Abstract
Objective(s):
Pyocyanin is a blue-greenish redox-active pigment, produced by Pseudomonas aeruginosa, with a wide range of biological and biotechnological applications. Pyocyanin biosynthesis is regulated by the quorum-sensing (QS) system in which the expression of QS genes and QS-controlled virulence genes may be affected by serum as a complex medium. In the current study, effects of adult bovine serum (ABS) and fetal bovine serum (FBS) on the production of pyocyanin were examined in order to develop it.
Materials and Methods:
The presence of pyocyanin-producing specific genes and proteins in clinical and soil isolates of P. aeruginosa was confirmed using PCR and SDS-PAGE. Isolates were inoculated to media containing different concentrations of complement-active/-inactivated ABS or FBS and pyocyanin concentration was measured by spectrophotometry. Extracted pigment was characterized by using UV-Visible spectrophotometry. Titration of ABS antibodies against studied isolates was performed by the tube agglutination test.
Results:
Adding ABS to P. aeruginosa culture medium decreased pyocyanin production compared to the control, while its production increased in FBS-containing media (113.21±2.581 vs. 55.26±0.827 μg.ml-1 and 126.80±2.036 vs. 30.56±0.382 μg.ml-1 of C11 and E8 pyocyanin concentration in the presence of 10% FBS vs. control, respectively).
Conclusion:
In this study, due to the presence of inhibitors such as complement proteins and antibodies in ABS samples, the use of FBS devoid of antibodies was effective to increase pyocyanin production in studied isolates.
Keywords: Adult bovine serum, Fetal bovine serum, PhzM, Pseudomonas aeruginosa, Pyocyanin
Introduction
Pseudomonas aeruginosa is an opportunistic Gram-negative bacterium and a pathogen for animals, nematodes, and plants (1). Complicated pathophysiology of P. aeruginosa infections is due to its ability in the production of several virulence factors such as phenazines, proteases, and rhamnolipids (2). Phenazines are secondary metabolites and a large family of tricyclic and nitrogen-containing redox active compounds including phenazine-1-carboxylic acid (PCA), pyocyanin, 1-hydroxy phenazine, and phenazine-1-carboxamide (3). Pyocyanin, a blue-green pigment and a derivative of PCA, is produced in the late exponential growth phase and stains cultures and sputum of cystic fibrosis patients colonized by P. aeruginosa (4).
Biosynthesis pathway of pyocyanin contains two homologous seven-gene operons (phzA1-G1 and phzA2-G2) and two additional genes (phzS and phzM) encoding specific proteins for the conversion of PCA to pyocyanin; phzM encodes a bacterial methyltrans-ferase-like protein with the molecular weight of 36.4 kDa, while phzS encodes a bacterial monooxygenase-like protein with the molecular weight of 43.6 kDa (5). Quorum-sensing (QS) system plays a key role in the regulation of pyocyanin biosynthesis in which LasI-LasR and RhlR-RhlI can compromise organisms to different environments (6).
Pyocyanin biological action is due to its ability in the generation of redox-cycle resulting in reactive oxygen species augmentation in cells (7, 8). This activity has provided pocyanin biological and biotechnological applications, such as decreased symptoms of plant diseases by toxic effects against the nematode Caenorhabditis elegans and the fruit fly Drosophila melanogaster (9, 10), bean resistance against Botrytis (11), and anti-fungi and anti-yeast activity with severe antagonistic effect on Candida albicans and Aspergillus fumigatus (12). Moreover, this compound has been utilized in microbial fuel cells due to its electron transferable nature and in the study carried out by Ohfuji et al. it was used in a glucose-sensor system (13, 14). Recent studies have also demonstrated cytotoxic effect of pyocyanin on different cancer cell lines (15, 16).
According to pyocyanin applications in industry and medicine, it is important to identify the factors by which pyocyanin production can amplify. Serum is a complex medium containing proteins and factors that may positively or negatively modulate QS and QS-controlling genes. It has been reported that P. aeruginosa can use adult bovine serum (ABS) factors to enhance its virulence by increased production of QS-controlled virulence factors (17). However, there are no studies reporting the effect of fetal bovine serum (FBS) on the production of pyocyanin. As a result, the aim of this study was to assess the influence of different concentrations of ABS and FBS on pyocyanin production in order to evaluate the application of these compounds as medium supplements.
Materials and Methods
Collection of clinical samples
A total of 11 P. aeruginosa isolates (10 isolates from wound specimens, and one isolate from urinary tract infection) were kindly donated by the lab of Shaheed Motahari Burns Hospital, Tehran, Iran and confirmed as P. aeruginosa by Gram staining and biochemical tests. The isolates were then cultured on cetrimide agar medium and incubated for 48 hr at 37°C to identify pyocyanin producer isolates and the isolate produced the darkest green color on the medium was chosen for further studies.
Collection of soil samples and bacterial isolation
Soil samples, consisting of 10 samples, were collected from the depth of 5-10 cm below the surface land and kept in sterile containers. Among soil samples, five of 10 were taken from agricultural fields, including mulberry (named S1), chili (S2), vegetables rhizosphere (S3 and S4), and humus-containing garden soil (S5) and the other five samples were taken from oil-hydrocarbons contaminated soil (S6-S10). All the soil samples were obtained from locations considered to have the lowest risk of hospital specimen contamination in Tehran, Iran. P. aeruginosa isolation procedure was performed by three methods containing dilution and pour-plate, surface culture of diluted samples, and bacterial enrichment accomplished as what follows: 1 g of each soil sample was mixed with 10 ml of sterile nutrient broth medium by vortexing for 1 min. The resulting suspension then settled for 20 min and incubated overnight at 37 °C with a 230-rpm shake in order to enrich the bacteria. After incubation time, the supernatant of each sample was cultured on the surface of cetrimide agar and incubated at 37 and 42 °C for 24 hr. Thereafter, P. aeruginosa (named E1-E10) was characterized by Gram staining and biochemical tests and after bacterial isolation, pyocyanin producing P. aeruginosa isolates were cultured on cetrimide agar with incubation conditions as 37 °C for 48 hr to choose the best pyocyanin producer isolate for further studies.
Bacterial growth curve
Bacterial suspensions of selected isolates (C11 and E8), adjusted to the McFarland 0.5 standard, were inoculated to brain heart infusion (BHI) broth medium (Merck) in order to gain the growth curve of each isolate by the measurement of optical density at 600 nm (OD600) from the inoculation time to four days in every 2 hr (sterile BHI broth as blank). Incubation conditions during the measurement were 230 rpm and 37 °C.
Molecular confirmation of pyocyanin production
Polymerase chain reaction (PCR) of phzM gene Genomic DNA of a standard P. aeruginosa strain (ATCC 9027), C11 and E8 isolates (greenish pigment producers), and C1 isolate (yellowish pigment producer) were extracted by a genomic DNA extraction kit. PCR mixture was provided in microtubes (Eppendorf) and PCR was performed by a thermocycler. PCR products was assessed for the presence of 1015 bp desired DNA segment by 1% agarose gel electrophoresis against 1 kb DNA marker (Metabion, mi-8201).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of PhzM and PhzS proteins
Two-day cultures of P. aeruginosa isolates (greenish pigment producers: C9, C11, E1, E7, E8 and yellowish pigment producer: C2) in BHI broth were centrifuged at 8000 rpm for 5 min, and the resulted cell pellet was then washed with 300 mM Tris. After a centrifugation at 8000 rpm for 5 min, 100 μl of lysis buffer added to cell pellet and the mixture was incubated an overnight at 4 °C. Thereafter, the cell pellet was removed by a centrifugation at 10000 rpm for 5 min. Sample buffer was added to the supernatant at the ratio of 1:4 and after a four-min boiling, the total bacterial protein separation continued by a 15% SDS-PAGE.
Pyocyanin production
A colony of an overnight culture of P. aeruginosa (C11 & E8) on cetrimide agar was inoculated on glycerol-alanine agar (per liter: 10 ml glycerol, 6 g L-alanine, 2 g MgSO4, 0.1 g K2HPO4, 0.018 g FeSO4, 20 g agar, final pH 7.2) and incubated for 48 hr at 37 °C.
Pyocyanin production in media containing different concentrations of active complement ABS (aABS)
ABS was separated from blood samples of three health-approved adult bovines (older than 12 months) provided to be slaughtered and then filter sterilized. Serum was added to glycerol-alanine broth as 5% (v/v) in the first group and as 10% (v/v) in the second group. Each group contained three subgroups for each serum sample and media lacking serum were considered as control. Bacterial suspension from an overnight C11 or E8 cultures on glycerol-alanine agar, adjusted to McFarland 0.5 standard, was added to the media and incubated at 37 °C with a 210-rpm shake for 72 hr. Pyocyanin concentration was then measured by a spectrophotometer in triplicate.
Pyocyanin production in media containing different concentrations of complement-inactivated ABS (inABS) and FBS
ABS complement was inactivated in a 60 °C-water bath for 60 min and then filter-sterilized. Ready to use FBS (Biosera, UK, the complement previously inactivated in a 56 °C-water bath for 30 min) as well as inABS were added to glycerol-alanine broth in concentrations of 2, 5, and 10% (v/v) and each in duplicate; one of which was inoculated by C11, while the other by E8 suspension. The media lacking serum were considered as control and incubation conditions were as the temperature of 37 °C and a 210-rpm shake for up to four days. Pyocyanin concentration (in triplicate) was measured by a spectrophotometer after 72 and 96 hr.
Pyocyanin extraction, characterization, and concen-tration measuring
The cultural contents were transferred into conical centrifuge tubes to be centrifuged at 4000 rpm for 15 min. The equal volume of the supernatant moved into new Falcons in triplicate after filter sterilization. Chloroform was then added to each at the ratio of 1:2 and after a momentary vertex, centrifugation was performed at 3000 rpm for 1 min. The upper phase was removed and 0.1 N HCl was added to the lower phase in the amount of 20% of initial supernatant volume. After a short vortex, a 1-min centrifugation at 3000 rpm was done in order to a red-pink superior phase formation. Thereafter, pyocyanin concentration was measured using a spectrophotometer and the resulted optical density at 520 nm (OD520) was multiplied to 17.072 (molar extinction coefficient) to gain the pyocyanin concentration in μg per ml (18).
The solution was then neutralized by NaOH and extraction steps repeated for three additional times to gain a pure pigment. To characterize extracted pigments as pyocyanin, absorption spectra of a standard pyocyanin (Sigma-Aldrich, P0046) as well as C11 and E8 pyocyanin dissolved in chloroform or 0.1 N HCl was measured by a UV-visible (UV-Vis) spectrophotometer (Cary 100 Bio) in the wavelength range of 300-800 nm.
ABS antibody titration
In order to assess the presence of antibodies against studied isolates in ABS samples, colonies of an overnight culture of C11 and E8 isolates were solved in physiological serum equal to 4.5 × 109 cells.ml-1 (OD600 = 3.00) and the bacteria were killed by a 85 °C water bath for 2 hr to get the bacterial antigens. Then, 30 μl of the resulted antigens was mixed with 100 μl of ABS on a glassy slide and in cases of positive agglutination, ABS antibodies titration was performed by tube agglutination test.
Statistical analysis
All data given as mean±SD was analyzed by Statistical Package for Social Sciences (SPSS 22.0) as analysis of variance (ANOVA) and T-test.
Results
Selection of the highest pyocyanin producer strains among clinical and soil isolates
One (C9) out of 10 P. aeruginosa strains isolated from burn specimens produced pyocyanin, while the other nine isolates produced yellowish pigment. However, an isolate (C11) from urinary tract infection was the highest pyocyanin producer amongst clinical isolates which entered in its stationary phase of growth after 25.5 hr of inoculation. Moreover, of 10 soil samples, four isolates in dilution and 13 isolates in the surface culture of diluted samples methods emerged on cetrimide agar that none of these isolates produced blue-green pigments. By using the enrichment method, however, 24 isolates were developed on cetrimide agar that 10 of which (E1-E10) were characterized as P. aeruginosa and four of these isolates (E1, E4, E7, E8) produced blue-greenish pigment on cetrimide agar, whereas the rest produced pigments in yellow or red (Table 1). Among soil isolates, an isolate obtained from oil-contaminated soil of a petroleum refinery in Tehran, Iran (E8) was the highest pyocyanin producer with initial stationary phase of growth at 26.5 hr after inoculation.
Table 1.
Number of soil isolates developed on cetrimide agar
| Soil Samples | Isolates | P. aeruginosa | Pyocyanin producer P. aeruginosa |
|---|---|---|---|
| S1 | 3 | 1 | 1 |
| S2 | 3 | 1 | − |
| S3 | − | − | − |
| S4 | 3 | 1 | − |
| S5 | 3 | 1 | 1 |
| S6 | 1 | − | − |
| S7 | 4 | 3 | 1 |
| S8 | 4 | 2 | 1 |
| S9 | 1 | − | − |
| S10 | 2 | 1 | − |
| Total | 24 | 10 | 4 |
Molecular identification of phzM
C11 and E8 isolates along with the standard strain showed a 1015 bp DNA fragment of desired gene, phzM, against DNA marker on gel agarose, but C1 isolate, a yellow pigment producer, could not present the desired DNA fragment. C11 and E8 isolates, additionally, encompassed 36.4 and 43.6 kDa protein bands for PhzM and PhzS respectively, while the yellowish pigment producer was PhzS-positive and PhzM-negative (Figure 1).
Figure 1.
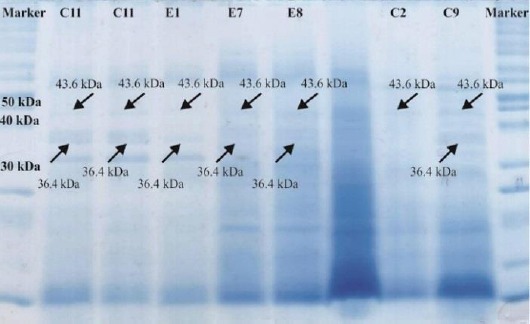
PhzM (36.4 kDa) and PhzS (43.6 kDa) protein bands on SDS-PAGE
Pyocyanin production
C11 and E8 isolates which produced greenish pyocyanin pigment on cetrimide agar could produce dark-blue pyocyanin pigment on glycerol-alanine agar.
Characterization of pyocyanin
UV-Vis spectra of pyocyanin in the range of 300 to 800 nm exhibited absorption maxima at 308 and 694 nm when dissolved in chloroform, whereas 387 and 518 nm when dissolved in 0.1 N HCl (Figure 2).
Figure 2.
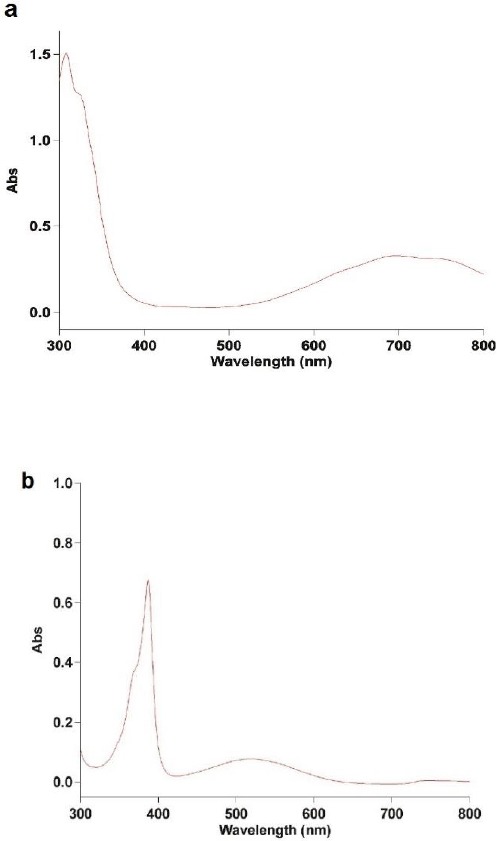
UV-Vis spectra of pyocyanin (a) in chloroform, (b) in 0.1 N HCl
Effect of aABS on pyocyanin production
Pyocyanin production in C11 and E8 isolates remarkably declined in the presence of 5 and 10% aABS in compare to the control and the more increase in aABS concentration, the greater reduction in pyocyanin production. Distinctive sera, moreover, showed relatively same effects on pyocyanin production when the serum complement was active (Figure 3).
Figure 3.
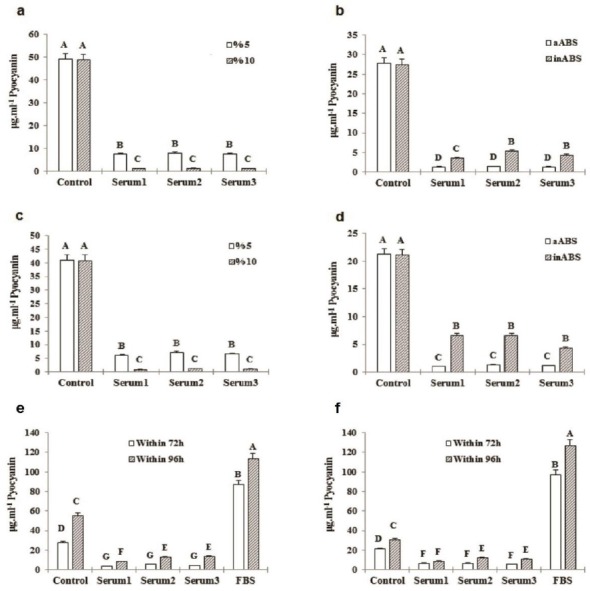
(a) C11 pyocyanin concentration in the presence of 5% different aABS in compare to those of the 10% after 72 hr, (b) C11 pyocyanin concentration in media complemented by 10% different aABS and also inABS against a control sample after 72 hr, (c) E8 pyocyanin concentration in the presence of 5% different aABS in compare to those of the 10% after 72 hr, (d) E8 pyocyanin concentration in media complemented by 10% different aABS and also inABS against a control sample after 72 hr, (e) C11 pyocyanin production within 72 hr compared with its production within 96 hr in the presence of 10% inABS and FBS, (f) E8 pyocyanin production within 72 hr compared with its production within 96 hr in the presence of 10% inABS and FBS. *Several letters indicate in-group and between-group significant difference (P<0.05)
Effect of inABS and FBS on pyocyanin production
As it has been shown in Figures 3-6, C11 and E8 pyocyanin production increased in the presence of inABS in compare to aABS, yet less than the control, while significantly enhanced by FBS resulting in highly increased pyocyanin concentration compared with the control (113.21±2.581 vs. 55.26±0.827 μg.ml-1 of C11 pyocyanin concentration in the presence of 10% FBS vs. the control and 126.80±2.036 vs. 30.56±0.382 μg.ml-1 of E8 pyocyanin concentration in the presence of 10% FBS vs. the control). This data also indicates that although pyocyanin production in E8 isolate was less than C11, the presence of 10% FBS led to higher concentration of E8 pyocyanin in compare to C11. As incubation period also increased, so did the pyocyanin production in all groups. In control groups, pyocyanin concentration rose from 27.44±0.346 and 21.09±0.81 μg.ml-1 (C11 and E8 respectively) at 72 hr to 55.28±0.827 and 30.56± 0.382 μg.ml-1 (C11 and E8 respectively) at 96 hr. In 10% FBS-containing groups, the concentrations of 87.17±948 and 96.76±0.820 μg.ml-1 at 72 hr, increased to 113.21±2.581 μg.ml-1 and 126.80±2.036 at 96 hr in C11 and E8, respectively. Furthermore, in contrast to ABS-containing samples, an increase in FBS concentration led to more pyocyanin production. As Table 2 also shows, all adult bovine sera contained antibodies against both strains.
Figure 4.
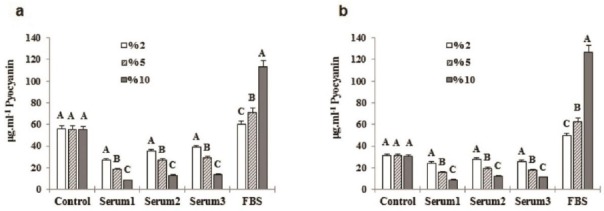
(a) C11 pyocyanin concentration in the presence of 2, 5, and 10% of inABS and FBS after 96 hr, (b) E8 pyocyanin concentration in the presence of 2, 5, and 10% of inABS and FBS after 96 hr of incubation. *Several letters represent between-group significant difference (P<0.05)
Figure 5.
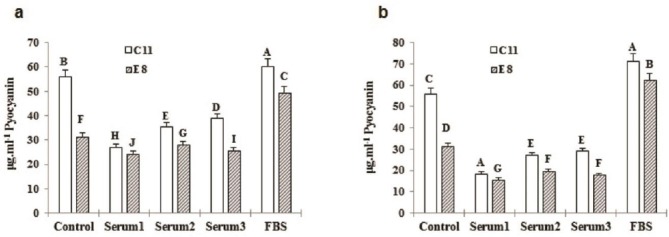
(a) Effect of 2% inABS and FBS on C11 pyocyanin production compared to E8 after 96 hr, (b) Effect of 5% inABS and FBS on C11 pyocyanin production compared to E8 after 96 hr incubation. *Various letters show in-group and between-group significant difference (P<0.05)
Figure 6.
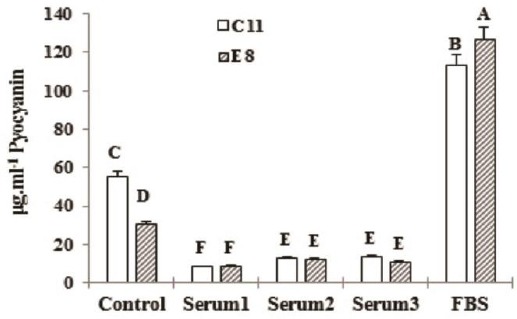
Effect of 10% inABS and FBS on C11 pyocyanin production compared to E8 after 96 hr of incubatin. *Different letters depict in-group and between-group significant difference (P<0.05)
Table 2.
ABS antibodies titre against C11 and E8 isolates of pseudomonas aeruginosa
| Samples | C11 | E8 |
|---|---|---|
| Serum 1 | 1:160 | 1:40 |
| Serum 2 | 1:40 | 1:20 |
| Serum 3 | 1:80 | 1:40 |
Discussion
P. aeruginosa is a Gram-negative bacillus dwelling in broad-spectrum environmental conditions including soil, water, plant, and animal tissues and considers noteworthy due to its role in human and plant diseases as well as the growing potential use in biotechnological applications (7, 19). P. aeruginosa ecological diversity is related to its well-metabolic compliance by which can utilize long-chain alkanes resulting in at least five years of survival in heavy oil (20). Approximately, 90 to 95% of P. aeruginosa strains produce pyocyanin with strong antibiotic activity particularly used in biological control (9). The aim of this study was to identify the effect of bovine serum on pyocyanin production as an enhancer agent.
In the present study, samples were collected from agricultural soils as well as those contaminated with petroleum hydrocarbons during the last days of autumn. The bacteria were isolated by using three methods including: dilution and pour-plate in cetrimide agar, surface culture of prepared dilution on cetrimide agar, and bacterial enrichment in nutrient broth medium followed by surface culture on cetrimide agar medium. The pour-plate method yielded four isolates and surface culturing of the prepared dilutions on cetrimide agar yielded 13 isolates, but none of these isolates produced blue-green pigments. However, the use of enrichment method resulted in the growth of 24 isolates on cetrimide agar medium that 10 (41.7%) of those were identified as P. aeruginosa and the rest belonged to other pseudomonas species or other genera of Gram-negative bacteria. Of 10 P. aeruginosa isolates, six (60%) belonged to samples that were contaminated with petroleum hydrocarbons indicating that petroleum-contaminated soil could be a richer source of P. aeruginosa than agricultural ones.
Results of this study are consistent with the results of Vives-Flórez et al. who used pour-plate method to obtain 50 isolates of 38 water and soil samples (nine oil-contaminated samples and 29 uncontaminated samples taken from Colombia) on cetrimide agar, and identified 19 (38%) of these isolates as P. aeruginosa. In their study, of 19 P. aeruginosa isolates, 16 belonged to samples that were contaminated with petroleum hydrocarbons. While P. aeruginosa was isolated from all nine oil-contaminated samples, only three uncontaminated samples (10.3%) contained P. aeruginosa. They also stated that those 23 samples that did not show P. aeruginosa may contain P. aeruginosa populations that cannot be identified by dilution method (21). Moreover, El-Amine et al. studied the hydrocarbon-contaminated soil in two gas processing stations in Mascara and also a forest that was charged by the residues of second station. This assessment was conducted through bacteria enrichment in nutrient broth medium. They were able to isolate 20 strains of P. aeruginosa from 15 collected soil samples (22). Al-Hinai et al. also isolated 20 strains of P. aeruginosa from 27 soil samples collected from cucumbers greenhouse in Barka, Oman (23). P. aeruginosa is an aerobic bacterium, so when using the pour-plate method, the bacteria positioned in lower levels of medium may fail to receive adequate aeration. Therefore, the more suitable approach for the isolation of P. aeruginosa strains from environmental samples would be to start the process with an enrichment broth and then proceed with surface culture.
After SDS-PAGE, those strains that produced green pigment showed 43.6 and 36.4 kDa bands, which are related to PhzS and PhzM proteins while strains that produced yellow pigment only had the 43.6 kDa PhzS band. These results are consistent with the results of Mavrodi et al. who have reported that two specific enzymes, PhzM and PhzS, are involved in the conversion of PCA to pyocyanin, and that in addition to pyocyanin production, PhzS is involved in the biosynthesis of 1-hydroxyphenazine (yellow product) in P. aeruginosa PAO1 (5). Thus, appearance of yellow pigment in P. aeruginosa is a result of the presence of PhzS, and appearance of green pigment in P. aeruginosa strains represents the presence of both PhzM and PhzS proteins.
In the present study, glycerol-alanine medium was used to increase the pyocyanin production. Those P. aeruginosa strains that produced green pigment in cetrimide agar were able to produce dark blue colored pyocyanin in the glycerol-alanine medium, where this color signifies the high concentration of pyocyanin. There are also several other studies that have used glycerol-alanine medium to increase pyocyanin production (24-26). The presence of alanine and glycerol as common substrates has a major impact on pyocyanin production and acts as a precursor to this process. This medium, which utilizes a shared substrate, is Frank and De Moss’s recommendation for rapid detection of P. aeruginosa and verification of pyocyanin (27).
To identify extracted pigments as pyocyanin, its UV-Vis spectra at wavelength ranges of 300-800 nm were evaluated against a standard pyocyanin. The peak absorptions of pyocyanin dissolved in chloroform were at 308 and 694 nm, and its maxima absorption when dissolved in 0.1 N HCl were at 387 and 518 nm. These results are consistent with the results of previous study (14). Moreover, El-Fouly et al. have reported that the peak absorptions of pyocyanin dissolved in 0.2 N HCl are at wavelengths of 300, 388, and 518 nm (28).
To study the effects of bovine serum on pyocyanin production, ABS was acquired from at least 12 months old bovines, while FBS package was acquired from the market. In one procedure, 5 and 10% (v/v) ABS with active complement were added to glycerol-alanine medium. In another procedure, ABS and FBS complement was deactivated by heating, and then 2, 5, and 10% (v/v) of resulting products were added to glycerol-alanine medium. Next, identical concentrations of P. aeruginosa suspensions were inoculated to all these media as well as a control (serum free) medium. These experiments were performed for both clinical and soil strains. After 72 to 96 hr, pyocyanin concentration of each sample was measured through chloroform-acid extraction and spectrophotometry. Results of data analysis showed that pyocyanin production increases as the bacteria approach the late stages of stationary phase of growth. All ABS-containing samples showed decreased pyocyanin production as compared to the control and pyocyanin concentration, additionally, in aABS groups was significantly lower than that of inABS groups. However, the FBS-containing samples showed significantly increased pyocyanin concentration in compare to the control. According to these results, as ABS concentration increased, pyocyanin concentration decreased; but as FBS concentration increased, so did the concentration of pyocyanin.
The results of this study contradict with the results of previous studies. Kruczek et al. cultured P. aeruginosa PAO1 in an LB medium supplemented with 10% (v/v) adult bovine serum. They used transcriptional analysis and enzyme assays to determine the effect of adult bovine serum on QS and QS-controlled virulence factors during early and late stationary phase of P. aeruginosa PAO1 growth. The serum suppressed the expression of QS-controlled genes, including phz in the early phase, but enhanced their expression in the late stationary phase. Comparing the serum and non-serum (control) samples, they concluded that the serum decreases the expression of QS genes and QS-controlled virulence genes in the early phase of growth and increases their expression in the late stationary phase (17). Juhas et al. also reported that QS and QS-controlled genes in P. aeruginosa PAO1 are among 113 genes that their expression increased by serum (29). Moreover, Wu et al. suggested that a specific binding between interferon gamma and outer membrane proteins, such as OmpF, initiates rhlI and rhlR effects in P. aeruginosa and leads to increased production of pyocyanin. In their study, the effect of interferon gamma was observed during the late stationary phase of growth (30).
The contradiction between this study and previous ones may arise from the immunization of native adult bovines against studied P. aeruginosa isolates. Accordingly, diminished pyocyanin production in the presence of ABS from Iranian bovines can attribute to serum inhibitors such as antibodies and complement proteins. This was confirmed by complement deactivation and comparing pyocyanin production in media containing active- and passive-complement ABS, the assessment of ABS antibody titre, and above all pyocyanin production in the presence of ABS compared with its production in the media containing FBS lacking any types of antibody.
Conclusion
FBS as a rich supplement is recommended to P. aeruginosa media aiming to enhance pyocyanin production. However, considering the ABS cost-effectiveness in compare to FBS, the effect of ABS on pyocyanin production requires further studies.
Acknowledgment
This work is a part of MSc student project supported by Tehran North Branch of Islamic Azad University, Tehran, Iran. The authors’ names have been ordered according to the contribution rates.
Conflict of interest
The authors declare that no conflict of interest exists.
References
- 1.Jander G, Rahme LG, Ausubel FM. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J Bacteriol. 2000;182:3843–3845. doi: 10.1128/jb.182.13.3843-3845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank DW. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 3.Budzikiewicz H. Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol Rev. 1993;104:209–228. doi: 10.1111/j.1574-6968.1993.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 4.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183:6454–6465. doi: 10.1128/JB.183.21.6454-6465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteley M, Lee KM, Greenberg E. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross H, Loper JE. Genomics of secondary metabolite production by pseudomonas spp. Nat Prod Rep. 2009;26:1408–1446. doi: 10.1039/b817075b. [DOI] [PubMed] [Google Scholar]
- 8.Muller M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radicals Biol Med. 2002;33:1527–1533. doi: 10.1016/s0891-5849(02)01087-0. [DOI] [PubMed] [Google Scholar]
- 9.Laursen JB, Nielsen J. Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chem Rev. 2004;104:1663–1686. doi: 10.1021/cr020473j. [DOI] [PubMed] [Google Scholar]
- 10.Rahme LG, Ausubel FM, Cao H, Drenkard E, Goumnerov BC, Lau GW, et al. Plants and animals share functionally common bacterial virulence factors. Proc Natl Acad Sci U S A. 2000;97:8815–8821. doi: 10.1073/pnas.97.16.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anjaiah V, Koedam N, Nowak-Thompson B, Loper JE, Höfte M, Tambong JT, et al. Involvement of phenazines and anthranilate in the antagonism of Pseudomonas aeruginosa PNA1 and Tn5 derivatives toward Fusarium spp. and Pythium spp. Mol Plant-Microbe Interact. 1998;11:847–854. [Google Scholar]
- 12.Kerr J, Taylor G, Rutman A, Høiby N, Cole P, Wilson R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J Clin Pathol. 1999;52:385–387. doi: 10.1136/jcp.52.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabaey K, Verstraete W. Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol. 2005;23:291–298. doi: 10.1016/j.tibtech.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Ohfuji K, Sato N, Hamada-Sato N, Kobayashi T, Imada C, Okuma H, et al. Construction of a glucose sensor based on a screen-printed electrode and a novel mediator pyocyanin from Pseudomonas aeruginosa. Biosens Bioelectron. 2004;19:1237–1244. doi: 10.1016/j.bios.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Hassani HH, Hasan HM, Al-Saadi A, Ali AM, Muhammad MH. A comparative study on cytotoxicity and apoptotic activity of pyocyanin produced by wild type and mutant strains of Pseudomonas aeruginosa. Eur J Exp Biol. 2012;2:1389–1394. [Google Scholar]
- 16.Zhao J, Wu Y, Alfred A, Wei P, Yang S. Anticancer effects of pyocyanin on HepG2 human hepatoma cells. Lett Appl Microbiol. 2014;58:541–548. doi: 10.1111/lam.12224. [DOI] [PubMed] [Google Scholar]
- 17.Kruczek C, Qaisar U, Colmer-Hamood JA, Hamood AN. Serum influences the expression of Pseudomonas aeruginosa quorum?sensing genes and QS?controlled virulence genes during early and late stages of growth. MicrobiologyOpen. 2014;3:64–79. doi: 10.1002/mbo3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essar D, Eberly L, Hadero A, Crawford I. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silby MW, Winstanley C, Godfrey SA, Levy SB, Jackson RW. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev. 2011;35:652–680. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- 20.Chaerun SK, Tazaki K, Asada R, Kogure K. Bioremediation of coastal areas 5 years after the Nakhodka oil spill in the Sea of Japan: isolation and characterization of hydrocarbon-degrading bacteria. Environ Int. 2004;30:911–922. doi: 10.1016/j.envint.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Vives-Flórez M, Garnica D. Comparison of virulence between clinical and environmental Pseudomonas aeruginosa isolates. Int Microbiol. 2006;9:247–252. [PubMed] [Google Scholar]
- 22.El-Amine Bendaha M, Mebrek S, Naimi M, Tifrit A, Belaouni H. Isolation and comparison of rhamnolipids production in Pseudomonas aeruginosa P.B:2 and Pseudomonas fluorescens P.V:10. Sci Rep. 2012;1:544. [Google Scholar]
- 23.Al-Hinai A, Al-Sadi A, Al-Bahry S, Mothershaw A, Al-Said F, Al-Harthi S, et al. Isolation and characterization of Pseudomonas aeruginosa with antagonistic activity against Pythium aphanidermatum. J Plant Pathol. 2010;92:653–660. [Google Scholar]
- 24.Karatuna O, Yagci A. Analysis of quorum sensing?dependent virulence factor production and its relationship with antimicrobial susceptibility in Pseudomonas aeruginosa respiratory isolates. Clin Microbiol Infect. 2010;16:1770–1775. doi: 10.1111/j.1469-0691.2010.03177.x. [DOI] [PubMed] [Google Scholar]
- 25.Mohammed HA, Yossef HS, Mohammad FI. The cytotoxicity effect of pyocyanin on human hepatocellular carcinoma cell line (HepG2) Iraqi J Sci. 2014;55:668–674. [Google Scholar]
- 26.Vinckx T, Wei Q, Matthijs S, Cornelis P. The Pseudomonas aeruginosa oxidative stress regulator OxyR influences production of pyocyanin and rhamnolipids: protective role of pyocyanin. Microbiology. 2010;156:678–686. doi: 10.1099/mic.0.031971-0. [DOI] [PubMed] [Google Scholar]
- 27.Subramaniam L. Rapid diagnosis of Pseudomonas aeruginosa infection by demonstration of pyocyanin & fluorescein. Indian J Med Res. 1985;81:561–566. [PubMed] [Google Scholar]
- 28.El-Fouly M, Sharaf A, Shahin A, El-Bialy HA, Omara A. Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J Radiat Res Appl Sci. 2015;8:36–48. [Google Scholar]
- 29.Juhas M, Wiehlmann L, Huber B, Jordan D, Lauber J, Salunkhe P, et al. Global regulation of quorum sensing and virulence by VqsR in Pseudomonas aeruginosa. Microbiology. 2004;150:831–841. doi: 10.1099/mic.0.26906-0. [DOI] [PubMed] [Google Scholar]
- 30.Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309:774–777. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]


