 | 12/14/14 | 15/19/20 | 20/18/18 |
| 46 | 22/22/20 | 24/28/28 | 18/18/16 | 20/18/16 | 18/18/16 |
| 47 | 28/28/28 | 28/28/28 | 18/18/18 | 18/18/18 | 22/22/22 |
| Gentamicin | 18 | 19 | 22 | 18 | NT |
| Fluconazole | NT | NT | NT | NT | 28 |
NT not tested
Moorthy et al. [5] synthesized a series of novel imidazolyl thiazolidinedione derivatives (Scheme 15) and screened them for their in vitro antimicrobial activity towards Gram-positive (S. aureus, S. epidermidis, M. luteus, B. cereus) and Gram-negative (E. coli, P. aeruginosa, K. pneumonia) bacteria and fungi (A.niger, A. fumigates). They were compared with standard drug ciprofloxacin and ketoconazole. Among the synthesized derivatives, compound 48 [Methyl-2-(4-((3-(2-methoxy-2-oxoethyl)-2,4-dioxothiazolidin-5-ylidene)methyl)1H-Imidazol-1-yl)acetate] showed potent activities towards S. aureus, S. epidermidis, E. coli, P. aeruginosa, A. niger and A, fumigates and 49 [Methyl-2-(2-((2,4-dioxothiazolidin-5-ylidene)methyl)-1H-imidazol-1-yl)acetate], 50 [Methyl-2-(2-((3-(2-methoxy-2-oxoethyl)2,4-dioxothiazolidin-5-yldiene)methyl)1H-imidazol-1-yl)acetate] and 51 [5-(4-Bromobenzylidene)thiazolidine-2,4-dione] showed good activity against all microorganism. The results of synthesized compounds presented in Table 15 (Moorthy et al. [5]).
Scheme 15.
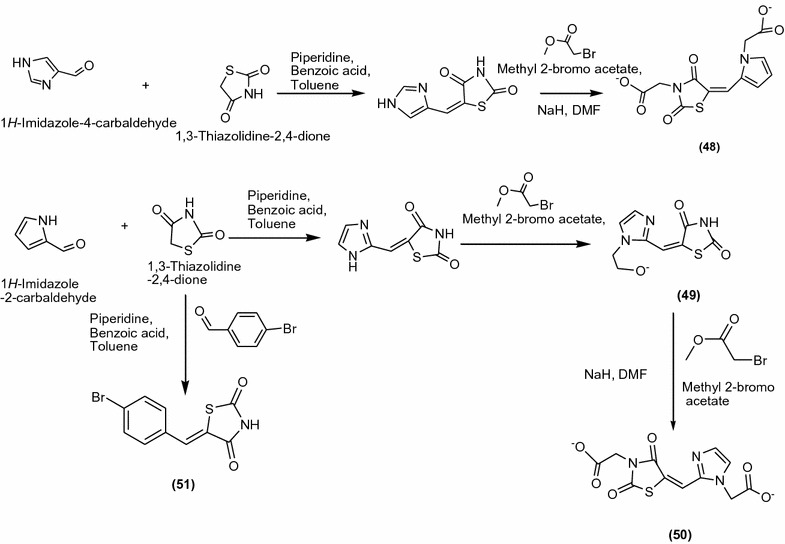
Synthesis of 5-(Substituted benzylidene)thiazolidine-2,4-dione and imidazolyl thiazolidinedione derivatives
Table 15.
In vitro activity zone of inhibition (mm) of compounds
| Compounds | Gram-positive bacteria | Gram-negative bacteria | Fungi | |||
|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | A. niger | A. fumigates | |
| 48 | 18 (1.9) | 16 (1.4) | 28 (1.6) | 28 (0.56) | 20 (8.8) | 26 (2.3) |
| 49 | 21 (22.1) | 27 (22.2) | 27 (21.5) | 21 (21.5) | 24 (20.7) | 20 (22.6) |
| 50 | 16 (2.7) | 18 (3.39) | 22 (9.2) | 16 (1.4) | 22 (8.2) | 26 (3.4) |
| 51 | 21 (22.1) | 25 (22.2) | 25 (21.5) | 21 (21.5) | 28 (21.6) | 25 (21.7) |
| Ciprofloxacin | 29 (0.2) | 31 (0.39) | 32 (0.2) | 33 (0.25) | – | – |
| Ketoconazole | – | – | – | – | 26 (6.1) | 24 (0.23) |
Alagawadi et al. [21] designed some novel derivatives of imidazole fused with thiazolidine-2,4-dione and evaluated them for their antibacterial activity against Gram-positive bacteria Staphylococcus aureus (S. a), Enterococcus faecalis (E. f) Gram-negative bacteria Escherichia coli (E. c.) Pseudomonas aeruginosa (P. a.) and antifungal activity Candida albicans (C.a.) Cryptococcus neoformans (C. n.) Aspergillus flavus (A. f.) and Aspergillus niger (A. n.) Among the screened compound the MIC value of compound 52 [5-{[2-(3,4,5-trimethoxyphenyl)-6-(4-bromophenyl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl]methylidene}-1,3-thiazolidine-2,4-dione], 53 [5-{[2-(3,4,5-trimethoxyphenyl)-6-(4-chlorophenyl)midazo[1-b][1,3,4]thiadiazol-5-yl]methylidene}-1,3-thiazolidine-2,4-dione] (Scheme 16), 54 [N-[-(dimethylamino)methylidene]-5-[(2,4-dioxo-1,3-thiazolidin-5-ylidene)methyl]-6-phenylimidazo[2,1-b][1, 3, 4]thiazolie-2-sulfonamide] and 55 [N-[-(dimethylamino)methylidene]-5-[-(2,4-dioxo-1,3-thiazolidin-5-ylidene)methyl]-6-(4-bromophenyl)-imidazo[2,1-b][1,3,4]thiazole-2-sulfonamide] (Scheme 17) were showed potent activity against Gram-positive, Gram-negative bacterial strain and fungal strains. The significant results of these compounds are presented in Table 16 (Alagawadi et al. [21]).
Scheme 16.
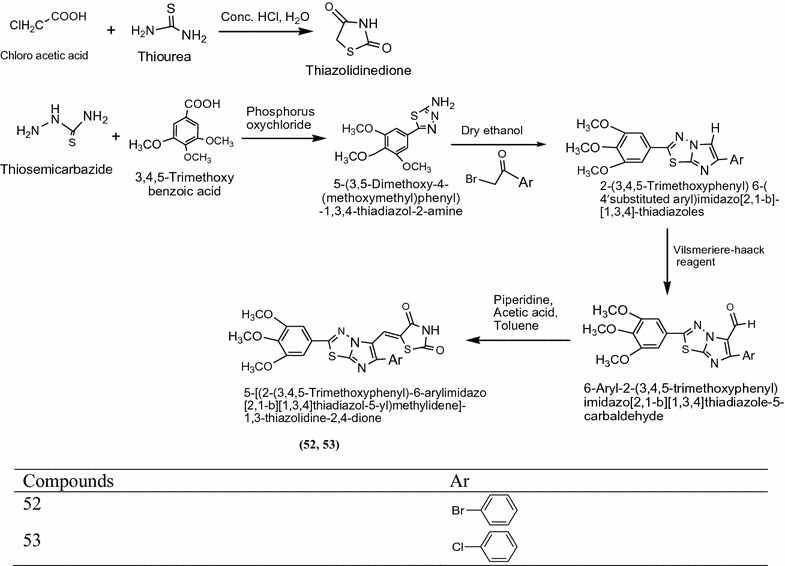
Synthesis of 5-[(2-(3,4,5-Trimethoxyphenyl)-6-arylimidazo[2,1-b][1,3,4]thiadiazol-5-yl)methylidene]-1,3-thiazolidine-2,4-dione
Scheme 17.
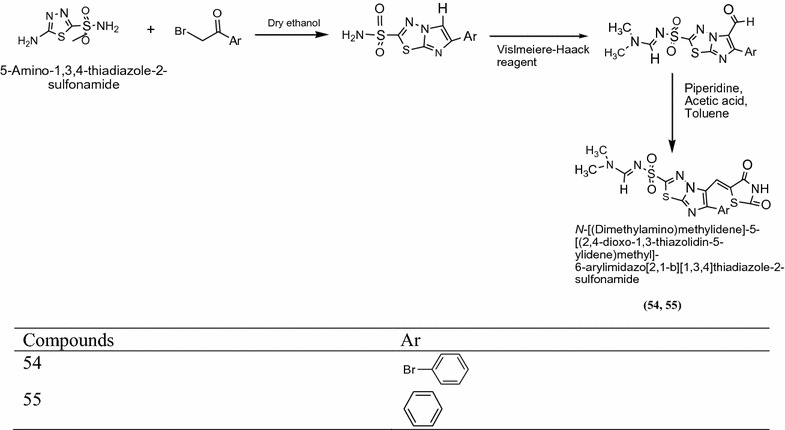
Synthesis of N-[(Dimethylamino)methylidene]-5-[(2,4-dioxo-1,3-thiazolidin-5-ylidene)methyl]-6-arylimidazo[2,1-b][1,3,4]thiadiazole-2-sulfonamide
Table 16.
Antimicrobial activities of synthesized compounds
| Compounds | Minimum inhibitory concentration (MIC) in μg/ml | |||||||
|---|---|---|---|---|---|---|---|---|
| E. c | P. a | S. a | E. f | C. a | C. n | A. f | A. n | |
| 52 | 256 | 256 | 32 | 32 | 4 | 8 | 4 | 4 |
| 53 | 128 | 64 | 32 | 32 | 4 | 8 | 32 | 32 |
| 54 | 128 | 32 | 8 | 4 | 1 | 2 | 4 | 4 |
| 55 | 64 | 64 | 8 | 8 | 4 | 8 | 4 | 4 |
| Ampicillin | 2 | 2 | 1 | 2 | – | – | – | – |
| Ketoconazole | – | – | – | – | 2 | 1 | 2 | 1 |
Khan et al. [22] designed some novel biphenyl tetrazole thiazolidinedione derivatives (Scheme 18) and evaluated for their antimicrobial activity against bacterial strain (Escherichia coli, Bacillus subtilis). Antimicrobial activity result indicated that among the synthesized derivatives 56 [(E)-3-((20-(1H)-tetrazol-5-yl)biphenyl-4-yl)methyl)-5-(4-chlorobenzylidene)thiazolidine-2,4-dione], 57 ((E)-3-((20-(1H-tetrazol-5-y)biphenyl-4-yl)methyl)-5-(2-chlorophenylbenzylidene)thiazolidine-2,4-dione) and 58 [(E)-3-((20-(1H-tetrazol—5-yl)biphenyl-4-yl)methyl)-5-(2,6-dichlorobenzylidene) thiazolidine-2,4-dione] showed potent in vitro antimicrobial activity. The results of most active derivatives showed in Table 17 (Khan et al. [22]).
Scheme 18.
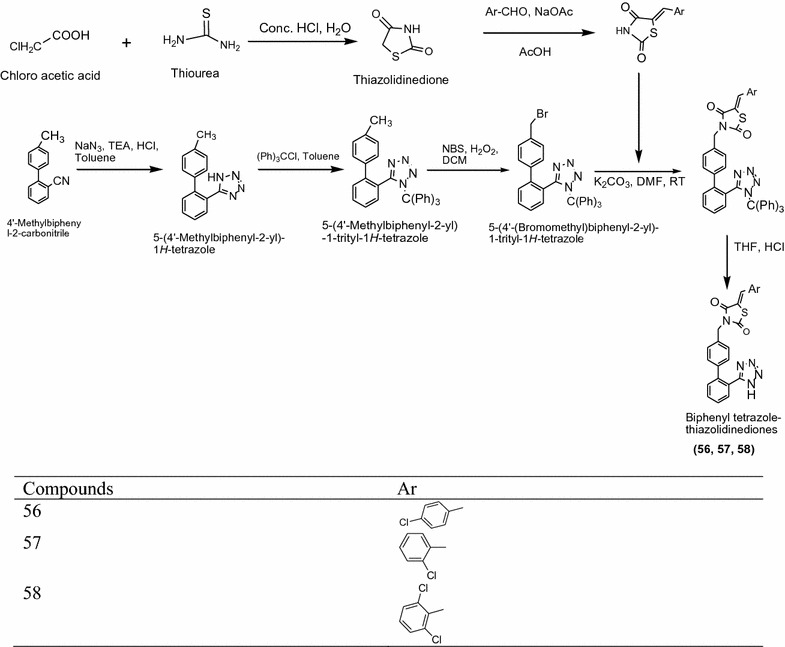
Synthesis of Biphenyl tetrazole-thiazolidinediones
Table 17.
Antibacterial activities of synthesized compounds
| Compounds | MIC ± SLM (μg/ml) | |
|---|---|---|
| E. coli | B. subtilis | |
| 56 | 20.75 ± 1.55 | 35.41 ± 2.41 |
| 57 | 19.41 ± 1.27 | 26.00 ± 1.96 |
| 58 | 8.58 ± 0.42 | 8.42 ± 0.51 |
| Ciprofloxacin | 25.00 ± 0.95 | 50.00 ± 1.75 |
Liu et al. [23] synthesized a series of new compound bearing 2,4-thiazolidinedione and benzoic moiety as presented in Scheme 19 and screened for their in vitro antimicrobial activity against bacterial strain (Staphylococcus aureus and Escherichia coli). Antimicrobial activity result indicated that among the synthesized derivatives, compounds 59, 60, 61, 62 and 63 showed highest in vitro growth of inhibition against bacterial strains. The results of synthesized compounds presented in Table 18 (Liu et al. [23]).
Scheme 19.
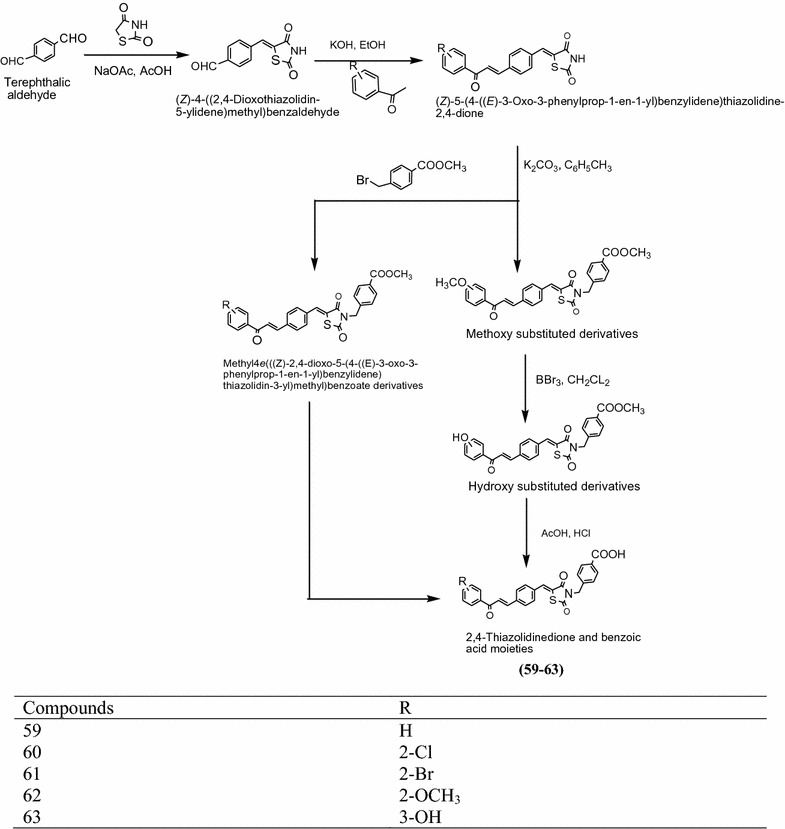
Synthesis of 4-(((Z)-5-((4-((E)-3-(Substituted)-3-oxoprop-1-en-1-yl)benzylidene)-2,4-dioxothiazolidin-3-yl)methyl)benzoic acid
Table 18.
Inhibitory activities of novel compounds against bacteria
| Compounds | S. aureus | E. coli | ||
|---|---|---|---|---|
| 4220 | 530 | 1356 | 1682 | |
| 59 | 1 | 2 | > 64 | > 64 |
| 60 | 1 | 2 | > 64 | > 64 |
| 61 | 2 | 4 | > 64 | > 64 |
| 62 | 2 | 4 | > 64 | > 64 |
| 63 | 2 | 4 | > 64 | > 64 |
| Norfloxacin | 2 | 2 | 16 | 16 |
| Oxacillin | 1 | 1 | > 64 | > 64 |
Purohit et al. [24] synthesized a series of novel 3,5-disubstituted thiazolidinediones derivatives (Scheme 20) and evaluated its antibacterial activity against Staphylococcus aureus, Enterococcus faecalis, Klebsiella pneumonia, Escherichia coli and antifungal activity was performed against Candia albicans, Aspergillus niger, Aspergillus flavus. The screening results were compared with ciprofloxacin, norfloxacin for antibacterial and fluconazole, griseofulvin for antifungal activity respectively. Among the synthesized compounds 64, 65, 66 and 67 showed highest antimicrobial potency and their structure were. The significant results of these compounds are presented in Table 19 (Purohit et al. [24]).
Scheme 20.
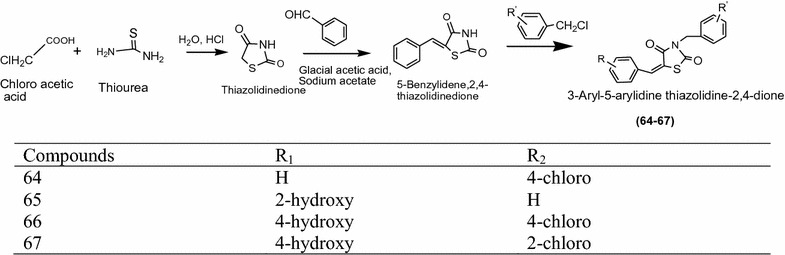
Synthesis of 3,5-Disubstituted thiazolidine-2,4-diones
Table 19.
Antimicrobial activities of synthesized compounds
| Compounds | Minimum inhibitory concentration (MIC μg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. faecalis | K. pneumonia | E. coli | C. albicans | A. niger | A. flavus | |
| 64 | 4 | 4 | 250 | 500 | 16 | 16 | 8 |
| 65 | 4 | 31.25 | 62.5 | 62.5 | 31.5 | 1 | 8 |
| 66 | 2 | 4 | > 500 | > 500 | 4 | 8 | 8 |
| 67 | 1 | 1 | 62.5 | 62.5 | 4 | 4 | 2 |
| Ciprofloxacin | 2 | 2 | 1 | 2 | – | – | – |
| Norfloxacin | 10 | 3.1 | 0.1 | 10 | – | – | – |
| Fluconazole | – | – | – | – | 16 | 8 | 8 |
| Griseofulvin | – | – | – | – | 500 | 100 | 7.5 |
Sharma et al. [25] synthesized a series of novel N-(-5-arylidene-2-(4-chlorophenyl)-4-oxothiazolidin-3-yl)isonicotnamide derivatives by knoevenagel condensation using Scheme 21 and assayed for antibacterial activity against Escherichia coli, Staphylococcus aureus, Bacillus subtilis and antifungal activity against Candida albicans, Aspergillus niger, Saccharomyces cervesia using turbidimetric method. Among the synthesized compounds 68 (N-(5-benzylidene-2-(4-chlorophenyl)-4-oxothiazolidin-3-yl)isonicotinamide), 69 (N-(2-(4-chlorophenyl)-5-(furan-2-ylmethylene)-4-oxothiazolidin-3-yl)isonicotinamide) and 70 (N-(5-(2-nitrobenzylidene)-2-(4-chlorophenyl)-4-oxothiazolidin-3-yl)isonicotinamide) result in wide spectrum antimicrobial activity against all the test bacteria and fungi using ciprofloxacin and clotrimazole as a standard drug respectively. The results of synthesized compounds presented in Table 20 (Sharma et al. [25]).
Scheme 21.
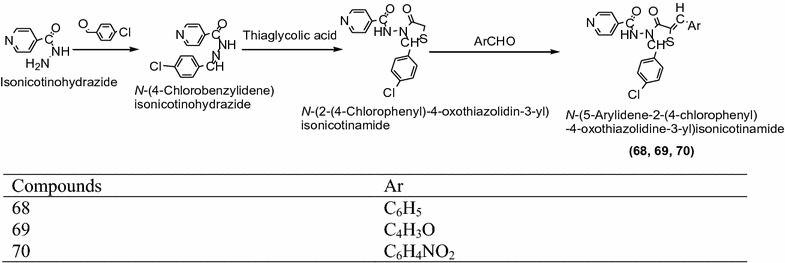
Synthesis of N-(5-Arylidene-2-(4-chlorophenyl)-4-oxothiazolidin-3-yl)isonicotinamide
Table 20.
Antimicrobial activities of synthesized compounds
| Compounds | Minimum inhibitory concentration (MIC) in μg/ml | |||||
|---|---|---|---|---|---|---|
| E. coli | B. subtilis | S. aureus | A. niger | C. albicans | S. cerevisiae | |
| 68 | 1.25 | 1.25 | 0.62 | 0.62 | 0.31 | 1.25 |
| 69 | 0.62 | 0.31 | 0.62 | 0.62 | 0.15 | 0.62 |
| 70 | 0.31 | 0.62 | 0.31 | 0.62 | 0.15 | 0.31 |
| Ciprofloxacin | 0.15 | 0.25 | 0.01 | – | – | – |
| Clotrimazole | – | – | – | 0.10 | 0.30 | 0.20 |
Thiazolidine-2,4-dione derivatives as anti-inflammatory agents
The future of anti-inflammatory compound lies in the development of orally active drugs that decreases production or activities of pro-inflammatory cytokines. Anti-inflammatory compounds are normally used for curing of different infectious conditions. Therefore, the rate of incidence of disease limits its clinical use. Thus here is requirement of designing advance drugs with improved activity and long term relieve from chronic inflammatory condition [26]. The complete knowledge and understanding of the pivotal role of inflammation in seemingly untreated diseases has resulted in development of novel anti-inflammatory agents [27].
Youssef et al. [26] synthesized some novel active pyrazolyl-2,4-thiazolidinedione derivatives (Scheme 22) followed by their in vitro anti-inflammatory evaluation. Among them, compounds 71 and 72 [(Z)-3-allyl-5-((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl(methylene)thiazolidine-2,4-dione] showed moderate to good anti-inflammatory activity using celecoxib as standard and turpentine oil as control. The results of potent derivatives presented in Tables 21, 22 and 23 (Youssef et al. [26]).
Scheme 22.
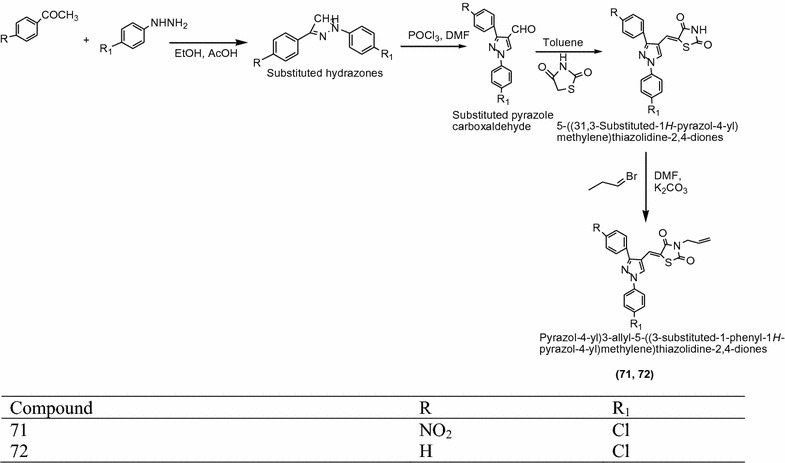
Synthesis of 3-Substituted benzyl-5-((3-substituted-1-phenyl-1H-pyrazol-4-yl)methylene)thiazolidine-2,4-diones
Table 21.
Cyclooxygenase inhibition activity of synthesized compound
| Compounds | Concentration (Um) (no. of experiments) | COX-1 activity (% inhibition) | COX-2 activity (% inhibition) |
|---|---|---|---|
| 71 | 10 (3) | 28.4 ± 11.6 | 19.4 ± 8.2 |
| 72 | 10 (3) | 26.5 ± 6 | 13.6 ± 1.1 |
| Celecoxib | 10 (3) | 0.3 ± 2.5 | 30.8 ± 5.9 |
Table 22.
Inflammation reduction results of synthesized compounds in Formalin induced rat paw edema bioassay
| Compounds | Volume of edema (ml) | ||||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | |
| 71 | 0.31 ± 0.001 | 0.44 ± 0.01 (24) | 0.44 ± 0.01 (46) | 0.46 ± 0.003 (68) | 0.46 ± 0.02 (68) |
| 72 | 0.33 ± 0.02 | 0.41 ± 0.01 (53) | 0.42 ± 0.01 (63) | 0.46 ± 0.01 (72) | 0.49 ± 0.01 (66) |
| Control | 031 ± 0.01 | 0.40 ± 0.01 | 0.55 ± 0.01 | 0.78 ± 0.01 | 0.78 ± 0.008 |
| Celecoxib | 0.31 ± 0.01 | 0.41 ± 0.005 (41) | 0.43 ± 0.02 (50) | 0.50 ± 0.005 (60) | 0.48 ± 0.03 (68) |
Table 23.
Inflammation reduction results of synthesized compounds in turpentine oil induced granuloma pouch bioassay in rat
| Compounds | Volume of exudates (ml) | % inhibition |
|---|---|---|
| 71 | 1.12 ± 0.06 | 51 |
| 72 | 1.12 ± 0.06 | 50 |
| Control | 2.28 ± 0.07 | – |
| Celecoxib | 1.05 ± 0.10 | 54 |
Ma et al. [28] synthesized a series of novel 5-benzylidene thiazolidine-2,4-dione derivatives as presented in Scheme 23 and screened for in vitro inflammation reduction activity. Among the synthesized derivatives, compounds 73 [(Z)-2-(4-((2,4-dioxothiazolidin-5-ylidene)methyl)phenoxy)-N-(3-fluorophenyl)acetamide], 74 [(Z)-N-(3-chlorophenyl)-2-(4-((2,4-dioxothiazolidin-5-ylidene)methyl)phenoxy)acetamide] and 75 [(Z)-2-(4-((2,4-dioxothiazolidin-5-ylidene)methyl)phenoxy)-N-(naphthalene-1-yl)acetamide] were found to be most active anti-inflammatory agent compared to indomethacin as the standard. The results of potent compounds are accessible in Table 24 (Ma et al. [28]).
Scheme 23.
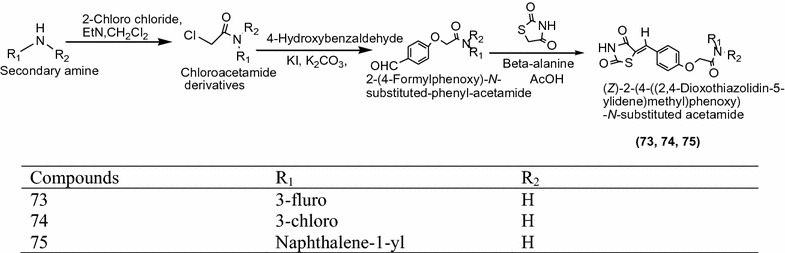
Synthesis of (Z)-2-(4-((2,4-Dioxothiazolidin-5-ylidene)methyl)phenoxy)-N-substituted acetamide
Table 24.
Anti-inflammatory activities of synthesized derivatives
| Compounds | No inhibition (%) ± SD |
|---|---|
| 73 | 41.5 ± 3.1 |
| 74 | 80.9 ± 5.0 |
| 75 | 70.9 ± 13.6 |
| Indomethacin | 63.2 ± 4.0 |
Thiazolidinedione derivatives as anticancer agents
Cancer is a genetic disorder that has always been a major threat all over the world and has been characterized by proliferation of abnormal cells and exhibiting an increasing mortality rate globally and being characterized by rapid formation of abnormal cells and spreading through metastasis to different organs [29, 30]. Currently available treatment (chemotherapy and radiotherapy) for most types of cancer only provide temporary therapeutic benefits as well as being limited by a narrow therapeutic index, remarkable toxicity and acquired resistance [31]. In recent times, advance in clinical researches for anticancer agents have been increased and as neoplastic cells are the anomalous proliferation of cells in the body which cause cancer, various effective compounds derived from natural products have been isolated and developed as anticancer agents. These chemical compounds are formulated with a view to create effective action with minimum side effects against cancer [32].
Patil et al. [33] developed a novel class of 5-benzylidene-2,4-thiazolidinediones using Scheme 24. The synthesized derivatives were screened for the anticancer activity against K-562 (human leukemia), MCF-7 (human breast cancer), HepG-2 (human hepatoma), PC-3 (human prostate cancer), GURAV (human oral cancer) and KB (human nasopharyngeal cancer) cell lines by SRB protein assay. Among this series, 76, 77, 78 and 79 displayed the most potent anticancer activity compared with doxorubicin. The results of synthesized compounds presented in Table 25 (Patil et al. [33]).
Scheme 24.
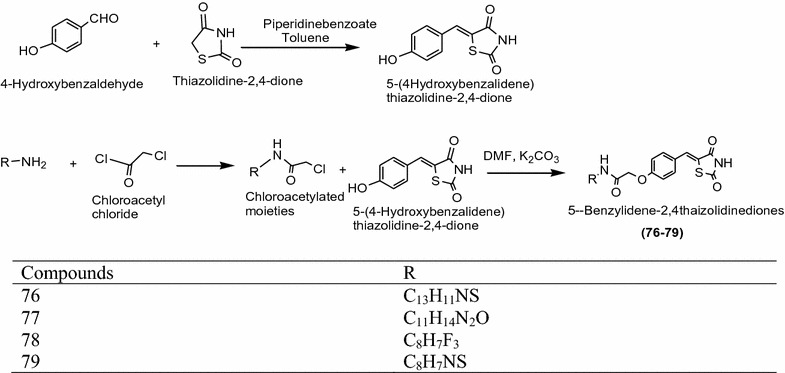
Synthesis of 5-Benzylidene-2,4-thiazolidinedione derivatives
Table 25.
Anti-tumor activities of synthesized derivatives in different cell lines
| Compounds | Diseases | Cancer cell line | Log GI50 (μM) | Log10 TGI (μM) |
|---|---|---|---|---|
| 76 | Leukemia | K-562 | > − 0.4 | > − 4.0 |
| Breast cancer | MCF-7 | − 4.53 | > 4.0 | |
| Hepatoma | HEPG-2 | > − 4.0 | > 4.0 | |
| NSC lung cancer | HOP-62 | − 6.72 | − 4.54 | |
| Prostate cancer | PC-3 | − 4.53 | > − 4.0 | |
| Oral cancer | GURAV | > − 4.0 | > − 4.0 | |
| Nasopharyngeal cancer | KB | > − 4.0 | > − 4.0 | |
| 77 | Leukemia | K-562 | > − 4.0 | > − 4.0 |
| Breast cancer | MCF-7 | > − 4.0 | > − 4.0 | |
| Hepatoma | HEPG-2 | > − 4.0 | > − 4.0 | |
| NSC lung cancer | HOP-62 | − 6.73 | > − 4.0 | |
| Prostate cancer | PC-3 | > − 4.0 | > − 4.0 | |
| Oral cancer | GURAV | > − 4.0 | > − 4.0 | |
| Nasopharyngeal cancer | KB | > − 4.0 | > − 4.0 | |
| 78 | Leukemia | K-562 | − 6.72 | > − 4.0 |
| Breast cancer | MCF-7 | − 6.71 | − 4.52 | |
| Hepatoma | HEPG-2 | > − 4.0 | > − 4.0 | |
| NSC lung cancer | HOP-62 | > − 4.0 | > − 4.0 | |
| Prostate cancer | PC-3 | − 5.60 | > − 4.0 | |
| Oral cancer | GURAV | − 6.73 | − 4.52 | |
| Nasopharyngeal cancer | KB | − 5.65 | > − 4.0 | |
| 79 | Leukemia | K-52 | > − 4.0 | > − 4.0 |
| Breast cancer | MCF-7-5 | − 4.60 | > − 4.0 | |
| Hepatoma | HEPG-2 | > − 4.0 | > − 4.0 | |
| NSC lung cancer | HOP-62 | − 6.77 | > − 4.0 | |
| Prostate cancer | PC-3 | − 4.55 | − 4.54 | |
| Oral cancer | GURAV | > − 4.0 | > − 4.0 | |
| Nasopharyngeal cancer | KB | > − 4.0 | > − 4.0 | |
| Doxorubicin | Leukemia | K-562 | − 5.59 | > − 4.0 |
| Breast cancer | MCF-7 | − 6.88 | − 5.68 | |
| Hepatoma | HEPG-2 | > − 7.0 | − 6.87 | |
| NSC lung cancer | HOP-62 | − 6.91 | − 4.45 | |
| Prostate cancer | PC-3 | − 6.96 | − 5.68 | |
| Oral cancer | GURAV | − 6.97 | − 6.80 | |
| Nasopharyngeal cancer | KB | > − 7.0 | − 6.85 |
Anh et al. [34] designed a chain of novel chromony thiazolidinediones derived from knoevenagel condensation reaction between 3-formyl-7-methoxy chromone with different thiazolidinedione derivatives as presented in Scheme 25. These synthesized derivatives were screened for their cytotoxic activity against Hep-G2 (heptocellular carcinoma), HC-60 (acute promyeloid carcinoma), KB (epidermoid carcinoma), LLC (lewis lung carcinoma), LNCaP (hormone dependent prostate carcinoma), MCF-7 (breast cancer), SW-480 (colon adenocarcinoma) cell lines using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay. In this series compounds 80, 81 and 82 showed highest cytotoxic activity against cancer cell lines. The results of potent compounds are presented in Table 26 (Anh et al. [34]).
Scheme 25.
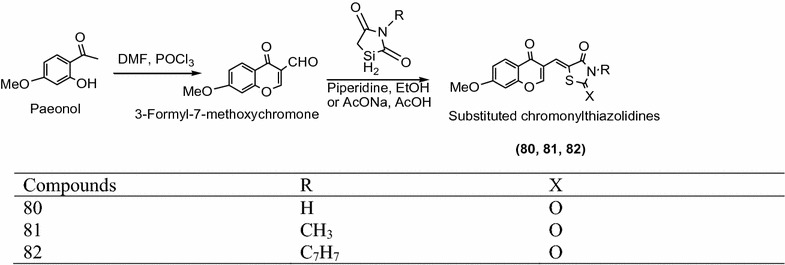
Synthesis of 5-((7-Methoxy-4-oxo-4H-chromen-3-yl)methylene) substituted thiazolidine-2,4-dione
Table 26.
Cytotoxicity of synthesized thiazolidinediones
| Compounds | IC50 (μg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| HepG2 | HC-60 | KB | LLC | LNCaP | LU-1 | MCF-7 | SW-480 | |
| 80 | > 100 | 82.2 ± 4.5 | 44.1 ± 3.6 | 87.4 ± 6.3 | 77.4 ± 5.8 | 52.9 ± 3.4 | 66.0 ± 2.7 | 71.4 ± 3.6 |
| 81 | 86.3 ± 6.4 | 75.3 ± 3.9 | 84.6 ± 4.2 | > 100 | 81.6 ± 6.3 | > 100 | 32.8 ± 1.4 | 90.1 ± 4.8 |
| 82 | 78.4 ± 5.8 | 92.3 ± 5.3 | 74.1 ± 5.1 | 90.1 ± 7.7 | 84.2 ± 4.1 | 65.5 ± 4.1 | 52.7 ± 3.6 | 85.4 ± 7.4 |
| Ellipticine | 1.45 ± 0.08 | 0.56 ± 0.04 | 0.43 ± 0.05 | 0.98 ± 0.04 | 0.86 ± 0.06 | 1.29 ± 0.11 | 0.49 ± 0.04 | 0.64 ± 0.05 |
Kumar et al. [35] synthesized a series of novel 3-(substituted aryl)-1-phenyl-1H-pyrazolyl-2,4-thiazolidinedione derivatives using Scheme 26. These synthesized derivatives were screened for their cytotoxic activity against lung and breast cancer cell lines using standard doxil. In this series 83 and 84 showed highest cytotoxic activity against cancer cell lines. The results of potent compounds are presented in Table 27 (Kumar et al. [35]).
Scheme 26.
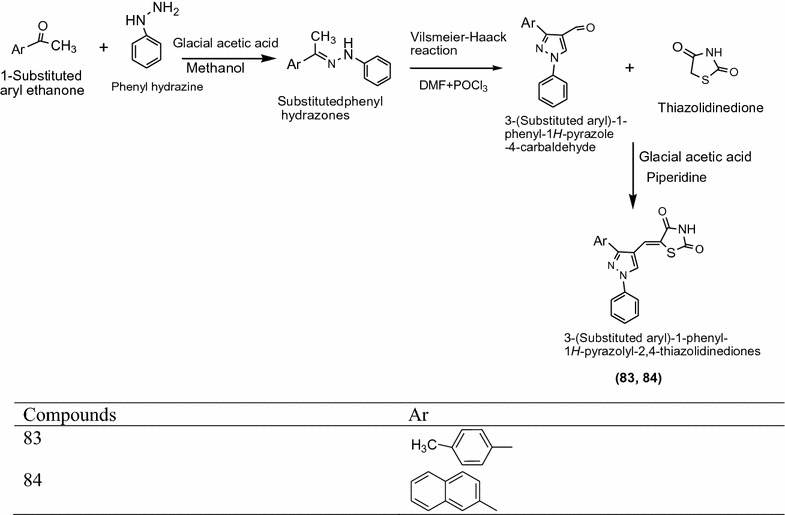
Synthesis of 3-(Substituted aryl)-1-phenyl-1H-pyrazolyl-2, 4-thiazolidinediones
Table 27.
IC50 value of synthesized derivatives against cancer cell lines
| Compounds | IC50 (μM) | ||
|---|---|---|---|
| A549 | MCF-7 | DU145 | |
| 83 | 05.12 | 09.16 | 43.17 |
| 84 | 06.83 | 4.44 | 59.29 |
| Doxil | 07.92 | 08.12 | 07.22 |
Thiazolidinedione derivatives as antioxidant agent
Free radicals produced in several biochemical reactions, cellular metabolism are negotiator for several infections and diseases like atherosclerosis, tumor as well as heart disease. Free radicals are not only formed by normal cellular processes but also produced by exposure of numerous chemical substances (polycyclic aromatic hydrocarbon, cadmium, lead, etc.), radiations, cigarette, smoke, and higher obese food. Usually free radical development is stopped by beneficial compounds known as antioxidant. Antioxidants deactivate free radicals before they attack the cell. Natural antioxidants are body detoxifiers and natural cleansers. They convert toxins of body to harmless waste products. They protect body from many diseases like cancer, heart attack and absorb bad cholesterol. Synthetic antioxidants such as BHT (butylated hydroxytoluene) and BHA (butylated hydroxyanisole), are effective as a antioxidants are also present and are used in several industries but there use has been limited because they can cause cancer as well as other side effects. So there use is decreased in food, cosmetic and pharmaceutical products. Thus, in present there is need for the oxidation inhibitor compounds [18, 36, 37].
Hossain et al. [37] synthesized a series of novel O-prenylated and O-geranylated derivatives of 5-benzylidene2,4-thiazolidinedione by knoevengeal condensation as showed in Scheme 27 and evaluated for their antioxidant activity. Among the synthesized derivatives, compounds 85, 86, 87, 88 and 89 were found to be most active antioxidant agent. The significant results of potent compounds are given in Table 28 (Hossain et al. [37]).
Scheme 27.
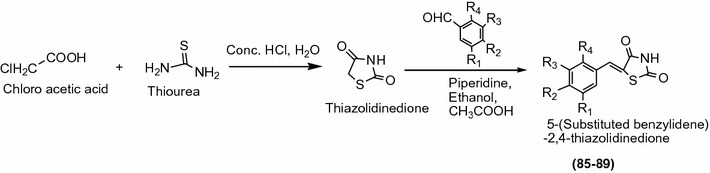
Synthesis of 5-Benzylidene 2,4-thiazolidinediones
Table 28.
Inhibition of DPPH radical by synthesized compounds
| Compounds | R1 | R2 | R3 | R4 | IC50 (μM) |
|---|---|---|---|---|---|
| α-Tocopherol | H | Hydroxyl | H | H | 2.3 |
| 85 | Methoxy | Hydroxyl | H | H | 2.49 |
| 86 | Methoxy | Hydroxyl | Methoxy | H | 2.85 |
| 87 | Methoxy | PRO | H | H | 17.89 |
| 88 | Methoxy | PRO | Methoxy | H | 4.08 |
| 89 | H | GRO | H | H | 9.8 |
DPPH 1,1-diphenyl-2-picrylhydrazyl
Lupascu et al. [4] designed a chain of novel thiazolidinediones containing xanthine moiety (Scheme 28) and evaluated for antioxidant potential using in vitro models such as DPPH radical scavenging assay and ABTS [2,2-azino-bis-(3-ethyl benzothiazoline-6-sulfonic acid] radical scavenging assay method. Among the synthesized derivatives 90, 91, 92 and 93 showed highest antioxidant activity. The results of potent derivatives are given in Table 29 (Lupascu et al. [4]).
Scheme 28.
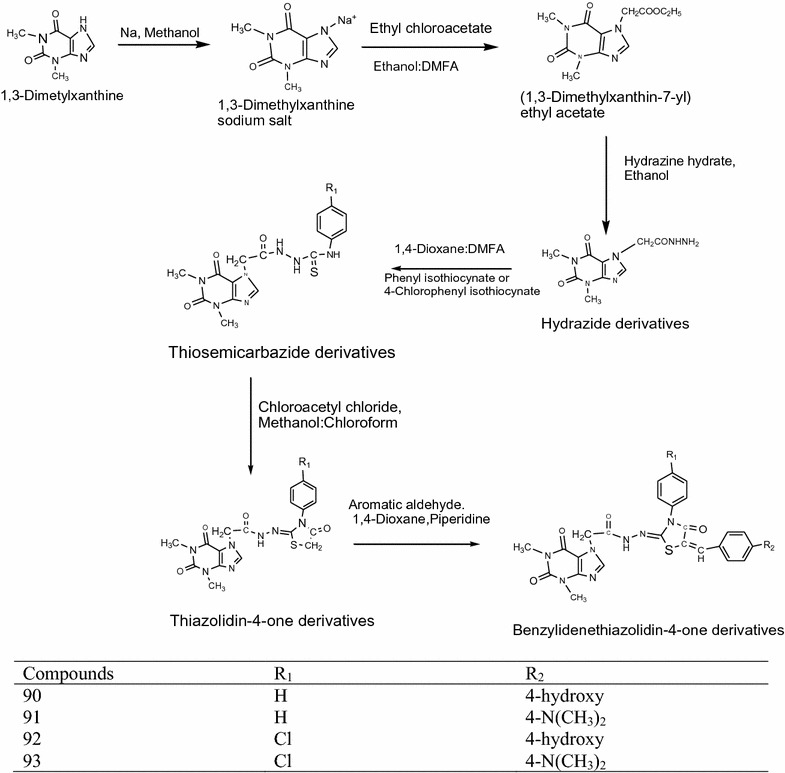
Synthesis of 2-{2-[2-(1,3-Dimethylxanthin-7-yl)acetyl]hydrazono}-3-(4-R1-phenyl-5-(R2-benzyliden)thiazolidin-4-ones
Table 29.
Antioxidant activities of the synthesized derivatives
| Compounds | EC50 mg/ml |
|---|---|
| 90 | 0.025 ± 0.0012 |
| 91 | 0.022 ± 0.0013 |
| 92 | 0.033 ± 0.0014 |
| 93 | 0.026 ± 0.0028 |
| Ascorbic acid | 0.0067 ± 0.0003 |
Thiazolidinedione derivatives as anti-tubercular agents
In present day, treatment of tuberculosis diseases (TB) is chief and challenging problem because of resistance to present regimen and also appearance of drug-resistance strains in tuberculosis like mycobacterium tuberculosis, is transmitted by air and can affected all organ of the body, especially the lungs [38]. The association of tuberculosis with HIV infection is so dramatic that in some cases, nearly two-third of the patients diagnosed with the tuberculosis is also HIV-1 seropositive [39]. The current drug therapy for TB is long and complex, involving multidrug combinations (usually isoniazid, rifampin, ethambutol, and pyrazinamide for the initial 2 months and rifampin and isoniazid for an additional 4 months) [40]. There is also an alarming increase in cases of TB caused by multidrug-resistant strains of M. tuberculosis. Thus, there is a need for new drugs targeting enzymes essential to mycobacterium survival [41, 42].
Chilamakuru et al. [42] synthesized a series of novel 3,5-disubstituted-2,4-thiazolidinediones as presented in Scheme 29 and appraised for anti-tubercular activities with pyrazinamide and streptomycin as the standard drug. Among all the synthesized derivatives, compounds 94, 95 [3-(2-amino-5-nitrophenyl)-5-(4-methoxybenzylidene)-1,3-thiazolidine-2,4-dione], 96 [3-tert-butyl-5-(4-methoxybenzylidene)-1,3-thiazolidine-2,4-dione] and 97 showed the maximum antitubercular activity against Mycobacterium tuberculosis H37Rv strain. The results of synthesized compounds presented in Table 30 (Chliamakuru et al. [42]).
Scheme 29.
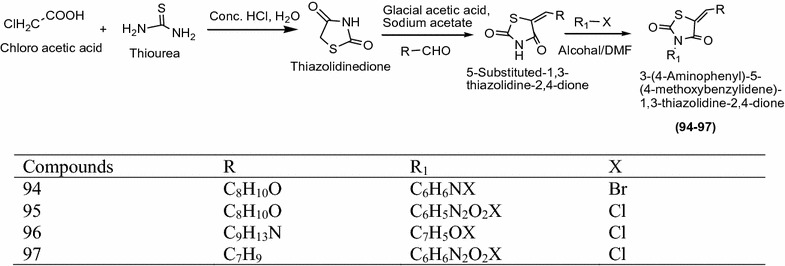
Synthesis of 3,5-Disubstituted-1,3-thiazolidine-2,4-dione
Table 30.
Anti-tubercular activity of synthesized derivatives
| Compounds | MIC μg/ml |
|---|---|
| 94 | 12.5 |
| 95 | 12.5 |
| 96 | 12.5 |
| 97 | 12.5 |
| Pyrazinamide | 3.125 |
| Streptomycin | 6.25 |
Pattan et al. [43] integrating a series of novel substituted thiazolidinediones via knoevenageal condensation reaction as presented in Scheme 30 and evaluated for their antitubercular activites by middle book 7H9 agar medium assay with streptomycin as the standard drug. Among all the synthesized derivatives, compounds 98 [(Z)-N-(3-(4-((2,4-dioxothiazolidin-5-ylidene)methyl)phenoxy)-2-oxopropyl)pyrazin-2-carboxamide] and 99 [(Z)-5-(4-methoxybenzylidene)-3-(2-oxo-2-(pyrazin-2-yl)ethyl)thiazolidine-2,4-dione] showed the maximum antitubercular activity against Mycobacterium tuberculosis H37Rv strain. The results of synthesized compounds presented in Table 31 (Pattan et al. [43]).
Scheme 30.
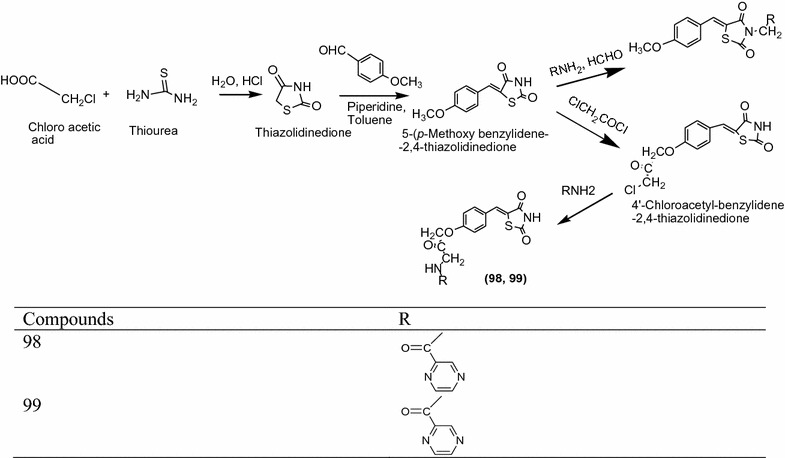
Synthesis of 4-Substitutedacetyl-benzylidene-2,4-thiazolidinediones
Table 31.
Antitubercular activity of synthesized derivatives
| Compounds | 25 μg/ml | 50 μg/ml | 100 μg/ml |
|---|---|---|---|
| 98 | Resistant | Resistant | Sensitive |
| 99 | Resistance | Resistance | Sensitive |
| Streptomycin | Sensitive | Sensitive | Sensitive |
Conclusion
Appraisal of literature reports reveals that thiazolidinediones and its derivatives represent an important class of compound in the medicinal field with various therapeutic potentials, i.e., antidiabetic, antimicrobial, anti-inflammatory, anticancer, antioxidant and antitubercular, antiviral, anti-malarial, anti-HIV and anti-convulsant activities etc. which created immense interest among researchers to synthesized variety of thiazolidinediones. This review focuses especially on synthesized active compounds of thiazolidinediones having different pharmacological activities playing an important role in the medicinal field. These most active thiazolidinediones derivatives may be taken as leads to discover novel agents with therapeutic potential in the future.
Authors’ contributions
PKV designed and finalized the scheme; SA performed review work and ST wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
Thanks to Head, Department of Pharmaceutical Sciences, M. D. University, Rohtak for kind support for providing internet facilities etc.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sucheta, Email: s.m.43singh@gmail.com.
Sumit Tahlan, Email: sumittahlan1989@gmail.com.
Prabhakar Kumar Verma, Email: vermapk422@rediffmail.com.
References
- 1.Yang Y, Hu X, Zhang Q, Zou R. Diabetes mellitus and risk of fall in older adult: a systematic review and meta-analysis. Age Ageing. 2016;45(6):761–767. doi: 10.1093/ageing/afw140. [DOI] [PubMed] [Google Scholar]
- 2.Pattan SR, Kekare P, Patil A, Nikalje A, Kittur BS. Studies on the synthesis of novel 2,4-thiazolidinedione derivatives with ant diabetic activity. Iran J Pharm Sci. 2009;5(4):225–232. [Google Scholar]
- 3.Rekha S, Shantharam U, Chandy V. Synthesis and evaluation of novel thiazolidinedione anti-inflammatory activity. Int Res J Pharm. 2011;2(9):81–84. [Google Scholar]
- 4.Lupascu FG, Dragostin OM, Foia L, Lupascu D. The synthesis and the biological evaluation of new thiazolidin-4-one derivatives containing a xanthine moiety. Lenuta Profire Mol. 2013;18:9684–9703. doi: 10.3390/molecules18089684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moorthy P, Ekambaram SP, Perumal SS (2014) Synthesis, characterization and antimicrobial evaluation of imidazolyl thiazolidinedione derivatives. Arabian J Chem 8:1–7
- 6.Unlusoy MC, Dundar OB, Alanlar N, Ertan R. Synthesis and antimicrobial activity of some new 3-substituted benzyl-5-(4-chloro-2-piperidin-1-ylthiazole-5-yl-methylene)-thiazolidine-2,4-dione derivatives. Turk J Chem. 2006;30:355–360. [Google Scholar]
- 7.Datar PA, Aher SB (2016) Desigh and synthesis of novel thiazoldine-2,4-dione as hypoglycemic agents. J Saudi Chem Soc 196–210
- 8.Swapna D, Sivagani B, Manasa K, Rajita G, Alagarsamy V (2016) Synthesis and evaluation of novel thiazolidinedione derivatives for antidiabetic activity. Int Res J Pharma 15–19
- 9.Badiger NP, Shashidhar N, Vaidya PN. Synthesis of novel 5-{[2-(4-fluorobenzyl)-6-arylimidazo[2,1-b] [1,3,4]thiadiazol-5-yl]methylene}thiazolidine-2,4-diones as potent antidiabetic agents. Int J Sci Eng Appl. 2015;4(2):24–29. [Google Scholar]
- 10.Patil SD, Nawale SL, Balasurbramaniyan V. Evaluation of thiazolidinedione derivatives for acute toxicity and potential antidiabetic activity. Der Pharm Chem. 2015;7(5):216–223. [Google Scholar]
- 11.Srikanth L, Raghunandan N, Srinivas P, Reddy GA. Synthesis and evaluation of newer quinoline derivatives of thiazolidinedione for their ant diabetic activity. Int J Pharm Bio Sci. 2010;1:120–131. [Google Scholar]
- 12.Nikaljea PGA, Choudharia S, Une H. Design, synthesis and hypoglycemic activity of novel 2-(4-((2, 4-dioxothiazolidin-5-ylidene) methyl)-2-methoxyphenoxy)-N-substituted acetamide derivatives. Pelagia Res Library. 2012;2:1302–1314. [Google Scholar]
- 13.Jiwane SK, Singh VK, Namdeo KP, Prajpap SK. Synthesis of some novel 2,4-thiazolidinedione derivatives and their biological screening as ant diabetic agents. Asian J Chem. 2009;21:5068–5072. [Google Scholar]
- 14.Grag A, Chawla P, Shubhini SA. Substituted-arylidene-3-substituted-benzyl-thiazolidine-2,4-dione analogues as anti-hyperglycemic agents. Int J Drug Dev Res. 2012;4(3):141–146. [Google Scholar]
- 15.Bhat BA, Ponnala S, SahuDP Tiwari P, Tripathi BK, Srivastava AK. Synthesis and antihyperglycemic activity profiles of novel thiazolidinedione derivatives. Bioorg Med Chem. 2004;12:5857–5864. doi: 10.1016/j.bmc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Jawale DV, Pratap UR, Rahuja N, Srivastava AK, Mane RA. Synthesis and antihyperglycemic evaluation of new 2,4-thiazolidinediones having biodynamic aryl sulfonylurea moieties. Bioorg Med Chem. 2012;22:436–439. doi: 10.1016/j.bmcl.2011.10.110. [DOI] [PubMed] [Google Scholar]
- 17.Vivekanand B, Mahendra Raj K, Mruthyunjayaswamy BHM. Synthesis, characterization, antimicrobial, DNA-cleavage and antioxidant activities of 3-((5-chloro-2-phenyl-1H-indol-3-ylimino)methyl)quinoline-2(1H)-thione and its metal complexes. J Mol Struct. 2015;1079:214–224. doi: 10.1016/j.molstruc.2014.08.033. [DOI] [Google Scholar]
- 18.Martin APM, Machado P, Piovesan LA, Flores AFC, De Campos MMA, Scheidt C, Bonacorso HG, Zanatta N. Microwave-assisted synthesis and antimicrobial activity of 5-trihalomethyl-3-arylisoxazoles. MonatshChem. 2008;139:985–990. doi: 10.1007/s00706-007-0849-1. [DOI] [Google Scholar]
- 19.Nawale SL, Dhake AS. Synthesis and evaluation of novel thiazolidinedione derivatives for antibacterial activity. Der Pharma Chemica. 2012;4(6):2270–2277. [Google Scholar]
- 20.Nastasa CM, Duma M, Pirnau A, Vlase L, Tiperciuc B, Oniga O. Development of new 5-(chromene-3-yl)methylene-2,4-thiazolidinediones as antimicrobial agents. Clujul Med. 2016;89(1):122–127. doi: 10.15386/cjmed-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alagawadi KR, Alegaon SG. Synthesis, characterization and antimicrobial activity evaluation of new 2,4-thiazolidinediones bearing imidazo[2,1-b][1,3,4]thiadiazole moiety. Arabian J Chem. 2011;4:465–472. doi: 10.1016/j.arabjc.2010.07.012. [DOI] [Google Scholar]
- 22.Khan FAK, Jadhav KS, Patil RH, Shinde DB, Arote RB, Sangshetti JN. Biphenyl tetrazole-thiazolidinediones as novel bacterial peptide deformylase inhibitors: synthesis, biological evaluations and molecular docking study. Biomed Pharmacother. 2016;83:1146–1153. doi: 10.1016/j.biopha.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Liu XF, Zheng CJ, Sun LP, Liu XK, Piao HR. Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. Eur J Med Chem. 2011;46:3469–3473. doi: 10.1016/j.ejmech.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Purohit SS, Alman A, Shewale J. Synthesis and antimicrobial activity of a new series of 3,5-disustitutedthiazolidine-2,4-diones. Int J Pharm Pharm Sci. 2012;4(3):273–276. [Google Scholar]
- 25.Sharma R, Vinay V. Synthesis and antimicrobial activity of thiazolidinedione derivatives. Int J Sci Res Rev. 2012;1(1):57–66. [Google Scholar]
- 26.Youssef AM, White MS, Villanueva EB, Ashmawy IM, Klegeris A. Synthesis and evaluation of novel pyrazolyl-2,4-thiazolidinediones a anti-inflammatory and neuroprotective agents. Bioorg Med Chem. 2010;18:2019–2028. doi: 10.1016/j.bmc.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L, Xie C, Ma Y, Liu J, Xiang M, Ye X, Zheng H, Chen Z. Synthesis and biological evaluation of novel 5-benzylidenethiazolidine-derivatives 2,4-dione derivatives for the treatment of inflammatory diseases. J Med Chem. 2011;54:211–235. doi: 10.1021/jm1010572. [DOI] [PubMed] [Google Scholar]
- 29.Dantu AS, Shankarguru P, Devi DR, Hari BNV. Evaluation of in vitro anticancer activity of hydroalcohalicextract of Tabernaemontana divaricata. Asain J Pharm Clin Res. 2012;5(3):50–61. [Google Scholar]
- 30.Arafa RK, Hegazy GH, Piazza GA, Abadi AH. Synthesis and in vitro antiproliferative effect of novel quinoline-based potential anticancer agents. Eur J Med Chem. 2013;63:826–883. doi: 10.1016/j.ejmech.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 31.El-Damasy AK, Seo SH, Cho NC, Kang SB, Pae AN, Kim KS, Keum G. Design, synthesis, in-vitro antiproliferative activity and kinase profile of new picolinamide based 2-amido and ureido quinoline derivatives. Eur J Med Chem. 2013;101:754–768. doi: 10.1016/j.ejmech.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Merina N, Chandra KJ, Jibon K. Medicinal plants with potential anticancer activities: a review. Int Res J Pharm. 2012;3(6):26–30. [Google Scholar]
- 33.Patil V, Tilekar K, Munj SM, Mohan R, Ramaa CS. Synthesis and primary cytotoxicity evaluation of new 5-benzylidene-2,4-thiazolidinedione derivatives. Eur J Med Chem. 2010;45:4539–4544. doi: 10.1016/j.ejmech.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Anh HLT, Cuc NT, Tai BH, Yen PH, Xuan N, Thao DT, Nam NH, Minh CV, Kiem PV, Kim YH. Synthesis of chromonylthiazolidines and their cytotoxicity to human cancer cell lines. Molecules. 2015;20:1151–1160. doi: 10.3390/molecules20011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar KS, Reddy BM, Babu VH. Synthesis of some novel 2,4-thiazolidinedione incorporated pyrazole derivatives as anticancer agents. Int J Pharm Sci. 2014;6(2):831–834. [Google Scholar]
- 36.Feng L, Lv K, Liu M, Wang S, Zhao J, You X, Li S, Cao J, Guo H. Eur J Med Chem. 2012;55:125–136. doi: 10.1016/j.ejmech.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Hossain SU, Bhattachary S. Synthesis of O-prenylared and O-geranylated derivatives of 5-benzylidene 2,4-thiazolidinediones and evaluation of their free radical scavenging activity as well s effect on some phase II antioxidant/detoxifying enzymes. Bioorg Med Chem Lett. 2007;17:1149–1154. doi: 10.1016/j.bmcl.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 38.Junior NS, Hyaric ML, Da Costa CF, Correa TA, Taveira AF, Araujo DP, Reis EFC, Lourenco MCS, Vicente FRC, De Almeida MV. Preparation and antitubercular activity of lipophilic diamines and amino alcohols. Med Inst Oswaldo Cruz. 2009;104(5):703–705. doi: 10.1590/S0074-02762009000500006. [DOI] [PubMed] [Google Scholar]
- 39.Illango K, Arunkumar S. Synthesis, antimicrobial and antitubercular activities of some novel trihydroxybenzamidoazetidin-2-one derivatives. Trop J Pharm Res. 2011;10(2):219–229. [Google Scholar]
- 40.Thompson AM, Blaser A, Anderson RF, Shinde SS, Franzblau SG, Ma Z, Denny WA, Palmer BD. Synthesis, reduction potential and anti-tubercular activity of ring A/B analogues of the bioreductive drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine. J Med Chem. 2008;52(3):637–645. doi: 10.1021/jm801087e. [DOI] [PubMed] [Google Scholar]
- 41.Bate AB, Kalin JH, Fooksman EM, Amorose EL, Price CM, Williams HM, Rodig MJ, Mitchell MO, Cho SH, Wang Y, Franzblau SG. Synthesis and antitubercular activity of quaternized promazine and promethazine derivatives. Bioorg Med Chem. 2006;17(5):1346–1348. doi: 10.1016/j.bmcl.2006.11.091. [DOI] [PubMed] [Google Scholar]
- 42.Chilamakuru N, Shankarananth V, Rajaskhar KK, Singirisetty T. Synthesis, characterization and anti-tubercular activity of some new 3,5-disubstituted-2,4-thiazolidinediones. Asian J Pharm Clin Res. 2013;6(5):29–33. [Google Scholar]
- 43.Pattan S, Kedar M, Pattan J, Dengale S, Sanap M, Gharate U, Shinde P, Kadam S. Synthesis and evaluation of some novel 2,4-thiszolidinedione derivatives for antibacterial, antitubercular and antidiabetic activities. Indian J Chem. 2012;51B:1421–1425. [Google Scholar]


