Abstract
Objective(s):
Arsenic, a toxic metal in drinking water and butyric acid (BA) is a free fatty acid found in many foods. These two can induce oxidative stress in some tissues. The present study investigated the protective effect of metformin against toxicity induced by Arsenic (As) and BA in isolated mice liver mitochondria and pancreatic islets.
Materials and Methods:
In this study, liver mitochondria were isolated by adopting different centrifugation methods and pancreatic islets isolated by a collagenase method. Mitochondria were incubated by BA (75 μM), As (100 μM) and metformin (0, 0.5, 1, 3, 10 mM) and the islets also incubated by BA (1000 μM), As (100 μM) and metformin (0, 1, 3, 10 mM) for 1 hr. At the end of study, mitochondrial viability (MTT), mitochondrial membrane potential (MMP), reactive oxygen species (ROS), malondial- dehyde (MDA), glutathione (GSH) and islets insulin secretion were measured employing specific relevant methods.
Results:
As and BA significantly increased ROS, MDA and ΔΨm levels and decreased GSH level, succinate dehydrogenase activity and insulin secretion. On the other hand, pretreatment with metformin, returned mitochondrial complex II activity, reduced ROS, MDA and ΔΨm levels and increased GSH level and insulin secretion of pancreatic islets.
Conclusion:
As and BA in combination or in isolation induce oxidative stress in liver mitochondria and decrease insulin secretion of pancreatic islets. Metformin has a protective effect probably caused by its antioxidant feature. The findings suggest the potential role of metformin in mitochondria therapy and insulin secretion in many diseases.
Keywords: Arsenic, Butyric acid, Islet insulin secretion, Liver mitochondrial, Metformin, Oxidative stress
Introduction
Arsenic is an element that occurs naturally in water, soil, human body and many parts of the world as organic and inorganic chemical compounds. Drinking water is the major source of inorganic arsenic, causing a public health problem in many world countries. Since this chemical element found in drinking water and foods causes acute and chronic disorders such as diabetes mellitus, peripheral vascular and cardiovascular diseases, hypertension, neurological diseases and increased rate in terms of different types of cancers (1-3). Mechanism of As toxicity is not completely known; however, one of the proposed mechanisms is the induction of ROS, followed by increased oxidative stress, increased lipid peroxidation, decreased GSH level and induction loss of mitochondrial membrane potential (MMP) (4, 5). On the other hand, it has been also reported that mitochondrial oxidative stress plays a key role in the progression of hyperglycemia and diabetes due to the increased production of free radicals and impaired antioxidant defenses (6, 7).
BA is a short-chain fatty acid, found in foods such as milk, cheese and butter and is also generated by non-digestible carbohydrates fermentation in the large intestine (8). Fatty acids in milk, such as BA, are immediately absorbed in the upper intestine and then rapidly taken up by the liver. The majority of BA remains in the liver and physiological concentrations of such a short chain fatty acid have been estimated to be about 375±70 mM in the portal blood of sudden death victims (9). Previous studies have indicated that daily administration of BA induces hepatotoxicity in bulls through enhancing the hepatic enzymes like aspartate aminotransferase, alanine aminotransferase and gamma-glutamyl transferase (10). Further, this fatty acid is an extracellular metabolite that is produced through the butyrate kinase and induced oxidative stress in the jugular blood (11, 12). Concerning human beings, in vivo and in vitro studies have demonstrated that acute exposure to fatty acids can cause non-glucose-stimulated insulin secretion and increase glucose, and chronic exposure to such acids can impair insulin sensitivity and lead to insulin resistance (13).
The biguanide metformin is an anti-diabetic drug, which acts through hepatic gluconeogenesis inhibition, insulin secretion stimulation and increased insulin sensitivity in muscles, adipose tissues, liver and possibly pancreatic β-cells. Metformin increases glucose oxidation but decreases FFA oxidation (14).
In vivo studies have indicated that the metformin has an anti-oxidative effect and significantly reduces tissue lipid peroxides as well as the generation of the ROS and increases the activity of mitochondrial aconitase. It is believed that this feature is critical in reducing the progression of type II diabetes (15).
In this study, the protective effect of metformin on insulin secretion was examined in isolated mice pancreatic islets. Furthermore, the oxidative stress factors were also concerned in isolated mice liver mitochondria after exposure to As and BA.
Materials and Methods
Chemicals
Sodium arsenite (NaAsO2), BA, 4-2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES), DMSO, D-mannitol, thiobarbituric acid (TBA), (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (MTT), dithiobis-2-nitrobenzoic acid (DTNB), tri chloro acetic acid (TCA), GSH, 2’,7’-dichlorofluorescein diacetate (DCFH-DA), Tetr amethoxypropane (TEP), sucrose, KCl, Na2HPO4, MgCl2, MnCl2, potassium phosphate, Rhodamine 123 (Rh 123), Coomassie blue, ethylene diamine tetra acetic acid (EDTA) and bovine serum albumin (BSA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Collagenase type P was purchased from Roch Company (Germany).
Animal’s preparation
Adult male Naval Medical Research Institute (NMRI) mice (30-35 g) were obtained from the animal facility of Ahvaz Jundishapur University of Medical Science (AJUMS), which is completely attributed by AJUMS animal care guidelines with an ethics committee grantee No. IR.AJUMS.RE.1395.636. They housed in an air-conditioned room with controlled temperature of 20±4 °C, humidity of 70-80% and maintained on a 12:12 hr light cycle with free access to food and water. Prior to the experiment, the protocols were confirmed to be in accordance with the Guidelines of Animal Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (16).
Preparation of mitochondria
Mice liver mitochondria were isolated by differential centrifugation according to Bermann and Hasting’s Method (17). Briefly, the animals were sacrificed and their livers were quickly removed, carefully washed with buffer and cut into small pieces. The liver pieces were homogenized in an ice-cold isolation buffer containing 1mM EGTA, 215 mM mannitol, 75 mM sucrose, 0.1% BSA and 20 mM HEPES. The homogenized tissue was then centrifuged at 800g for 10 min. at 4 °C in order for the nuclei, unbroken cells and other non-subcellular tissues to be removed. The supernatant was removed and centrifuged at 13,000 g for 15 min. The packed lower layer (heavy mitochondrial fraction) was then resuspended in cold isolation buffer and the previous steps were repeated twice. Finally, the heavy mitochondrial sediments were resuspended in the storage buffer and the protein concentration was measured by using the Coomassie blue protein-binding method, in which the BSA was concerned as the standard sample. In all experiments, the mitochondrial preparation was performed as fresh and immediately placed on ice to guarantee the isolation of high-quality mitochondrial preparation. After Bradford test, the calculated mito-chondrial protein amounted to 0.5 mg/ml for each test. Isolated liver mitochondria were incubated by metformin (0, 0.5, 1, 3 and 10 mM) at 37 °C for 1 hr and then were incubated by As (100 μM) and BA (75 μM) (18), separately and in combination, at 37 °C for 1 hr. The concentrations of BA, As and metformin were selected in accordance with previous studies (19-21).
MTT assessment (mitochondrial Succinate dehydrogenase activity or complex II activity)
The mitochondrial total dehydrogenase activity was assayed by measuring reduced MTT to formazan ratio. In short, 100 μl of mitochondrial suspensions (0.5 mg protein/ml) was incubated with different concentrations of As, BA and metformin. After being washed and suspended in the mitochondria buffer, they were centrifuged at 1000 g for 20 min at 4 °C. Then, 50 μl MTT 0.4% was added to each tube and incubated at 37 °C for 30 min. After re-centrifuging at 1000g for 20 min, DMSO (1ml) was added to each tube and the tubes were shacked well. Then each sample was poured in glass spectrophotometer cuvette and its color intensity was measured by spectrophotometer reader. Ultimately, succinate dehydrogenase (SDH) activity was assessed as a percentage of the control (21).
Mitochondrial membrane potential assessment
The MMP was measured by using mitochondrial uptake of cationic fluorescent probe rhodamine 123. The mitochondrial fractions (0.5 mg protein/ml) were incubated by different concentrations of As, BA and metformin. Then, 10 μM of Rh 123 was added to the mitochondrial solution. The capacity of mitochondria for the Rh 123 uptake was calculated as differences between control and treated mitochondria through spectrofluorometerical measurement of fluorescence intensity (22).
Mitochondrial ROS assessment
The ROS levels were measured by adding 1 ml of DCFH-DA (3.32 M) to 1 ml of isolated mitochondria sample. DCFH-DA enters into mitochondria and hydrolyzes to non-fluorescent DCFH. Then, it oxidized to form fluorescent 2 and 7-dichlorofluorescein through reacting with the ROS. Further, the fluorescence intensity was measured by a fluorescence spectrophotometer (22).
Lipid peroxidation measurement
The content of MDA in mitochondria samples was determined in term of thiobarbituric acid reactive substances (TBARS) formation. The mitochondrial fractions (0.5 mg protein/ml) were incubated with various concentrations of As, BA and metformin. Then, 1ml of the isolated mitochondrial solution was mixed with 250 µl TCA (0.8%) and then centrifuged at 3000 g for 15 min. The supernatant was added to 1 ml TBA (70%) and placed in a boiling water bath for 30 min. The absorbance was read on the spectrophotometer at 532 nm. Values were expressed as nM/mg protein. Since 99% of the TBARS was MDA, the TBARS concentrations of the samples were calculated based on a standard curve using tetramethoxypropane (23).
Mitochondrial GSH measurement
GSH was analyzed by using the DTNB as an indicator. To sum up, the mitochondrial sample (0.5 mg protein/ml) was incubated with various concentrations of As, BA and metformin. Then, 0.1 ml of mitochondrial fractions was added to phosphate buffer (0.1 M) and DTNB (0.04%) in a total volume of 3 ml (pH 7.4). The developed yellow color was read at 412 nm using a spectrophotometer (24).
Islet isolation
Mice pancreatic islets were isolated by a collagenase method (25). The animals’ pancreatic tissues were removed and transferred into a petri dish containing Krebs-bicarbonate buffer and centrifuged at 100 g for 5 min. To purify the isolated islets from exocrine tissues, the collagenase type P was added to the solution and placed in a shaking water bath 800 oscillations shake at 37 °C for 5-10 min. Then, 15 ml of cold Krebs-bicarbonate buffer was added to stop digestion and then centrifuged at 500 g for 5 min. The supernatant of sample was transferred to a blackened petri dish. Finally, islets dissection was carried out manually using drawn-out glass pipette under stereomicroscope observation (26).
Islet insulin secretion
Ten isolated islets were transferred to 2 ml micro tubes containing Krebs-bicarbonate buffer with 5.6 mM of d-Glucose concentrations (similar to fasting blood glucose) (27). The different concentrations of metformin (0, 1, 3, 10 mM) was added to the islets medium and incubated at 37 °C for 1 hr. Then BA (1000 μM) and AS (100 μM) were separately and jointly added to medium and incubated at 37 °C for 90 min. After incubation, the samples were centrifuged at 100g for 5 min and 0.9 ml of sample supernatant was kept at −70 °C until the insulin secretion assay was performed (27). Each micro tube contained 10 islets and this in vitro protocol was repeated 8 times for each concentration. Insulin secretion was measured using enzyme-linked immunosorbent assay (ELISA) method and its commercial assay kit (Lance Research, St. Charles, MO) (28). The concen-trations of the As, BA and metformin were selected based on previous studies (18, 29, 30).
Statistical analysis
All the results were presented as means ± SE for three different experiments and were analyzed using Graph Pad Prism (version 6.01). The data were normally distributed. One-way ANOVA followed by the Tukey’s post hoc test was used to determine the statistical significance of the differences. Further, the differences were considered statistically significant at P<0.05.
Results
Effects of As, BA and metformin exposure on liver mitochondrial viability
MTT as mitochondrial viability variable was assessed with respect to the activity of succinate dehydro-genase (complex II). As shown in Figure 1, the separate exposure to As (100 µM) and BA (75 µM) decreased complex II activity (P<0.05); however, the results of BA 75 µM in combination with As 100 µM revealed an additive effect on the MTT reduction in comparison to the control scenario (P<0.01). The results of metformin administration (with dose of 10 mM) indicated a significant increase in MTT level, following the separate exposure to As and BA (P<0.05) and combined exposure to metformin with doses of 1, 3 and 10 mM) revealed an additive effect on induction, compared to the control scenario in each group (metformin 0) (P<0.01).
Figure 1.
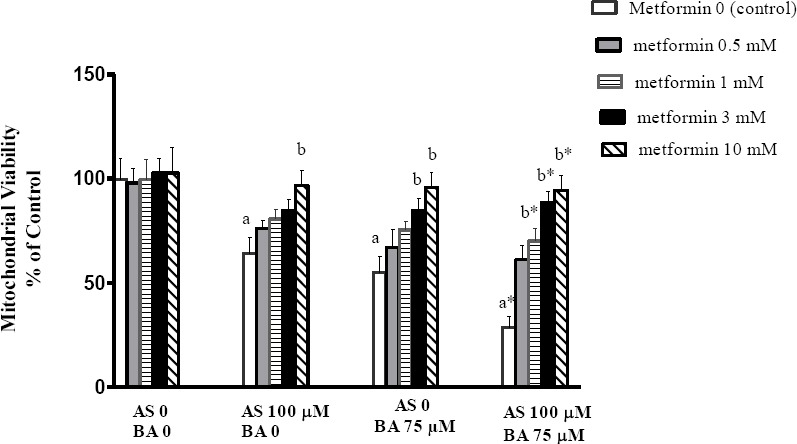
Effects of butyric acid, arsenic and metformin on liver mitochondrial viability (MTT). Data are expressed as the mean ± SEM for 6 mice in each group a: compared to control group (As0, BA0, metformin 0), b: compared to control in each group (metformin 0), a and b P<0.05, a* and b* P<0.01
Effect of BA, As and metformin exposure on mitochondrial membrane damage
MMP is a variable of the mitochondrial membrane damage and is known as ΔΨm. In this regard, the present data indicated that the combined exposure to As and BA induce a significant increase in ΔΨm levels, compared to the control scenario (P<0.05). Also shown in Figure 2, 10 mM metformin significantly decreased the MMP in the treatment group receiving As or BA separately; however, metformin with doses of 3 and 10 mM decreased this variable in the treatment group receiving a combination of As and BA, in comparison to the control scenario in each group (P<0.05).
Figure 2.
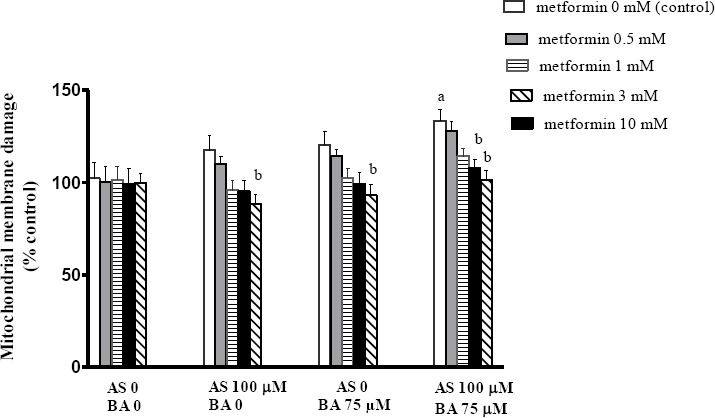
Effects of butyric acid, arsenic and metformin on liver mitochondrial membrane potential collapse (ΔΨm). Data are expressed as the mean±SEM for 6 mice in each group a: compared to control (As0, BA0, metformin 0), b: compared to control in each group (metformin 0), a and b P<0.05
Effect of BA, As and metformin exposure on mitochondrial oxidative stress
Oxidative stress was assessed by measuring the ROS throughout DCFH-DA oxidation. The results suggested that separate and combined exposure to As and BA increases the ROS levels, in comparison to the control group. Moreover, metformin with doses of 3 and 10 mM significantly decreased the ROS formation in the treatment group being separately exposed to As (P<0.05). Metformin with doses of 1 and 3 (P<0.05) and 10 mM (P<0.01) decreased the ROS formation in the treatment group being separately exposed to BA. Moreover, metformin with doses of 1 (P<0.05), 3 and 10 mM (P<0.01) significantly decreased the ROS formation in the treatment group receiving a combination of As and BA, compared with the control scenario in each group (Figure 3).
Figure 3.
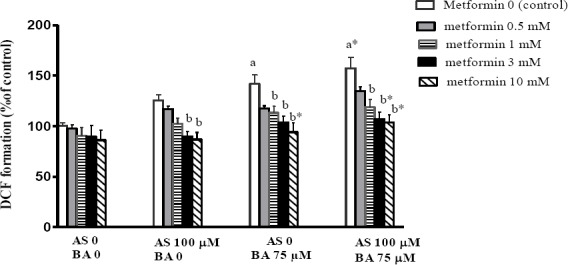
Effects of butyric acid, arsenic and metformin on liver mitochondrial ROS formation. Data are expressed as the mean±SEM for 6 mice in each group a: compared to control (As0, BA0, metformin 0), b: compared to control in each group (metformin 0), a and b P<0.05, a* and b* P<0.01
Effect of BA, As and metformin exposure on mitochondrial MDA level
MDA, as a mitochondrial lipid peroxidation factor, revealed a significant increase following the separate and combined exposure to As and BA in comparison to the control scenario. Also the results revealed that metformin with doses of 3 and 10 mM significantly decreased the MDA level in treatment group receiving the BA separately (P<0.01); even though, it at doses of 1 (P<0.05), 3 and 10 mM (P<0.01) significantly decreased the MDA level in the group receiving a combination of As and BA, compared with the control scenario in each group (Figure 4).
Figure 4.
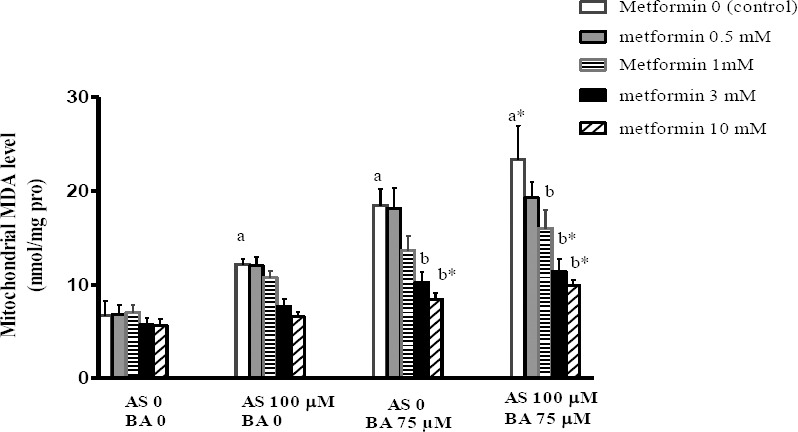
Effects of butyric acid, arsenic and metformin on liver mitochondrial MDA level. Data are expressed as the mean±SEM for 6 mice in group a: compared to control (As0, BA0, metformin 0), b: compared to control in each group (metformin 0), a and b P<0.05, a* and b* P<0.01
Effect of BA, As and metformin exposure on mitochondrial GSH level
GSH is the first antioxidant defense against ROS formation and is determined spectrophotometerically using DTNB as an indicator. Present results showed a significant decrease in mitochondrial GSH levels after separate and combined exposure to the As and BA, when compared to the control scenario. Metformin (10 mM) significantly increased the GSH level in the As group (P<0.05) and it with doses of 3 and 10 mM increased the same variable in the BA group (P<0.05). It, however, with doses of 1 (P<0.05), 3 and 10 mM (P<0.01) produced an additive effect on the GSH content in the treatment group receiving a combination of As and BA, compared with the control scenario in each group (P<0.01) (Figure 5).
Figure 5.
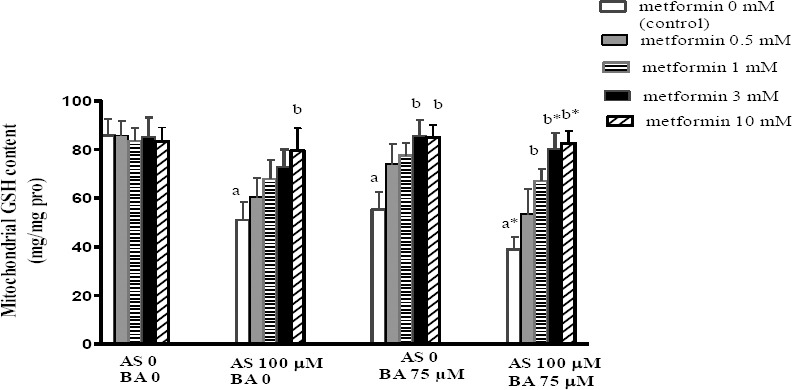
Effects of butyric acid, arsenic and metformin on liver mitochondrial GSH level. Data are expressed as the mean±SEM for 6 mice in each group a: compared to control (As0, BA0, metformin 0), b: compared to control in each group (metformin 0), a and b P<0.05, a* and b* P<0.01
Effects BA, As and metformin exposure on insulin secretion
Pancreatic insulin secretion, known as beta cell function, was measured after 1hr incubation by BA, As and metformin. The findings revealed a significant decrease in islets insulin secretion following the separate and combined exposure to As and BA in comparison to the control scenario. According to Figure 6, 10 mM metformin significantly increased the islet insulin secretion in the group receiving the BA or As separately and in combination, compared to the control scenario in each group (p<0.05).
Figure 6.
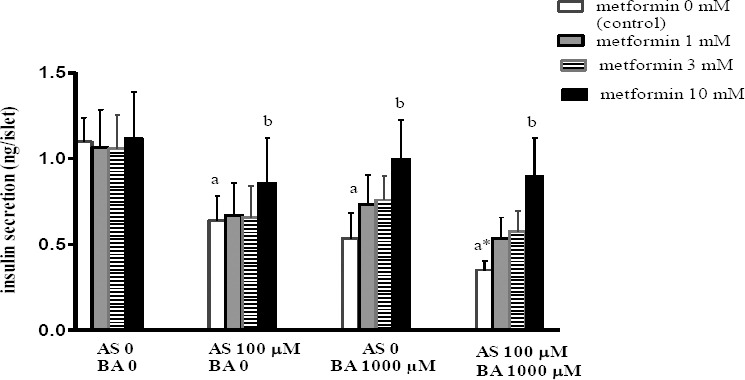
Effects of butyric acid, arsenic and metformin on pancreatic islets insulin secretion. Data are expressed as the mean ± SEM for 6 mice in each group. a: compared to control (As0, BA0, metformin 0); b: compared to control (metformin 0). a P<0.05, b P<0.01
Discussion
Present data reflected that separate administration of BA and As induced liver mitochondrial oxidative stress through increasing MDA, and ROS and decreasing GSH levels and also reduced the pancreatic islet insulin secretion. Hence, the findings regarding the BA and As co-administration indicated that the combination of these two enhanced the likelihood of increasing mitochondrial oxidative stress and decreasing pancreatic islets insulin secretion as an additive effect. In addition, the current study showed that metformin has protective effect against oxidative stress induced by As and BA in isolated mice liver mitochondria and pancreatic isolated islets.
Liver is a vital tissue exposed to ROS attack and the mitochondria of hepatic cells can produce ROS in association with the liver fatty acid oxidation. In mammals’ liver, an antioxidant system such as GSH is developed to maintain the redox homeostasis. This homeostasis would be disturbed by excessive ROS production, resulting in oxidative stress as a factor affecting the liver diseases. Further, tissue MDA level as a lipid peroxidation indicator showed the severity of the disease (4, 31).
In addition, liver is a major organ targeted by As toxicity. In this regard, several studies have demonstrated that the As exposure induces severe toxic effects on liver by generating free radicals (32). Also previous studies have reported that the As induces oxidative stress by increasing the ROS production and lipid peroxidation and decreasing the liver’s GSH content (33). Other studies reported that the As induces oxidative stress by changing the antioxidant system in the liver and free fatty acids also leads to increased ROS production. Further, it is indicated that the GSH as an important oxidative stress biomarker is the first line of antioxidant defense against the oxidative damages induced by As (34). In line with these studies, Dutta, et al. showed that the As caused liver tissue damage by the induction of oxidative stress, lipid peroxidation and decreased GSH. It is thus suggested that animals that treated by the As aggravating the liver mitochondrial damage through increased oxidative stress and reduced GSH level (4). Hence, the current study showed that the liver mitochondrial incubation with the As could induce effects on mitochondrial destruction, similar with that for in vivo administration in chronic exposure.
Zgorzynska et al. showed that the BA induced oxidative stress by over-generation of ROS, reduction of cell viability and mitochondrial membrane potential in primary human fibroblasts from gingival tissue (35). Cueno et al. revealed that high (1 and 5 mM) BA concentrations induced oxidative stress and altered calcium homeostasis (12). Ahangarpour et al. found that the BA has oxidative stress effects at dose of 75 µM on isolated mice liver mitochondria (18). In addition, the findings with regard to the BA and As and co-administration presented that the combination of these two suppresses the mitochondrial oxidative stress, as an additive effect. Accordingly, the As and BA induced more oxidative stress through increasing the liver mitochondrial MDA level, ROS production and MMP and decreasing liver mitochondrial GSH and succinate dehydrogenase contents.
Some in vivo studies reported that the generation of ROS influences the onset and development of type II diabetes. They also suggested that metformin treatment decreases hyperglycemia through decreasing the ROS (36). Previous studies have showed that metformin inhibits the mitochondrial enzymatic activity of complex I and consequently, reduces the oxygen consumption and the mitochondrial membrane potential. The findings confirmed that the metformin inhibits the generation of superoxide by mitochondria (37, 38). The present study came to the conclusion that the metformin has a protective effect on the induction of ROS production, lipid peroxidation and mitochondrial membrane permeabilization, reduction of mitochon-drial Succinate dehydrogenase (complex II) and GSH in isolated liver mitochondria after being exposed to As and BA. It is also suggested in this study that the anti-oxidative effect of metformin can possibly be considered as an important mechanism to prevent the development of type II diabetes induced by As and high fat diet.
Since beta cells have limited defense against the excessive ROS production, they are more susceptible to oxidative damage, compared with many other cell types (39). It is well-documented that the islets are more susceptible to lipotoxicity damage at normal glucose levels (40). Low sub-toxic concentrations of the As and its methylated trivalent metabolites may induce adverse effects on the pancreatic beta cells and inhibit glucose stimulated insulin secretion (41). Additionally, some other studies have concluded that the As causes impairment in the stimulated insulin secretion from β-cells through promoting the ROS production (42). Previous studies also suggested that high-fat intake results in the accumulation of fatty acids or fatty acid derivatives in muscles and liver, subsequently leading to insulin resistance (43). Recently, majority of studies argued that fat-rich diets have pro-oxidant and pro-inflammatory compounds that are linked to impaired insulin sensitivity (44). BA is a short-chain free fatty acid that acute exposure with it causes non-glucose-stimulated insulin secretion and chronic exposure with it causes β-cell apoptosis, impaired insulin sensitivity and enhanced insulin resistance. In contrast, Itoh et al. study reported that the BA at dose of 10 µM do not make a significant change in islet insulin secretion (29); however, Ahangarpour et al. revealed that the BA at dose of 1000 µM has toxic effect on the insulin secretion in islets (18). On the other hand, BA and As have similar impact on the induction of liver mitochondrial oxidative stress and reduce islets insulin secretion and their co-administration generate additive effects through imbalanced redox system or lipotoxicity.
Concerning the previous studies, metformin along with glucose increases insulin release in mice isolates pancreatic islets in a time-dependent manner (45). Masini et al claimed that the metformin significantly prevents the functional, biochemical and ultra-structural abnormalities in human islet cells exposed to glucotoxic condition and that this drug has significant effects on the secretion of human pancreatic beta cells (13). Present study showed that metformin modified the secretory irregularities induced by As and BA in isolated mice pancreatic islets and this confirms the findings of previous results.
Conclusion
The administration of BA and As induced oxidative stress in liver mitochondria and decreased insulin secretion in pancreatic islets. Metformin has protective effect that may be caused by its antioxidant properties in mitochondria and pancreatic islets. Furthermore, these findings suggested the potential role of metformin in treating impairments observed in disorders and diseases regarding mitochondria and insulin secretion. Further studies are recommended to delve into the mechanisms regulating such reactions.
Acknowledgment
This study was supported by a project (NO. AJUMS (94s14) by Student Research Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Conflict of interest
All authors declared no conflict of interest in present manuscript.
References
- 1.Abejón A, Garea A, Irabien A. Arsenic removal from drinking water by reverse osmosis: Minimization of costs and energy consumption. Sep Purif Technol. 2015;144:46–53. [Google Scholar]
- 2.Kumar S, Yedjou CG, Tchounwou PB. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J Exp Clin Cancer Res. 2014;33:42–53. doi: 10.1186/1756-9966-33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miltonprabu S, Sumedha N. Arsenic-induced hepatic mitochondrial toxicity in rats and its amelioration by diallyl trisulfide. Toxicol Mech Methods. 2014;24:124–135. doi: 10.3109/15376516.2013.869778. [DOI] [PubMed] [Google Scholar]
- 4.Dutta M, Ghosh D, Ghosh AK, Bose G, Chattopadhyay A, Rudra S, et al. High fat diet aggravates arsenic induced oxidative stress in rat heart and liver. Food Chem Toxicol. 2014;66:262–277. doi: 10.1016/j.fct.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Zheng CY, Lam SK, Li YY, Ho JC-M. Arsenic trioxide-induced cytotoxicity in small cell lung cancer via altered redox homeostasis and mitochondrial integrity. Int J Oncol. 2015;46:1067–1078. doi: 10.3892/ijo.2015.2826. [DOI] [PubMed] [Google Scholar]
- 6.Kahya MC, Nazıroğlu M, Övey İS. Modulation of diabetes-induced oxidative stress, apoptosis, and Ca2+ entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol. 2017;54:2345–2360. doi: 10.1007/s12035-016-9727-3. [DOI] [PubMed] [Google Scholar]
- 7.Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal. 2017;26:501–518. doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 9.Qaisrani S, Van Krimpen M, Kwakkel R, Verstegen M, Hendriks W. Diet structure, butyric acid, and fermentable carbohydrates influence growth performance, gut morphology, and cecal fermentation characteristics in broilers. Poult Sci. 2015;94:2152–2164. doi: 10.3382/ps/pev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu H, Lu L, Xu ZC, Lu YJ, Zhao B, Zhuang L, et al. Tauroursodeoxycholic acid and 4-phenyl butyric acid alleviate endoplasmic reticulum stress and improve prognosis of donation after cardiac death liver transplantation in rats. Hepatob Pancreatic Dis. 2014;13:586–593. doi: 10.1016/s1499-3872(14)60269-1. [DOI] [PubMed] [Google Scholar]
- 11.Cueno ME, Imai K, Tamura M, Ochiai K. Butyric acid-induced rat jugular blood cytosolic oxidative stress is associated with SIRT1 decrease. Cell Stress Chaperones. 2014;19:295–298. doi: 10.1007/s12192-013-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cueno ME, Kamio N, Seki K, Kurita-Ochiai T, Ochiai K. High butyric acid amounts induce oxidative stress, alter calcium homeostasis, and cause neurite retraction in nerve growth factor-treated PC12 cells. Cell Stress Chaperones. 2015;20:709–713. doi: 10.1007/s12192-015-0584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masini M, Anello M, Bugliani M, Marselli L, Filipponi F, Boggi U, et al. Prevention by metformin of alterations induced by chronic exposure to high glucose in human islet beta cells is associated with preserved ATP/ADP ratio. Diabetes Res Clin Pract. 2014;104:163–170. doi: 10.1016/j.diabres.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Huang W, Wang J, Xu Z, He J, Lin X, et al. Metformin plays a dual role in MIN6 pancreatic βcell function through AMPK-dependent autophagy. Int J Biol Sci. 2014;10:268–277. doi: 10.7150/ijbs.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mousavi SM, Niazmand S, Hosseini M, Hassanzadeh Z, Sadeghnia HR, Vafaee F, et al. Beneficial effects of Teucrium polium and metformin on diabetes-induced memory impairments and brain tissue oxidative damage in rats. Int J Alzheimers Dis. 2015;2015:1–8. doi: 10.1155/2015/493729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul DS, Walton FS, Saunders RJ, Stýblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect. 2011;119:1104–1109. doi: 10.1289/ehp.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mashayekhi V, Eskandari MR, Kobarfard F, Khajeamiri A, Hosseini MJ. Induction of mitochondrial permeability transition (MPT) pore opening and ROS formation as a mechanism for methamphetamine-induced mitochondrial toxicity. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:47–58. doi: 10.1007/s00210-013-0919-3. [DOI] [PubMed] [Google Scholar]
- 18.Ahangarpour A, Oroojan AA, Rezaei M, Khodayar MJ, Alboghobeish S, Zeinvand M. Effects of butyric acid and arsenic on isolated liver mitochondria and pancreatic islets of male mouse. Gastroenterol Hepatol Bed Bench. 2017;10:44–53. [PMC free article] [PubMed] [Google Scholar]
- 19.Hickmann FH, Cecatto C, Kleemann D, Monteiro WO, Castilho RF, Amaral AU, et al. Uncoupling, metabolic inhibition and induction of mitochondrial permeability transition in rat liver mitochondria caused by the major long-chain hydroxyl monocarboxylic fatty acids accumulating in LCHAD deficiency. BBA Biomemb. 2015;1847:620–628. doi: 10.1016/j.bbabio.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Hassani S, Yaghoubi H, Khosrokhavar R, Jafarian I, Mashayekhi V, Hosseini MJ, et al. Mechanistic view for toxic effects of arsenic on isolated rat kidney and brain mitochondria. Biologia. 2015;70:683–689. [Google Scholar]
- 21.Mehrabadi AR, Jamshidzadeh A, Rashedinia M, Niknahad H. Study of the effects of ATP suppliers and thiol reductants on toxicity of pioglitazone in isolated rat liver mitochondria. Iran J Pharm Res. 2015;14:825–832. [PMC free article] [PubMed] [Google Scholar]
- 22.Keshtzar E, Khodayar M, Javadipour M, Ghaffari M, Bolduc D, Rezaei M. Ellagic acid protects against arsenic toxicity in isolated rat mitochondria possibly through the maintaining of complex II. Hum Exp Toxicol. 2016;35:1060–1072. doi: 10.1177/0960327115618247. [DOI] [PubMed] [Google Scholar]
- 23.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Xin X, Tian Q, Yin G, Chen X, Zhang J, Ng S, et al. Reduced mitochondrial and ascorbate–glutathione activity after artificial ageing in soybean seed. J Plant Physiol. 2014;171:140–147. doi: 10.1016/j.jplph.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Amaral AG, Rafacho A, de Oliveira CAM, Batista TM, Ribeiro RA, Latorraca MQ, et al. Leucine supplementation augments insulin secretion in pancreatic islets of malnourished mice. Pancreas. 2010;39:847–855. doi: 10.1097/MPA.0b013e3181d37210. [DOI] [PubMed] [Google Scholar]
- 26.O'Dowd JF. The isolation and purification of rodent pancreatic islets of Langerhans. Type 2 Diabetes: Methods & Protocols. 2009;560:37–42. doi: 10.1007/978-1-59745-448-3_3. [DOI] [PubMed] [Google Scholar]
- 27.Machado de Oliveira CA, Ferreira Paiva M, Alencar Soares Mota C, Ribeiro C, Curiacos de Almeida Leme JA, Luciano E, et al. Exercise at anaerobic threshold intensity and insulin secretion by isolated pancreatic islets of rats. Islets. 2010;2:240–246. doi: 10.4161/isl.2.4.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahangarpour A, Heidari H, Fatemeh RAA, Pakmehr M, Shahbazian H, Ahmadi I, et al. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type2 diabetic patients. J Diabetes Metab Disord. 2014;13:29–33. doi: 10.1186/2251-6581-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, et al. Free fatty acids regulate insulin secretion from pancreatic βcells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 30.Yen CC, Ho TJ, Wu CC, Chang CF, Su CC, Chen YW, et al. Inorganic arsenic causes cell apoptosis in mouse cerebrum through an oxidative stress-regulated signaling pathway. Arch Toxicol. 2011;85:565–575. doi: 10.1007/s00204-011-0709-y. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Tan H-Y, Wang N, Zhang Z-J, Lao L, Wong C-W, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash C, Kumar V. Chronic Arsenic Exposure-Induced Oxidative Stress is Mediated by Decreased Mitochondrial Biogenesis in Rat Liver. Biol Trace Elem Res. 2016;173:87–95. doi: 10.1007/s12011-016-0622-6. [DOI] [PubMed] [Google Scholar]
- 33.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellinsworth DC. Arsenic, reactive oxygen, and endothelial dysfunction. J Pharmacol Exp Ther. 2015;353:458–464. doi: 10.1124/jpet.115.223289. [DOI] [PubMed] [Google Scholar]
- 35.Zgorzynska E, Wierzbicka-Ferszt A, Dziedzic B, Witusik-Perkowska M, Zwolinska A, Janas A, et al. Docosahexaenoic acid attenuates oxidative stress and protects human gingival fibroblasts against cytotoxicity induced by hydrogen peroxide and butyric acid. Arch Oral Biol. 2015;60:144–153. doi: 10.1016/j.archoralbio.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, et al. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:1088–1099. doi: 10.1038/cddis.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462:475–587. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutter GA, Pullen TJ, Hodson DJ, Martinez-Sanchez A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem J. 2015;466:203–218. doi: 10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- 39.Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Lin GC, Mather KJ, et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J Cli Endocrinol Metab. 2014;99:1983–1992. doi: 10.1210/jc.2014-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prentki M, Nolan CJ. Islet βcell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang CF, Yang CY, Chan DC, Wang CC, Huang KH, Wu CC, et al. Arsenic exposure and glucose intolerance/insulin resistance in estrogen-deficient female mice. Environ Health Perspect. 2015;123:1138–1144. doi: 10.1289/ehp.1408663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padmaja Divya S, Pratheeshkumar P, Son YO, Vinod Roy R, Andrew Hitron J, Kim D, et al. Arsenic induces insulin resistance in mouse adipocytes and myotubes via oxidative stress-regulated mitochondrial Sirt3-FOXO3a signaling pathway. Toxicol Sci. 2015;146:290–300. doi: 10.1093/toxsci/kfv089. [DOI] [PubMed] [Google Scholar]
- 43.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat–fed mice are linked to high glycotoxin intake. Diabetes. 2005;54:2314–2319. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- 45.Hashemitabar M, Bahramzadeh S, Saremy S, Nejaddehbashi F. Glucose plus metformin compared with glucose alone on β?cell function in mouse pancreatic islets. Biomed Rep. 2015;3:721–725. doi: 10.3892/br.2015.476. [DOI] [PMC free article] [PubMed] [Google Scholar]


