Abstract
Background:
Piper guineense (PG) and Sesamum indicum (SI) have been shown to be rich sources of antioxidants and other health benefits; hence, we evaluated the impact of its consumption in hypercholesterolemic model on lipid metabolism.
Methods:
Forty-eight animals were divided into eight groups of six rats each. Rats were given cholesterol (40 mg/0.3ml), PG and SI extract (100 and 200 mg/kg), and Questran (0.26 g/kg) orally, five times a week for 28 days. Lipid profile, hepatic antioxidant status, biomarkers of liver toxicity, and tissue histopathology were examined. Data were analyzed using one-way ANOVA and P<0.05 were considered statistically significant.
Results:
Cholesterol feeding caused 100% gain in weight, significantly increased AST, LPO (P=0.41 and 0.002) but significantly decreased SOD (P=0.003) compared to control. CHPG(1)/(2) and CHSI(1)/(2) caused a significant decrease (P=0.01, 0.005, 0.003, and 0.023) in cholesterol-induced body-weight gain and decreased serum total cholesterol by 20-30% compared to untreated-hypercholesterolemic rats. Triglyceride and LDL-c decreased with extract administration and specifically HDL-c increased significantly (P<0.001) by CHSI(1) compared to untreated-hypercholesterol rats. Furthermore, an increase in HDL-c was higher (P=0.04 and 0.002) by SI compared to PG at both doses.
Conclusion:
These results indicate that PG and SI exerts a hypolipidemic effect, reduces cholesterol intake induced body weight gain, and increases the body’s antioxidant defense system in experimental hypercholesterolemia.
Keywords: Antioxidants, Hypercholesterolemia, Hyperlipidemias, Piper guineense, Sesamum indicum
What’s Known
Piper guineense (PG) stimulates the digestive enzymes of pancreas and protects against oxidative damage/lipid.
Sesame oil has estrogenic, anti-inflammatory, and lipid-lowering properties.
What’s New
Aqueous extract of PG and SI at the tested doses exerted hypolipidemic effects, reversed the elevations witnessed in serum aminotransferase activities and lipid peroxidation levels, and ameliorated enzymatic and non-enzymatic antioxidant status in hypercholesterolemic rats.
Our findings regarding the protective role of PG and SI lend support to the claims for the traditional usage of these plants.
Introduction
Evidence abounds to substantiate the fact that diet offers protection against various diseases, especially those related to aging. Diets rich in fruits, vegetables, whole grains, and spices have been known to contribute to increased level of antioxidant.1 Seeds have nutritive and calorific values, but could be used as spices to add aroma and improve the taste of food. Spices are necessary condiments in foods and many have been shown to be good sources of antioxidants. Food consumption plays a crucial role in the management of disease, especially those related to dietary indiscretion, aside from medication. Hypercholesterolemia is one of the major risk factors for coronary heart disease (CHD) and atherosclerosis.2 However age, estrogen replacement therapy, family history of CHD, cigarette smoking, hypertension, and diabetes mellitus among others could affect cholesterol level.
Plant-based remedies have also been recognized as therapeutic agents with little or minimal side effects. Several herbal preparations have been used to treat various ailments, such as diabetes, rheumatoid arthritis, atherosclerosis3,4 and some have been used to modulate cholesterol level.1,5
Piper guineense (PG) or African black pepper, a climbing perennial plant of the family Piperaceae, is used as spice, food preservative, insecticide, herbal medicine, and as fragrance in the cosmetic industry.6 PG is used for the treatment of cough, bronchitis, intestinal diseases, and rheumatism.7 PG stimulates the digestive enzymes, lowers lipid peroxidation, prevents oxidative damage, and inflammation.8 Sesamun indicum (SI), locally known as Beeni seed in Nigeria, belong to the family Pedaliaceae. It is a staple food in both the Middle Belt and Northern Nigeria and often sold as antioxidants-rich health food. Sesame oil has been reported to have estrogenic, anti-inflammatory and lipids lowering properties.9-12 The acclaimed antioxidant and lipid-lowering properties of these seeds led us to evaluate the effect of the aqueous extracts in hypercholesterolemic rats and to compare the effect with a known hypocholesterolemic drug (Questran®). We demonstrate that the aqueous extract of PG and SI at the tested doses exerts hypolipidemic effect, reversed the elevations witnessed in serum aminotransferase activities as well as LPO level and ameliorated enzymatic and non-enzymatic antioxidant status in hypercholesterolemic rats. This complemented previously identified protective roles of PG and SI; also lends support to the claims for traditional usage of these plants.
Materials and Methods
Plant Extract
Seeds of SI and PG were purchased from Ojoo market (Ibadan, Nigeria) and were identified and authenticated in Botany Department Herbarium, University of Ibadan, Nigeria.6,12 Extraction of seeds were as stated earlier.5
Animals
Forty-eight male Wistar albino rats weighing between 85-130g were obtained from IMRAT and were housed in the Animal House of Biochemistry Department, University of Ibadan (Ibadan, Nigeria) at ambient conditions. Rats were acclimatized for two weeks on standard rat chow and were allowed free access to food and water ad libitum. Rats were randomly placed into eight groups of six rats each. Group CTRL (control) received only distilled water. Group QU (positive control) had Questran only. Group CH (untreated-hypercholestrolemic) had only cholesterol. Group CHQU received cholesterol and Questran. Groups CHPG(1) and CHPG(2) received cholesterol (40 mg/0.3ml/animal) and PG extracts (100 or 200 mg/kg), respectively, while CHSI(1) and CHSI(2) received cholesterol (40 mg/0.3ml/animal) and SI extracts (100 or 200 mg/kg), respectively. Distilled water served as vehicle for extract administration; dietary cholesterol and Questran were given at doses of 40 mg/0.3ml/animal and 0.26 g/kg body weight, respectively. Drugs were administered by oral gavage once daily for 28 days. Animal experiments conformed to the protocols for the Care and Use of Laboratory Animals established by the National Institutes of Health (NIH Publication, number 85-23, revised 1985) and the protocol was approved by the Institutional Animal Care and Use Committee of Afe Babalola University.
Sample Collection
Animals were fasted for 24 h after the last dose of drugs and were sacrificed by cervical dislocation. Serum, tissue homogenate, and sections of liver and kidney for histology were as reported earlier.5
Biochemical Assays
Lipid profile was assayed using kits. Catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH), malonyldialdehyde (MDA), and protein concentration were assayed spectrophotometrically using the established methods6 and Randox assay kits were used for aspartate aminotransferase (AST) and alanine aminotransferase (ALT).
Statistical Analysis
All data are presented as the mean±SD (standard deviation). The data were analyzed by one-way ANOVA followed by student t-test using SPSS software package version 17.0 (SPSS Inc., Chicago, IL, USA). The differences were considered significant at P<0.05.
Results
Body/Organ Weights in Cholesterol SI and PG Administered Rat
Tables 1 and 2 show data on organ, body weight (bwt), and multiple-comparison. Cholesterol feeding caused almost 100% increase in CH compared to CTRL and was ameliorated significantly P=0.01, 0.05, 0.03, and 0.023 by CHPG(1), CHGP(2), CHSI(1) and CHSI(2), respectively.
Table 1.
Comparison of effects of Piper guineense (PG), Sesamum indicum (SI), Cholesterol (CH) and Questran (Q) on the body/organ weights and liver index
| Group | Initial body weight (IBWT) | Final body weight (FBWT) | Weight gain (WG) | Liver weight (LG) | Liver index (LINX) |
|---|---|---|---|---|---|
| CTRL | 101±3.0 | 130±16.7 | 29±16.7 | 3.9±0.7 | 3.8±0.3 |
| QU | 106±13.9 | 139±20.7 | 33±18.3 | 4.5±1.1 | 3.5±0.3 |
| CH | 116±9.6 | 238±27.8 | 122±20.8 | 7.5±0.8 | 2.9±0.6 |
| CHQU | 106±19.19 | 165±28.5 | 59±14.8 | 6.9±0.6 | 3.8±0.9 |
| CHPG (1) | 118±8.4 | 145±13.2 | 27±11.5 | 7.1±1.5 | 4.9±0.7 |
| CHPG (2) | 127±2.7 | 153±33.3 | 26±31.7 | 7.2±1.2 | 4.8±0.4 |
| CHSI (1) | 118±7.6 | 146±15.2 | 28±17.5 | 7.8±0.5 | 5.4±0.4 |
| CHSI (2) | 110±8.2 | 153±10.8 | 43±7.5 | 7.2±0.9 | 3.7±0.3 |
Table 2.
Multiple-comparison of data in table 1 (P levels)
| MComp | Groups | ||||||
|---|---|---|---|---|---|---|---|
| QU | CH | CHQU | CHPG (1) | CHPG (2) | CHSI (1) | CHSI (2) | |
| IBWT | |||||||
| QU | 0.321 | 0.587 | 0.033* | 0.859 | 0.872 | 0.979 | |
| CHPG (1) | 0.022* | 0.047* | 0.031* | ||||
| FBWT | |||||||
| CTRL | 0.258 | 0.304 | 0.558 | 0.020* | 0.312 | 0.005* | 0.278 |
| CHQU | 0.074 | 0.667 | 0.021* | 0.165 | |||
| WG | |||||||
| CH | 0.123 | 0.010* | 0.005* | 0.003* | 0.023* | ||
| CTRL | 0.228 | 0.102 | 0.026* | 0.485 | 0.029* | 0.375 | 0.907 |
| LW | |||||||
| CHQU | 0.115 | 0.96 | 0.164 | 0.034* | |||
| CHPG (2) | 0.179 | 0.038* | |||||
| LINX | |||||||
| QU | 0.12 | 0.08 | 0.359 | 0.994 | 0.624 | 0.023* | |
| CHPG (2) | 0.629 | 0.023* | |||||
Significant at P<0.05
Data on proteins, serum lipids and its multiple-comparison are shown in tables 3 and 4. Cholesterol intake caused a significant increase (P=0.002) in total-cholesterol; elevated triglyceride, LDL-cholesterol, lipid-peroxidation and decreased protein level and HDL-cholesterol compared to control. CHSI(1) and CHSI(2) caused a significant increase in HDL-c (P=0.000 and 0.010), respectively compared to CH rats.
Table 3.
Comparison of effects of Piper guineense (PG), Sesamum indicum (SI), Cholesterol (CH) and Questran (Q) on protein (PROT), total cholesterol (TCH), HDLc, triglyceride, lipid peroxidation
| Group | Protein (mg/100mL) | Triglyceride (mg/dL) | TCH (mg/dL) | Lipid peroxidation (mg/dL) | HDLc (mg/dL) |
|---|---|---|---|---|---|
| CTRL | 1.2±0.2 | 2.1±0.3 | 4.7±0.4 | 27.3±1.6 | 1.8±0.2 |
| QU | 1.2±0.1 | 3.8±0.3 | 2.9±1.6 | 29.3±1.9 | 5.2±1.2 |
| CH | 0.8±0.4 | 3.8±0.9 | 4.9±1.8 | 80.5±2.2 | 2.4±0.5 |
| CHQU | 1.4±0.2 | 2.3±0.7 | 4.1±0.1 | 54.5±2.7 | 4.3±2.2 |
| CHPG (1) | 1.5±0.8 | 2.2±0.1 | 3.3±0.4 | 66.2±1.0 | 6.3±2.8 |
| CHPG (2) | 1.4±0.2 | 2.5±0.1 | 3.3±0.3 | 50.1±2.8 | 5.5±1.0 |
| CHSI (1) | 1.7±0.7 | 2.6±0.2 | 3.9±0.7 | 62.6±1.9 | 12.1±1.9 |
| CHSI (2) | 1.7±0.2 | 1.5±0.2 | 3.5±0.4 | 51.4±2.5 | 7.9±0.8 |
Table 4.
Multiple-comparison for table 3 (P levels)
| MComp | Groups | ||||||
|---|---|---|---|---|---|---|---|
| QU | CH | CHQU | CHPG (1) | CHPG (2) | CHSI (1) | CHSI (2) | |
| PROT | |||||||
| CHPG (1) | 0.415 | 0.007* | 0.055 | ||||
| CHPG (2) | 0.049* | 0.257 | |||||
| TRIG | |||||||
| QU | 0.1 | 0.111 | 0.138 | 0.079 | 0.147 | 0.019* | |
| LPO | |||||||
| CTRL | 0.957 | 0.002* | 0.749 | 0.269 | 0.066 | 0.453 | 0.59 |
| QU | 0.002* | 0.709 | 0.247 | 0.059 | 0.486 | 0.554 | |
| CH | 0.006* | 0.04* | 0.183 | 0.000* | 0.010* | ||
| CHPG (2) | 0.012* | 0.185 | |||||
| CHSI (1) | 0.201* | ||||||
| HDLc | |||||||
| CTRL | 0.42 | 0.846 | 0.86 | 0.234 | 0.325 | 0.000* | 0.087 |
| QU | 0.539 | 0.327 | 0.696 | 0.858 | 0.001* | 0.352 | |
| CH | 0.711 | 0.317 | 0.429 | 0.000* | 0.126 | ||
| CHQU | 0.173 | 0.248 | 0.000* | 0.06 | |||
| CHPG (1) | 0.832 | 0.004* | 0.587 | ||||
| CHPG (2) | 0.002* | 0.451 | |||||
| CHSI (1) | 0.017* | ||||||
Significant at P<0.05
Cholesterol intake caused a decrease in liver GSH, SOD, GST activity compared with other treatment groups (table 5) and table 6 shows multiple-comparison data. There was an elevation in both AST and ALT in the serum of cholesterol only fed group compared to control.
Table 5.
Comparison of effects of Piper guineense (PG), Sesamum indicum (SI), Cholesterol (CH) and Questran (Q) on AST, ALT, CAT, GSH, GST and SOD
| Group | Serum AST (mg/dL) | Serum ALT (mg/dL) | CAT activity (unit/mg protein) | GSH (µg/mL) | GST activity (µmol/mg protein) | SOD activity (unit/mg protein) |
|---|---|---|---|---|---|---|
| CTRL | 8.0±0.5 | 30.2±1.5 | 5.3±0.4 | 2.8±0.3 | 26.2±0.1 | 9.0±2.8 |
| QU | 8.0±1.2 | 34.1±1.3 | 6.2±0.6 | 4.0±0.7 | 31.1±0.1 | 9.0±1.8 |
| CH | 20.7±1.5 | 45.5±3.0 | 3.8±0.6 | 2.1±0.1 | 18.1±0.3 | 2.0±2.1 |
| CHQU | 6.0±0.2 | 24.0±1.3 | 4.1±0.7 | 3.2±1.4 | 28.4±0.3 | 6.0±1.3 |
| CHPG (1) | 12.1±1.9 | 36.5±2.7 | 3.3±0.5 | 4.8±1.4 | 22.1±0.2 | 4.0±1.0 |
| CHPG (2) | 6.6±0.2 | 31.9±5.0 | 3.2±0.4 | 5.5±0.7 | 27.1±0.2 | 7.0±0.9 |
| CHSI (1) | 6.5±4.7 | 38.3±1.8 | 3.2±0.3 | 4.1±1.6 | 25±0.2 | 4.0±2.8 |
| CHSI (2) | 5.0±1.4 | 35.3±2.1 | 3.5±0.1 | 3.9±0.4 | 28.1±0.1 | 6.0±2.2 |
Table 6.
Multiple-comparison for table 5 (P levels)
| MComp | Groups | ||||||
|---|---|---|---|---|---|---|---|
| QU | CH | CHQU | CHPG (1) | CHPG (2) | CHSI (1) | CHSI (2) | |
| AST | |||||||
| CTRL | 0.86 | 0.041* | 0.701 | 0.185 | 0.477 | 0.851 | 0.736 |
| ALT | |||||||
| 0.017* | 0.452 | 0.007* | 0.062 | 0.019* | |||
| CHPG (1) | 0.045* | 0.252 | 0.099 | ||||
| CAT | |||||||
| QU | 0.102 | 0.017* | 0.015* | 0.16 | 0.002* | 0.036* | |
| GSH | |||||||
| CTRL | 0.18 | 0.845 | 0.424 | 0.024* | 0.329 | 0.496 | 0.456 |
| CH | 0.321 | 0.015* | 0.243 | 0.382 | 0.348 | ||
| GST | |||||||
| CHPG (1) | 0.543 | 0.512 | 0.017* | ||||
| SOD | |||||||
| CTRL | 0.543 | 0.016 | 0.277 | 0.324 | 0.604 | 0.030* | 0.275 |
| QU | 0.003* | 0.094 | 0.115 | 0.263 | 0.007* | 0.093 | |
| CH | 0.0162* | 0.135* | 0.052 | 0.79 | 0.164 | ||
Significant at P<0.05
Discussion
Hypercholesterolemia is a metabolic disorder characterized by high blood cholesterol level, a risk factor in the development of atherosclerosis and cardiovascular diseases (CVD).13,14 Hypercholesterolemia is a global problem and there is a close correlation between CVD and lipid abnormalities.15,16 Cholesterol level is regulated by its absorption, synthesis, and excretion. Dietary cholesterol affects total cholesterol, LDLc, and free radical generation in humans and animals. In recent years, CVD have been described as the leading cause of death worldwide. The enormous and growing burdens of CVD, especially in the developing countries can be attributed to the increasing prevalence of atherosclerotic diseases, urbanization, and other predisposing risk factors such as obesity, diabetes, dyslipidamia, and hypertension. Hypercholesterolemia has been reported as a common risk factor for CVD. Therefore, management of hypercholesterolemia may attenuate the pathogenesis of cardiovascular disease.17,18
The present study demonstrated that cholesterol intake remarkably increased both body and liver weights in male rats, elevated serum lipids, increased LPO, but decreased SOD, GST, GSH and HDL-c. All these biochemical parameters were significantly attenuated in hypercholesterolemic-treated with PG and SI, respectively. The role of medicinal plants in the management of hypercholesterolemia and its role in the prevention of CVD have been documented in the literature.19,20 In this study, cholesterol feeding caused a significant increase in body weight of animals relative to control. Upon treatment with Piper guineense (PG) or Sesamum indicum (SI) and Questran, there was a significant reduction in body weight gain in treated-hypercholesterolemic animal compared to hypercholesterolemic control. It was also observed that liver weights of hypercholesterolemic rats were significantly increased when compared to normal control. Similar results were obtained by Harnafi et al. (2009).21 Increased body weight by cholesterol intake may be due to high rate of lipid deposition in the adipose tissues.
On the other hand, cholesterol intake caused an increase in total-cholesterol and triglyceride, this increase was associated with a concurrent decrease in HDL-cholesterol; thus, indicating cholesterol feeding induced hypercholesterolemia.22 Linear correlation has been established between dietary cholesterol intake and mortality due to coronary heart disease.23 Lipid close-packing structure, composition, configuration, plus high fat and cholesterol intakes affect plasma lipid level24 and these are risk markers for obesity, diabetes, and coronary heart diseases. PG and SI caused a significant increase in HDL-c when compared with untreated hypercholesterolemic rats. SI had more than 100% increased at 100 mg/kg bwt than the reference drug, thus indicating cholesterol-lowering potential of plant extracts.25 This result is in agreement with the previously documented report.26
Added to this, there was a slight reduction in the protein concentration in untreated hypercholesterolemic rats compared to the control. A similar finding was revealed by Olorunnisola and co-worker in 2012. They reported that the declined level of total protein might be attributed to decrease in protein uptake from the intestine due to high-calorie lipid diet. This is an indication of diminished synthetic function of the liver resulting probably from hepatocellular injury or stress resulting from the increased metabolic need for tissue repair and free radical neutralization occasioned by the high-fat diet. The ability of the plant extracts to prevent reduction in total protein after cholesterol administration may be attributed to its free radical scavenging properties.27
As observed in the current study, the increase in lipid peroxidation in hypercholesterolemic rats is an indication of increased oxidative stress. Similar results were obtained in previous studies in rats.28 A possible explanation for the increased oxidative stress associated to hypercholesterolaemia could be the enhanced production of free radicals as a consequence of the higher cholesterol content of erythrocytes, polymorphonuclear cells, and platelets initiating a series of reactions that lead to ROS formation and the subsequent peroxidation of macromolecules.29
Endogenous antioxidant such as SOD, CAT, GSH, GPx, GST, and non-enzymatic antioxidants such as tocopherols, carotenoids, and ascorbic acid can neutralize free radicals. Feeding cholesterol to rats in this study caused a decrease in hepatic SOD, CAT and GST activities as well as GSH level compared with the control. In agreement with previous studies,30 the antioxidant status was restored in hypercholesterolemic rats treated with PG, SI, or Questran. The antioxidant defense system in the body can change with feed-intakes.31 This depletion in antioxidants might lead to the generation of reactive oxygen species and oxidative stress with a cascade of effects, thereby affecting the membrane, causing lipid peroxidation and various pathological conditions that may include atherogenesis.32,33
The liver plays an essential role in lipid metabolism, several stages of lipid synthesis and transportation. ALT is primarily localized to the liver, but the AST is present in tissues like the heart, skeletal muscle, kidney, brain, and liver.34,35 AST activities in serum at any moment reflects the relative rate at which it enters and leaves circulation. In the present study, there was an elevation in both AST and ALT in the serum of untreated-hypercholesterolemic group compared to control. Our result is in agreement with previous study who reported that pomegranate extract decreased serum ALT and AST in rats.36 An increase in the levels of serum marker enzymes is generally regarded as one of the most sensitive index of the hepatic damage. The plant extract significantly suppressed the increase of transaminases and MDA, suggesting a protective role of the plant extract against oxidative stress. Photomicrographs of histopathology result of liver tissue are shown in figure 1. Histologically, the results of this study showed no visible lesions in the control groups, but showed lesions in untreated-hypercholesterolemic rats. Hypercholesterolemic rats co-treated with either Questran, PG and SI showed mild lesions.
Figure 1.
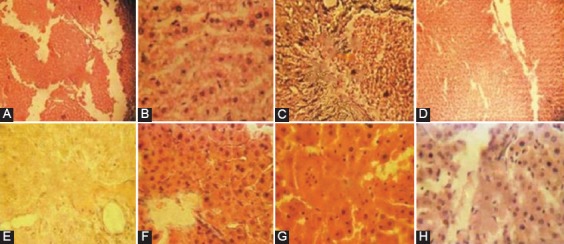
Photomicrographs of liver tissue (section stained with H&E, ×400) (A) CTRL: No visible lesions, (B) QU: Diffuse hydropic degeneration of the hepatocytes with very prominent sinusoids, (C) CH: Marked portal congestion and diffuse vacuolar degeneration of the hepatocytes, (D) CHQU: Mild Kupffer cell proliferation with moderate hepatic vacuolar degeneration, associated with moderate periportal cellular infiltration by mononuclear cells, (E) CHPG(1): Mild congestion of the portal vessels, with very mild periportal, hydropic hepatic degeneration, (F) CHPG(2): Mild portal congestion and mononuclear cellular infiltration, mild periportal and hydropic degeneration, (G) CHSI(1): Marked portal congestion and cellular infiltration by mononuclear cells, (H) CHSI(2): Portal canal congestion moderately infiltrated by mononuclear cells.
Conclusion
Taken together, the results from the present study indicate that the aqueous extract of PG and SI exerts a hypolipidemic effect, reduces cholesterol-induced body weight gain, and increases the body’s antioxidant defense system in experimental hypercholesterolemia in rats. The mechanism has pointed towards inhibiting lipid peroxidation and mitigating oxidative stress by diminishing free radical generation, especially reactive oxygen species and ameliorating the endogenous antioxidants.
Acknowledgement
We are indebted to Mr. Eric Sabo for technical assistance
Conflict of Interest: None declared.
References
- 1.Onyenibe NS, Fowokemi KT, Emmanuel OB. African Nutmeg (Monodora Myristica) Lowers Cholesterol and Modulates Lipid Peroxidation in Experimentally Induced Hypercholesterolemic Male Wistar Rats. Int J Biomed Sci. 2015;11:86–92. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 2.Mensink RP, Aro A, Den Hond E, German JB, Griffin BA, ten Meer HU, et al. PASSCLAIM - Diet-related cardiovascular disease. Eur J Nutr. 2003;42(Suppl 1):I6–27. doi: 10.1007/s00394-003-1102-2. [DOI] [PubMed] [Google Scholar]
- 3.Sharma R. Nutraceuticals and nutraceutical supplementation criteria in cancer: a literature survey. Open Nutraceuticals J. 2009;2:92–106. [Google Scholar]
- 4.Said O, Fulder S, Khalil K, Azaizeh H, Kassis E, Saad B. Maintaining a physiological blood glucose level with ‘glucolevel’, a combination of four anti-diabetes plants used in the traditional arab herbal medicine. Evid Based Complement Alternat Med. 2008;5:421–8. doi: 10.1093/ecam/nem047. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mothana RA, Abdo SA, Hasson S, Althawab FM, Alaghbari SA, Lindequist U. Antimicrobial, antioxidant and cytotoxic activities and phytochemical screening of some yemeni medicinal plants. Evid Based Complement Alternat Med. 2010;7:323–30. doi: 10.1093/ecam/nen004. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nwozo SO, Orojobi BF, Adaramoye OA. Hypolipidemic and antioxidant potentials of Xylopia aethiopica seed extract in hypercholesterolemic rats. J Med Food. 2011;14:114–9. doi: 10.1089/jmf.2008.0168. [DOI] [PubMed] [Google Scholar]
- 7.Sarah ON, Adeyinka AA, Babatunji EO. Hepatoprotective effect of Piper guineense aqueous extract against ethanol-induced toxicity in male rats. J Exp Integr Med. 2011;2:71–6. [Google Scholar]
- 8.Ogunniran KO. Antibacterial effects of extracts of Ocimum gratissimum and piper guineense on Escherichia coli and Staphylococcus aureus. African Journal of Food Science. 2009;3:77–81. [Google Scholar]
- 9.Stohr JR, Xiao PG, Bauer R. Constituents of Chinese Piper species and their inhibitory activity on prostaglandin and leukotriene biosynthesis in vitro. J Ethnopharmacol. 2001;75:133–9. doi: 10.1016/s0378-8741(00)00397-4. [DOI] [PubMed] [Google Scholar]
- 10.Coulman KD, Liu Z, Hum WQ, Michaelides J, Thompson LU. Whole sesame seed is as rich a source of mammalian lignan precursors as whole flaxseed. Nutr Cancer. 2005;52:156–65. doi: 10.1207/s15327914nc5202_6. [DOI] [PubMed] [Google Scholar]
- 11.Hsu DZ, Li YH, Chien SP, Liu MY. Effects of sesame oil on oxidative stress and hepatic injury after cecal ligation and puncture in rats. Shock. 2004;21:466–9. doi: 10.1097/00024382-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 12.El Wakf AM, Hassan HA, Gharib NS. Osteoprotective effect of soybean and sesame oils in ovariectomized rats via estrogen-like mechanism. Cytotechnology. 2014;66:335–43. doi: 10.1007/s10616-013-9580-4. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyinloye BE, Ajiboye BO, Ojo OA, Nwozo SO, Kappo AP. Cardioprotective and Antioxidant Influence of Aqueous Extracts from Sesamum indicum Seeds on Oxidative Stress Induced by Cadmium in Wistar Rats. Pharmacogn Mag. 2016;12:S170–4. doi: 10.4103/0973-1296.182155. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rerkasem K, Gallagher PJ, Grimble RF, Calder PC, Shearman CP. Managing hypercholesterolemia and its correlation with carotid plaque morphology in patients undergoing carotid endarterectomy. Vasc Health Risk Manag. 2008;4:1259–64. doi: 10.2147/vhrm.s3729. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matos SL, Paula Hd, Pedrosa ML, Santos RCd, Oliveira ELd, Chianca Júnior DA, et al. Dietary models for inducing hypercholesterolemia in rats. Braz Arch Biol Technol. 2005;48:203–9. [Google Scholar]
- 16.Ramachandran H, Narasimhamurthy K, Raina P. Modulation of cholesterol induced hypercholesterolemia through dietary factors in Indian desert gerbils (Meriones hurrianae) Nutrition Research. 2003;23:245–56. [Google Scholar]
- 17.Olorunnisola O, Bradley G, Afolayan A. Anti-hyperlipidemic and biochemical effect of extract of Tulbaghia violacea rhizomes on high cholesterol diet fed rats. Afr J Biotechnol. 2012;11:13498–505. doi: 10.5897/AJB12.1604. [DOI] [Google Scholar]
- 18.Kreatsoulas C, Anand SS. The impact of social determinants on cardiovascular disease. Can J Cardiol. 2010;26(Suppl C):8C–13C. doi: 10.1016/s0828-282x(10)71075-8. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruthappan V, Shree KS. Effects of Phyllanthus reticulatus on lipid profile and oxidative stress in hypercholesterolemic albino rats. Indian J Pharmacol. 2010;42:388–91. doi: 10.4103/0253-7613.71923. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasanthi HR, ShriShriMal N, Das DK. Phytochemicals from plants to combat cardiovascular disease. Curr Med Chem. 2012;19:2242–51. doi: 10.2174/092986712800229078. [DOI] [PubMed] [Google Scholar]
- 21.Harnafi H, Aziz M, Amrani S. Sweet basil (Ocimum basilicum L.) improves lipid metabolism in hypercholesterolemic rats. E Spen Eur E J Clin Nutr Metab. 2009;4:e181–e6. doi: 10.1016/j.eclnm.2009.05.011. [DOI] [Google Scholar]
- 22.Qinna NA, Kamona BS, Alhussainy TM, Taha H, Badwan AA, Matalka KZ. Effects of prickly pear dried leaves, artichoke leaves, turmeric and garlic extracts, and their combinations on preventing dyslipidemia in rats. ISRN Pharmacol. 2012;2012:167979. doi: 10.5402/2012/167979. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abolaji A, Adebayo H, Odesanmi O. Effects of ethanolic fruit extract of Parinari polyandra (Rosaceae) on serum lipid profile and some electrolytes in pregnant rabbits. Research Journal of Medicinal Plant. 2007;1:121–7. [Google Scholar]
- 24.Griffin JD, Lichtenstein AH. Dietary Cholesterol and Plasma Lipoprotein Profiles:Randomized-Controlled Trials. Curr Nutr Rep. 2013;2:274–82. doi: 10.1007/s13668-013-0064-0. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogier N, Amiot MJ, George S, Maillot M, Mallmann C, Maraninchi M, et al. LDL-cholesterol-lowering effect of a dietary supplement with plant extracts in subjects with moderate hypercholesterolemia. Eur J Nutr. 2013;52:547–57. doi: 10.1007/s00394-012-0357-x. [DOI] [PubMed] [Google Scholar]
- 26.Balamurugan G, Shantha A. Effect of Erythrina variegata seed extract on hyperlipidemia elicited by high-fat diet in wistar rats. J Pharm Bioallied Sci. 2010;2:350–5. doi: 10.4103/0975-7406.72139. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olorunnisola OS, Bradley G, Afolayan AJ. Protective effect of T. violacea rhizome extract against hypercholesterolemia-induced oxidative stress in Wistar rats. Molecules. 2012;17:6033–45. doi: 10.3390/molecules17056033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lecumberri E, Goya L, Mateos R, Alia M, Ramos S, Izquierdo-Pulido M, et al. A diet rich in dietary fiber from cocoa improves lipid profile and reduces malondialdehyde in hypercholesterolemic rats. Nutrition. 2007;23:332–41. doi: 10.1016/j.nut.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Bravo L, Mateos R, Sarria B, Baeza G, Lecumberri E, Ramos S, et al. Hypocholesterolaemic and antioxidant effects of yerba mate (Ilex paraguariensis) in high-cholesterol fed rats. Fitoterapia. 2014;92:219–29. doi: 10.1016/j.fitote.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Zidan Y, Bouderbala S, Bouchenak M. Portulaca oleracea aqueous extract reduces oxidative stress in erythrocytes and tissues, in rats fed enriched-cholesterol diet. J Exp Integr Med. 2016;6:21–5. doi: 10.5455/jeim.260116.or.145. [DOI] [Google Scholar]
- 31.Perlemuter G, Davit-Spraul A, Cosson C, Conti M, Bigorgne A, Paradis V, et al. Increase in liver antioxidant enzyme activities in non-alcoholic fatty liver disease. Liver Int. 2005;25:946–53. doi: 10.1111/j.1478-3231.2005.01126.x. [DOI] [PubMed] [Google Scholar]
- 32.Khan HBH, Vinayagam KS, Kumar S, Palanivelu S, Panchanadham S. Anihypercholesterolemic effect of Semecarpus anacardium Linn nut milk extract in high-cholesterol-fed rats. Comp Clin Path. 2014;23:875–84. doi: 10.1007/s00580-013-1706-8. [DOI] [Google Scholar]
- 33.Subramaniam S, Khan HBH, Palanivelu S, Panchanatham S. Antihyperlipidemic and antiinflammatory effect of Bhallataka nuts in ameliorating the alterations in lipid metabolism and inflammation in diabetes-induced cardiac damage in rats. Comp Clin Path. 2014;23:1593–601. doi: 10.1007/s00580-013-1828-z. [DOI] [Google Scholar]
- 34.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 35.Martins J, Azzalis A, de Oliveira C, Fernandes L, Tufik S, D’Almeida V. Effects of Chow and Liquid Diet on Liver Integrity and Antioxidant Defense in Sleep-Deprived Male Rats. Nutrition & Food Sciences. 2013 doi: 10.4172/2155-9600.1000228. [DOI] [Google Scholar]
- 36.Sadeghipour A, Eidi M, Ilchizadeh Kavgani A, Ghahramani R, Shahabzadeh S, Anissian A. Lipid Lowering Effect of Punica granatum L. Peel in High Lipid Diet Fed Male Rats. Evid Based Complement Alternat Med. 2014;2014:432650. doi: 10.1155/2014/432650. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


