Abstract
The extirpation of native wildlife species and widespread establishment of livestock farming has dramatically distorted large mammal herbivore communities across the globe. Ecological theory suggests that these shifts in the form and the intensity of herbivory have had substantial impacts on a range of ecosystem processes, but for most ecosystems it is impossible to quantify these changes accurately. We address these challenges using species-level biomass data from sub-Saharan Africa for both present day and reconstructed historical herbivore communities. Our analyses reveal pronounced herbivore biomass losses in wetter areas and substantial biomass increases and functional type turnover in arid regions. Fire prevalence is likely to have been altered over vast areas where grazer biomass has transitioned to above or below the threshold at which grass fuel reduction can suppress fire. Overall, shifts in the functional composition of herbivore communities promote an expansion of woody cover. Total herbivore methane emissions have more than doubled, but lateral nutrient diffusion capacity is below 5% of past levels. The release of fundamental ecological constraints on herbivore communities in arid regions appears to pose greater threats to ecosystem function than do biomass losses in mesic regions, where fire remains the major consumer.
Introduction
Large mammal herbivore populations have been radically altered across the globe since the late Pleistocene1. Recent research has highlighted the staggering extent of herbivore extinctions, particularly of the largest-bodied species, and how human pressures have devastated populations of surviving species2–5. This loss of native species has wide-ranging ecological consequences6,7, and has produced a surge of interest in how rewilding initiatives can restore herbivores and their cascading effects on ecosystems8–10. However, livestock densities in many parts of the world often approach or exceed herbivore densities before the Pleistocene extinctions11,12. This raises the important question of whether livestock restore ecological processes, by serving as functionally comparable replacements for extirpated species, or if their influence converts landscapes into novel ecosystems13.
Africa was least affected by the Pleistocene extinctions14, and has the largest remaining area of untransformed (uncultivated) land15. However, livestock have been progressively introduced into herbivore communities over thousands of years, with pastoralism practiced throughout the continent from at least 2,000 years ago16,17. Over the last few centuries colonial hunters have decimated indigenous herbivore and predator communities18, with the simultaneous increase in human population, and improved disease and vector control measures (e.g. tsetse fly suppression), meaning that livestock now vastly outnumber wildlife19,20. Today, most African herbivore communities have a much narrower range of body sizes and diets than in the past, which in turn reduces the range in the amount, quality, and type of plant material that can be consumed in particular ecosystems21. As a consequence, the spatial distribution of herbivore biomass across the continent will also have been altered22.
Recent studies have emphasised how large mammal herbivores can strongly modify ecosystems through effects on fire prevalence23–25, woody cover26–28 and biogeochemical cycling29–31. However, all of these processes are subject to environmental control, and with herbivore communities also being structured by environmental gradients22,32, responses will inevitably be contingent on factors like rainfall and soils19,20,33,34. A full appreciation of these impacts, and their use as drivers of rewilding programs and climate mitigation policies, requires that we quantify them. Fire management policies, sustainable woodland harvesting rates, and greenhouse gas accounting efforts all require that we have some understanding of how these processes operated in the past ecosystems which we have so dramatically transformed35–37. Africa is arguably the continent best suited to exploring these effects, because although it is no less ‘distorted’ than other ecosystems globally, there is a rich knowledge about the ecology of both wildlife and livestock on the continent across a range of environments (this is one of the few places where quantifiable data on the impacts of near-intact megafaunal assemblages are possible). Moreover, this information has been used to reconstruct and map past herbivore densities on the continent12, providing an opportunity to quantify some of the ecological impacts of these trophic disruptions.
Here we explore how the form and the intensity of herbivory pressure has changed across Africa by contrasting livestock-dominated present day herbivore communities with reconstructed past herbivore communities. The past biomass surface comprises pre-colonial era (i.e. about 1,000 years ago) species-level biomass estimates for 92 extant native herbivores12. Cattle, goats and sheep were included in the present day livestock biomass estimates38, with remnant wildlife populations approximated by filtering past biomass using information on a region’s conservation status, human footprint index39, and extent of area converted to cropland20. Species-level information on body size, diet, gut type and water dependence12 were used to gauge how shifts in herbivore community composition would influence ecological processes such as fire, tree-grass dynamics, carbon emissions and nutrient distribution. Broad patterns of global relevance emerge from our analysis of how herbivore community turnover is shaped by environmental conditions. The results provide a more nuanced model for understanding these changes across other continents, and represent essential validation data for global vegetation models which aim to reproduce the ecological impacts of herbivores40.
Results and Discussion
Herbivore biomass change
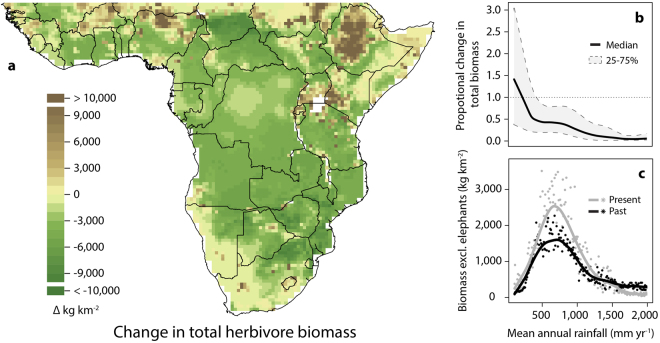
Total herbivore biomass has decreased across most of Africa (Fig. 1a), with maximum declines exceeding 10,000 kg km−2. The only regions where herbivore biomass has increased are arid – below 500 mm yr−1 – with average changes only being positive below 250 mm yr−1 (Fig. 1b). These arid regions are also the least predictable, with either large increases or declines observed in many ecosystems. This is attributable to low historical biomass in these arid regions, but is also because humans can support artificially high herbivore abundance in dry areas by providing supplementary water and forage11. The average current biomass of herbivores falls below 10% of historical levels in the wettest parts of Africa ( > 1,300 mm yr−1). Elephants once dominated herbivore biomass in these regions12, and their extirpation by hunting is largely responsible for these overall declines. Excluding elephants from the analysis (Fig. 1c) reveals that livestock match or exceed past wildlife biomass in areas with rainfall up to about 1,500 mm yr−1, but cannot compensate for wildlife biomass in wetter regions.
Figure 1.
Patterns of herbivore biomass change in Africa. (a) Absolute change in herbivore biomass (present – past), and (b) proportional change (present/past) in herbivore biomass in relation to mean annual rainfall. (c) Herbivore biomass excluding elephants in relation to mean annual rainfall. Points (c) represent median values for 10 mm yr−1 rainfall intervals and are shown with locally weighted scatterplot smoothing regression lines (b,c). Figure 1(c) is similar to Fig. 1 in Hempson et al.12, but differs by present biomass including both livestock and wildlife, and by being quantified at 0.5° spatial resolution. The map was generated using R version 3.3.393 (www.R-project.org) and QGIS 2.4.0.94 (www.qgis.org).
Herbivore functional turnover
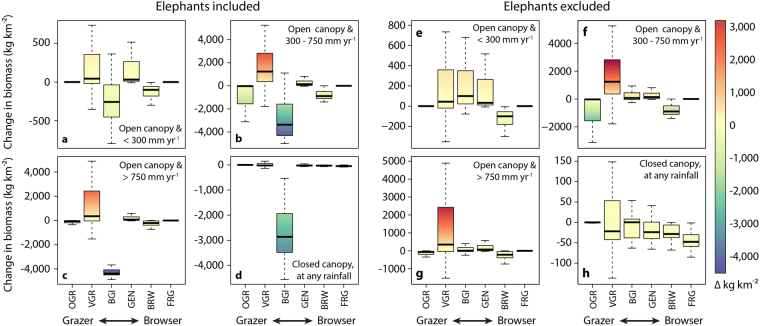
Three livestock species dominate African rangelands, accounting for over 90% of current day herbivore biomass. The vast majority of the continent has thus experienced a substantial contraction of herbivore trait diversity. Fortuitously, cattle (water-dependent grazers), and goats and sheep (both medium-sized social mixed diets), are not novel herbivore functional types in African ecosystems12. Nonetheless, the relative abundance of e.g. different herbivore diet types (Fig. 2) has shown dramatic and varied shifts across rainfall and vegetation type categories (Supplementary Figure S1). Cattle, which are variable grazers (i.e. consuming 60–90% grass41), dominate herbivore biomass in regions with open vegetation types and rainfall < 300 mm yr−1 (Fig. 2b,c,f,g). The greatest relative contribution of sheep and goats to increased herbivore biomass is in the driest parts of the continent ( < 300 mm yr−1), reflecting their lower water dependence and more varied diets (Fig. 2a; goat effect is evident in Fig. 2e which excludes elephants). Closed canopy vegetation types have lost herbivore biomass across all dietary categories (Fig. 2d,h), although the decline in elephant populations makes the effect on browser-grazer intermediates particularly pronounced. Elephants appear to have strong effects on the density and diversity of saplings in African forests42, and also act as important seed dispersers43, and their widespread loss may thus have broad implications for forest community dynamics. The lack of an elephant analogue – and specialist browser and frugivore diet types – among livestock species also appears to hold important consequences for open-canopied African vegetation communities, for example by potentiating bush encroachment33,44,45 or reducing seed dispersal46–48.
Figure 2.
Herbivore biomass change by diet type. Biomass change is shown for open vegetation types for three mean annual rainfall categories: (a,e) < 300 mm yr−1, (b,f) 300–750 mm yr−1 and (c,g) > 750 mm yr−1. All closed canopy vegetation types are shown in (d,h). Elephants are browser-grazer intermediates and historically dominated herbivore biomass across the continent; changes in biomass are shown both including (a–d) and excluding (e–h) elephants. Livestock diet classifications: cattle = variable grazers, goats = generalists and sheep = browser-grazer intermediates41. Boxes show the median and interquartile range, and whiskers extend to the most extreme data point that is no further than the interquartile range. Median values are significantly different from zero (p = 0.05) in all cases. Dietary classifications follow Gagnon & Chew41: OGR = obligate grazer ( > 90% monocots, not variable), VGR = variable grazer (60–90% monocots, variable), BGI = browser-grazer intermediate (30–70% dicots and monocots, < 20% fruits), GEN = generalist ( > 20% of monocots, dicots and fruits), BRW = browser ( > 70% dicots) and FRG = frugivore ( > 70% fruits, little or no monocots).
Fire-grazer competition
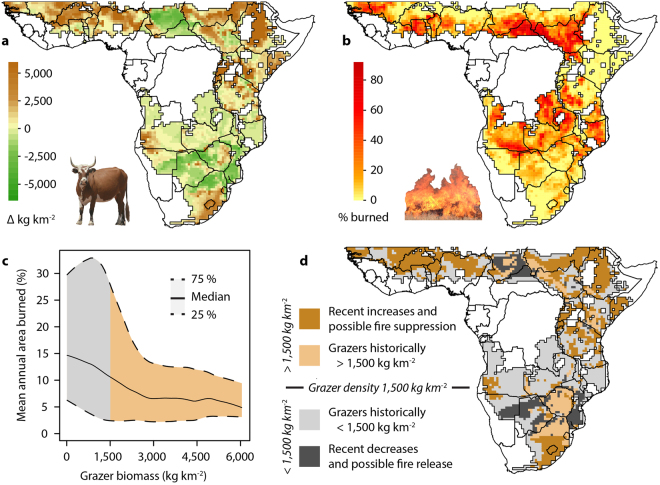
Conceptually, grazers can suppress fires when they consume so much grassy material in the wet season that there is insufficient fuel to carry fires in the dry season. The effect of grazers is therefore dependent both on the number of grazers and the productivity of the grass layer (i.e. more and/or larger-bodied grazers are needed in wetter areas to suppress fire49. At continental scales grazers have clear negative effects on fire prevalence in savannas above a density of 1,500 kg km−2 20 (Fig. 3c). This suggests that current fire patterns are substantially influenced by changes in grazer communities (Fig. 3a) – both positively and negatively (Fig. 3d). The close match between regions exceeding this threshold (Fig. 3d) and patterns of annual burned area (Fig. 3b) suggests that fire has been strongly suppressed across vast expanses of Africa that previously would have had too few grazers to suppress grassy fires (dark brown regions in Fig. 3d). This large-scale evidence for fire-grazer interactions supports paleoecological data showing that losing large mammal grazers could lead to higher fire prevalence23,24. An important caveat, however, is that environmental conditions are important in shaping large mammal effects on ecosystems: grazers are predicted to have the greatest potential to alter grassland structure in a manner that excludes fire (e.g. by creating grazing lawns) where rainfall lies between 400–850 mm yr−1 50. Consistent with this is the lack of evidence for change in fire prevalence in the mesic Miombo regions (>1,000 mm yr−1) spanning Angola to northern Mozambique (Fig. 3b,d). The most pronounced effects of fire release on other continents are thus likely to have occurred where fire has replaced herbivores as the dominant consumer in xeric savannas20,51, although the rainfall threshold at which this occurs may vary52.
Figure 3.
Fire prevalence in relation to grazer biomass. (a) Grazer biomass change (kg km−2), (b) mean annual burned area (%) and (c) the relationship between present day grazer biomass and mean annual burned area for savannas with annual rainfall between 300–1,300 mm yr−1. Panel (d) indicates whether grazer biomass has crossed a threshold value of 1,500 kg km−2 (corresponding to the colour bands in (c), above which fire-grazer competition has greater potential to reduce mean burned area20 (analysis restricted to areas with <40% woody cover). Regions with enhanced present day fire-grazer competition (i.e. dark brown areas in d) correspond closely with patterns of mean annual burned area (i.e. the match with lightly shaded areas in b), particularly in East and West Africa. Panel (c) was derived by fitting locally weighted scatterplot smoothing regression lines to median grazer biomass values at 20 kg km−2 intervals. The maps were generated using R version 3.3.393 (www.R-project.org) and QGIS 2.4.094 (www.qgis.org).
Woody cover
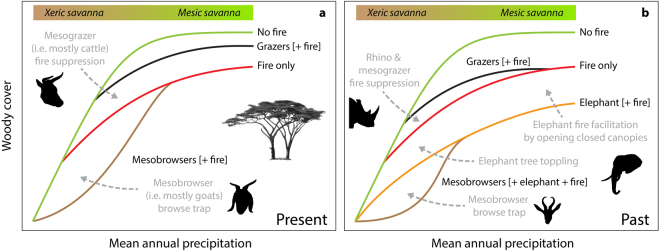
Herbivores can have direct effects on woody cover, but can also act indirectly by altering local fire regimes53,54. Rainfall, and to a lesser extent soil nutrient status, mediates the influence of different herbivore functional groups via productivity-linked effects on woody plant growth rates55 and the quantity and quality of grass biomass56. We predict that the general pattern of herbivore community distortion should enhance woody cover in African savannas (Fig. 4), over and above the influence of elevated CO2-levels that are a global driver of woody encroachment57–59. The substantial contraction of elephant distribution ranges and population sizes is a well-recognised direct release on woody cover that has occurred widely across the continent33,45,53. This loss equates to reduced tree toppling26, but also includes indirect effects on fire by facilitating grass and thus fire spread into closed canopy vegetation types60, and an increase in the susceptibility of damaged adult trees to fire61,62. Moreover, the widespread increase in grazer densities (Fig. 3a) has likely reduced fire across much of the continent (Fig. 3d), resulting in a lower severity fire-trap that otherwise can limit woody recruitment63 (Fig. 4). Grazers may also promote woody recruitment by reducing grass competition53. However, there are also conditions where herbivore community distortion might have decreased woody cover: small-stock farming across the continent has increased the biomass of mesobrowsers (Fig. 2), which may suppress woody cover via an enhanced browse-trap effect53,63. The loss of white rhino from ecosystems and the associated increase in fire in the general landscape may also constrain tree cover in mesic savannas49, and suggests that losing megaherbivores might not always result in higher tree cover7,27,28.
Figure 4.
Conceptual model of the effects of herbivore community distortion on woody cover. Shifts in the biomass and functional composition of herbivore communities are likely to mean that their net effect on woody cover is different now (a) to in the past (b). The green line represents the maximum tree cover along a rainfall gradient in the absence of fire or herbivory following Sankaran et al.95. The red line represents the putative effect of fire on tree cover in the absence of herbivory. Grazers (black line) suppress fire by reducing fuel loads thereby increasing woody cover, while elephants (orange line) and mesobrowsers (brown line) have direct consumptive effects that reduce woody cover while also enhancing tree vulnerability to fire. Xeric to mesic savanna transitions are broadly aligned with the 650 mm yr−1 threshold revealed by Sankaran et al.95. Grey arrows associate particular herbivore influences with relevant parts of the rainfall-woody cover conceptual space. Two of the herbivore icons make a reappearance after previously featuring in Hempson et al.12.
Greenhouse gas emissions
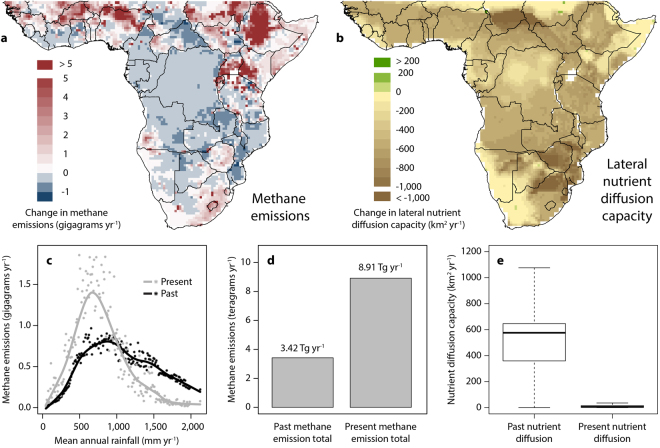
The vegetation changes above will alter the carbon cycle and atmospheric CO2 levels through altering both above and below-ground biomass. Soil organic carbon has been shown to be remarkably resilient to grazing in tropical grassy ecosystems64 – only dropping off at extremely high grazing intensity. On the other hand, woody thickening due to replacement of browsers with grazers can increase above-ground carbon stocks. Neither of these impacts are well quantified spatially. It is easier to quantify the methane impacts of herbivore distortion, and as methane has 28 times the warming potential of CO2 65, these are arguably more significant. The transition to livestock-dominated African herbivore communities has led to a net increase in methane emissions by enteric fermentation (Fig. 5). Herbivore methane emissions are strongly contingent on gut type (i.e. ruminant vs. non-ruminant) and body size: ruminants produce considerably more methane per unit body mass than non-ruminants, while larger animals produce more methane per unit body mass37. These functional trait details are important for understanding how a ruminant, cattle-dominated community – often with lower total biomass – can exceed methane emissions of a non-ruminant, elephant-dominated historical biomass in regions receiving rainfall below 1,000 mm yr−1 (Fig. 5c). Our analyses suggest that although approximately 50% of the sub-Saharan Africa land surface has experienced a decrease in methane emission levels (Fig. 5a), this is outweighed by substantial increases across large parts of the Sahel, Ethiopia and East Africa that combined produce a net increase from 3.4 to 8.9 Tg yr−1 (Fig. 5d). This estimate is broadly in line with recently revised estimates of methane emissions from livestock66 for the entire African continent (9.9 Tg), and constitutes approximately 15% of current global methane emissions (67.2 Tg). These calculations build on exciting recent attempts to quantify shifts in herbivore contributions to global carbon cycling31, but there remains much scope for improving these estimates with validation from detailed local studies. Our analysis differs from previous assessments20,31,37 by using considerably more nuanced herbivore biomass data, and by downscaling the contribution of elephants by using a body mass estimate that better reflects typical herd age structure (i.e. from ~4000 kg to 1725 kg67). Overall, our calculations suggest that Smith et al.’s31 global estimates of end-Pleistocene methane emission levels are probably too high, but highlight the clear need for further research to better understand herbivore contributions of this important non-CO2 greenhouse gas68.
Figure 5.
Herbivore effects on biogeochemical cycling. Shifts in herbivore community composition have altered (a) methane emissions and (b) lateral nutrient diffusion capacity across sub-Saharan Africa (both at 0.5° spatial grain). The relationship between rainfall and methane emissions (c) broadly reflects the overall dependence of herbivore biomass on rainfall (Fig. 1), with cattle-dominated communities now producing a higher peak in drier regions than in the past, but falling below past levels in wetter areas that were historically dominated by elephants. Total methane emissions for sub-Saharan Africa (d) are now considerably higher than in the past, while there have been marked decreases in nutrient diffusion capacity (e). In (e), box shows the median and interquartile range of nutrient diffusion capacity for all sub-Saharan Africa 0.5° cells, and whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range. The maps were generated using R version 3.3.393 (www.R-project.org) and QGIS 2.4.0.94 (www.qgis.org).
Lateral nutrient diffusion capacity
Recent theoretical studies have provided intriguing insights into how animals can disperse nutrients across ecosystems29,30,36. Importantly, animals can transport nutrients against passive movement gradients (e.g. upslope) and away from nutrient hotspots, thereby increasing overall landscape-level fertility patterns69. We extended recent allometric equation based approaches30,36 to quantify changes in nutrient diffusion capacity in Africa, but substituted the population density term in the models with our own independently-derived herbivore abundance surfaces to produce spatially-explicit estimates across the continent. Furthermore, we modified our present day nutrient diffusion estimates by scaling the daily movement range term for all species by an index of human influence39, with a further 50% reduction in day movement range imposed on livestock species. These modifications attempt to account for land use transformation effects on habitat connectivity, and also the effects of fences and night corralling on livestock movement (see Supplementary Figure S2 for estimates without day range constraints). The resulting surface confirms the widespread loss of the nutrient dispersing ecosystem service provided by large mammal herbivores in Africa29,30,36 (Fig. 5b), but projects a far more dramatic decline than in these previous estimates. Our results suggest that nutrient diffusion levels for sub-Saharan Africa are < 5% of past levels (Fig. 5e), and thus contrast strongly with Doughty et al.’s36 global assessment that suggests Africa retains 46% of its Pleistocene nutrient diffusion capacity. This is made all the more striking because previous assessments did not include contributions from livestock (further enhancing discrepancies between the studies), and suggests that while allometric estimates of herbivore population density may provide a useful first approximation of their influences, future projections should aim to incorporate information on how environmental conditions shape population densities.
Ecosystem susceptibility
Mean annual rainfall plays a clear role in determining the form and extent of herbivore community distortion that occurs in African ecosystems. Mesic regions are most prone to overall biomass losses, while many arid regions have experienced considerable increases in herbivore biomass. Which end of this spectrum is of most concern? The associated turnover in herbivore functional type composition experienced in arid regions, largely in response to human interventions through water and supplemental feed provisioning and predator and disease control11, suggests that many of these regions may now be entering a novel state following the release of fundamental ecological constraints. Associated with the shifts in herbivore biomass and composition, populations are now also likely to be more sedentary, posing a further perturbation to vegetation dynamics that are generally more seasonally pulsed70. For example, year-round trampling and grazing may increase grass tuft mortality71,72, leading to increased soil erosion by wind and water, and reduced water infiltration due to soil compaction and greater run-off73. A global meta-analysis of the indirect effects of large mammal herbivores on ecosystems suggest these to be greatest in low productivity regions, despite the lower herbivore biomass in these regions74. This is explained by large mammal herbivores having the greatest potential to modify vegetation and hence habitats and food resources for other animal species in these regions75. Herbivore community distortion in arid regions may thus have many consequences beyond those of fire suppression and woody encroachment that we have explored here.
Mesic regions may be more buffered against herbivore losses due to fire having been the major consumer in these ecosystems over evolutionary timescales20,51,76. Despite general decreases in grazer biomass, historically low grazer abundance in these regions suggests that their impact on fire is likely to always have been minimal, meaning that fire prevalence is probably largely unaffected. The extirpation of elephants holds more substantial ecological implications, notably through enhanced woody thickening, and reduced lateral nutrient diffusion capacity in these typically highly leached, nutrient poor ecosystems77. On the other hand – humans are replacing some of the functions of elephants in these mesic systems through fuelwood harvesting78, and currently woody biomass is declining over much of the region in Africa35 – and in some instances to a state potentially more similar to what it was before elephant hunting79. Human hunting pressure is likely to remain the greatest threat to large mammal herbivores in Africa’s tropical forest ecosystems80, exacerbating current effects on seed dispersal, recruitment and vegetation structure in the forest understory42,46,81.
The general shift from migratory to resident herbivore populations, due to fences and land use change that act as barriers to movement, constitutes a ubiquitous but poorly quantified distortion of large mammal herbivore ecology. Only a handful of ecosystems now retain the diversity of functional seasonal resources necessary to support large, migratory herbivore populations that can exhibit the adaptive movement responses needed to persist at high densities in inherently variable ecosystems82,83. Populations that cannot migrate are more susceptible to droughts71,72 – periods during which intense defoliation of vegetation occurs – and will have lower mean population sizes with higher interannual variation83. These effects are most pronounced in climatically variable arid regions, but also highlight the considerable natural fluctuation in herbivore densities that occurs at local scales84, due to both movement and the intrinsic and extrinsic factors that regulate population size32,85.
Implications
Currently there are several global initiatives afoot for carbon mitigation through reforestation (e.g. REDD + 86), altered fire regimes87, and livestock methane management37,88. The spatial data presented here for Africa give a slightly different perspective on the ecological appropriateness of some of these schemes – implying not only in the past there was extensive wood harvesting by elephant across large areas that are currently considered ‘deforested’, but that fire has been suppressed by livestock in a significant area of the continent. Moreover, rewilding schemes are likely to have consequences for national methane budgets, as large parts of Africa currently have methane ‘credits’ from the rampant destruction of their megaherbivores in the last few centuries1,18. Current African landscapes represent a continuum from pure livestock to pure wildlife. While livestock have been, and should continue to be an intrinsic part of African ecology, our data identify which processes and which environments are degraded by this shift in herbivore functional attributes, which should enable better interventions to restore these essential functions. Multispecies wildlife-livestock production systems are one such consideration that have long been advocated as a means to reinforce ecological resilience19 and support the unique biodiversity of the disturbance-maintained ecosystems that account for over half of Africa’s land surface51,89,90.
Final remarks
Africa is effectively the world’s last laboratory for testing the effects of native large mammal herbivores on ecosystems. Even so, herbivore communities across Africa are massively transformed, with livestock now dominating the continent’s large mammal biomass. This raises an important question going forward as to where, globally, livestock serve to restore ecological processes, and where their influence converts landscapes into novel ecosystems13. Our knowledge about the spatial distributions of past herbivore communities is scarce, but although our results presented here for Africa remain necessarily coarse, they clearly demonstrate that using spatially-invariant mean values can result in under-, or over-estimation of how herbivore extinctions have altered important ecological processes. Merging insights from Africa may thus help to considerably refine projections of herbivore influences across other continents. The mounting evidence that large mammals matter should heighten support for Dirzo et al.’s5 call to elevate ‘defaunation’ to a status commensurate with that of ‘deforestation’ in wider society.
Methods
Herbivore biomass and environmental variables
Past biomass estimates were recalculated at quarter degree grid square resolution (0.5° × 0.5°) following the methods used in Hempson et al.12. Present day biomass estimates were updated from Archibald & Hempson20, and include both livestock38 and remnant wildlife population estimates. Livestock population estimates in Archibald & Hempson20 were updated with finer grained FAO data (0.0083° vs. 0.05°), which report higher livestock numbers for sub-Saharan Africa that better reflect total estimates for the region. Additional details are provided in the Supplementary Information. Change in herbivore biomass was calculated by subtracting past biomass values from present day biomass values, and was assessed for a range of different species and functional type groupings: total (Fig. 1a,b), total excluding elephants (Fig. 1c), diet type (following Gagnon & Chew41, and shown for four vegetation canopy cover-rainfall categories [Supplementary Figure S1]; with elephants included [Fig. 2a-d] and excluded [Fig. S2e-h]) and for grazer species (i.e. the combined biomass of obligate and variable grazers [Fig. 3]). Biomass change values are shown in relation to mean annual rainfall (WorldClim database, www.worldclim.org; accessed June 2013; Figs 1b,c and 5c). Mean annual area burned (Fig. 3b,c) was derived from the Global Fire Emissions Database 4.1 (GFED) 3.1 following van der Werf et al.91. Vegetation canopy cover classifications used in Fig. 2 were based on White92, with evergreen forests (mapping units: 1a, 2, 3, 4, 6, 8, 9), forest-grassland mosaics (50% of area of mapping units: 11a, 12, 17, 19a, 19b, 20, 65, 66) and mangrove forests (mapping unit 77) scored as closed canopy vegetation types and the remainder as open canopy systems. One-sample t-tests or else non-parametric sign tests were used to test whether the mean or median change in biomass was different from zero for each diet type in each rainfall × vegetation type category in Fig. 2.
Methane emissions and lateral nutrient diffusion capacity
Methane emissions (kg indvidual−1 yr−1) were estimated separately for ruminant and non-ruminant species using body mass-methane output relationships from Smith et al.37: ruminant methane emissions = 10−0.619 + 0.812 x log10(BM)^1.171, and non-ruminant methane emissions = 10−4.564 + 3.278 x log10(BM)^0.592, where BM = body mass in kilograms. Lateral nutrient diffusion capacity (km2 yr−1) was calculated at 0.5° spatial grain based on the revised equation in Doughty et al.36, which is reformulated from the allometric relationships and the random walk-process outlined in Wolf et al.30 and Doughty et al.29 i.e. nutrient diffusion capacity = MR*PD*(DD*PR)2/(2*PR), where MR = metabolic rate (0.021*BM 0.716), PD = population density, DD = daily movement range (0.453*BM 0.368) and PR = food passage time (0.29*BM 0.26). We substituted our independently derived estimates of population density into the model instead of using the allometric relationship for PD, and used a body mass estimate of 1725 kg for elephants67. Daily movement range was rescaled (DD r) for present day nutrient diffusion capacity estimates using the human influence index39 (HII) i.e. DD r = DD*(1–HII/72), where 72 is the maximum possible HII value. Livestock daily movement ranges (DD rl) were further reduced by 50% to account for the effects of fences and night corralling i.e. DD rl = DD*(1–HII/72)*0.5. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
GPH was supported by South African DST/NRF Global Change Grand Challenge Grant #92464. SA was supported by the USAID/NAS programme Partnerships for Enhanced Engagement in Research (Sub-Grant 2000004946, Cycle 3). Tristan Charles-Dominique provided valuable feedback on an earlier version of the manuscript.
Author Contributions
G.P.H. and S.A. conceived the project and supplied and analysed the data. G.P.H. led the writing with contributions from S.A. and W.J.B.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17348-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ripple W, et al. Collapse of the world’s largest herbivores. Sci. Adv. 2015;1:e1400103. doi: 10.1126/sciadv.1400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin, P. S. Quaternary extinctions: a prehistoric revolution, chap. Prehistoric overkill: the global model, 354–403 (University of Arizona Press, Tucson, 1984).
- 3.Ceballos G, Ehrlich PR. Mammal population losses and the extinction crisis. Sci. 2002;296:904–907. doi: 10.1126/science.1069349. [DOI] [PubMed] [Google Scholar]
- 4.Karanth, K. K., Nichols, J. D., Karanth, K. U., Hines, J. E. & Christensen, N. L. The shrinking ark: patterns of large mammal extinctions in India. Proc. Royal Soc. Lond. B rspb20100171 (2010). [DOI] [PMC free article] [PubMed]
- 5.Dirzo R, et al. Defaunation in the Anthropocene. Sci. 2014;345:401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 6.Keesing, F. & Young, T. P. Cascading consequences of the loss of large mammals in an African savanna. BioScience biu059 (2014).
- 7.Malhi Y, et al. Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. 2016;113:838–846. doi: 10.1073/pnas.1502540113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seddon PJ, Griffiths CJ, Soorae PS, Armstrong DP. Reversing defaunation: restoring species in a changing world. Sci. 2014;345:406–412. doi: 10.1126/science.1251818. [DOI] [PubMed] [Google Scholar]
- 9.Lorimer J, et al. Rewilding: Science, practice, and politics. Annu. Rev. Environ. Resour. 2015;40:39–62. doi: 10.1146/annurev-environ-102014-021406. [DOI] [Google Scholar]
- 10.Svenning J-C, et al. Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proc. Natl. Acad. Sci. 2016;113:898–906. doi: 10.1073/pnas.1502556112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oesterheld M, Sala OE, McNaughton SJ. Effect of animal husbandry on herbivore-carrying capacity at a regional scale. Nat. 1992;356:234–236. doi: 10.1038/356234a0. [DOI] [PubMed] [Google Scholar]
- 12.Hempson GP, Archibald S, Bond WJ. A continent-wide assessment of the form and intensity of large mammal herbivory in Africa. Sci. 2015;350:1056–1061. doi: 10.1126/science.aac7978. [DOI] [PubMed] [Google Scholar]
- 13.Mack RN, Thompson JN. Evolution in steppe with few large, hoofed mammals. Am. Nat. 1982;119:757–773. doi: 10.1086/283953. [DOI] [Google Scholar]
- 14.Owen-Smith N. Contrasts in the large herbivore faunas of the southern continents in the late Pleistocene and the ecological implications for human origins. J. Biogeogr. 2013;40:1215–1224. doi: 10.1111/jbi.12100. [DOI] [Google Scholar]
- 15.Alexandratos, N. & Bruinsma, J. World agriculture towards 2030/2050: the 2012 revision. Tech. Rep., ESA Working paper Rome, FAO (2012).
- 16.Hanotte O, et al. African pastoralism: genetic imprints of origins and migrations. Sci. 2002;296:336–339. doi: 10.1126/science.1069878. [DOI] [PubMed] [Google Scholar]
- 17.Pleurdeau D, et al. “Of sheep and men”: earliest direct evidence of caprine domestication in southern Africa at Leopard Cave (Erongo, Namibia) PloS One. 2012;7:e40340. doi: 10.1371/journal.pone.0040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinage CA. A review of ivory exploitation and elephant population trends in Africa. Afr. J. Ecol. 1973;11:281–289. doi: 10.1111/j.1365-2028.1973.tb00093.x. [DOI] [Google Scholar]
- 19.Du Toit JT, Cumming DHM. Functional significance of ungulate diversity in african savannas and the ecological implications of the spread of pastoralism. Biodivers. Conserv. 1999;8:1643–1661. doi: 10.1023/A:1008959721342. [DOI] [Google Scholar]
- 20.Archibald S, Hempson GP. Competing consumers: contrasting the patterns and impacts of fire and mammalian herbivory inAfrica . Philos. Transactions Royal Soc. B. 2016;371:20150309. doi: 10.1098/rstb.2015.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demment MW, Van Soest PJ. A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. Am. Nat. 1985;125:641–672. doi: 10.1086/284369. [DOI] [Google Scholar]
- 22.Olff H, Ritchie ME, Prins HHT. Global environmental controls of diversity in large herbivores. Nat. 2002;415:901–904. doi: 10.1038/415901a. [DOI] [PubMed] [Google Scholar]
- 23.Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Sci. 2009;326:1100–1103. doi: 10.1126/science.1179504. [DOI] [PubMed] [Google Scholar]
- 24.Rule S, et al. The aftermath of megafaunal extinction: ecosystem transformation in Pleistocene Australia. Sci. 2012;335:1483–1486. doi: 10.1126/science.1214261. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CN. Fire, people and ecosystem change in Pleistocene Australia. Aust. J. Bot. 2016;64:643–651. doi: 10.1071/BT16138. [DOI] [Google Scholar]
- 26.Asner GP, Vaughn N, Smit IP, Levick S. Ecosystem-scale effects of megafauna in African savannas. Ecography. 2016;39:240–252. doi: 10.1111/ecog.01640. [DOI] [Google Scholar]
- 27.Doughty CE, Faurby S, Svenning J-C. The impact of the megafauna extinctions on savanna woody cover in South America. Ecography. 2016;39:213–222. doi: 10.1111/ecog.01593. [DOI] [Google Scholar]
- 28.Bakker ES, et al. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl. Acad. Sci. 2016;113:847–855. doi: 10.1073/pnas.1502545112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doughty CE, Wolf A, Malhi Y. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat. Geosci. 2013;6:761–764. doi: 10.1038/ngeo1895. [DOI] [Google Scholar]
- 30.Wolf A, Doughty CE, Malhi Y. Lateral diffusion of nutrients by mammalian herbivores in terrestrial ecosystems. Plos One. 2013;8:e71352. doi: 10.1371/journal.pone.0071352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith FA, et al. Exploring the influence of ancient and historic megaherbivore extirpations on the global methane budget. Proc. Natl. Acad. Sci. 2016;113:874–879. doi: 10.1073/pnas.1502547112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopcraft JGC, Olff H, Sinclair ARE. Herbivores, resources and risks: alternating regulation along primary environmental gradients in savannas. Trends In Ecol. & Evol. 2010;25:119–128. doi: 10.1016/j.tree.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Stevens N, Erasmus B, Archibald S, Bond W. Woody encroachment over 70 years in South African savannahs: overgrazing, global change or extinction aftershock? Philos. Transactions Royal Soc. B. 2016;371:20150437. doi: 10.1098/rstb.2015.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fritz H, Duncan P, Gordon IJ, Illius AW. Megaherbivores influence trophic guilds structure in African ungulate communities. Oecologia. 2002;131:620–625. doi: 10.1007/s00442-002-0919-3. [DOI] [PubMed] [Google Scholar]
- 35.Ryan CM, et al. Quantifying small-scale deforestation and forest degradation in African woodlands using radar imagery. Glob. Chang. Biol. 2012;18:243–257. doi: 10.1111/j.1365-2486.2011.02551.x. [DOI] [Google Scholar]
- 36.Doughty CE, et al. Global nutrient transport in a world of giants. Proc. Natl. Acad. Sci. 2016;113:868–873. doi: 10.1073/pnas.1502549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith FA, Lyons SK, Wagner PJ, Elliott SM. The importance of considering animal body mass in IPCC greenhouse inventories and the underappreciated role of wild herbivores. Glob. Chang. Biol. 2015;21:3880–3888. doi: 10.1111/gcb.12973. [DOI] [PubMed] [Google Scholar]
- 38.Robinson TP, et al. Mapping the global distribution of livestock. PloS One. 2014;9:e96084. doi: 10.1371/journal.pone.0096084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanderson EW, et al. The human footprint and the last of the wild: the human footprint is a global map of human influence on the land surface, which suggests that human beings are stewards of nature, whether we like it or not. BioScience. 2002;52:891–904. doi: 10.1641/0006-3568(2002)052[0891:THFATL]2.0.CO;2. [DOI] [Google Scholar]
- 40.Pachzelt A, Forrest M, Rammig A, Higgins SI, Hickler T. Potential impact of large ungulate grazers on African vegetation, carbon storage and fire regimes. Glob. Ecol. Biogeogr. 2015;24:991–1002. doi: 10.1111/geb.12313. [DOI] [Google Scholar]
- 41.Gagnon M, Chew AE. Dietary preferences in extant African Bovidae. J. Mammal. 2000;81:490–511. doi: 10.1644/1545-1542(2000)081<0490:DPIEAB>2.0.CO;2. [DOI] [Google Scholar]
- 42.Terborgh J, et al. Megafaunal influences on tree recruitment in African equatorial forests. Ecography. 2016;39:180–186. doi: 10.1111/ecog.01641. [DOI] [Google Scholar]
- 43.Blake S, Deem SL, Mossimbo E, Maisels F, Walsh P. Forest elephants: tree planters of the Congo. Biotropica. 2009;41:459–468. doi: 10.1111/j.1744-7429.2009.00512.x. [DOI] [Google Scholar]
- 44.Moleele N, Ringrose S, Matheson W, Vanderpost C. More woody plants? The status of bush encroachment in Botswana’s grazing areas. J. Environ. Manag. 2002;64:3–11. doi: 10.1006/jema.2001.0486. [DOI] [PubMed] [Google Scholar]
- 45.Skowno, A. L. et al. Woodland expansion in South African grassy biomes based on satellite observations (1990–2013): general patterns and potential drivers. Glob. Chang. Biol. (2016). [DOI] [PubMed]
- 46.Vanthomme H, Belle B, Forget P-M. Bushmeat hunting alters recruitment of large-seeded plant species in Central Africa. Biotropica. 2010;42:672–679. doi: 10.1111/j.1744-7429.2010.00630.x. [DOI] [Google Scholar]
- 47.Doughty CE, et al. Megafauna extinction, tree species range reduction, and carbon storage in Amazonian forests. Ecography. 2016;39:194–203. doi: 10.1111/ecog.01587. [DOI] [Google Scholar]
- 48.Bunney K, Bond WJ, Henley M. Seed dispersal kernel of the largest surviving megaherbivore–the African savanna elephant. Biotropica. 2017;49:395–401. doi: 10.1111/btp.12423. [DOI] [Google Scholar]
- 49.Waldram MS, Bond WJ, Stock WD. Ecological engineering by a mega-grazer: white rhino impacts on a South African savanna. Ecosyst. 2008;11:101–112. doi: 10.1007/s10021-007-9109-9. [DOI] [Google Scholar]
- 50.Hempson GP, et al. Ecology of grazing lawns in Africa. Biol. Rev. 2015;90:979–994. doi: 10.1111/brv.12145. [DOI] [PubMed] [Google Scholar]
- 51.Charles-Dominique T, et al. Spiny plants, mammal browsers, and the origin of African savannas. Proc. Natl. Acad. Sci. 2016;113:E5572–E5579. doi: 10.1073/pnas.1607493113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehmann CE, et al. Savanna vegetation-fire-climate relationships differ among continents. Sci. 2014;343:548–552. doi: 10.1126/science.1247355. [DOI] [PubMed] [Google Scholar]
- 53.Sankaran M, Ratnam J, Hanan N. Woody cover in African savannas: the role of resources, fire and herbivory. Glob. Ecol. Biogeogr. 2008;17:236–245. doi: 10.1111/j.1466-8238.2007.00360.x. [DOI] [Google Scholar]
- 54.Holdo RM, Holt RD, Fryxell JM. Grazers, browsers, and fire influence the extent and spatial pattern of tree cover in the Serengeti. Ecol. Appl. 2009;19:95–109. doi: 10.1890/07-1954.1. [DOI] [PubMed] [Google Scholar]
- 55.Bond WJ. What limits trees in C-4 grasslands and savannas? Annu. Rev. Ecol. Evol. Syst. 2008;39:641–659. doi: 10.1146/annurev.ecolsys.39.110707.173411. [DOI] [Google Scholar]
- 56.McNaughton SJ. Ecology of a grazing ecosystem: the Serengeti. Ecol. Monogr. 1985;55:259–294. doi: 10.2307/1942578. [DOI] [Google Scholar]
- 57.Wigley BJ, Bond WJ, Hoffman MT. Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Glob. Chang. Biol. 2010;16:964–976. doi: 10.1111/j.1365-2486.2009.02030.x. [DOI] [Google Scholar]
- 58.Bond WJ, Midgley GF. Carbon dioxide and the uneasy interactions of trees and savannah grasses. Philos. Transactions Royal Soc. B. 2012;367:601–612. doi: 10.1098/rstb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buitenwerf R, Bond WJ, Stevens N, Trollope WSW. Increased tree densities in South African savannas: > 50 years of data suggests CO2 as a driver. Glob. Chang. Biol. 2012;18:675–684. doi: 10.1111/j.1365-2486.2011.02561.x. [DOI] [Google Scholar]
- 60.Beuchner H, Dawkins H. Vegetation change induced by elephants and fire in Murchion Falls National Park, Uganda. Ecol. 1961;42:752–766. doi: 10.2307/1933504. [DOI] [Google Scholar]
- 61.Moncrieff GR, Kruger LM, Midgley JJ. Stem mortality of Acacia nigrescens induced by the synergistic effects of elephants and fire in Kruger National Park, South Africa. J. Trop. Ecol. 2008;24:655–662. doi: 10.1017/S0266467408005476. [DOI] [Google Scholar]
- 62.Shannon G, et al. Relative impacts of elephant and fire on large trees in a savanna ecosystem. Ecosyst. 2011;14:1372–1381. doi: 10.1007/s10021-011-9485-z. [DOI] [Google Scholar]
- 63.Staver AC, Bond WJ. Is there a ‘browse trap’? Dynamics of herbivore impacts on trees and grasses in an African savanna. J. Ecol. 2014;102:595–602. doi: 10.1111/1365-2745.12230. [DOI] [Google Scholar]
- 64.Ritchie ME. Plant compensation to grazing and soil carbon dynamics in a tropical grassland. PeerJ. 2014;2:e233. doi: 10.7717/peerj.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saunois, M et al. The Global Methane Budget 2000–2012. Earth System Science Data. 10.5194/essd-8-697-2016 (2016)
- 66.Wolf J, Asrar GR, West TO. Revised methane emissions factors and spatially distributed annual carbon fluxes for global livestock. Carbon Balance Manage. 2017;12:16. doi: 10.1186/s13021-017-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laws R. Age criteria for the African elephant. Afr. J. Ecol. 1966;4:1–37. doi: 10.1111/j.1365-2028.1966.tb00878.x. [DOI] [Google Scholar]
- 68.Ripple WJ, et al. Ruminants, climate change and climate policy. Nat. Clim. Chang. 2014;4:2–5. doi: 10.1038/nclimate2081. [DOI] [Google Scholar]
- 69.Doughty, C. E. Herbivores increase the global availability of nutrients over millions of years. Nat. Ecol. Evol. 1. 10.1038/s41559-017-1 (2017). [DOI] [PubMed]
- 70.Austin AT, et al. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia. 2004;141:221–235. doi: 10.1007/s00442-004-1519-1. [DOI] [PubMed] [Google Scholar]
- 71.Walker BH, Emslie RH, Owen-Smith N, Scholes RJ. To cull or not to cull: lessons from a southern African drought. J. Appl. Ecol. 1987;24:381–401. doi: 10.2307/2403882. [DOI] [Google Scholar]
- 72.Buitenwerf R, Swemmer AM, Peel MJS. Long-term dynamics of herbaceous vegetation structure and composition in two African savanna reserves. J. Appl. Ecol. 2011;48:238–246. doi: 10.1111/j.1365-2664.2010.01895.x. [DOI] [Google Scholar]
- 73.van de Koppel J, Rietkerk M, Weissing FJ. Catastrophic vegetation shifts and soil degradation in terrestrial grazing systems. Trends In Ecol. & Evol. 1997;12:352–356. doi: 10.1016/S0169-5347(97)01133-6. [DOI] [PubMed] [Google Scholar]
- 74.Daskin JH, Pringle RM. Does primary productivity modulate the indirect effects of large herbivores? A global meta-analysis. J. Animal Ecol. 2016;85:857–868. doi: 10.1111/1365-2656.12522. [DOI] [PubMed] [Google Scholar]
- 75.Pringle RM, Young TP, Rubenstein DI, McCauley DJ. Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc. Natl. Acad. Sci. 2007;104:193–197. doi: 10.1073/pnas.0609840104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maurin O, et al. Savanna fire and the origins of the ‘underground forests’ of Africa. New Phytol. 2014;204:201–214. doi: 10.1111/nph.12936. [DOI] [PubMed] [Google Scholar]
- 77.Bell, R. H. The Ecology of Tropical Savannas, chap. The effect of soil nutrient availability on community structure in African ecosystems, 193–216 (Springer, Heidelberg, 1982).
- 78.Campbell, B. The Miombo in Transition: Woodlands and Welfare in Africa (Center for International Forestry Research (CIFOR), Bogor, Indonesia, 1996).
- 79.Cumming DHM, et al. Elephants, woodlands and biodiversity in southernAfrica. South Afr. J. Sci. 1997;93:231–236. [Google Scholar]
- 80.Brashares JS, et al. Bushmeat hunting, wildlife declines, and fish supply in WestAfrica. Sci. 2004;306:1180–1183. doi: 10.1126/science.1102425. [DOI] [PubMed] [Google Scholar]
- 81.Abernethy K, Coad L, Taylor G, Lee M, Maisels F. Extent and ecological consequences of hunting in Central African rainforests in the twenty-first century. Philos. Transactions Royal Soc. B. 2013;368:20120303. doi: 10.1098/rstb.2012.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fynn RW, Bonyongo MC. Functional conservation areas and the future of Africa’s wildlife. Afr. J. Ecol. 2011;49:175–188. doi: 10.1111/j.1365-2028.2010.01245.x. [DOI] [Google Scholar]
- 83.Illius A, O’Connor T. Resource heterogeneity and ungulate population dynamics. Oikos. 2000;89:283–294. doi: 10.1034/j.1600-0706.2000.890209.x. [DOI] [Google Scholar]
- 84.Ellis JE, Swift DM. Stability of African pastoral ecosystems - alternate paradigms and implications for development. J. Range Manag. 1988;41:450–459. doi: 10.2307/3899515. [DOI] [Google Scholar]
- 85.Bonenfant C, et al. Empirical evidence of density-dependence in populations of large herbivores. Adv. In Ecol. Res. 2009;41:313–357. doi: 10.1016/S0065-2504(09)00405-X. [DOI] [Google Scholar]
- 86.Agrawal A, Nepstad D, Chhatre A. Reducing emissions from deforestation and forest degradation. Annu. Rev. Environ. Resour. 2011;36:373–396. doi: 10.1146/annurev-environ-042009-094508. [DOI] [Google Scholar]
- 87.Russell-Smith, J. et al. Managing fire regimes in north Australian savannas: applying Aboriginal approaches to contemporary global problems. Front. Ecol. Environ. 11 (2013).
- 88.Gerber, P. J. et al. Tackling climate change through livestock: a global assessment of emissions and mitigation opportunities. (Food and Agriculture Organization of the United Nations (FAO), 2013).
- 89.Bond WJ, Woodward FI, Midgley GF. The global distribution of ecosystems in a world without fire. New Phytol. 2005;165:525–537. doi: 10.1111/j.1469-8137.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 90.Bond WJ. Ancient grasslands at risk. Sci. 2016;351:120–122. doi: 10.1126/science.aad5132. [DOI] [PubMed] [Google Scholar]
- 91.Van der Werf GR, et al. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009) Atmospheric Chem. Phys. 2010;10:11707–11735. doi: 10.5194/acp-10-11707-2010. [DOI] [Google Scholar]
- 92.White, F. The vegetation of Africa - a descriptive memoir to accompany the UNESCO/AETFAT/UNSO vegetation map of Africa (1983).
- 93.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2017).
- 94.QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation (2009). URL http://qgis.osgeo.org.
- 95.Sankaran M, et al. Determinants of woody cover in African savannas. Nat. 2005;438:846–849. doi: 10.1038/nature04070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.