Abstract
Objective(s):
Bill duct ligation (BDL) is a representative model of biliary cholestasis in animals. Curcumin has a protective effect on the liver; however, its underlying mechanisms are not completely known. This study explored the hepatoprotective activity of curcumin on hepatic damage via measuring the expression of sirtuin3 (SIRT3), AMP-activated protein kinase (AMPK), carnitine palmitoyltransferase 1A (CPT-1A), isocitrate dehydrogenase2 (IDH2) and manganese superoxide dismutase (MnSOD) as well as the level of serum lipid profile in the BDL fibrotic rat model.
Materials and Methods:
The study consisted of four groups (n=8 for each of Wistar rats): sham group, sham+curcumin (sham+Cur) group (received curcumin 100 mg/kg/day), BDL+Cur group, and BDL group. Transcription levels of SIRT3, AMPK, CPT-1A, IDH2, MnSOD and protein expression level of SIRT3 were measured by real-time PCR and Western blotting, respectively.
Results:
It was identified that SIRT3, AMPK, CPT-1A, IDH2 and MnSOD expression significantly decreased in BDL rats compared to sham rats; however, in the curcumin treatment of BDL rats, the expression of these factors increased significantly compared to BDL (P<0.05). It was, moreover, observed that treatment of BDL rats with curcumin reduced liver injury as verified by a reduction in the levels of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein (LDL) and increase in high-density lipoprotein (HDL) (P <0.05).
Conclusion:
Curcumin reduced liver damage and oxidative stress in the liver tissue of BDL rats through up-regulation of SIRT3, AMPK, CPT-1A, IDH2 and MnSOD as well as changing the level of serum lipid profile.
Keywords: Curcumin, Gene expression, Liver cirrhosis, Rats, SIRT3 protein
Introduction
Biliary duct ligated (BDL) is an experimental model, which induces cholestasis and increases hepato cellular damage and finally results in liver fibrosis (1). Hyperlipidemia occurs in cholestasis liver disease and is characterized by marked changes in the level of lipid profile (2, 3). During liver fibrogenesis, quiescent hepatic stellate cells (HSCs) become active and undergo a transformation that is associated with enhanced cell proliferation and overproduction of extra cellular matrix (ECM).
In liver fibrogenesis, cell proliferation increases and many ECM are produced, which can lead to transformation and activation of HSCs (4, 5). Oxidative stress is an imbalance between antioxidant capacity and the level of cell reactive oxygen species (ROS) (6). Oxidative stress induces HSC to transform and increasingly activates HSCs. This state is named myofibroblasts (MFs), which contributes to fibrosis (7). Several studies reveal that BDL causes oxidative stress, which can have a significant role in the development of hepatic fibrosis (8).
ROSs and accumulation of triglycerides in the hepatocytes are important factors of the liver fibrosis (9, 10). Increased sirtuin3 (SIRT3) expression has been reported in response to oxidative stress (11). SIRT3 (Silent mating type information regulation 2, homolog3) is a member of the sirtuin family with NAD+-dependent deacetylase activity and ADP-ribosyltransferase activity (12). Sirtuins play a crucial role in the regulation of cellular activities such as metabolism, cell death, cell growth, and cellular responses to oxidative stress by deacetylation of histones and a wide range of non-histone proteins such as enzymes and transcription factors (13). The SIRT3 expression is highest in metabolically active tissues including the brain, heart, liver, brown adipose tissue, and skeletal muscles (11).
As some studies have revealed, SIRT3 can serve as a vital diagnostic and therapeutic target in human health/aging and disease (12). Several studies have indicated that SIRT3 plays a pivotal role in fatty acid oxidation and reduction of cell ROS in the liver (14). Studies showed that SIRT3 can reduce lipid accumulation via AMP-activated protein kinase (AMPK) in human hepatic cells (15). In liver fibrosis, the expression of AMPK and carnitine palmitoyltransferase 1A (CPT-1A) is reduced and this contributes to the activation of lipogenic pathways and inhibition of fatty acid oxidation pathways (16). Under oxidative stress conditions, the antioxidant enzymes such as manganese superoxide dismutase (MnSOD) remove the extra ROS to maintain homeostasis (17). SIRT3 reduces cellular ROS levels depending on MnSOD. Meanwhile, SIRT3 overexpression is accompanied by MnSOD upregulation (18, 19). SIRT3 mediates protective effects against oxidative stress by promoting the mitochondrial antioxidant system through regulation of isocitrate dehydrogenase2 (IDH2) (20).
Curcumin (diferuloylmethane) has been known as a phenolic antioxidant found in the turmeric spice (21). It is driven from the rhizome of the plant Curcuma longa and shows antioxidant, anti-inflammatory, and anti-cancer properties; however, its mechanisms are not completely understood (21, 22). Curcumin has varying pharmacological effects on different disorders such as asthma, and hepatic diseases and has been used as an effective and public remedy. Curcumin is currently undergoing clinical testing in humans (23).
The aim of this study is to investigate the effects of curcumin on SIRT3, AMPK, CPT-1A, IDH2 and MnSOD gene expression in BDL fibrotic rat model.
Materials and Methods
Animals and experimental procedures
Matured male Wistar rats (200-250 g, Pasteur Institute, Tehran, Iran) were used in this research. Rats were housed in an air-conditioned room under standard conditions (25 °C) with a 12 hr darkness/light cycle and had easy access to rat food diet and drinking water. A total of the study’s protocols performed were in accordance with the present ethical considerations of the aboriginal ethical committee of animal use.
All of 32 rats were randomly allocated in two groups: sham group and BDL group. Each group was divided into two subgroups and treated with curcumin 100 mg/kg/day (Sigma Chemicals Co Purity (HPLC)>80%, USA) suspended in 5% carboxymethyl cellulose (CMC) or the same volume/weight of the 5% CMC vehicle by oral gavage once a day from the day after surgery for 28 days (24, 25). BDL was performed briefly under general anesthesia by ketamine (90 mg/kg) and xylazine (10 mg/kg) intraperitoneally (IP); the current bile duct was performed by a midline abdominal cutting under the sterile situation. It was then ligated in two positions with a silk thread and sectioned between the ligatures and cefazolin antibiotic was used to inhibit infection.
At the end of the 4-week period, blood samples were collected by puncturing the heart under deep anesthesia and they were centrifuged at 3000 g for 15 min. The serum was isolated for further experiments. Liver tissues were divided into two parts; the first part was frozen in liquid nitrogen for RNA extraction and the second part was kept at -70 °C to make homogenized tissue for SIRT3 Western blot analyses. In this study, we used the serum samples and the liver tissues that were cirrhotic by BDL method performed by Ghoreshi et al (17). The pathology and oxidative stress results have previously been reported (17).
Western blot analysis of liver tissue SIRT3 levels
Liver tissue samples (30 mg) were homogenized for protein extraction using phosphate saline buffer containing 100 mM Tris–HCl, protease inhibitor cocktail 1:100 (Sigma, Saint Louis, MO, USA), 150 mM NaCl, 0.1% SDS and 1% NP-40; pH 7.4 by incubation on ice for 30 min and subsequent centrifuge at 15000 g (4 °C, 30 min). By using Bradford method, protein concentrations were determined in the supernatants. The extracted proteins (100 µg) were separated on 8% SDS-polyacrylamide gel and transferred from the gel to a nitrocellulose membrane. Bovine serum albumin (BSA) 5% in Tris-buffered saline with Tween-20-0.05% (TBS/T) pH 7.4 blocked the nitrocellulose membrane for 2 hr and this membrane was probed with polyclonal rabbit anti-SIRT3 primary antibody (Abcam, Cambridge, UK) and polyclonal rabbit anti-β-actin (Abcam, Cambridge, UK) as a reference at 4 °C overnight. The membranes were incubated with a goat anti-rabbit secondary antibody (2:4000) conjugated with horseradish peroxidase (cell signaling, Munich, Germany) for 2 hr. The predicted sizes for SIRT3 (28 KDa) and β-actin (42 kDa) were checked using molecular weight size markers. Specific bands were visualized by an enhanced chemiluminescence reagent (GE) on a ChemiDoc system (Syngene GBOX, 680X) and densitometric quantification of these bands was performed with the Quantity Image j program (SynGene, V4.1).
Analysis of mRNA levels by quantitative real-time PCR (qPCR)
The cellular RNA was isolated from liver samples using a Fast Pure RNA Kit (TakaRa, Japan). Concentrations of RNA were identified by measuring the absorbance at 260 nm, and the purity of RNA was also assessed by 260/280 nm absorbance ratio (Eppendorf, Hamburg, Germany). Forward and reverse primer sequences are shown in Table 1. One microgram of RNA is typically used to convert RNA into cDNA using MuLV RT enzyme (Fermentas), dNTP and random primer hexamers in a total volume of 20 μl. The cDNA samples were diluted 1/10 and aliquots were stored at −70 °C until use. Quantitative real-time PCR was performed in triplicate using SYBR-green with a Rotor-Gene system (Corbett Research 2004, Australia). Data normalization was carried out against β-actin, and the relative quantity of gene expression was analyzed based on ΔCt method and the results were calculated as 2-ΔΔCt.
Table 1.
Primer sequences used for RT-PCR analysis
| GENE | Forward primer | Reverse primer |
|---|---|---|
| SIRT3 | 5’- TGCACGGTCTGTCGAAGGTC -3’ | 5’- TGTCAGGTTTCACAACGCCAG-3’ |
| AMPK | 5’- TGTGGCTCGCCCAATTATG-3’ | 5’- GACCCCGCTGCTCCAGAT-3’ |
| CPT-1A | 5’- CGGTTCAAGAATGGCATCATC-3’ | 5’- TCACACCCACCACCACGAT-3’ |
| IDH2 | 5’- CCCATCACCATTGGCAGACAC-3’ | 5’- CCTCCGGCAGGGAAGTTATACA-3’ |
| MnSOD | 5’-ATTAACGCGCAGATCATGCA-3’ | 5’ CCTCGGTGACGTTCAGATTGT -3’ |
| Β-actin | 5’-CGTTGACATCCGTAAAGACCTC-3’ | 5’-AGCCACCGATCCACACAGA-3’ |
Lipid analyses
Serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) concentrations were measured enzymatically using commercially available kits (Pars Azmon Diagnostic Co; Iran) and a Roche BT 3000 Auto Analyzer. The assays were performed in accordance with the manufacturer’s instruction.
Statistical analyses
A one-way analysis of variance (ANOVA) followed by Tukey-Kramer test for group comparison was conducted with the statistical software program, GraphPad Prism 5 to determine the differences between the obtained values (mean±SEM). The differences less than 0.05 were considered statistically significant.
Results
Protein and gene expression of SIRT3 was increased in curcumin administered group
Twenty four days after BDL surgery and treatment with curcumin, we measured the level of protein/gene expression of SIRT3 in liver tissue of four groups (sham, sham+Cur, BDL, and BDL+Cur) by the Western immunoblotting and qPCR method. It was observed that SIRT3 protein/gene expressions decreased in liver tissue of BDL rats compared with the sham group (P-value<0.05). However, treatment with curcumin significantly increased protein/gene expression of SIRT3 in the liver tissue of BDL+Cur group compared with BDL group as shown in Figure 1.
Figure 1.
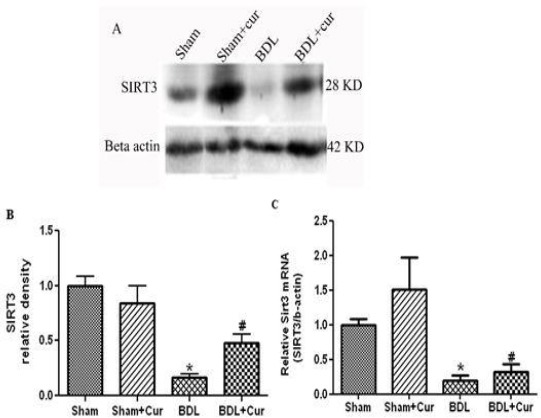
Western blotting pattern of sirtuin3 (Sirt3) proteins expression (Figure 1A), the relative density of protein expression levels of Sirt3 (Figure 1B) and gene expression of SIRT3 (Figure 1C) in four studied groups (Sham, Sham+Cur, BDL, BDL+Cur) analyzed by one-way ANOVA. Significant differences between groups are indicated by symbols (*P-value<0.05 compared with the Sham group and #P-value < 0.05 compared with the bill duct ligation (BDL) group)
mRNA expression of AMPK, CPT-1A, IDH2, and MnSOD was increased in curcumin administered group
We measured the mRNA expression levels of AMPK, CPT-1A, IDH2, and MnSOD in liver tissue in all groups (sham, sham+Cur, BDL, and BDL+Cur) by qPCR method. It was observed that AMPK, CPT-1A, IDH2, and MnSOD gene expressions significantly decreased in liver tissues of BDL rats compared with the sham group (P-value<0.05). However, treatment with curcumin increased gene expression of these factors in the liver tissue of BDL+Cur group compared with the BDL group as shown in Figure 2.
Figure 2.
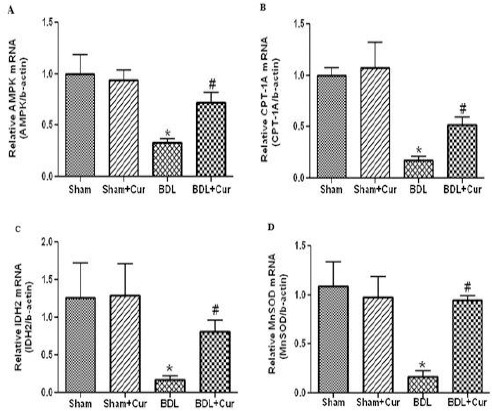
Gene expression of AMP-activated protein kinase (AMPK) (Figure 2A), carnitine palmitoyltransferase 1A (CPT-1A) (Figure 2B), isocitrate dehydrogenase2 (IDH2) (Figure 2C) and manganese superoxide dismutase (MnSOD) (Figure 2D) in four studied groups (Sham, Sham+Cur, BDL, BDL+Cur) analyzed by one-way ANOVA. Significant differences between groups are indicated by symbols (*P-value<0.05 compared with the Sham group and #P-value<0.05 compared with the bill duct ligation (BDL) group)
Effect of curcumin on lipid profile in BDL-induced hepatic injury
Table 2 represents the results of the curcumin effect on serum lipid profile. The serum levels of TC, TG and LDL were significantly elevated in BDL rats (P-value <0.05). The high levels of these factors in BDL rats were significantly decreased after their treatment with curcumin (100 mg/kg/day) (P-value <0.05). It was observed that HDL level significantly decreased in liver tissue of BDL rats compared with the sham group (P-value<0.05). However, treatment with curcumin increased the level of HDL in the liver tissue of BDL+Cur group compared with the BDL group as shown in Table 2.
Table 2.
Effect of curcumin on lipid profile in bill duct ligation (BDL)-induced hepatic injury
| Sham | Sham+Cur | BDL | BDL+Cur | |
|---|---|---|---|---|
| TC (mg/dl) | 87.5±2.56 | 89.2±4.52 | 144.2±4.17# | 101.5±3.79* |
| TG (mg/dl) | 53.5±4.23 | 61±4.74 | 171.2±6.45# | 110.5±3.27* |
| LDL (mg/dl) | 42.26±3.14 | 38.35±1.65 | 139.4±2.94# | 120.5±3.01* |
| HDL (mg/dl) | 33.53±1.52 | 30.08±1.95 | 12.92±1.34# | 23.05±1.22* |
Data are expressed as mean±SEM. Analyzed by one-way ANOVA;
Represents P<0.05, comparison between BDL and Sham groups;
Represents P<0.05, comparison between BDL+Cur and BDL groups. Tc: Total cholestrol; TG: triglyceride; LDL: low-density lipoprotein; HDl: high-density lipoprotein
Discussion
The present study investigated the hepatoprotective effect of curcumin through evaluation of protein/gene expression profile of SIRT3 in BDL fibrotic rat model. As far as we know, this is to date the first study to determine the hepatoprotective effect of curcumin by evaluating the protein/gene expression level of SIRT3 in BDL rats. Our findings showed that curcumin treatment reduced liver injury through up-regulation of either mRNA or protein expression of SIRT3. To assess the process of improving hepatic tissue, the mRNA expression of different cellular factors including AMPK, CPT-1A, IDH2 and MnSOD was measured in liver tissue of the four groups. Compared to the sham group, BDL rats demonstrated a significant decrease in the mRNA expression of AMPK, CPT-1A, IDH2, and MnSOD, but the treatment of BDL rats with curcumin led to an increase in the mRNA expression of these factors.
Curcumin is a polyphenol derived from the yellowish pigments of turmeric plant. It has some useful properties like antioxidant, anti-inflammatory, and anti-cancer effects, although its mechanisms as to inhibiting liver fibrosis are not completely understood (21, 22).
BDL is a representative model of biliary cholestasis in animals, which results in oxidative damage and hyperlipidemia (2). In this study, we used the liver tissues that were fibrotic by BDL method. The fibrosis of these tissues was confirmed through hematoxylin and eosin (H&E) staining of hepatic sections of BDL rats, evaluating the mRNA expression of alpha smooth muscle actin (α-SMA), collagen I and transforming growth factor beta 1 (TGF-β1) as well as measuring hepatic enzymes including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP). BDL rats demonstrated a significant increase in the expression of α-SMA, collagen I and TGF-β1, but the treatment of BDL rats with curcumin led to a reduction in these markers and an increase in the activity of hepatic enzymes (17).
SIRT3 is a mitochondrial and cytosolic deacetylase, and studies have shown that both forms of the enzyme are functional and show enzymatic activities (26, 27). In this study, the changes in the expression level of SIRT3 was evaluated because this factor in the liver could be envisaged for therapeutic purposes and the expression level of SIRT3 fluctuated in response to diet signals (11, 14). SIRT3 regulates mitochondrial function by deacetylation of mitochondrial proteins (13). SIRT3 is a central player in hepatic metabolism (14). In our study, we showed that SIRT3 protein/gene expressions significantly decreased in liver tissue of BDL rats compared with the sham group. However, treatment with curcumin increased protein/gene expression of SIRT3 in the liver tissue of BDL+Cur group compared with the BDL group. Grabowska et al have reported that curcumin can promote the activity of sitruin by inducing AMPK and increasing the level of NAD+ (28). Chen et al have shown a decrease of SIRT3 protein level in BDL rats compared with the sham group (29), which is in accord with the results of ours.
Hepatic fibrosis is a pathologic process characterized by fatty acid synthesis and the accumulation of TG in the hepatocytes (15). AMPK and SIRT3 play a key role in the regulation of hepatic energy metabolism and fatty acid metabolism (11, 30, 31). In BDL fibrotic rats, the rate of AMPK expression significantly decreased, and this decrease was in accordance with the reduction in the expression of SIRT3 compared with the sham group. However, these rates increased after treatment of BDL fibrotic rats with curcumin (BDL+Cur compared to BDL group). Shi et al showed that SIRT3 can reduce lipid accumulation via AMPK in human hepatic cells (15). Furthermore, in another study, it was identified that curcumin can increase the activity of AMPK by reducing the level of ATP in the cells and regulate lipid metabolism-related genes, leading to the suppression of hepatic lipid accumulation. During energy depletion, AMPK inhibits de novo fatty acid synthesis by stimulating fatty acid oxidation through up-regulating the expression of CPT-1A (30). Studies indicated that CPT-1A can catalyze the transfer of long-chain fatty acids from acyl-CoA to carnitine for translocation across the mitochondrial membranes and it was an initiating step in the mitochondrial oxidation of long-chain fatty acids (32). CPT-1A expressions significantly decreased in liver tissue of BDL rats compared with the sham group. However, treatment with curcumin increased gene expression of CPT-1A in the liver tissue of BDL+Cur group compared with the BDL group. In line with our findings, You et al. have reported that in liver fibrosis, the expression of AMPK and CPT1A declines, which leads to activation of lipogenic pathways and inhibition of fatty acid oxidation pathways (16).
BDL induces cholestasis (1). Cholestasis is the intrahepatic accumulation of potentially toxic bile acids that occurs in several chronic liver diseases as an effect of obstruction or destruction of bile ducts and affects several pathways involved in lipoprotein metabolism as well as lipoprotein secretion (33). Hyperlipidemia occurs in cholestatic liver disease and is characterized by marked changes in the level of lipid profile. Upon BDL and chronic cholestasis, the total and low-density lipoprotein cholesterol levels increased but the high-density lipoprotein cholesterol level decreased (2, 3). Curcumin has hypocholesterolemic effect on rats. Findings have shown that serum TC, LDL and TG are elevated in cholesterol-fed rats, whereas HDL is decreased compared with the sham group. Moreover, TC, TG, and LDL in curcumin-fed groups were significantly lower than those in the control group. Also serum HDL in curcumin-fed groups was significantly higher than that of the control group (34). In our study, it was demonstrated that curcumin has some effect on serum lipid profile in BDL rats. The serum levels of TC, TG, and LDL significantly raised in BDL rats. The high levels of these factors in BDL rats significantly decreased after treatment with curcumin (100 mg\kg\day). HDL level significantly decreased in liver tissue of BDL rats compared with the sham group. However, treatment with curcumin increased the level of HDL in the liver tissue of BDL+Cur group compared with the BDL group.
Evidence has shown the role of oxidative stress in liver damage. Oxidative stress is an imbalance between oxidant-antioxidant substances resulting in potential cellular damage. The imbalance can happen from an absence of antioxidant capacity caused by disturbances in production and distribution, or by an excess of ROS from other factors (35, 36). The accumulation of toxic hydrophobic bile acids in hepatocytes induces substantial modification in the redox state and in mitochondrial function and promotes mitochondrial production of ROS that, in turn, may lead to cell death (33, 37). Increased SIRT3 expression has also been reported in response to oxidative stress. Interestingly, the SIRT3 activity can reduce ROS levels by directly modulating key antioxidant enzymes thereby acting as a shield against oxidative damage. To date, the known antioxidant effects of SIRT3 are mediated by the interaction of this protein with MnSOD and IDH2 (11).
Likewise, it was observed that, compared to the sham group, the expression levels of IDH2 and MnSOD in the BDL group significantly reduced. However, the expression of these factors increased in BDL rats after they were treated with curcumin resulting in a protection against hepatic fibrosis through elevation of the antioxidant capacity.
Someya et al. indicated that under oxidative stress conditions, SIRT3 promotes the mitochondrial antioxidant system through regulation of IDH2 (38). Zhong et al found that overexpression of MnSOD protects the liver against the injury caused by cholestasis (39). In other ways, SIRT3 reduces cellular ROS levels depending on MnSOD, a major mitochondrial antioxidant enzyme; in addition, Sundaresan et al showed that overexpression of SIRT3 increased mRNA expression of the antioxidant gene MnSOD (19). On the other hand, Schiffman and Martin found that cells treated with curcumin show an increased expression of MnSOD compared to the control group (40).
Conclusion
Our results revealed that curcumin may reduce liver damage thereby suggesting that curcumin probably exerts its anti-hepatofibrotic effects via up-regulation of SIRT3, AMPK, CPT-1A, IDH2, and MnSOD.
Acknowledgment
The results described in this paper were part of student thesis and this article was supported by Shahid Sadoughi University of Medical Sciences in Yazd, Iran.
Conflict of interest
The authors declare that no conflict of interest.
References
- 1.Teixeira C, Franco E, Oliveira PA, Colaco B, Gama A, Carrola J, et al. Effects of nebivolol on liver fibrosis induced by bile duct ligation in Wistar rats. In vivo. 2013;27:635–640. [PubMed] [Google Scholar]
- 2.Ji H, Jiang JY, Xu Z, Kroeger EA, Lee SS, Liu H, et al. Change in lipid profile and impairment of endothelium-dependent relaxation of blood vessels in rats after bile duct ligation. Life Sci. 2003;73:1253–1263. doi: 10.1016/s0024-3205(03)00423-5. [DOI] [PubMed] [Google Scholar]
- 3.Longo M, Crosignani A, Podda M. Hyperlipidemia in chronic cholestatic liver disease. Curr Treat Options Gastroenterol. 2001;4:111–114. doi: 10.1007/s11938-001-0022-6. [DOI] [PubMed] [Google Scholar]
- 4.George J. Ascorbic acid concentrations in dimethylnitrosamine-induced hepatic fibrosis in rats. Clin Chim Acta. 2003;335:39–47. doi: 10.1016/s0009-8981(03)00285-7. [DOI] [PubMed] [Google Scholar]
- 5.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 9.Natarajan SK, Thomas S, Ramamoorthy P, Basivireddy J, Pulimood AB, Ramachandran A, et al. Oxidative stress in the development of liver cirrhosis: a comparison of two different experimental models. J Gastroenterol Hepatol. 2006;21:947–957. doi: 10.1111/j.1440-1746.2006.04231.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Bengmark S, Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD) Lipids Health Dis. 2010;9:1. doi: 10.1186/1476-511X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci. 2013;5:48. doi: 10.3389/fnagi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward Jr, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klishadi MS, Zarei F, Hejazian SH, Moradi A, Hemati M, Safari F. Losartan protects the heart against ischemia reperfusion injury: sirtuin3 involvement. J Pharm Pharm Sci. 2015;18:112–123. doi: 10.18433/j3xg7t. [DOI] [PubMed] [Google Scholar]
- 14.Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J. 2012;444:1–10. doi: 10.1042/BJ20120030. [DOI] [PubMed] [Google Scholar]
- 15.Shi T, Fan GQ, Xiao SD. SIRT3 reduces lipid accumulation via AMPK activation in human hepatic cells. J Dig Dis. 2010;11:55–62. doi: 10.1111/j.1751-2980.2009.00416.x. [DOI] [PubMed] [Google Scholar]
- 16.You M, Jogasuria A, Taylor C, Wu J. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2014;4:88–100. doi: 10.3978/j.issn.2304-3881.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghoreshi ZA, Kabirifar R, Safari F, Karimollah A, Moradi A, Eskandari-Nasab E. Hepatoprotective effects of curcumin in rats after bile duct ligation via downregulation of Rac1 and NOX1. Nutrition. 2017;36:72–78. doi: 10.1016/j.nut.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhury M, Jonscher KR, Friedman JE. Reduced mitochondrial function in obesity-associated fatty liver: SIRT3 takes on the fat. Aging (Albany NY) 2011;3:175–178. doi: 10.18632/aging.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Rath PC. Curcumin, a natural antioxidant, acts as a noncompetitive inhibitor of human RNase L in presence of its cofactor 2-5A in vitro. BioMed Res Int. 2014;2014 doi: 10.1155/2014/817024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M-E, Chen Y-C, Chen I-S, Hsieh S-C, Chen S-S, Chiu C-H. Curcumin protects against thioacetamide-induced hepatic fibrosis by attenuating the inflammatory response and inducing apoptosis of damaged hepatocytes. J Nutr Biochem. 2012;23:1352–1366. doi: 10.1016/j.jnutbio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Zheng J, Wu C, Lin Z, Guo Y, Shi L, Dong P, et al. Curcumin up?regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA?mediated control of DNA methylation–a novel mechanism suppressing liver fibrosis. FEBS J. 2014;281:88–103. doi: 10.1111/febs.12574. [DOI] [PubMed] [Google Scholar]
- 24.Park EJ, Jeon CH, Ko G, Kim J, Sohn DH. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J Pharm Pharmacol. 2000;52:437–440. doi: 10.1211/0022357001774048. [DOI] [PubMed] [Google Scholar]
- 25.Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, et al. Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: role of TGF-beta modulation and oxidative stress. Fundam Clin Pharmacol. 2008;22:417–427. doi: 10.1111/j.1472-8206.2008.00611.x. [DOI] [PubMed] [Google Scholar]
- 26.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci. 2002;99:13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir) 2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide–dependent deacetylase. J Cell Biol. 2002;158:647–657. doi: 10.1083/jcb.200205057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grabowska W, Suszek M, Wnuk M, Lewinska A, Wasiak E, Sikora E, et al. Curcumin elevates sirtuin level but does not postpone in vitro senescence of human cells building the vasculature. Oncotarget. 2016 doi: 10.18632/oncotarget.8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Qing W, Sun M, Lv L, Guo D, Jiang Y. Melatonin protects hepatocytes against bile acid-induced mitochondrial oxidative stress via the AMPK-SIRT3-SOD2 pathway. Free Radic Res. 2015;49:1275–1284. doi: 10.3109/10715762.2015.1067806. [DOI] [PubMed] [Google Scholar]
- 30.Um MY, Hwang KH, Ahn J, Ha TY. Curcumin Attenuates Diet?Induced Hepatic Steatosis by Activating AMP?Activated Protein Kinase. Basic Clin Pharmacol Toxicol. 2013;113:152–157. doi: 10.1111/bcpt.12076. [DOI] [PubMed] [Google Scholar]
- 31.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song S, Attia RR, Connaughton S, Niesen MI, Ness GC, Elam MB, et al. Peroxisome proliferator activated receptor α(PPARα) and PPAR gamma coactivator (PGC-1α) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol Cell Endocrinol. 2010;325:54–63. doi: 10.1016/j.mce.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serviddio G, Bellanti F, Stanca E, Lunetti P, Blonda M, Tamborra R, et al. Silybin exerts antioxidant effects and induces mitochondrial biogenesis in liver of rat with secondary biliary cirrhosis. Free Radic Biol Med. 2014;73:117–126. doi: 10.1016/j.freeradbiomed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Ghada Z. Effect of curcumin, mixture of curcumin and piperine and curcum (turmeric) on lipid profile of normal and hyperlipidemic rats. EJHM. 2005;21:145–161. [Google Scholar]
- 35.Tseilikman V, Pankov N, Pankova N, Filimonova T, Sinitskii A, Kozochkin D, et al. Correlation between circulating corticosterone and protein carbonylation in the liver after short-term hypokinesia. Bull Exp Biol Med. 2013;156:188. doi: 10.1007/s10517-013-2307-x. [DOI] [PubMed] [Google Scholar]
- 36.De Waal EM, Liang H, Pierce A, Hamilton RT, Buffenstein R, Chaudhuri AR. Elevated protein carbonylation and oxidative stress do not affect protein structure and function in the long-living naked-mole rat: a proteomic approach. Biochem Biophys Res Commun. 2013;434:815–819. doi: 10.1016/j.bbrc.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Serviddio G, Pereda J, Pallardó FV, Carretero J, Borras C, Cutrin J, et al. Ursodeoxycholic acid protects against secondary biliary cirrhosis in rats by preventing mitochondrial oxidative stress. Hepatology. 2004;39:711–720. doi: 10.1002/hep.20101. [DOI] [PubMed] [Google Scholar]
- 38.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong Z, Froh M, Wheeler MD, Smutney O, Lehmann TG, Thurman RG. Viral gene delivery of superoxide dismutase attenuates experimental cholestasis-induced liver fibrosis in the rat. Gene Ther. 2002;9:183–191. doi: 10.1038/sj.gt.3301638. [DOI] [PubMed] [Google Scholar]
- 40.Schiffman SC, Li Y, Martin RC. The association of manganese superoxide dismutase expression in barrett's esophageal progression with MnTBAP and curcumin oil therapy. J Surg Res. 2012;176:535–541. doi: 10.1016/j.jss.2011.11.1013. [DOI] [PubMed] [Google Scholar]


