Abstract
Chronic wasting disease (CWD) is a rapidly spreading prion disorder affecting captive and free-ranging cervids. The zoonotic potential of CWD is unknown, as well as the mechanism for its highly efficient transmission. A top priority to minimize further spreading of this disease and its potential impact on environmental prion contamination is the development of a non-invasive, sensitive, and specific test for ante-mortem detection of infected animals. Here, we optimized the protein misfolding cyclic amplification (PMCA) assay for highly efficient detection of CWD prions in blood samples. Studies were done using a blind panel of 98 field-collected samples of whole blood from codon 96 glycine/glycine, captive white-tailed deer that were analyzed for prion infection post-mortem by immunohistochemistry (IHC). The results showed a sensitivity of 100% in animals with very poor body condition that were IHC-positive in both brain and lymph nodes, 96% in asymptomatic deer IHC-positive in brain and lymph nodes and 53% in animals at early stages of infection that were IHC-positive only in lymph nodes. The overall mean diagnostic sensitivity was 79.3% with 100% specificity. These findings show that PMCA might be useful as a blood test for routine, live animal diagnosis of CWD.
Introduction
Prion diseases are fatal infectious diseases affecting humans and various species of mammals, including sheep, goats, mink, cervids, cattle and felines. Although prion diseases are rare in humans, the reported animal-to-human transmission and the increasing prevalence of some animal diseases present important problems for public and animal health. The most common animal prion disease is scrapie, a disorder of sheep and goats that has been recognized for centuries and has become an endemic problem nearly worldwide. Currently the most worrisome animal diseases are bovine spongiform encephalopathy and chronic wasting disease (CWD). CWD is the only known prion disease of wild animals; it is highly contagious and the exact origin, prevalence and mechanisms for transmission remain incompletely understood1–3. The disease has been rapidly expanding geographically and now affects 24 states in the USA, two Canadian provinces, South Korea and Norway. The risk of transmission of CWD to other animal species is unknown and surveillance to detect the infection in non-cervid species is limited. Much effort has been dedicated to analyze the potential for CWD transmission to humans4. To date, no evidence of CWD transmission to humans has been documented. Nevertheless, the US Centers for Disease Control and Prevention (CDC) strongly advise to keep cervid derived prions out of the human food chain. The potential transmission of CWD prions to humans has no clear answer yet, due to limitations with animal models and the lack of detailed knowledge concerning the factors in the host and the mechanisms controlling the species barrier.
CWD affects various species of captive and free-ranging cervids, including mule deer, white-tailed deer, elk, reindeer, sika, muntjac and moose3. The clinical symptoms include progressive weight loss, difficulties in movement, behavioral changes, decreased interactions with other animals, lethargy, lowering of the head, tremors, nervousness and repetitive walking in set patterns5. As with other prion diseases, brain abnormalities include spongiform degeneration, neuronal loss, glial activation and the accumulation of a protease-resistance version of the prion protein, termed PrPSc. PrPSc is mostly found in the CNS, but minute levels have also been detected in various peripheral tissues6. Compelling evidence indicates that PrPSc is the sole component of the infectious agent. Therefore, the hallmark event in the disease is the conversion of the normal isoform of the prion protein (termed PrPC) into the misfolded form, which is templated by PrPSc present in the infectious material7,8.
The efficient propagation of CWD in captive and free-ranging cervids suggests that transmission may involve retention and enrichment of infectious prions in the environment9–12. Studies have reported instances of CWD recurrence upon reintroduction of animals to premises previously exposed to infected animals many years before13. Infectious prions can enter the environment through saliva, feces, urine, blood or placenta tissue from infected animals, as well as by decomposing carcasses from dead animals14–16. Importantly, CWD prion shedding in excreta has been shown to occur many months before onset of clinical disease and the cumulative amount of prions released from infected, but not yet sick, animals could be very large11. Various reports have shown that infectious prions bind tightly to soil and remain infectious after years in this material17–19, suggesting that environmental contamination may play an important role in CWD spreading. This conclusion is further supported by recent findings showing that prions bind to a variety of components of the environment, including plants, and remain infectious by oral administration20. Genetic analyses have shown that various polymorphisms in the gene encoding the prion protein (Prnp) affects CWD susceptibility in cervid populations. In white-tailed deer, a polymorphism at position 96, either a glycine (G) or a serine (S), influences CWD susceptibility, rate of disease progression and the extent of peripheral distribution of PrPSc (ref.21). The most common genetic makeup of wild white-tailed deer affected by CWD is GG at position 96, with the presence of at least one copy of the S-allele reducing the disease susceptibility21.
An important goal to minimize the further spreading of CWD is the development of an assay for highly sensitive, non-invasive, and inexpensive ante-mortem detection of prions in infected, but not clinically sick animals. Currently, CWD is definitively diagnosed by post-mortem examinations of brain, brain stem and lymphoid tissues3. For this purpose, brain, brain stem at the obex, and medial retropharyngeal lymph nodes (MRPLN) samples are collected post-mortem, and analyzed by immunohistochemistry (IHC), ELISA, and/or western blot (WB) to detect PrPSc (ref.22). It is estimated that post-mortem IHC analysis (considered the gold standard for CWD regulatory testing in the USA) can identify the presence of infectious prions in the MRPLN and obex, as early as three and six to nine months after infection, respectively23. Recently, IHC detection of PrPSc in rectal biopsy was evaluated as an ante-mortem test24–26. The diagnostic sensitivity of this assay was variable depending on the genotype of the animal and the disease progression at the moment of sample collection, ranging from 36% to 100%25. Particularly, low sensitivity was observed in animals at the early stage of infection when the obex was negative for PrPSc, and positive staining was only detected in MRPLN25. Furthermore, although the rectal biopsy is relatively simple, it is an invasive, and expensive procedure.
Like scrapie, CWD has a wide distribution of prion infection in peripheral tissues and biological fluids. Indeed, infectivity bioassays in deer or transgenic mice expressing cervid prion protein (PrP) have shown the presence of infectious materials in a large variety of tissues, including CNS tissues, peripheral nerves, lympho-reticular organs, gastro-intestinal tissues and skeletal muscle2. Infectivity was also found in various biological and excretory fluids, including blood, saliva, urine and feces2,3. These findings offer hope for the development of an assay that can detect prions in easily accessible biological fluids. However, it is likely that the quantity of PrPSc present in these fluids is very small, orders of magnitude under the level of sensitivity of the commonly used WB and ELISA assays. In recent years, the implementation of techniques for extremely high sensitive detection of prions in biological fluids has been a major breakthrough in diagnosing various prion diseases27–30. These techniques rely on the amplification of PrPSc and include the protein misfolding cyclic amplification (PMCA) and a variant of it termed real-time quaking induced conversion (RT-QuIC)31,32. Both of these procedures take advantage of the PrPSc capacity to seed the conversion of PrPC into the abnormal form and employ a mechanical force to fragment the PrPSc aggregates leading to the cyclic amplification of the prion replication process. These procedures enable specific detection of very small quantities of PrPSc in tissues and biological fluids, likely approaching the levels of single particles of PrPSc. Both PMCA and RT-QuIC have been used to detect with high sensitivity and specificity CWD prions in various tissues, fluids and excreta14,15,20,33–37. Arguably, blood offers the best opportunity for non-invasive and routine testing of large populations of cervids for potential prion infection. PMCA has been extensively used in the past to detect various human and animal prions in blood samples collected at both the symptomatic and asymptomatic stages of the disease38–44. However, until now, it has not been used to detect CWD prions in blood samples collected from naturally infected deer. The main goal of this study was to optimize PMCA for sensitive detection of CWD prions in blood and study the utility of the technique in field collected samples for ante-mortem diagnosis of CWD-infected animals.
Results
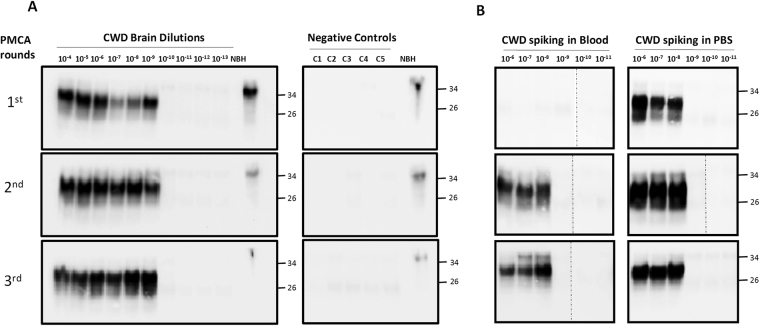
To optimize PMCA for ultra-sensitive detection of CWD PrPSc and analyze the limit of detection of the assay, we first serially diluted a 10% brain homogenate from a deer with confirmed CWD and performed serial rounds of PMCA. Under our optimized conditions, maximum amplification was obtained after only 1 round of 144 PMCA cycles, with a limit of detection equivalent to a 10−9 dilution of the brain extract (Fig. 1A). The exact limit of detection depends on the amount of PrPSc present in the particular animal and on the brain region utilized, which usually ranges between 10−8 and 10−11. This sensitivity is similar to that reported previously for rodent and human prion samples43,45–47 and is on the range of detection of single PrPSc particles45,46. None of the unseeded PMCA assays gave any protease-resistant PrPSc signal (Fig. 1A). Next, we spiked the same dilutions of CWD brain homogenate into healthy, CWD-free deer blood, processed the samples through sarkosyl precipitation (as indicated in the Methods section) and performed the PMCA assay. Maximum amplification was achieved in the second round of PMCA (Fig. 1B), indicating that despite the sample processing the presence of blood components still interfered with the assay. Nevertheless, the limit of detection was the same as the samples spiked in buffer (Fig. 1B).
Figure 1.
PMCA of CWD prions. (A) To optimize CWD PrPSc amplification by PMCA and determine the limit of detection, brain extracts from CWD sick animals was serially diluted (10−4 to 10−13) in buffer and subjected to various consecutive rounds of 144 PMCA cycles. As negative controls, 5 tubes (C1 to C5) in which PMCA was done without CWD brain homogenate were used to control for possible cross-contamination. After each PMCA round, an aliquot of 10 µL was taken to analyze for PrPSc signal by western blot using the 6H4 anti-PrP antibody. All samples, except the normal brain homogenate (NBH), were treated with 10 µg/mL of PK for 1 h at 37 °C, before western blotting to differentiate PrPSc from PrPC. (B) Whole blood from a healthy deer was spiked with CWD brain homogenate at distinct final dilutions (10−6 to 10−11). The same dilutions were spiked in buffer (PBS) as control (right panel). After processing by high-speed centrifugation in the presence of sarkosyl (as described in Methods), samples were subjected to three consecutive rounds of PMCA. The PrPSc signal was assessed by Western blot analysis after PK digestion. NBH refers to the transgenic normal (healthy) brain homogenate, used as migration control marker. Dashed lines in some of the blots indicate splicing, done to remove unrelated lanes. Numbers in the right indicate the position of molecular weight markers.
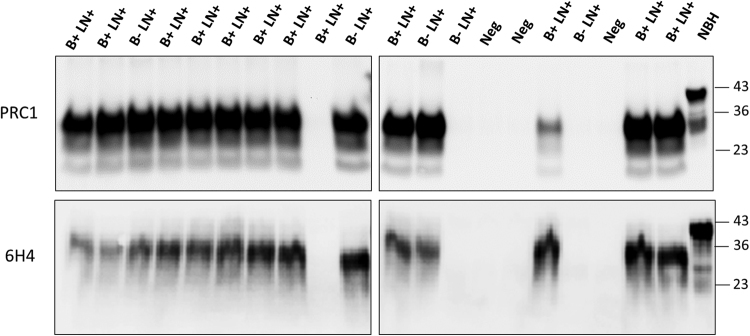
We analyzed blood samples from five deer showing very poor body condition, which were positive in both obex and MRPLN by IHC, along with three replicates coming from a pool of blood from seven animals with negative IHC. The results showed a clear detection of PrPSc signal in all of the samples from CWD-infected animals and none in the controls (Fig. 2 shows representative samples from this set), indicating a 100% sensitivity and specificity in this small group of samples. Next, we analyzed a panel of 93 whole blood samples provided blindly by the United States Department of Agriculture (USDA) scientists from captive white-tailed deer. For the studies included in this article we focused exclusively on deer harboring the most common polymorphic variant (GG at position 96). These samples were from animals which did not show any gross clinical or behavioral abnormalities and included 10 samples collected from farms free of CWD, 49 that were IHC positive in both obex and MRPLN and 34 that were not detected in obex and IHC-positive only in MRPLN. Samples were processed independently by two different investigators who ran each sample in duplicate. Figure 3 shows the results for representative samples from each group, which were detected using two different anti-PrP antibodies. For the samples from animals that were PrPSc positive in obex and MRPLN we detected most of them (47 out of 49) as positives after two or three rounds of PMCA, whereas none of the 10 samples coming from CWD-free deer gave any signal (Fig. 3). Conversely, 18 of the 34 deer that were only IHC positive in the MRPLN gave a positive signal in the PMCA assay. Overall, the assay reached a sensitivity of 100% in symptomatic animals, 96% in deer positive in obex and MRPLN and 53% in animals that were only positive in MRPLN (Table 1). The overall mean diagnostic sensitivity was 79.3% and specificity was 100%.
Figure 2.
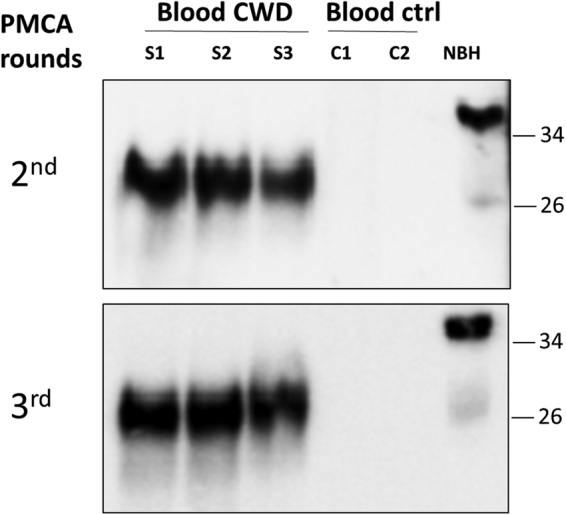
PrPSc detection in blood of animals showing clinical symptoms of CWD. Representative samples of whole blood (80 µL) from three white-tailed deer positive for PrPSc in obex and MRPLN as examined by IHC and presenting with poor body condition (S1, S2, S3), were analyzed by PMCA. As controls, blood from two deer from a CWD-free control herd (C1 and C2) were analyzed in parallel. Samples were subjected to three serial rounds of PMCA and the results of rounds 2 and 3 are shown. The PRC1 anti-PrP antibody was used for western blotting immunodetection. All samples were treated with PK, except the transgenic mice normal brain homogenate (NBH), used as migration control. Numbers in the right indicate the position of molecular weight markers.
Figure 3.
Blind study of PrPSc detection in blood of CWD-infected, but asymptomatic animals. Representative samples of whole blood (200 µL) from asymptomatic white-tailed deer, were collected from either CWD-free or CWD-infected herds. Samples analyzed included 49 animals that were positive for PrPSc-staining in both the MRPLN and the obex via IHC (B + LN + ), 34 animals that were positive for PrPSc-staining only in the MRPLN via IHC (B-LN + ) and 10 that were negative in both brain and MRLPN. The figure shows representative samples from 12 B + LN + , 5 B-LN + and 3 B-LN- (Neg). The entire set of samples was analyzed independently by two different investigators in duplicate. Top and bottom panels show the results from the two different investigators which developed their western blots using two distinct anti-PrP antibodies (PRC1 and 6H4). Samples were subjected to three serial rounds of PMCA and the results obtained in the third round are shown. All samples were treated with PK, except the transgenic mice normal brain homogenate (NBH), used as migration control. Numbers in the right indicate the position of molecular weight markers.
Table 1.
Summary of the results obtained in a blind study of detection of CWD prions in blood samples from white-tailed deer at various stages of CWD.
| CWD status | Total number of animals | PMCA positive1 | % Correct diagnosis |
|---|---|---|---|
| Symptomatic (B + LN + ) | 5 | 5 | 100% |
| Asymptomatic (B + LN + ) | 49 | 47 | 97% |
| Asymptomatic (B- LN + ) | 34 | 18 | 53% |
| Negative (B- LN-) | 10 | 0 | 100% |
1Samples were declared as positive in PMCA if at least one of the replicates gave a protease-resistant PrPSc signal in any of the PMCA rounds analyzed.
As we previously described for the detection of human PrPSc in blood from vCJD patients, it is possible to eliminate the time-consuming and effort-demanding pre-cleaning step to remove inhibitors of the PMCA reaction by using small volumes of blood samples43. In order to evaluate whether this strategy may also work with CWD blood, we took five random samples from those previously shown to be positive with the standard assay, along with two negatives, and added different small volumes of whole blood directly into the PMCA reaction. As shown in Fig. 4, using 10 µl of blood showed perfect sensitivity and specificity in this set of samples analyzed, whereas larger or smaller volumes only enabled correct detection in some of the samples. These results may permit the implementation of a simpler procedure for PrPSc detection in CWD blood, leading to substantial savings of time and samples, but the robustness of the simplified assay needs to be confirmed by blind analysis of a larger number of samples.
Figure 4.
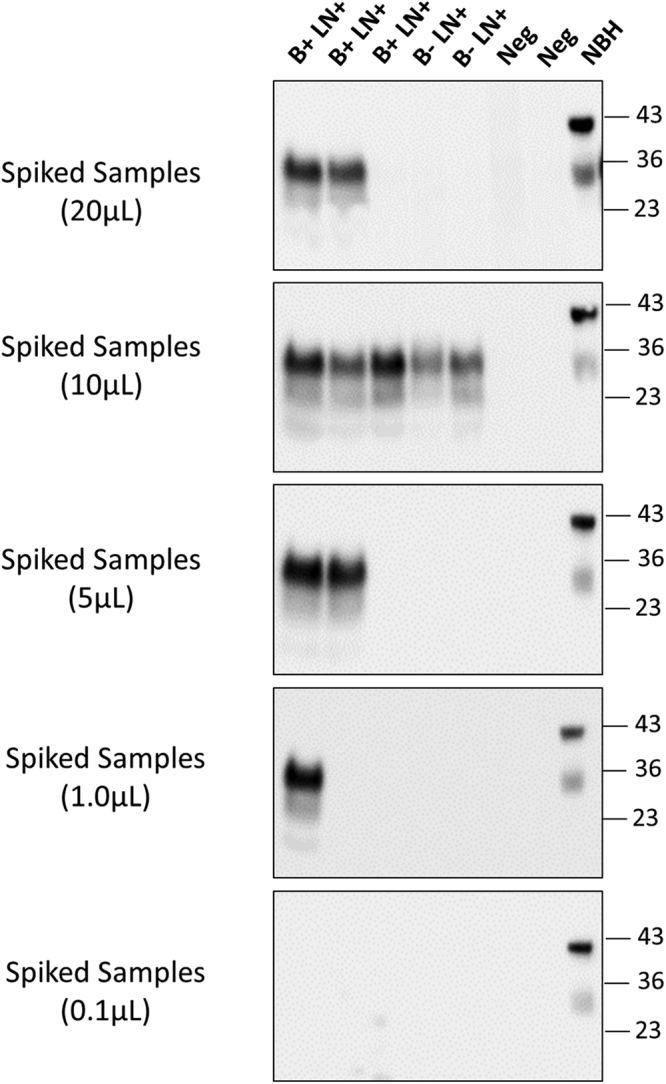
Detection of PrPSc in small volume of blood and removal of pre-cleaning step. To estimate the minimum amount of blood needed for detection, different volumes (20, 10, 5, 1 and 0.1 µL) of whole blood from five representative samples that were positive in the blinded study (3 B + LN + and 2 B-LN + ), were directly added to a 10% brain homogenate from cervid transgenic mice. As controls we used two samples from CWD negative animals. Samples were subjected to three sequential rounds of PMCA and PrPSc detected by Western blot using the PRC1 antibody. The figure shows the results of the third round of PMCA. As before, all samples were treated with PK, except the transgenic mice normal brain homogenate (NBH), used as migration control. Numbers in the right indicate the position of molecular weight markers.
Discussion
A safe, non-invasive, sensitive and specific live animal test to identify cervids infected with CWD prions is a top priority to control further spreading of this emerging infectious disease. Availability of such a test could have many important applications, including: (i) screening of deer farms to remove infected animals before they can spread substantial amounts of prions into the environment; (ii) monitoring the efficacy of prion decontamination procedures; (iii) monitoring the extent of environmental prion contamination; and (iv) whole herd screenings for determination of CWD status.
Currently, the only definitive way to diagnose CWD is by post-mortem examination of brain and lymphoid tissues for the presence of PrPSc by IHC, the current gold standard for prion detection. Various attempts have been made to develop a test for ante-mortem detection of prions during the long, silent period between prion infection and the onset of clinical signs of the disease, which can encompass several months or years. One strategy that has been evaluated is palatine tonsil biopsy followed by IHC analysis48. However, this method is invasive and time consuming, and requires anesthesia that may lead to complications. An alternative possibility for ante-mortem CWD detection, is rectal biopsy followed by IHC analysis for protease resistant PrPSc. Various independent studies have shown the efficacy of this procedure for pre-clinical detection of prions in CWD-infected animals24–26. In a collaborative study between the Canadian Food Inspection Agency and the USDA, a detailed analysis was done for the sensitivity and specificity of IHC detection of PrPSc in rectal biopsy samples25. For this study, samples were collected post-mortem from white-tailed deer in four CWD-infected herds. PrPSc detection was compared to the CWD infection rate estimated by IHC analysis of the obex and MRPLN. The overall diagnostic sensitivity of this assay was estimated at 68%, including all stages of the disease and all polymorphic variants analyzed25. The study also showed a clear correlation between disease progression, genotype at codon 96 and the presence of PrPSc signal in rectal biopsy samples. Disease progression was estimated from the presence and degree of PrPSc signal in obex as well as prion detection in MRPLN. In early disease progression (low obex grades), the diagnostic sensitivity of the rectal biopsy assay was 36% in samples from animals that did not have PrPSc in brain, but were positive in MRPLN25. The genotype at codon 96 also strongly influenced the outcome of the rectal biopsy IHC, with GG animals having a higher detection rate than GS and SS animals25. Sensitivity was slightly increased by using RT-QuIC instead of IHC, reaching an overall diagnostic sensitivity (including all disease stages and polymorphisms) of 70% in samples obtained by rectal biopsy36. In this study, sensitivity for RT-QuIC in rectal samples from animals negative in the obex was 25%, compared to only 8% using IHC. However, several false positives were found using this technique, decreasing specificity to 94%36. The same authors also analyzed the efficacy of RT-QuIC using nasal brushing as a less invasive sample collection compared to rectal biopsy. The results showed a diagnostic sensitivity of only 34%49, suggesting that this strategy is significantly less effective for cervid samples than human sCJD samples50.
In our blind study using field collected blood samples from white-tailed deer, we found a mean overall diagnostic sensitivity of 79.3%, with 100% specificity. Sensitivity for blood samples from CWD-infected animals at the pre-clinical stage of the disease was 96%, when were IHC positive in both the obex and the MRPLN. For similar samples in which only the MRPLN was IHC positive, sensitivity was reduced to 53%. Provided that the infection status of these animals was correctly determined by IHC and because of the extremely high sensitivity of PMCA, which is able to detect few particles of PrPSc aggregates45,46, these results suggest that at some stages of the pre-clinical disease, infected animals may not have PrPSc in blood. Since obtaining blood samples is relatively non-invasive, a much more accurate ante-mortem laboratory diagnosis of CWD could be done by repeating the PMCA test multiple times in live animals. This scheme is useful because sensitivity approaches 100% as the disease progress. Nevertheless, further studies need to be done employing larger number of samples in which the status of prion infection has been confirmed by IHC and a biochemical procedure (western blot or ELISA), as well as analyzing the efficacy of the test in animals harboring the less frequent Prnp genotypes (such as GS and SS at position 96). Studies should also be done in blood samples longitudinally collected from experimentally infected deer to determine the earliest time in which PrPSc can be detected by PMCA and the dynamic changes on the PrPSc levels present in blood during the incubation period. Our results suggest that PMCA may provide a suitable platform for rapid prion diagnosis in farmed and wild cervids. Early and non-invasive CWD prion detection could not only help to control severely affected premises, but also be useful for surveillance of areas where CWD cases are not yet known to occur.
Materials and Methods
Study Populations
The samples utilized in the present study were collected by scientists from the USDA from captive, codon 96 GG (glycine, glycine), white-tailed deer (Odocoileus virginianus) from a CWD-free control herd or depopulated CWD-infected herds within the United States. Blood samples were collected venously in EDTA tubes ante-mortem, either from animals within a chute, or when chemically immobilized for euthanasia. Samples from a total of 98 deer were utilized, ten of which were from a CWD-free research herd. 34 animals were positive for PrPSc-staining only in the MRPLN via IHC, while asymptomatic and appearing to be in good health. 54 animals were positive for PrPSc-staining in both the MRPLN and the obex via IHC. These animals were also asymptomatic and in good body condition, with the exception of five individuals that were potentially at the beginning of the symptomatic disease phase (very poor body condition, poor coat and extreme emaciation).
Sample Collection
After euthanasia, animals from the CWD-infected herds were aged either by herd records or by estimation based on visual inspection of the teeth by a wildlife biologist. Brainstem, at the level of the obex and MRPLN, were collected post-mortem utilizing standard USDA regulatory sample collection methods (For information, see https://www.aphis.usda.gov/animal_health/animal_diseases/cwd/downloads/cwd_program_standards_2014.pdf). Blood was collected from the jugular vein in commercial EDTA blood tubes and stored at −80 °C until used. Regulatory samples of obex and MRPLN were collected and stained post-mortem from the euthanized animals for protease-resistant PrP by IHC, as previously described51.
Processing of Blood Samples
Frozen samples of whole blood were processed to reduce proteins and other components that interfered with the PMCA reaction, as previously described28. Briefly, 200 µL of sample were mixed and incubated with 1 volume of 20% sarkosyl for 1 h at room temperature. Thereafter, samples were centrifuged at 100,000 × g for 1 h at 4 °C, supernatant was discarded and pellet washed in 400 µL of PBS. Tubes were centrifuged again at 100,000 × g for 30 min at 4 °C. The pellet was resuspended directly in 10% brain homogenate from transgenic mice expressing cervid PrPC, as described below. Each sample was simultaneously processed in four independent replicates by two different investigators. For some experiments, 10 µL of whole blood were directly added to the PMCA reaction without sarkosyl processing.
PMCA Assay
The PMCA reaction was carried out as previously described28,47, using brain homogenate from mice expressing cervid PrPC (Tg1536) as substrate. These animals were kindly provided by Dr. Glenn Telling (Colorado State University) and a colony was established in our facility. Brain substrate was prepared at a concentration of 10% (weight/volume) in conversion buffer (PBS supplemented with 150 mM NaCl and 1% Triton X-100, 0.025% Digitonin (Invitrogen #BN20061) and 3mM EDTA (Promega Cat V4231)) with protease inhibitors (Complete, Roche). Debris were removed by a low speed centrifugation (800 × g, 1 min, 4 °C) and brain homogenates were stored frozen at −80 °C until further use.
For PMCA, samples were subjected to 144 cycles of PMCA in 0.2 mL tubes (Eppendorf, cat. N. 951010022) containing 3 teflon beads (Hoover precision products) by either resuspending sarkosyl extraction pellets in PMCA substrate or direct spiking of brain homogenates or blood samples. Each cycle consisted of a 29 min and 40 s incubation at 37 °C followed by a 20 s pulse of sonication set at a potency of 110–120 W, using a Misonix microsonicator (Model S4000) equipped with a titanium horn. Subsequent rounds of 96 PMCA cycles were done by taking an aliquot of the amplified material, which was diluted 10-fold into fresh transgenic mice brain homogenate. After each round of PMCA, samples were taken for detection of PrPSc with the 6H4 or the PRC1 anti-PrP antibodies via Western blot after digestion with proteinase K, as described28,47. All precautions were taken to avoid cross-contamination as illustrated in previous publications28.
Ethics Statement
This project does not involve animal experimentations. Blood samples from white-tailed deer were collected previously for other purposes by Dr Tracy Nichols. We have USDA approval to receive and work with these samples. As substrate for PMCA, we used brains from transgenic mice expressing deer PrPC. The procedures for breeding, manipulations and euthanasia of these transgenic mice was performed following NIH guidelines and approved by the Animal Welfare Committee of the University of Texas Medical School at Houston (protocol AWC-16-0057).
Acknowledgements
The authors would like to thank Drs. Mitchell Palmer and Rebecca Cox from the National Animal Disease Center, Agricultural Research Service, USDA, and Drs. Thomas Gidlewski, Justin Fischer, and Michael Lavelle from the National Wildlife Research Center at the USDA for help with field sample collection, and Dr Glenn Telling (Colorado State University) for providing the transgenic mice colony and the PRC1 antibody. We also thank Dr Charles Mays (University of Texas Medical School at Houston) for reading and editing the manuscript. This work was funded by grants from the National Institutes of Health P01AI07774 to CS and R01AI132695 to RM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
C.K. carried out most of the PMCA experiments, analyzed results, and prepared the figures. S.P. participated in designing the study, analyzed the data and supervised the execution of some experiments. A.L. contributed in some of the PMCA experiments and helped in the preparation of the figures. T.N. provided the blinded samples of CWD-infected blood. R.M. designed the experiments, analyzed the data, supervised the work, coordinated with USDA to receive the samples and was responsible for funding. C.S. is the principal investigator and was responsible for coordinating research activity, experimental design, data analysis, funding and writing the manuscript. All authors reviewed and corrected the manuscript.
Competing Interests
Dr. Soto is inventor on several patents related to the PMCA technology and is currently Founder, Chief Scientific Officer and Vice-President of Amprion Inc, a biotech company focusing on the commercial utilization of PMCA for diagnosis of various neurodegenerative diseases. Dr. Morales is listed as an inventor in one patent related to the PMCA technology.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rodrigo Morales, Email: Rodrigo.MoralesLoyola@uth.tmc.edu.
Claudio Soto, Email: Claudio.Soto@uth.tmc.edu.
References
- 1.Sigurdson CJ, Aguzzi A. Chronic wasting disease. Biochim. Biophys. Acta. 2006;1772:610–618. doi: 10.1016/j.bbadis.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno, J. A. & Telling, G. C. Molecular Mechanisms of Chronic Wasting Disease Prion Propagation. Cold Spring Harb. Perspect. Med.(2017). [DOI] [PMC free article] [PubMed]
- 3.Haley NJ, Hoover EA. Chronic wasting disease of cervids: current knowledge and future perspectives. Annu. Rev. Anim Biosci. 2015;3:305–325. doi: 10.1146/annurev-animal-022114-111001. [DOI] [PubMed] [Google Scholar]
- 4.Waddell, L. et al. Current evidence on the transmissibility of chronic wasting disease prions to humans-A systematic review. Transbound. Emerg. Dis. (2017). [DOI] [PubMed]
- 5.Williams ES, Miller MW. Chronic wasting disease in deer and elk in North America. Rev. Sci. Tech. 2002;21:305–316. doi: 10.20506/rst.21.2.1340. [DOI] [PubMed] [Google Scholar]
- 6.Williams ES, Young S. Neuropathology of chronic wasting disease of mule deer (Odocoileus hemionus) and elk (Cervus elaphus nelsoni) Vet. Pathol. 1993;30:36–45. doi: 10.1177/030098589303000105. [DOI] [PubMed] [Google Scholar]
- 7.Prusiner SB. Prions. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soto C. Prion Hypothesis: The end of the Controversy? Trends in Biochemical Sciences. 2011;36:151–158. doi: 10.1016/j.tibs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartelt-Hunt SL, Bartz JC. Behavior of prions in the environment: implications for prion biology. PLoS. Pathog. 2013;9:e1003113. doi: 10.1371/journal.ppat.1003113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gough KC, Maddison BC. Prion transmission: prion excretion and occurrence in the environment. Prion. 2010;4:275–282. doi: 10.4161/pri.4.4.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathiason CK, et al. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS. ONE. 2009;4:e5916. doi: 10.1371/journal.pone.0005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders SE, Bartelt-Hunt SL, Bartz JC. Prions in the environment: occurrence, fate and mitigation. Prion. 2008;2:162–169. doi: 10.4161/pri.2.4.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 2004;10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haley NJ, et al. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J. Virol. 2011;85:6309–6318. doi: 10.1128/JVI.00425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS. ONE. 2009;4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathiason CK, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CJ, et al. Prions Adhere to Soil Minerals and Remain Infectious. PLoS. Pathog. 2006;2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidel B, et al. Scrapie Agent (Strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS. ONE. 2007;2:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. Oral Transmissibility of Prion Disease Is Enhanced by Binding to Soil Particles. PLoS. Pathog. 2007;3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritzkow S, et al. Grass plants bind, retain, uptake, and transport infectious prions. Cell Rep. 2015;11:1168–1175. doi: 10.1016/j.celrep.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson C, et al. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J. Gen. Virol. 2006;87:2109–2114. doi: 10.1099/vir.0.81615-0. [DOI] [PubMed] [Google Scholar]
- 22.Spraker TR, et al. Distribution of protease-resistant prion protein and spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. Vet. Pathol. 2002;39:546–556. doi: 10.1354/vp.39-5-546. [DOI] [PubMed] [Google Scholar]
- 23.Fox KA, Jewell JE, Williams ES, Miller MW. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus) J. Gen. Virol. 2006;87:3451–3461. doi: 10.1099/vir.0.81999-0. [DOI] [PubMed] [Google Scholar]
- 24.Spraker TR, et al. Antemortem detection of PrPCWD in preclinical, ranch-raised Rocky Mountain elk (Cervus elaphus nelsoni) by biopsy of the rectal mucosa. J. Vet. Diagn. Invest. 2009;21:15–24. doi: 10.1177/104063870902100103. [DOI] [PubMed] [Google Scholar]
- 25.Thomsen BV, et al. Diagnostic accuracy of rectal mucosa biopsy testing for chronic wasting disease within white-tailed deer (Odocoileus virginianus) herds in North America: effects of age, sex, polymorphism at PRNP codon 96, and disease progression. J. Vet. Diagn. Invest. 2012;24:878–887. doi: 10.1177/1040638712453582. [DOI] [PubMed] [Google Scholar]
- 26.Monello RJ, et al. Efficacy of antemortem rectal biopsies to diagnose and estimate prevalence of chronic wasting disease in free-ranging cow elk (Cervus elaphus nelsoni) J. Wildl. Dis. 2013;49:270–278. doi: 10.7589/2011-12-362. [DOI] [PubMed] [Google Scholar]
- 27.Saa, P. & Cervenakova, L. Protein misfolding cyclic amplification (PMCA): Current status and future directions. Virus Res.(2014). [DOI] [PubMed]
- 28.Morales R, Duran-Aniotz C, Diaz-Espinoza R, Camacho MV, Soto C. Protein misfolding cyclic amplification of infectious prions. Nat. Protoc. 2012;7:1397–1409. doi: 10.1038/nprot.2012.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orru CD, Caughey B. Prion seeded conversion and amplification assays. Top. Curr. Chem. 2011;305:121–133. doi: 10.1007/128_2011_184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orru CD, Wilham JM, Vascellari S, Hughson AG, Caughey B. New generation QuIC assays for prion seeding activity. Prion. 2012;6:147–152. doi: 10.4161/pri.19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 32.Atarashi R, et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat. Methods. 2008;5:211–212. doi: 10.1038/nmeth0308-211. [DOI] [PubMed] [Google Scholar]
- 33.Kurt TD, et al. Efficient in vitro amplification of chronic wasting disease PrPRES. J. Virol. 2007;81:9605–9608. doi: 10.1128/JVI.00635-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulford B, et al. Detection of PrPCWD in feces from naturally exposed Rocky Mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. J. Wildl. Dis. 2012;48:425–434. doi: 10.7589/0090-3558-48.2.425. [DOI] [PubMed] [Google Scholar]
- 35.Hoover, C. E. et al. Pathways of Prion Spread during Early Chronic Wasting Disease in Deer. J. Virol.(2017). [DOI] [PMC free article] [PubMed]
- 36.Haley NJ, et al. Antemortem Detection of Chronic Wasting Disease Prions in Nasal Brush Collections and Rectal Biopsy Specimens from White-Tailed Deer by Real-Time Quaking-Induced Conversion. J. Clin. Microbiol. 2016;54:1108–1116. doi: 10.1128/JCM.02699-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng YC, et al. Early and Non-Invasive Detection of Chronic Wasting Disease Prions in Elk Feces by Real-Time Quaking Induced Conversion. PLoS One. 2016;11:e0166187. doi: 10.1371/journal.pone.0166187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castilla J, Saa P, Soto C. Detection of prions in blood. Nat. Med. 2005;11:982–985. doi: 10.1038/nm1286. [DOI] [PubMed] [Google Scholar]
- 39.Saa P, Castilla J, Soto C. Presymptomatic detection of prions in blood. Science. 2006;313:92–94. doi: 10.1126/science.1129051. [DOI] [PubMed] [Google Scholar]
- 40.Thorne L, Terry LA. In vitro amplification of PrPSc derived from the brain and blood of sheep infected with scrapie. J. Gen. Virol. 2008;89:3177–3184. doi: 10.1099/vir.0.2008/004226-0. [DOI] [PubMed] [Google Scholar]
- 41.Tattum MH, Jones S, Pal S, Collinge J, Jackson GS. Discrimination between prion-infected and normal blood samples by protein misfolding cyclic amplification. Transfusion. 2010;50:996–1002. doi: 10.1111/j.1537-2995.2010.02595.x. [DOI] [PubMed] [Google Scholar]
- 42.Lacroux C, et al. Preclinical detection of variant CJD and BSE prions in blood. PLoS Pathog. 2014;10:e1004202. doi: 10.1371/journal.ppat.1004202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Concha-Marambio L, et al. Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med. 2016;8:370ra183. doi: 10.1126/scitranslmed.aaf6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bougard D, et al. Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med. 2016;8:370ra182. doi: 10.1126/scitranslmed.aag1257. [DOI] [PubMed] [Google Scholar]
- 45.Saa P, Castilla J, Soto C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem. 2006;281:35245–35252. doi: 10.1074/jbc.M603964200. [DOI] [PubMed] [Google Scholar]
- 46.Chen B, Morales R, Barria MA, Soto C. Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat. Methods. 2010;7:519–520. doi: 10.1038/nmeth.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moda F, et al. Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N. Engl. J. Med. 2014;371:530–539. doi: 10.1056/NEJMoa1404401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wild MA, Spraker TR, Sigurdson CJ, O’Rourke KI, Miller MW. Preclinical diagnosis of chronic wasting disease in captive mule deer (Odocoileus hemionus) and white-tailed deer (Odocoileus virginianus) using tonsillar biopsy. J. Gen. Virol. 2002;83:2629–2634. doi: 10.1099/0022-1317-83-10-2629. [DOI] [PubMed] [Google Scholar]
- 49.Haley NJ, et al. Seeded Amplification of Chronic Wasting Disease Prions in Nasal Brushings and Recto-anal Mucosa-Associated Lymphoid Tissues from Elk by Real-Time Quaking-Induced Conversion. J. Clin. Microbiol. 2016;54:1117–1126. doi: 10.1128/JCM.02700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orru CD, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N. Engl. J. Med. 2014;371:519–529. doi: 10.1056/NEJMoa1315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nichols TA, et al. Dietary magnesium and copper affect survival time and neuroinflammation in chronic wasting disease. Prion. 2016;10:228–250. doi: 10.1080/19336896.2016.1181249. [DOI] [PMC free article] [PubMed] [Google Scholar]