Abstract
Background:
The adjuvanticity potential of Lactobacillus casei was first suggested in an old survey. The present study was designed to investigate the efficacy of a new immunotherapy against breast cancer made by mixing an extract of heated 4T1 mammary carcinoma cell line and a heat-killed preparation of Lactobacillus casei.
Methods:
Female BALB/c mice (6–8 weeks old, n=40) were challenged subcutaneously in the right flanks with 4T1 cells. When all the animals developed a palpable tumor, they were allocated to 4 equal groups and immunotherapy was initiated. The tumor-bearing mice in the experimental groups received the extract of heated 4T1 or heated Lactobacillus casei and/or a combination of both, twice at a 1-week interval. The mice in the control group received phosphate-buffered saline. One week after the last immunotherapy, one half of the mice were euthanized to determine the immune response profile. The remaining animals were kept until death occurred spontaneously.
Results:
The animals receiving the combined treatment significantly showed more favorable survival curves and slower rates of tumor development than the tumor-bearing mice receiving only the heated 4T1 and/or the negative control mice. The combined immunization significantly amplified the production of nitric oxide and the cytotoxicity of natural killer cells in the spleen cell culture of the tumor-bearing mice. Moreover, the combined immunotherapy significantly increased the secretion of IFN-γ and conversely diminished the secretion of IL-4 and TGF-β in the splenocyte population compared to the splenocytes from the other groups.
Conclusion:
The combined immunotherapy with heated 4T1 cells and heated Lactobacillus casei conferred beneficial outcomes in our mouse model of breast cancer.
Keywords: 4T1 cell line, Breast neoplasms, Lactobacillus casei, Immunotherapy
What’s Known
Adjuvanticity potential of Lactobacillus casei was suggested in an old survey.
There is no/or limited information about the possible adjuvant benefit of the killed preparation of Lactobacillus casei in tumor vaccines.
What’s New
Mice with mammary tumors treated with combined immunization showed a more favorable survival curve and slower rate of tumor development than mice with tumors receiving only heated 4T1 and/or negative control mice.
Combined immunization significantly amplified the respiratory burst potential and the secretion of IFN-γ and, conversely, diminished the secretion of IL-4, IL-10, and TGF-β in the splenocyte population compared to the splenocytes from the other groups.
Combined immunotherapy with heated 4T1 cells and heated Lactobacillus casei promoted beneficial outcomes in our mouse model of breast cancer.
Introduction
In spite of valuable progress in the treatment of breast cancer, many women with breast cancer have a less-than-perfect response.1 It is well-recognized that innate and adaptive immune responses possess the ability to identify and eliminate some tumor cells, at least in the early stage of their development.2 Nevertheless, many tumors are frequently able to grow and evade destruction by the immune system due to their low immunogenicity or the production of immunoregulatory substances. Thus, improving immunity responses against tumor cells is a useful approach to control malignancy.2-4 Therapeutic cancer vaccines are one of the suitable strategies to elicit an appropriate anti-tumor immune response.3 It is obvious that the cellular arm of immunity like the Th1 response plays a pivotal role in the defense against tumor cells.5 Adjuvants are advantageous materials to polarize immune responses into the desired pathway.6,7 Alum is the only adjuvant licensed worldwide for human use. However, alum is only able to promote the Th2-specific response.8 Unfortunately, strong Th1 adjuvants such as complete Freund’s adjuvant cannot be tolerated by humans.9 Therefore, investigation of new and safe adjuvants for the induction of robust cellular immunity is a logical decision.
The 4T1 mammary carcinoma is an easily transplantable, highly tumorigenic, and invasive tumor cell line that can be used as an experimental animal model for human mammary cancer.10 In contrast to most tumor models, 4T1 cell lines can spontaneously metastasize from the primary tumor in the mammary gland to multiple distant sites like lymph nodes, blood, liver, lung, brain, and bone in a manner very similar to human mammary cancer.10
Probiotics are organisms that are believed to improve health when consumed. Lactobacillus casei (L. casei) is one of the Lactobacillus bacteria considered a safe probiotic.11,12 Although several beneficial effects have been attributed to probiotic bacteria, the anticancer activity is the most interesting and controversial property of these bacteria.12 It has also been shown that some strains of this family such as L. casei can modify immune responses against solid tumors when administrated orally.13,14 For example, it has been reported that the daily intake of L. casei can improve the cytotoxicity of natural killer cells and adoptive immune responses in mice bearing invasive ductal carcinoma.14 On the other hand, the adjuvanticity potential of a killed preparation of L. casei was suggested in an old survey.15 However, there is precious little information on the possible adjuvant benefits of the killed preparation of L. casei in a tumor vaccine. Accordingly, the current study was conducted to investigate the efficacy of a new therapeutic vaccine against breast cancer made by mixing an extract of heated 4T1 cells and a heat-killed preparation of L. casei.
Materials and Methods
Reagents
Griess reagent kit, Dulbecco’s modified eagle medium (DMEM), and fetal calf serum (FCS) were purchased from GIBCO/Life Technologies Inc. (Gaithersburg, MD). Nitrotetrazolium blue tetrazolium (NBT), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), phytohemagglutinin (PHA), dioxin, and phosphate-buffered saline (PBS) were obtained from Sigma-Aldrich (St. Louis, MO). Enzyme-linked immunosorbent assay (ELISA) kits were procured from Qiagen (Hilden, Germany).
Bacterial Strains and Growth Conditions
L. casei (ATCC: 393) was obtained from Pasteur Institute of Iran. The bacteria were cultivated in a Rogosa’s medium at 37 °C for 24 hours, washed with PBS, heated at 56 °C for 60 minutes, and lyophilized.
Cell and Culture Conditions
4T1 cells were provided by Pasteur Institute of Iran. The cells were cultured at 37°C humidified atmosphere with 5% CO2 and maintained in monolayer cultures in DMEM supplemented with 10% FBS.
Mice and Tumor Induction
Female inbred BALB/c mice, between 6 and 8 weeks of age, were procured from Pasteur Institute of Iran. All the experimental procedures were conducted in compliance with the regulations of the Ministry of Health, I. R. Iran and were approved by the Medical Ethics Committee for Animal Studies of Urmia University. The mice were housed under standardized conditions of temperature (22–24 °C) and 12-hour light/dark cycles and received standard food and water ad libitum. All the animals were acclimatized to their new surroundings for 1 week prior to the experimental procedures and then challenged subcutaneously in the right flanks with 1× 104 viable tumor cells in 50 µL of PBS. Tumor growth was evaluated every 5 days with a caliper. Tumor volume (mm3) was computed using the formula of an ellipsoid (length×width×height×0.5236).
Vaccination of the Mice
Vaccination was started once all the animals had developed a palpable tumor. Thereafter, the mice were randomly divided into 4 groups. Each group contained 10 tumor-bearing animals, and all the vaccines were administrated via subcutaneous injections in the left flank. The 1st group (control mice) received 100 µL of PBS twice at a 1-week interval. The 2nd group (heated 4T1-treated mice) was immunized at a 1-week interval with a freeze-and-thaw extract of 104 heated 4T1cells in a 100-µL volume, twice. The heated 4T1 cells were provided by the exposure of the 4T1 cell lines to nonlethal heat shock (43 °C, 30 min). The 3rd group (heated L. casei-treated group) was treated twice with 2×108 CFU/mL of heated L. casei bacteria in a 100-µL volume at a 1-week interval. Finally, the 4th group (combined L. casei and heated 4T1-treated mice) was vaccinated twice at a 1-week interval with a freeze-and-thaw extract of 104 heated 4T1cells and 2×108 heated L. casei bacteria in a 100-µL volume.
Splenocyte Proliferation and Cytokine Assay
One week after the last immunotherapy, one half of the animals were euthanized to determine the cytokine milieu induced in splenocytes. In brief, splenocytes were aseptically isolated from the mice, and single-cell suspensions of the splenocytes were prepared in a DEMEM medium supplemented with 10% FCS and red blood cells (RBCs) were omitted by RBC lysis buffer.16 Next, the cell suspensions (2×106 cells/mL) were incubated in 24-well plates and pulsed with antigens derived from the tumor cells by freeze and thaw (20 μg/mL). Tumor antigen was prepared as was described previously.17 The culture supernatants were collected after 72 hours. The production of IFN-γ, IL-4, IL-10, and TGF-β was assessed via the ELISA according to the manufacturer’s instructions.
The proliferation potential of lymphocytes in the splenocyte population was checked using the MTT assay. The splenocytes were plated in 96-well flat-bottomed plates in a DEMEM medium supplemented with 10% FCS (1×105 cells/100 μL/well) and stimulated with antigens derived from the tumor cells by freeze and thaw (20 μg/mL). After 72 hours of incubation, the cultures were pulsed with 20 μL of the MTT solution (5 mg/mL) for 4 hours at 37 °C. Then, 150 mL of DMSO was added and shaken vigorously to dissolve formazan crystal. The optical density (OD) at 550 nm was measured using a microplate reader (Dynatech, Denkendorf, Germany). The experiments were done in triplicate sets. The results are expressed as the proliferation index according to the ratio of OD550 of the stimulated cells with PHA to OD550 of the non-stimulated cells.16
Cytotoxicity Assay
Cytotoxic activity was assessed using a lactate dehydrogenase (LDH) cytotoxicity detection kit (Takara Company, Tehran, Iran). This assay is a simple and fast colorimetric assay for quantifying cytotoxicity by determining LDH activity released from damaged cells. LDH is a stable cytoplasmic enzyme which is available in most cells. The splenocytes were used as effector cells, and the 4T1 cell lines were used as target cells. The effector and target cells were washed with the assay medium (RPMI1640 with 1% bovine serum albumin) and co-cultured at a ratio of 50 effector cells to 1 target cell in 96-well round-bottomed plates for 6 hours at 37 °C. Subsequently, the plates were centrifuged and the supernatants were transferred to 96-well flat-bottomed plates. Thereafter, 100 μL of the LDH detection mixture was added to each well and incubated for 30 minutes at room temperature. The OD was measured using a microplate reader (Dynatech, Denkendorf, Germany) at 490 nm. The percentage of cell-mediated cytotoxicity was determined by the following equation:
cytotoxicity (%)=(experimental release–spontaneous target release–spontaneous effector release)/(maximal target release–spontaneous target release)×100%.
Evaluation of Nitric Oxide in the Splenocyte Population
The potential of nitric oxide production was determined by assaying the nitrite levels of the splenocyte culture supernatants using the Griess reagent. After the splenocytes were cultured, as was described above, the cell-free supernatants (50 μL) were collected and mixed with 50 μL of Griess reagent (0.1% sulfanilamide, 3% phosphoric acid, and 0.1% naphthyl-ethylenediamine) and incubated at room temperature for 10 minutes in the dark. After the incubation, absorbance was read at 540 nm on a microplate reader (Dynatech, Denkendorf, Germany). The nitrite concentration was estimated based on a standard curve.
Statistical Analysis
The statistical analyses were performed using the Kruskal–Wallis test, followed by pair-wise comparisons, using the Mann–Whitney U-test with the Bonferroni adjustment. The Kaplan–Meier estimator was used to evaluate the survival function from lifetime data. The results are shown as means±SDs. A P<0.05 was considered statistically significant.
Results
Following tumor induction, the mice were monitored every 5 days for the first sign of a palpable tumor. All the immunization protocols were started on day 12 after tumor induction, when individual mice developed a palpable tumor, and continued until day 62, when all the mice died. As is illustrated in figure 1, the mice receiving the combined immunotherapy showed a more favorable survival curve than the mice in the other groups. At least 20% of the mice in the combined immunization group were alive until day 61 after tumor induction, while all the mice in the group treated with heated 4T1 alone died 45 days after tumor induction. The survivability rates of the control tumor-bearing mice and the tumor-bearing mice receiving heated L. casei were very poor compared to the other groups; indeed, all the mice in these groups died on days 36 and 38 after tumor induction, respectively (figure 1).
Figure 1.
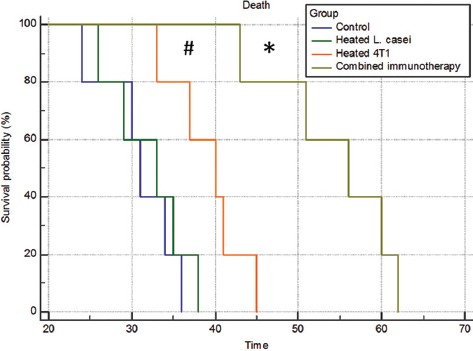
Comparison of the Kaplan–Meier survival curves of the BALB/c mice challenged with 4T1 cells after the immunotherapy. The immunotherapy was started once all the animals had developed a palpable tumor. A combined extract of heated 4T1 and a heat-killed preparation of Lactobacillus casei led to a favorable outcome compared to the other groups (*P<0.01 vs. other groups; #P<0.05 vs. the control tumor-bearing mice and/or the tumor-bearing mice receiving only the heat-killed preparation of Lactobacillus casei).
Tumors also developed at a significantly slower rate in the mice receiving the combined immunotherapy than in the other groups (figure 2). The mice in this group showed a significant reduction in the tumor growth rate from day 27 after tumor induction compared to the other groups. Tumor volume alteration was not statistically different between the mice receiving each of the individual immunotherapies and the control tumor-bearing mice (figure 2).
Figure 2.
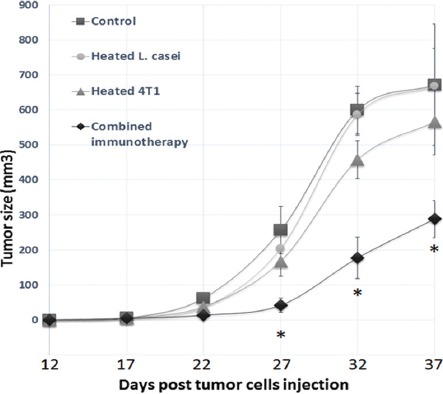
Evaluation of the mammary tumor size after the immunotherapy. The tumors were evaluated every 5 days with a caliper. Tumor volume (mm3) was computed using the formula of an ellipsoid (length×width×height×0.5236). The combined immunotherapy considerably decreased the tumor growth rate compared to the other groups (*P<0.01 vs. the control tumor-bearing mice and/or the tumor-bearing mice receiving only the monotherapy).
Ex vivo cytokine assay demonstrated that the combined immunization significantly upregulated the secretion of IFN-γ and conversely downregulated the secretion of IL-4, IL-10, and TGF-β in the splenocyte population compared to the splenocytes from the control (vehicle-treated) tumor-bearing mice (figure 3). Although a similar pattern of change in the secretion of these cytokines was observed in the heated L. casei-immunized mice compared to the control group, these changes, except for TGF-β, were not statistically significant (figure 3). Also, compared to the cells from the vehicle-treated group, a significant decrease in IL-4, TGF-β, and IL-10 production was found in the cells from the heated 4T1-treated groups. Of note, the combination treatment attenuated IL-4 and TGF-β secretion more prominently than the heated 4T1 immunotherapy, compared with the vehicle-treated tumor-bearing mice (figure 3).
Figure 3.
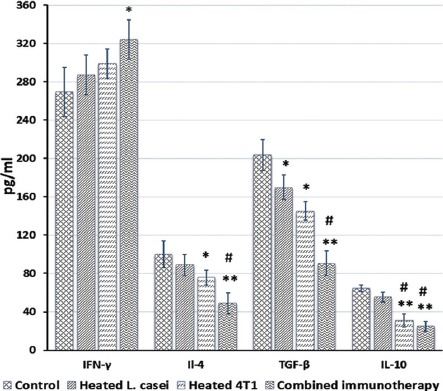
Effects of the immunotherapy on cytokine production in the splenocyte population. One half of the mice in each group were euthanized 1 week after the last immunotherapy, and the splenocytes were isolated and cultured for 72 hours under the described materials and methods (*P<0.01 **P<0.001 vs. the control tumor-bearing mice and/or the tumor-bearing mice receiving only the monotherapy; #P<0.01 vs. the heat-killed preparation of Lactobacillus casei).
Moreover, a significant increase in the splenocyte proliferation was observed only in the tumor-bearing mice receiving the combined heated 4T1 and heated L. casei compared to the other animals (table 1).
Table 1.
Effects of the immunotherapy on the proliferation index, cytotoxicity, and nitric oxide production in the splenocyte population
| Index | Control | Heated L. casei | Heated 4T1 | Combined immunotherapy | P value |
|---|---|---|---|---|---|
| Proliferation index | 1.44±0.16 | 1.5±0.14 | 1.69±0.18 | 2.41±0.19* | 0.003 |
| Cytotoxicity (%) | 48.12±5 | 49.01±3.45 | 54.34±4.2 | 70.1±5.32* | 0.001 |
| Nitric oxide (µmol/L) | 22.2±4.31 | 40.43±4.61$ | 43.64333±5.13S | 72.3±4.98$$,# | 0.004 |
One half of the mice in each group were euthanized 1 week after the last immunotherapy, and splenocytes were isolated and cultured as under the described materials and methods (
P<0.001 vs. the control tumor-bearing mice and/or the tumor-bearing mice receiving only the monotherapy;
P<0.01,
P<0.001 vs. the control tumor-bearing mice;
P<0.01 vs. the tumor-bearing mice receiving only the monotherapy)
The effects of the immunotherapy on natural killer cell-mediated cytotoxicity were explored by assessing the release of LDH from the tumor cells after challenge with the natural killer cells in the splenocyte population. The attained data showed that natural killer cell cytotoxicity was only significantly increased in the tumor-bearing mice receiving the combined heated 4T1 and heated L. casei compared to the other mice (table 1).
As is shown in table 1, nitric oxide production by the splenocytes was significantly increased in the splenocytes from the combined immunized mice with mammary tumors and those receiving the monotherapy compared to the control tumor-bearing mice. Nevertheless, this increase was much more significant in the tumor-bearing mice receiving the combination immunotherapy than in the animals receiving only the extract of heated 4T1.
Discussion
None or low immunogenic cell lines such as 4T1cells are able to grow rapidly and evade destruction by the immune system.18,19 Therefore, designing new strategies to enhance the immunogenicity of these tumor cell lines is a logical approach to control induced malignancy.
The induction of heat shock proteins (HSPs) is a proper therapeutic approach to enhance the immunogenicity of tumor cells.20 HSPs are highly-conserved housekeeping proteins expressed in all organisms. Their expressions are increased under a wide variety of physiological and pathological stimuli such as heat shock.20,21 Extracellular and membrane-bound HSPs, especially Hsp70 families, chaperone tumor antigens and mediate their uptake into antigen presenting cells (APCs). HSP-antigen complexes can be conducted toward the conventional exogenous MHC class II pathway. On the other hand, HSP-antigen complexes may promote the cross-presentation of antigens by APCs. The cross-presentation of phagocyted antigens is essential for the generation of cytotoxic T subsets.22,23 It has been found that the exposure of 4T1 cell lines to nonlethal heat shock promotes the surface expression of Hsp72, a member of the Hsp70 family, and diminishes the growth and metastatic potential of these cells in vivo.24 Accordingly, the 4T1 cells used in the present survey were sublethally heated before undergoing the freeze-and-thaw procedure. On the other hand, adaptive immune responses are initiated only when a dangerous signal is sensed by the pattern recognition receptors (PRRs) of APCs. In response to sensing the danger signals by PRRs such as toll-like receptors, APCs produce co-stimulatory molecules and polarizing cytokines that promote adaptive immunity into the best responses against the danger signals.25,26 Tumors may fail to induce appropriate effector immune responses because most tumor cells do not induce co-stimulatory molecules or appropriate polarizing cytokines.27 In this regard, we used a heat-killed preparation of L. casei to induce danger signals for the induction of robust cellular immunity against tumor antigens. Prior evidence has suggested that L. casei affects the phenotype of the immune system through the upregulation of surface MHC class II and CD86 on dendritic cells (co-stimulatory molecules) and the promotion of IL-12 secretion (a potent polarizing cytokine toward Th1 immune responses). Likewise, L. reuteri, another analog of L. casei, reduced L. casei-induced upregulation of CD86 and secretion of IL-12, IL-6, and TNF-α.28 Based on our results, the utilization of the heat-killed preparation of L. casei alone could not alter the outcome of the tumor-bearing mice. Immunotherapy with the extract of heated 4T1 cells was able to reduce the tumor growth rate and increase the survivability of the mice with breast tumors; however, these changes were not significant. Nevertheless, the vaccine made with the extract of heated 4T1 cells and heated L. casei as an adjuvant significantly diminished the tumor growth rate and significantly increased the survivability of these mice. In this regard, the combined heated 4T1 cells and the heat-killed preparation of L. casei significantly increased the proliferative response of the splenocytes more markedly than the treatment with heated 4T1 cells alone.
Tumors are able to evade immunity responses by secreting some mediator-like IL-4, TGF-β, and IL-10. These cytokines can suppress the essential arms of anti-tumor immunity, including inflammatory macrophages and Th1 responses.29-31 IL-4 can directly promote tumor cell growth in human breast cancer.29 TGF-β and IL-10 tend to suppress the proliferation and activation of lymphocytes and macrophages and, therefore, suppress cell-mediated immunity, which is needed to control tumor growth.30,31 Both cytokines can induce the development of regulatory T cells, which have been found in a variety of tumors, and may suppress the responses of T cells to tumors. Interestingly, regulatory T cells are the other source of TGF-β and IL-10.16,32 Our data demonstrated that the combined 4T1 and heated L. casei significantly inhibited the levels of TGF-β, IL-10, and IL-4 by comparison with the levels in the control tumor-bearing mice and/or the tumor-bearing mice receiving heated L. casei. Moreover, the obtained results showed that the combined immunotherapy more significantly lessened IL-4 and TGF-β secretion by lymphocytes than the heated 4T1 immunotherapy.
Previous data have suggested that the level of IFN-γ is correlated with anti-tumor responses.4 Nonetheless, our results indicated that the level of IFN-γ did not show any significant differences between the mice in the control group and the mice receiving each of the immunotherapies alone. However, the attained data showed that IFN-γ was significantly increased only in the tumor-bearing mice receiving the combined heated 4T1 and heated L. casei compared to the other mice.
Natural killer cells and macrophages are 2 important innate immune effector cells involved in the defense against malignant cells.33,34 In vitro and in vivo studies have indicated that natural killer cells can eliminate tumor cells. Natural killer cells are capable of mounting an immune response against tumor cells by the secretion of cytokines such as IFN-γ and the direct induction of apoptosis in tumor cells.33 Our results demonestated that natural killer cell cytotoxicity was agumented only in the tumor-bearing mice receiving the combined immunotherapy.
The activity of tumor-associated macrophages is controversial as there is surprising evidence for their role in both cancer progression and anti-tumor functions.34 Be that as it may, it has been revealed that macrophages are remarkably plastic and, based on their environments, can undergo a reprogramming, which can lead to the emergence of a spectrum of distinct functional phenotypes. Previous studies have demonstrated that M1 macrophages display various anti-tumor functions such as profound production of nitric oxide, a cytostatic cytotoxic factor for tumors. Unfortunately, malignant tumors by the promotion of macrophages toward the M2 anti-inflammatory phenotype confer a local state for tumor growth.34 A previous study showed that heat-killed L. casei, when administered intravenously to normal mice, stimulated the production of inflammatory macrophages in the bone marrow and spleen of mice.35 Our findings showed that the production of nitric oxide increased more significantly in the tumor-bearing mice treated with the combined immunotherapy than in tumor-bearing mice receiving each of the immunotherapies alone.
Of note, 4T1 cells are low-immunogenic tumor cell lines.18,19 Therefore, the better outcomes by the combination of the extract of heated 4T1 and the heat-killed preparation of L. casei are remarkable. However, the present survey is a preliminary study and further studies should be undertaken, especially in a larger group of mice. Moreover, some additional evaluation such as histopathology is also required.
Conclusion
The combined immunotherapy with heated 4T1 cells and heated L. casei promoted beneficial outcomes in our mouse model of breast cancer. Additionally, the current study demonstrated that the beneficial effects of the combination might be partly due to immune deviation from anti-inflammatory cytokines (such as IL-4 and TGF-β) to pro-inflammatory cytokine IFN-γ and the induction of innate anti-tumor immunity arm, which was more pounced than that observed from the monotherapy.
Acknowledgment
This study was fully sponsored by Urmia University, Urmia, Iran.
Conflict of Interest: None declared.
References
- 1.Rohan TE, Xue X, Lin HM, D’Alfonso TM, Ginter PS, Oktay MH, et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju136. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raval RR, Sharabi AB, Walker AJ, Drake CG, Sharma P. Tumor immunology and cancer immunotherapy: summary of the 2013 SITC primer. J Immunother Cancer. 2014;2:14. doi: 10.1186/2051-1426-2-14. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corthay A. Does the immune system naturally protect against cancer? Front Immunol. 2014;5:197. doi: 10.3389/fimmu.2014.00197. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maueroder C, Munoz LE, Chaurio RA, Herrmann M, Schett G, Berens C. Tumor immunotherapy: lessons from autoimmunity. Front Immunol. 2014;5:212. doi: 10.3389/fimmu.2014.00212. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood LM, Paterson Y. Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol. 2014;4:51. doi: 10.3389/fcimb.2014.00051. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Lombard M, Pastoret PP, Moulin AM. A brief history of vaccines and vaccination. Rev Sci Tech. 2007;26:29–48. doi: 10.20506/rst.26.1.1724. [DOI] [PubMed] [Google Scholar]
- 8.De Gregorio E, Tritto E, Rappuoli R. Alum adjuvanticity: unraveling a century old mystery. Eur J Immunol. 2008;38:2068–71. doi: 10.1002/eji.200838648. [DOI] [PubMed] [Google Scholar]
- 9.Claassen E, de Leeuw W, de Greeve P, Hendriksen C, Boersma W. Freund’s complete adjuvant: an effective but disagreeable formula. Res Immunol. 1992;143:478–83. doi: 10.1016/0923-2494(92)80057-r. discussion 572. [DOI] [PubMed] [Google Scholar]
- 10.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hori T, Kiyoshima J, Yasui H. Effect of an oral administration of Lactobacillus casei strain Shirota on the natural killer activity of blood mononuclear cells in aged mice. Biosci Biotechnol Biochem. 2003;67:420–2. doi: 10.1271/bbb.67.420. [DOI] [PubMed] [Google Scholar]
- 12.Rizzello V, Bonaccorsi I, Dongarra ML, Fink LN, Ferlazzo G. Role of natural killer and dendritic cell crosstalk in immunomodulation by commensal bacteria probiotics. J Biomed Biotechnol. 2011;2011:473097. doi: 10.1155/2011/473097. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwivedi A, Nomikou N, Nigam PS, McHale AP. The effects of microencapsulated Lactobacillus casei on tumour cell growth In vitro and in vivo studies. Int J Med Microbiol. 2012;302:293–9. doi: 10.1016/j.ijmm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Soltan Dallal MM, Yazdi MH, Holakuyee M, Hassan ZM, Abolhassani M, Mahdavi M. Lactobacillus casei ssp.casei induced Th1 cytokine profile and natural killer cells activity in invasive ductal carcinoma bearing mice. Iran J Allergy Asthma Immunol. 2012;11:183–9. doi: 011.02/ijaai.183189. [PubMed] [Google Scholar]
- 15.Bloksma N, de Heer E, van Dijk H, Willers JM. Adjuvanticity of lactobacilli. I. Differential effects of viable and killed bacteria. Clin Exp Immunol. 1979;37:367–75. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 16.Abtahi Froushani SM, Delirezh N, Hobbenaghi R, Mosayebi G. Synergistic effects of atorvastatin and all-trans retinoic acid in ameliorating animal model of multiple sclerosis. Immunol Invest. 2014;43:54–68. doi: 10.3109/08820139.2013.825269. [DOI] [PubMed] [Google Scholar]
- 17.Ramakrishnan S, Laxminarayan S, Wesensten NJ, Kamimori GH, Balkin TJ, Reifman J. Dose-dependent model of caffeine effects on human vigilance during total sleep deprivation. J Theor Biol. 2014;358:11–24. doi: 10.1016/j.jtbi.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Shilling DA, Smith MJ, Tyther R, Sheehan D, England K, Kavanagh EG, et al. Salmonella typhimurium stimulation combined with tumour-derived heat shock proteins induces potent dendritic cell anti-tumour responses in a murine model. Clin Exp Immunol. 2007;149:109–16. doi: 10.1111/j.1365-2249.2007.03393.x. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morecki S, Yacovlev E, Gelfand Y, Trembovler V, Shohami E, Slavin S. Induction of antitumor immunity by indomethacin. Cancer Immunol Immunother. 2000;48:613–20. doi: 10.1007/s002620050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmood K, Jadoon S, Mahmood Q, Irshad M, Hussain J. Synergistic effects of toxic elements on heat shock proteins. Biomed Res Int. 2014;2014:564136. doi: 10.1155/2014/564136. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed K, Zaidi SF. Treating cancer with heat: hyperthermia as promising strategy to enhance apoptosis. J Pak Med Assoc. 2013;63:504–8. [PubMed] [Google Scholar]
- 22.Ciocca DR, Cayado-Gutierrez N, Maccioni M, Cuello-Carrion FD. Heat shock proteins (HSPs) based anti-cancer vaccines. Curr Mol Med. 2012;12:1183–97. doi: 10.2174/156652412803306684. [DOI] [PubMed] [Google Scholar]
- 23.Milani V, Noessner E, Ghose S, Kuppner M, Ahrens B, Scharner A, et al. Heat shock protein 70: role in antigen presentation and immune stimulation. Int J Hyperthermia. 2002;18:563–75. doi: 10.1080/02656730210166140. [DOI] [PubMed] [Google Scholar]
- 24.Bausero MA, Page DT, Osinaga E, Asea A. Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis. Tumour Biol. 2004;25:243–51. doi: 10.1159/000081387. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong E, Lee JY. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med J. 2011;52:379–92. doi: 10.3349/ymj.2011.52.3.429. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolle LB, Standiford TJ. Danger-associated molecular patterns (DAMPs) in acute lung injury. J Pathol. 2013;229:145–56. doi: 10.1002/path.4124. [DOI] [PubMed] [Google Scholar]
- 27.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185–98. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–8. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 29.Nagai S, Toi M. Interleukin-4 and breast cancer. Breast Cancer. 2000;7:181–6. doi: 10.1007/BF02967457. [DOI] [PubMed] [Google Scholar]
- 30.Austenaa LM, Carlsen H, Ertesvag A, Alexander G, Blomhoff HK, Blomhoff R. Vitamin A status significantly alters nuclear factor-kappaB activity assessed by in vivo imaging. FASEB J. 2004;18:1255–7. doi: 10.1096/fj.03-1098fje. [DOI] [PubMed] [Google Scholar]
- 31.Wobke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol. 2014;5:244. doi: 10.3389/fphys.2014.00244. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heppner GH, Miller FR, Shekhar PM. Nontransgenic models of breast cancer. Breast Cancer Res. 2000;2:331–4. doi: 10.1186/bcr77. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2017;222:11–20. doi: 10.1016/j.imbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Josephs DH, Bax HJ, Karagiannis SN. Tumour-associated macrophage polarisation and re-education with immunotherapy. Front Biosci (Elite Ed) 2015;7:293–308. doi: 10.2741/E735. [DOI] [PubMed] [Google Scholar]
- 35.Nanno M, Shimizu-Takeda T, Mike A, Ohwaki M, Togashi Y, Suzuki R, et al. Increased production of cytotoxic macrophage progenitors by Lactobacillus casei in mice. J Leukoc Biol. 1989;46:89–95. doi: 10.1002/jlb.46.2.89. [DOI] [PubMed] [Google Scholar]


