Abstract
Objective:
The role of growth factors, including vascular endothelial growth factor of activated omentum on mitosis is clearly known, though not on all the aspects of in vitro oocyte maturation. This study was designed to assess the effect of activated-omental extract (AOE) on in vitro maturation (IVM) of rat cumulus-oocyte complexes (COCs).
Materials and Methods:
In this experimental study, the COCs were incubated in Ham’s F-10 supplemented with either 20% AOE, 20% fetal bovine serum (FBS) or serum-free media. Post-culture COCs were studied according to the cumulus cells (CCs) expansion, nuclear maturation and cytoplasmic maturation. Cumuli expansion was evaluated by inverted microscope without staining; nuclear maturation was assessed by aceto-orcein staining (light microscope) and cytoplasmic maturation was also observed by TEM.
Results:
Expansion of CCs and nuclear maturation of the oocytes in in vitro for 24 hr was significantly higher in AOE- and FBS-supplemented groups (P=0.000 and 0.013) and (P=0.004 and 0.014), respectively, compared to serum-free group. At ultra-structural level, after 24 hr, both FBS and AOE-supplemented media showed uniformly wide perivitelline space (PVS). After 12 hr, the cortical granules were found in the oocytes cultured in FBS and AOE-supplemented media. Within 24 hr, both granules and mitochondria were large without any detectable topographic tendency across the ooplasm. In AOE and FBS-supplemented oocytes, the number and size of microvilli were more than those in serum-free one.
Conclusion:
Although AOE supplementation induced a higher rate of the CCs expansion, and resuming meiosis, it was not as potent as FBS to provide cytoplasmic maturation of rat oocytes.
Keywords: Cumulus cells, Cytoplasm, In vitro oocyte maturation, Nucleus, Omentum, Rats
Introduction
In mammals, in vivo oocyte maturation is started mainly by the surge of gonadotropins, especially luteinizing hormone (LH), but an in vitro oocyte maturation onset occurs just by the elimination of inhibitory effect of granulosa cells (1, 2). Edwards in the 1960s constructed a great biologic innovation by reporting that mammalian oocytes not only survived in vitro condition but would also reach metaphase-II, if the appropriate attention is paid (3). However, in vitro maturation (IVM) outcome still remains inferior compared to conventional in vitro fertilization (IVF) (4). The redistribution of ooplasmic organelles along with oocyte maturation is well documented (5-7). Migration of cortical granules (CGs) during oocyte maturation has repeatedly been confirmed (5, 6). Sub-cortical arrangement of CGs can be used to evaluate cytoplasmic maturation of oocytes. By tracing CGs translocation across ooplasm, it is reported that supplementation of vascular endothelial growth factor (VEGF) enhances the cytoplasmic maturation (7).
Moreover, this has prompted widespread acknowledgment of ooplasmic ultra-structural alterations, which can be employed as morphologic criteria to assess cytoplasmic maturation of oocytes. For instance, microvilli have been shown to increase in both size and number during maturation of oocytes. The cumulus cell projection endings (CCPEs) diminish along with oocyte maturation. Mitochondria have also been reported to show different micro-topographic tendency in oocytes of different maturity stages (6).
Improvement in quality of in vitro matured oocytes is performed chiefly in two different manners: co-culture and additive supplements. Addition of insulin-like growth factor (IGF), epidermal growth factor (EGF), VEGF, LH, follicle stimulating hormone (FSH) and growth hormone have been studied on IVM of mammalian oocytes (4, 7-9). Nevertheless, the most commonly used IVM media are those supplemented with hormones, serum or albumin (4). Fetal bovine serum (FBS) is still preferred as the gold standard supplement in laboratories all around the world (10).
Besides mammalian sera, tissue extracts have long been proved to be full of proteins, antioxidants and growth factors, which make them potentially useful as supplements. It is reported that “human adipose tissue extract induces angiogenesis and adipogenesis in vitro” (11).
Omentum has been suggested to have a critical role in wound healing events such as limiting the spread of inflammation, vascularization and regeneration. Activated omentum of rat contains mesenchymal stem cells with the ability to produce growth factors such as VEGF and fibroblast growth factors (FGFs) (12). In the present study, the greater omentum was used as the source of tissue extract. The greater omentum has always attracted researcher’s curiosity because of its strange and vital characteristics. Without knowing the underlying mechanism, the greater omentum has been applied for wound healing (13). There are extensive reports of the greater omentum as a rich source of growth factors, angiogenic and even neurogenic factors (12-15). Litbarg et al. reported that “activated omentum becomes rich in factors that promote healing and tissue regeneration” (14). They documented that VEGF content was 70-fold higher in activated omentum compared to omentum in naive status. In addition, it has been suggested that decellularized omentum can be used as a novel biological scaffold in regenerative medicine (16). The role of growth factors, including VEGF, of activated omentum on mitosis is well known (17). Although Araujo et al. reported that VEGF-A165 led to progress the development of goat preantral follicle (18), no study is available on the role of omentum growth factors in IVM of oocyte in rats. As mentioned above, activated-omental extract (AOE) is a potential source of VEGF, our assumption is that the AOE may be improved the three aspects of in vitro oocyte maturation. Therefore, this study was designed to assess the effect of AOE on in vitro nuclear and cytoplasmic maturation and cumulus cells (CCs) expansion of rat cumulus-oocyte complexes (COCs).
Materials and Methods
Except where otherwise indicated, all chemicals were obtained from the Sigma Company.
Oocyte collection
The female adult rats were treated according to the guidelines (photoperiod of 12 hr and free access to food and water) approved by the Ethics Committee of Shiraz University of Medical Sciences (SUMS). The rats were deeply anesthetized and their ovaries were removed and placed on a petri dish that contained 50 µl droplets of Ham’s F10. The ovaries were carefully dissected using a pair of insulin needles under stereo-microscope. The extruded COCs were recognized morphologically and only the ones having at least 2 or 3 layers of cumuli and homogenous ooplasm were transferred to another petri dish (19).
In vitro maturation
Each COCs was placed in a 10 µl droplet of Ham’s F10, covered with mineral oil and incubated at 37°C and 5% CO2 for predefined time-periods; 6, 12 and 24 hr. The experimental group was incubated in 20% AOE-supplemented media (the concentration was achieved from a pilot study) and the control groups were cultured in 20% FBS-supplemented (20) as positive and serum-free media as negative control groups.
Greater omentum activation
According to Litbarg et al., a group of female rats weighing 200-250 g received intra-peritoneal injection of 5 µl polydextran particle slurry (Biogel P-60, 120 µM; Biorad Laboratories, Richmond, CA; 1:1in normal saline) to activate their greater omentum (14). One omental piece of every single animal was controlled morphologically by hematoxylin and eosin staining to ensure that the activation was imposed. Notable changes can be found in morphology of an activated omentum compared to naive omentum. These changes include reduction in density of mesothelial and adipose cells and appearance of large cavitation, which indicate that polydextran particles have been surrounded by mesothelial cells (Figure 1). Moreover, the protein yield of the AOE was quantified though dye-binding Bradford protein assay (21).
Figure 1.
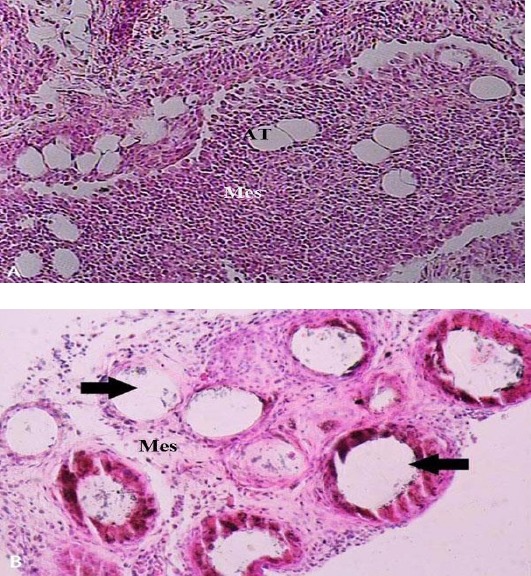
A) Morphology of a normal omentum. Densely arranged mesothelial cells with sparse adipose cells between them can be observed in a normal omentum micrograph. X150. B) Morphology of an activated omentum. Pathologic cavitation (arrows) indicates the activation of omentum in response to the injection of polydextran particles, which has been washed away during tissue processing. Loosely arranged mesothelial cells with almost no adipose tissue can be observed in an activated omentum micrograph X200. (H & E staining). Mes. (Mesothelium); AT (adipose tissue)
Greater omentum extract preparation
To remove the omentum, it was released from surrounding adipose tissue, cut into pieces, washed twice by phosphate buffered saline (PBS) and kept at 4 °C. Three ml of Trypsin / EDTA mixture was added to the tissue (trypsin acts as a protease and EDTA as a chelator). The petri dish (containing tissue pieces) was placed in a 37°C incubator for 20 min. Trypsin/EDTA mixture was replaced with cold PBS. The tissue pieces were transferred into cryotubes then snap frozen. The samples were thawed and washed twice in cold PBS and exposed to DTT-containing lysis buffer. Then, the samples were centrifuged at 800 g for 10 min and the supernatant was discharged. A mixture of DTT-containing lysis buffer and protease inhibitor 0.1%, approximately the size of the pellet were added and the suspension was then kept at 4°C for 45 min. Subsequently, the suspension was sonicated 7 times for 45 sec durations (0.5 pulse at 25%). The sonicated suspension was centrifuged at 15000 g for 15 min. Finally, the supernatant was collected as AOE (22).
Morphologic scoring of cultured oocytes Cumuli expansion
The CCs expansion was controlled and scored in 262 post-culture COCs using inverted microscope. The COCs were recognized as belonging to one of four different stages of cumuli expansion (23):
Expanded: absolute expansion was observed in the cumuli in a colloidal matrix with no cumulus cell contacting the oocyte (Figure 2A).
Figure 2.
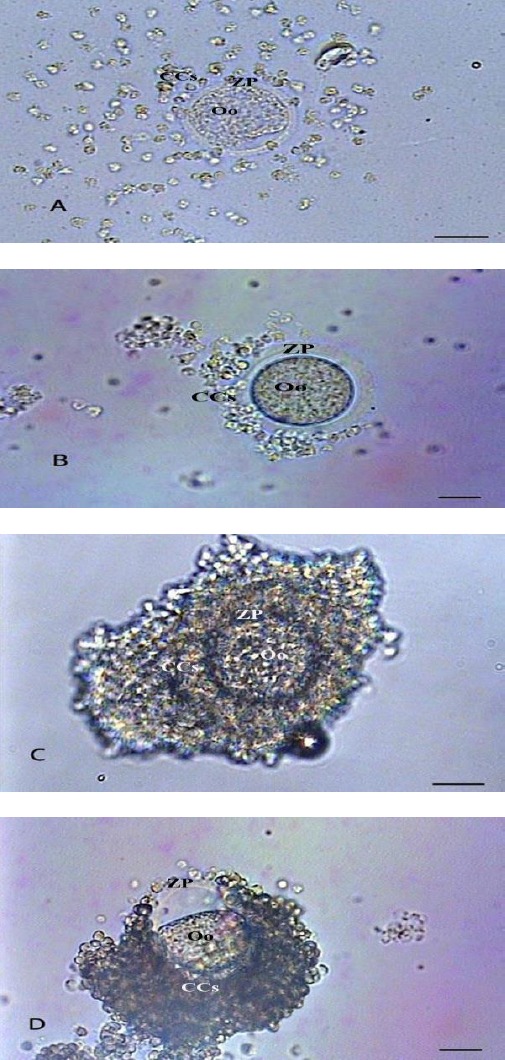
Photomicrographs of post-culture cumulus oocyte complexes at different cumuli expansion levels: expanded (A), semi-expanded (B), non-expanded (C) and degenerated (D) (Bars=5 µm). Oo (oocyte); ZP (zona pellucida); CCs (cumulus cells)
Semi-expanded: relative expansion with some cumuli still in the neighborhood of the oocyte (Figure 2B).
Non-expanded: no expansion with at least 2 or 3 layers of cumuli still surrounding the oocyte (Figure 2C).
Degenerated: irregular appearance of the oocyte with the cumuli either expanded or not expanded (Figure 2D).
Meiotic phases
The post-culture COCs were put in a 50 µl droplet of hyaluronidase to make the adjacent cumuli spread off. Then denuded oocytes were washed twice in physiologic serum 0.9% and fixed in Carnoy’s solution for 24 hr. Denuded oocytes were put on a glass slide and settled under a cover glass having four droplets of paraffin-vaseline mixture in its four corners. The oocytes were put in Carnoy’s solution for 12 hr. In order to stain oocytes, a few droplets of 1% aceto-orcein solution were injected under the cover glass. Immediately, the oocytes were screened under light microscope and their meiotic stage was determined according to the appearance of chromatin (1):
Germinal vesicle (GV): round vesicular nucleus.
Germinal vesicle breakdown (GVBD): non-vesicular dense chromatin.
Meiosis I (MI): duplicated patchy chromatin.
Anaphase-telophase (A-T): duplicated chromatin in bipolar appearance.
Meiosis II (MII): extruded polar body.
Degenerated (Deg.): distorted ooplasm and / or irregular outline.
Transmission electron microscopic (TEM) study
To prepare oocytes for TEM study, cultured COCs were washed in 50 µl droplets of physiologic serum 0.9%, primary-fixed in buffered glutaraldehyde 2.5%, washed in sodium cacodylate three times, post-fixed in 1% buffered osmium tetroxide and then washed in sodium cacodylate three times and an additional time in deionized distilled water. The COCs were dehydrated in an ascending series of ethanol (30-100%), embedded in resin (agar 100) and polymerized at 60°C overnight. Thin sections (60-90 nm) were contrasted with uranyl acetate and lead citrate and examined by TEM (1).
Statistical analysis
Discrete variables were reported as percentage. The normality condition of the quantitative variables was investigated using the Shapiro-Wilks test. Kruskal–Wallis with Mann-Whitney post hoc tests and One-way ANOVA with Tukey’s HSD post hoc tests were used to evaluate differences in continuous or ordinal variables across multiple groups. P-value of less than 0.05 was considered as significant. Data was analyzed using SPSS 23.0 software.
Results
Degeneration
Degeneration rates of COCs were significantly higher in serum-free group compared to both FBS- and AOE-supplemented groups after 12 and 24 hr of incubation (P=0.001) (Figure 3). While there was no significant difference in the degeneration rates of AOE-supplemented group compared to FBS-supplemented group after 12 hr of culture; after 24 hr of incubation, the COCs matured in AOE-supplemented medium were more degenerated compared to their counterparts cultured in FBS-supplemented medium (P<0.001) (Figure 2-D and Figure 3).
Figure 3.
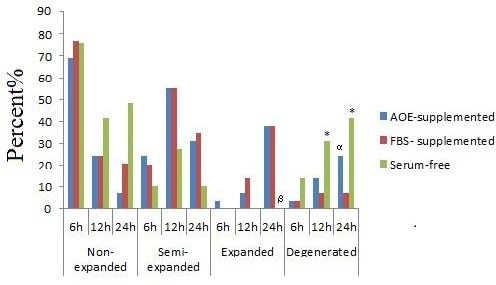
Cumulus cells expansion in AOE- and FBS-supplemented and serum-free groups. AOE; Activated-omental extract, FBS; Fetal bovine serum
* Serum-free versus AOE-supplemented and FBS-supplemented groups (P=0.001)
α AOE-supplemented versus FBS- supplemented group (P<0.001)
ß Serum-free versus AOE-supplemented and FBS-supplemented groups (P=0.000 and 0.013)
Cumulus cells expansion
In 6 hr of culture, about 3.45% of COCs showed complete expansion in the AOE-supplemented group, while no complete expansion was observed in serum-free and FBS- supplemented groups. However, no significant difference was observed between the groups.
The FBS-supplemented group showed a higher percentage of expanded COCs in 12 hr of culture compared with the AOE-supplemented group (13.80% vs. 6.90%); however, there was no significant difference.
The percentage of complete expansion was the same in the FBS-supplemented (37.90%) and AOE-supplemented (37.95%) groups in 24 hr, while no expanded COCs were observed in serum-free group in 6, 12 and 24 hr. The COCs from AOE and FBS- supplemented groups showed a significantly (P=0.000 and P=0.013) higher rate of expansion compared to their counterparts from the serum-free group. Figure 3 shows the expansion of CCs.
Nuclear maturation
Although no significant difference was detected between groups after 12 hr of culture, following 24 hr of incubation the oocytes in serum-free medium showed a significantly poor nuclear maturation as compared to both FBS-supplemented (P=0.014) and AOE-supplemented (P=0.004) groups. However, no significant difference was observed in the nuclear maturation outcome of FBS-supplemented and AOE-supplemented media (Figure 4). No significant difference was observed between the degeneration rate of FBS and AOE-supplemented groups, though both of them were significantly (P-value<0.001) less than serum-free group (Figure 4).
Figure 4.
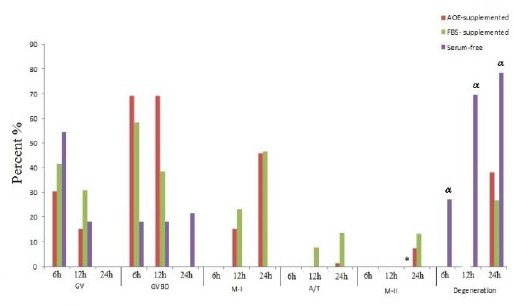
Nuclear maturation in AOE- and, FBS-supplemented and serum-free groups. AOE; Activated-omental extract, FBS; Fetal bovine serum, GV: Germinal vesicle; GVBD: Germinal vesicle breakdown: MI: Meiosis I; A/T: Anaphase-telophase: M-II; Meiosis II
* Serum-free group versus AOE-supplemented (P=0.014) and FBS- supplemented groups (P=0.004)
α Serum-free group versus AOE-supplemented (P=0.014) and FBS- supplemented groups (P<0.001)
Cytoplasmic maturation Cumulus cell projection endings
The COCs from all the different media showed extensive CCPEs after 6 hr of culture without any considerable difference among groups. However, after 12 hr of culture some alterations were observed in FBS- and AOE-supplemented (Figure 5A) groups compared with serum-free group.
Figure 5.
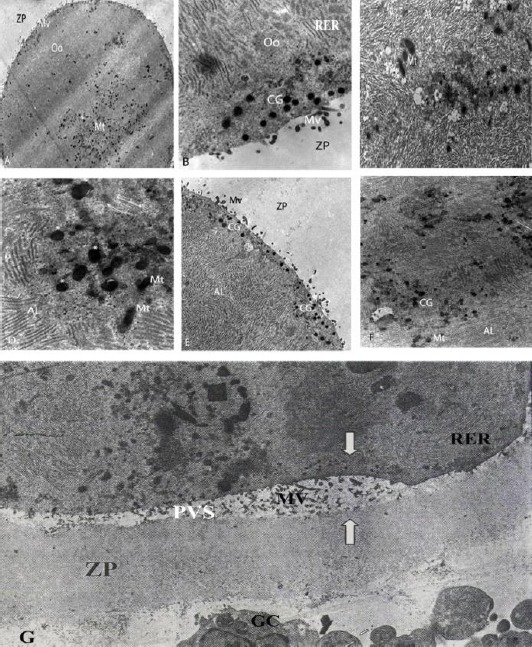
Electron micrographs of cytoplasmic characteristics: A) cultured for 12 hr in activated omental extract-supplemented medium (×1200); B) cultured for 24 hr in FBS-supplemented medium (×11300); C) cultured for 6 hr in serum-free medium (×10400); D) cultured for 24 hr in activated omental extract-supplemented medium (stars show CG) (×11000); E) cultured for 24 hr in activated omental extract-supplemented medium (×7500); F) cultured for 6 hr in FBS-supplemented medium (×5800); G) cultured for 24 hr in serum-free medium, arrows show uneven ZP and PVS (×3400). AL (annullate lamellae); CG (cortical granule); GC (granolosa cell); Mt (mitochondria); Mv (microvilli); Oo (oocyte); PVS (perivitelline space); RER (rough endoplasmic reticulum); ZP (zona pellucida)
While numerous CCP free endings were detected in thickness of zona pellucida (ZP) in FBS-supplemented group, only slight reduction in the density of CCPEs was detected in AOE-supplemented group (Figure 5A). In other words, contraction of CCPEs was much more obvious in FBS-supplemented group than in AOE-supplemented group. After 24 hr of culture, a notable difference was also recognized in the morphology of CCPE-mediated interconnections between CCs and oocytes. The CCPEs remained extensive in serum-free group after 24 hr of culture (Figure 5G). Numerous retracted forms of CCPEs were observed in AOE-supplemented group after 24 hr of culture, while at the same time, almost no CCPE was detected in COCs cultured in FBS-supplemented medium, (Figure 5B).
Perivitelline space
After 6 hr of culture, an extremely narrow perivitelline space (PVS) was detected in the oocytes cultured in FBS-supplemented medium (Figure not shown), while ZP and oolemma were intimately coupled in both serum-free and AOE-supplemented groups leaving no PVS. Following 12 hr of culture, the PVS became wider in FBS-supplemented group, while no detectable space existed between ZP and oolemma in AOE-supplemented and serum-free groups. After 24 hr of culture, wide and extremely uneven PVSs were observed in oocytes matured in serum-free medium (Figure 5G). Their counterparts in FBS-supplemented and AOE-supplemented media showed uniformly wide PVSs, while they were broader in FBS-supplemented group (Figure 5B). Asymmetrical appearance of PVS in serum-free group was accompanied by distorted outline of oocytes and impaired ZP structures (Figure 5G).
Ultra-structure of the oocytes incubated in a serum-free medium for 6 hr showed that microvilli were rare in both size and number with free ending in inner ZP (Figure 5C), and they were still scant after 24 hr (Figure 5G). In FBS-treated oocytes, after 6 hr microvilli were few, while they considerably increased in both size and number after 12 hr. After 24 hr, the microvilli penetrated into the ZP thickness. Oocytes treated with AOE showed very slight microvilli in the thickness of ZP after 6 hr. After 12 hr and 24 hr, the number and size of microvilli increased and were found in the ZP thickness.
Cortical granules
After 6 hr of culture, completely divergent morphologies of granules were observed in the different groups. Multiple granules were observed in FBS-supplemented group, (Figure 5F) with random distribution across ooplasm, while they were small and scanty, and primarily in mid-ooplasmic clusters in AOE-supplemented and serum-free groups. After 12 hr of culture, an increase in overall granular density was observed in all groups.
They were frequently found in cortical region in the oocytes cultured in FBS and AOE-supplemented media, while no recognizable change was observed in their location in oocytes cultured in serum-free medium. Following 24 hr of culture, various conditions of the granules distribution were observed in the different media. Oocytes cultured in serum-free medium showed numerous tiny granules, primarily in mid-ooplasmic areas. Oocytes from FBS-supplemented medium showed relatively large granules in cortical arrangement, and their counterparts from AOE-supplemented group showed relatively large granules without any detectable topographic tendency across the ooplasm, (Figure 5B, E).
Mitochondria
Following 6 hr of culture, mitochondria were extremely small and few in the central region of the oocytes cultured in serum-free medium. In the oocytes grown in FBS-supplemented medium, mitochondria were notably unleashed from their centrally located clusters (Figure 5F). Oocytes from this group were ovoid-shaped and larger than their counterparts in serum-free group (Figure 5C). Mitochondria in oocytes from AOE-supplemented group were piled up at the center of the oocytes and mainly had short, ovoid contour. After 12 hr of culture, no considerable change was detected in the ultra-structure of the mitochondria in either serum-free or FBS-supplemented groups. However, mitochondria were obviously detected larger under AOE-supplemented group, although they were still clustered in the central region of oocytes (Figure 5A). While there were no considerable changes in mitochondrial ultra-structure in oocytes cultured in serum-free medium, their counterparts in FBS-supplemented group were shown larger and gained significant tendency toward peripheral regions. Bulky mitochondria were also observed in the oocytes from AOE-supplemented group. However, they were randomly distributed across the ooplasm (Figures 5A, 5D).
Protein content of activated-omental extract
According to the Bradford dye-binding protein assay, the mean protein content in AOE, FBS-supplemented and serum-free groups was 24.9±1.2, 33.4±0.9 and 11.2±1.4 µg/ml, respectively. One-way ANOVA showed that mean protein content among groups was statistically significant (P-value<0.001). The Tukey’s-HSD post hoc test showed that the protein yield in AOE was significantly lower than those in FBS-supplemented medium (P-value=0. 188).
Discussion
In the present study, the data analysis showed that changes in expansion rate of CCs and nuclear maturation were detected just 24 hr after exposing the COCs to FBS and AOE-supplemented media when compared with serum-free COCs. Despite the fact that the AOE has less protein, it can compete with FBS as a gold standard medium, but nuclear maturation rate is not concomitant with CCs expansion in both FBS and AOE-supplemented media. Degeneration rates in both FBS and AOE-supplemented groups were significantly lower compared to the serum-free group. However, the degeneration rate in AOE-supplemented group is slightly lower than the FBS-supplemented group in nuclear maturation study, which could indicate the adequate growth factors in AOE, although in this study, the exact types and amounts of proteins and growth factors have not been evaluated, which are the main limitations for this study.
Since the morphologic milestones for maturation process are initiated by the expansion of CCs, we postulated that earlier maturation took place in the COCs cultured in AOE-supplemented group. It is well documented that resumption of meiosis in mammalian oocytes is a result of CCs expansion (2, 24). It is documented that interconnection between oocyte GVBD and CCs expansion concisely follows a cyclic pattern: Pre-ovulatory gonadotropin surge triggered oocyte to signal glycosaminoglycans (GAG) synthesis in CCs. The GAG production in CCs, in turn, nullifies the factor that has been keeping oocytes in meiotic arrest (24). In a study performed by Salustri et al. CCs expansion was significantly greater in FBS-supplemented medium. They reported that FBS helps COCs retain hyaluronic acid through unknown mechanisms (25). Although the punctual mechanism underlying expansion of cumulus cell has yet to be unveiled, it may be suggested that a more efficient GAG synthesis takes place in CCs when AOE is added to the maturation medium. However, it may be difficult to determine why the COCs that had an early maturation outbreak yielded approximately the same proportion of matured nucleus after 24 hr of culture as did the FBS- supplemented group. Moreover, it is complicated to explain, despite the higher rate of degeneration in FBS- supplemented and AOE-supplemented group especially, the COCs is enough qualified to resume meiosis.
One of the possible mechanisms of acceleration of IVM is the presence of VEGF in AOE. The maximum amount of VEGF in follicular fluid has been reported in bovine preovulatory oocytes and both oocyte and CCs expresses VEGF receptor (26). It is reported that VEGF increases intracellular glutathione during porcine (27), but not in bovine (26) oocyte maturation, and it is well known that glutathione is needed for the CCs expansion (28) and meiotic spindle maintenance (29). VEGF has also been found to improve oocyte maturation isolated from small size follicle (30), blastocyst formation rate (26), completion of second meiotic division (31) and fertilization rate (32). The presence of VEGF in follicular fluid indicates the critical role of such a growth factor in oocyte maturation (33). Besides, VEGF prevents oocyte apoptosis through inhibitions of caspase-3 activation (34). Also, VEGF can activate MAPK signaling pathway and in this way it can accelerate the formation of the first polar body (34). The other mechanism may be the presence of FGFs in AOE. FGFs also accelerate cumulus cell expansion (35).
In bovine, it has been demonstrated that FGFs are important in final phase of oocyte maturation and FSH induces the CCs to express FGF receptor (36).
Since degeneration rate was higher in AOE-supplemented group compared to FBS-supplemented group during the investigation of CCs expansion, it can be concluded that despite acceleration at the onset of maturation, the AOE-supplemented medium was not potent enough to yield more healthy oocytes in a shorter time-period. This may also be due to the undissolved form of some chemical and molecular components of omental extract or lack of O2 in culture medium (37) in AOE-supplemented group, which was not measured in this study and needs to be described in another study.
The fine structure of oocyte and redistribution of ooplasmic organelles is a determining factor of cytoplasmic maturation (6). In the study performed by Yang et al. extensive complex of CCPEs was reduced by oocyte maturation and as the authors claimed, these projections were withdrawn from ZP following IVM (6). They demonstrated that COCs from all the different media showed extensive CCPEs, suggesting that they possessed pre-experimental homogeneity and also showed that after 6 hr of culture, no detectable change occurred in the morphology of cumulus-oocyte interconnections. This result is in accordance with our records on CCs expansion following 6 hr of culture. Numerous retracted forms of CCPEs were observed in AOE-supplemented group after 24 hr of culture, while almost no CCPE was detected in COCs cultured in FBS-supplemented medium. Considering the CCs expansion records in which COCs were cultured for 24 hr in AOE-supplemented medium, the CCs expansion was started earlier than their counterparts in FBS-supplemented group. It can be concluded that AOE-supplemented medium has probably yielded more expanded forms though a direct effect on CCs intercellular attachments.
Special attention was also paid to morphologic characteristics of PVS. Narrow PVS was detected in the oocytes cultured in FBS-supplemented medium after 6 hr and became wider after 12 hr. These findings suggest that PVS formation had an earlier outbreak in FBS-supplemented medium compared to the other two media. After 24 hr of culture, wide and extremely uneven PVS was observed in oocytes matured in serum-free medium. Since the irregular appearance of PVS in serum-free group was accompanied by distorted outline of oocytes and impaired ZP structures, and also with an obviously apoptotic cumuli complex, all of these can be acknowledged as degenerative consequences in this group of oocytes.
In the present study, oocytes from different culture media were similarly poor in their surface microvilli density after 6 hr of culture. Following 12 hr of culture, an increase in both size and number of microvilli was observed in FBS and AOE-supplemented groups. Following 24 hr of culture, retracted microvilli were recognized in FBS-supplemented group by their free endings in ZP thickness. It can be concluded that mature form of microvilli was achieved in oocytes cultured in FBS-supplemented group, while it was not gained in AOE-supplemented group after 24 hr of culture.
The dynamics of secretory granules have always been an issue of interest among reproductive biologists. In rat and mouse, CGs are first observed in the unilaminar follicle, whereas in the human, monkey, hamsters, and rabbit, they first appear in multilayered follicles. During the early stages of follicular growth, Golgi complexes swell and proliferate, and the CGs dispatch from Golgi complexes at this stage (38). These organelles are important not only for oocytes maturation assessment but also for post-fertilization changes in ZP and prevention of polyspermy. Liu in a detailed review on the dynamics of these organelles in mammalian oocytes confirmed that “the migration of CGs is an important step in cytoplasmic maturation and has been used routinely as a criterion in assessing the maturity and organelle organization of developing oocytes” (38). In the present study, secretory granules were meticulously pursued and recorded as one of the determining factors of cytoplasmic maturation. After 6 hr of culture, completely divergent morphologies were observed in the different groups. After 12 hr of culture, an increase in overall granular density was observed in all groups. Following 24 hr of culture, various conditions of granules distribution were observed in the different media. Oocytes from FBS-supplemented medium showed relatively large granules in cortical arrangement and their counterparts from AOE-supplemented group showed relatively large granules without any detectable topographic tendency across ooplasm. It can be concluded that, for unknown reasons, the desired cortical rearrangement did not occur in AOE-supplemented medium. Despite the larger growth of CGs that indicated the progress of oocyte maturation, their translocation did not occur properly that caused infertile oocyte.
In the mammalian oocyte, mitochondria are believed to grow large and move outward during oocyte maturation (6). Since there are numerous similar reports on mitochondrial redistribution and its role in determining oocytes cytoplasmic maturation, we also followed changes in the fine structure of mitochondria in the present study. After 6 hr of culture, in the oocytes belonging to FBS-supplemented medium mitochondria were notably unleashed from their centrally located clusters. The mitochondria in oocytes from AOE-supplemented group were piled up at the center of the oocytes. After 12 hr of culture, mitochondria obviously grew larger in AOE-supplemented group although they were still clustered in central region of oocytes. Their counterparts in FBS-supplemented group grew larger and gained significant tendency toward peripheral regions. Bulky mitochondria were also observed in the oocytes from AOE-supplemented group. However, they were randomly distributed across the ooplasm. From these findings, it can be deduced that mitochondria were efficient in oocytes from AOE-supplemented medium but for unknown reasons translocation was defective or at least delayed.
The limitations of this study include the lack of using VEGF as a positive control to find whether the beneficial effects of the extract were due to the presence of such a growth factor, and also lack of measuring especial growth factors such as VEGF in the AOE.
Conclusion
Comparing to negative control, AOE-supplemented medium was potent enough to induce CCs expansion, resume meiosis, change the mitochondria distribution pattern and increase the number of microvilli within PVS after 24 hr. However, in the same period of time, the degeneration rate was higher in AOE-compared to FBS-supplemented medium. The overall ooplasmic architecture shows that cross-ooplasmic transportation did not occur properly in AOE-supplemented group. However, the crude AOE was not enough to induce in vitro oocyte maturation. This is a preliminary study that focused on the assessment of the role of AOE on in vitro oocyte maturation. Therefore, to overcome its limitations, it is worthy to continue studies on the potentials of growth factors and proteins of AOE, in order to optimize its composition and suggest AOE as a supplementation factor for culture media.
Acknowledgment
This paper is the result of research project [number 85-2824] of an MSc student thesis. The authors would like to thank the Vice Chancellery for Research of Shiraz University of Medical Sciences for financial support, and Mrs. Roohangeez Jafarpour for the electron microscopy technique and Mr Izad Noori for performing the histological procedure.
Conflict of interest
There is no conflict of interest that could be declared.
References
- 1.Mesbah F, Moslem M, Vojdani Z, Mirkhani H. Does metformin improve in vitro maturation and ultrastructure of oocytes retrieved from estradiol valerate polycystic ovary syndrome-induced rats. J Ovarian Res. 2015;8:874–880. doi: 10.1186/s13048-015-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Stetina JR, Orr-Weaver TL. Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb Perspect Biol. 2011;3:a005553. doi: 10.1101/cshperspect.a005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards RG. Maturation in vitro of human ovarian oocytes. Lancet. 1965;2:926–929. doi: 10.1016/s0140-6736(65)92903-x. [DOI] [PubMed] [Google Scholar]
- 4.Ellenbogen A, Shavit T, Shalom-Paz E. IVM results are comparable and may have advantages over standard IVF. Facts View Vis Obgyn. 2014;6:77–80. [PMC free article] [PubMed] [Google Scholar]
- 5.Coticchio G, Dal Canto M, Fadini R, Mignini Renzini M, Guglielmo MC, Miglietta S, et al. Ultrastructure of human oocytes after in vitro maturation. Mol Hum Reprod. 2016;22:110–118. doi: 10.1093/molehr/gav071. [DOI] [PubMed] [Google Scholar]
- 6.Yang YJ, Zhang YJ, Li Y. Ultrastructure of human oocytes of different maturity stages and the alteration during in vitro maturation. Fertil Steril. 2009;92:396e1–6. doi: 10.1016/j.fertnstert.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Zhou P, Luo H, Zhao Y, Shi G. The effect of VEGF on the temporal-spatial change of alpha-tubulin and cortical granules of ovine oocytes matured in vitro. Anim Reprod Sci. 2009;113:236–250. doi: 10.1016/j.anireprosci.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Rated M, Ketoja E, Pitkanen T, Ahola V, Kananen K, Peippo J. In vitro maturation supplements affect developmental competence of bovine cumulus-oocyte complexes and embryo quality after vitrification. Cryobiology. 2011;63:245–255. doi: 10.1016/j.cryobiol.2011.09.134. [DOI] [PubMed] [Google Scholar]
- 9.Marchal R, Caillaud M, Martoriati A, Gérard N, Mermillod P, Goudet G. Effect of growth hormone (GH) on in vitro nuclear and cytoplasmic oocyte maturation, cumulus expansion, hyaluronan synthases, and connexins 32 and 43 expression, and gh receptor messenger RNA expression in equine and porcine species. Biol Reprod. 2009;69:1013–1022. doi: 10.1095/biolreprod.103.015602. [DOI] [PubMed] [Google Scholar]
- 10.Abedelahi A, Salehnia M, Allameh AA. The effects of different concentrations of sodium selenite on the in vitro maturation of preantral follicles in serum-free and serum supplemented media. J Assist Reprod Genet. 2008;25:483–488. doi: 10.1007/s10815-008-9252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkanan JR, Kaila V, Mannerström B, Raty S, Kuokkanen H, Miettinen S, Ylikomi T. Human adipose tissue extract induces angiogenesis and adipogenesis in vitro. Tissue Eng Part A. 2012;18:17–25. doi: 10.1089/ten.TEA.2010.0712. [DOI] [PubMed] [Google Scholar]
- 12.Shah S, Lowery E, Braun RK, Martin A, Huang N, Medina M, et al. iCellular basis of tissue regeneration by omentum. PLoS One. 2012;7:e38368. doi: 10.1371/journal.pone.0038368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Li C, Deng G, Xu X, Shu L, Liu X, et al. Value of the pedicle omentum transfer for the healing of large skin wound in dog. Int J Appl Res Vet Med. 2012;10:300–304. [Google Scholar]
- 14.Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JAL, Dunea G, Singh AK. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res. 2007;328:487–497. doi: 10.1007/s00441-006-0356-4. [DOI] [PubMed] [Google Scholar]
- 15.Karimi I, Bigham-Sadegh A, Oryan A. Concurrent use of greater omentum with Persian Gulf coral on bone healing in dog: a radiological and histopathological study. Iran J Vet Sur. 2013;8:35–42. [Google Scholar]
- 16.Porzionato A, Sfriso MM, Macchi V, Rambaldo A, Lago G, Lancerotto L, et al. Decellularized omentum as novel biologic scaffold for reconstructive surgery and regenerative medicine. Eur J Histochem. 2013;57:e4. doi: 10.4081/ejh.2013.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XW, Tao WU, Zou H, Kun W, Song-Quan H. Effect of adipose tissue extract of greater omentum on human fibroblasts in vitro. Chin J Hepat Sur. 2011;17:261–263. [Google Scholar]
- 18.Araujo VR, Silva MG, Duarte AB, Magalhaes DM, Almeida AP, Goncalves RF, et al. Vascular endothelial growth factor-A165 (VEGF-A) stimulates the in vitro development and oocyte competence of goat preantral follicles. Cell Tissue Res. 2011;346:273–281. doi: 10.1007/s00441-011-1251-1. [DOI] [PubMed] [Google Scholar]
- 19.Eimani H, Tahaei LS, Parivar KA, Rezazadeh MO, Kazemi S, Shahverdi A, et al. Effect of ethanol on maturation and development of immatur mouse oocytes. J Iran Anatom Sci. 2007;4:367–376. [Google Scholar]
- 20.Puri G, Chaudhary SS, Singh VK, Sharma AK. Effects of fetal bovine serum and estrus buffalo serum on maturation of buffalo (Bubalus bubalis) oocytes in vitro. Vet World. 2015;8:143–146. doi: 10.14202/vetworld.2015.143-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford MM. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Neri T, Monti M, Rebuzzini P, Merico V, Garagna S, Redi CA, et al. Mouse fibroblasts are reprogrammed to Oct-4 and Rex-1 gene expresion and alkaline phosphatase activity by embryonic stem cell extracts. Cloning Stem Cells. 2007;9:394–406. doi: 10.1089/clo.2006.0011. [DOI] [PubMed] [Google Scholar]
- 23.Varnosfaderani SR, Ostadhosseini S, Hajian M, Hosseini SM, Khashouei EA, Abbasi H, et al. Importance of the GDF9 signaling pathway on cumulus cell expansion and oocyte competency in sheep. Theriogenology. 2013;80:470–478. doi: 10.1016/j.theriogenology.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Assidi M, Dieleman SJ, Sirard MA. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: potential early markers of oocyte competence. Reproduction. 2010;140:835–852. doi: 10.1530/REP-10-0248. [DOI] [PubMed] [Google Scholar]
- 25.Salustri A, Yanagishita M, Hascall VC. Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH-stimulated cumulus cells. Devel Biol. 1990;138:26–32. doi: 10.1016/0012-1606(90)90173-g. [DOI] [PubMed] [Google Scholar]
- 26.Anchordoquy JM, Anchordoquy JP, Testa JA, Sirini MÁ, Furnus CC. Influence of vascular endothelial growth factor and Cysteamine on in vitro bovine oocyte maturation and subsequent embryo development. Cell Biol Int. 2015;39:1090–1098. doi: 10.1002/cbin.10481. [DOI] [PubMed] [Google Scholar]
- 27.Dibyendu B, Jeon YB, Kim G, Jeung E, Hyun S. Effect of vascular endothelial growth factor on in vitro porcine oocyte maturation and subsequent developmental competence after parthenogenesis. J Anim Vet Adv. 2010;9:2924–2931. [Google Scholar]
- 28.Nagyova E. Regulation of cumulus expansion and hyaluronan synthesis in porcine oocyte-cumulus complexes during in vitro maturation. Endocr Regul. 2012;46:225–235. doi: 10.4149/endo_2012_04_225. [DOI] [PubMed] [Google Scholar]
- 29.Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev. 2003;64:106–112. doi: 10.1002/mrd.10214. [DOI] [PubMed] [Google Scholar]
- 30.Bui TM, Nguyên KX, Karata A, Ferré P, Trén MT, Wakai T, et al. Presence of vascular endothelial growth factor during the first half of IVM improves the meiotic and developmental competence of porcine oocytes from small follicles. Reprod Fertil Dev. 2017;29:1902–1909. doi: 10.1071/RD16321. [DOI] [PubMed] [Google Scholar]
- 31.Yan L, Luo H, Gao X, Liu K, Zhang Y. Vascular endothelial growth factor-induced expression of its receptors and activation of the MAPK signaling pathway during ovine oocyte maturation in vitro. Theriogenology. 2012;78:1350–1360. doi: 10.1016/j.theriogenology.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Biswas D, Hyun SH. Supplementation with vascular endothelial growth factor during in vitro maturation of porcine cumulus oocyte complexes and subsequent developmental competence after in vitro fertilization. Theriogenology. 2011;76:153–160. doi: 10.1016/j.theriogenology.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Ntala V, Asimakopoulos B, Veletza S, Alexandris E, Karachaiou V, Nikolettos N. Follicular fluid levels of vascular endothelial growth factor and its receptors and pregnancy outcome of women participating in intracytoplasmic sperm injection cycles. Clin Exp Obstet Gynecol. 2015;42:437–441. [PubMed] [Google Scholar]
- 34.Kere M, Siriboon C, Liao JW, Lo NW, Chiang HI, Fan YK, et al. Vascular endothelial growth factor A improves quality of matured porcine oocytes and developing parthenotes. Domest Anim Endocrinol. 2014;49:60–69. doi: 10.1016/j.domaniend.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Machado MF, Caixeta ES, Sudiman J, Gilchrist RB, Thompson JG, Lima PF, et al. Fibroblast growth factor 17 and bone morphogenetic protein 15 enhance cumulus expansion and improve quality of in vitro-produced embryos in cattle. Theriogenology. 2015;84:390–398. doi: 10.1016/j.theriogenology.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 36.Zhang K, Ealy AD. Disruption of fibroblast growth factor receptor signaling in bovine cumulus-oocyte complexes during in vitro maturation reduces subsequent embryonic development. Domest Anim Endocrinol. 2012;42:230–238. doi: 10.1016/j.domaniend.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JG, Brown HM, Kind KL, Russell DL. The ovarian antral follicle: living on the edge of hypoxia or not? Biol Reprod. 2015;92:153. doi: 10.1095/biolreprod.115.128660. [DOI] [PubMed] [Google Scholar]
- 38.Liu M. The biology and dynamics of mammalian cortical granules. Reprod Biol Endocrinol. 2011;9:149. doi: 10.1186/1477-7827-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]


