Abstract
Objective(s):
Apoptotic effect of apoptin has been demonstrated in numerous studies. However, its tumor specificity has been questioned by some reports. The aim of this study was to confine the expression of apoptin in the prostate tumor cells by inducing its gene expression under the control of a chimeric enhancer composing of prostate-specific membrane antigen (PSMA) and prostate-specific antigen (PSA) regulatory elements (PSES). Furthermore, we investigated the effects of apoptin expression on LNCaP prostate carcinoma cell survival and apoptosis using MTT assay and annexin V/7-AAD flow cytometry assay.
Materials and Methods:
Recombinant plasmids containing apoptin gene under the control of PSES/PSA promoter or Cytomegalovirus (CMV) promoter were constructed. Tumor cell lines including LNCaP cells and HeLa cells, and LX-2 cells (as a normal control) were transfected with the plasmids and the expression of apoptin was evaluated by real time-PCR and western blot analyses. The effects of apoptin expression on cell survival and apoptosis were then investigated using MTT and annexinV/7-AAD flow cytometry assay, respectively.
Results:
Western blot and real time PCR analyses confirmed the specific expression of apoptin under the control of PSES/PSA regulatory element in the LNCaP cells, while CMV promoter caused apoptin expression in both tumor and normal cell lines. Apoptin expression significantly increased cell death and apoptosis in tumor cells when compared with the normal cells (P<0.001).
Conclusion:
These results suggest that PSES/PSA regulatory element may be considered as an efficient approach for specific expression of apoptin gene in prostate tumor cells and treatment of prostate cancer.
Keywords: Apoptin protein, Apoptosis, Prostate-specific antigen, Prostate-specific-membrane antigen Prostate cancer
Introduction
Prostate carcinoma (PCa) is among the leading causes of cancer deaths in men worldwide. The incidence of PCa has been increased in recent years, which may be due to changes in human life style, environmental factors, and population aging (1). The common therapeutic modalities that are currently available for the treatment of PCa include radical prostatectomy, radiotherapy, androgen deprivation, or a combination of them (2, 3). Because of some limitations of these traditional approaches, other strategies including immunotherapy and gene therapy are being considered as complementary therapies (4). Impaired cellular apoptosis is a characteristic of many tumors including PCa. Thus, gene therapy in which the increased expression of apoptotic protein or destruction of anti-apoptotic proteins is targeted has been received great attention in treatment of PCa (5). However, increase in tumor specificity in which the killing effects of the transduced gene confine to tumor cells, still remains a major challenges in this area. Use of tumor specific promoters and enhancers that allow the specific expression of apoptotic genes in tumor cells could be an effective approach to overcome this problem (6).
Apoptin (VP3) is a chicken anemia virus (CAV) protein with 121 amino acids, which has a significant apoptotic activity in various tumor cell lines including glioblastoma (7), breast (8), lung (9), leukemia (10), osteosarcoma (11) , hepatoma (12), and bladder cancer cells (13). P53 independency, bcl2 insensitivity, and tumor specificity are among major desirable features of apoptin that make it an ideal protein for cancer gene therapy (12). Numerous studies have shown that apoptin specifically induced apoptosis in tumor cells while it did not induce apoptosis in normal cells. This specific effect is due to nuclear localization of apoptin in tumor cells, which is determined by its phosphorylated status. Phosphorylation of apoptin at Thr 108 by Akt –activated cyclin-dependent kinase2 and protein kinase C leads to accumulation of apoptin in the nuclei of the tumor cells, an event that does not occur in normal cells (14). In normal cells, unphosphorylated apoptin remains in the cytoplasm where it is aggregated and degraded subsequently. However, He et al. (15) demonstrated that adenovirus-mediated expression of apoptin in normal cells caused G2-M cell cycle arrest and chromatin condensation, which did not correlate with its nuclear localization. Furthermore, the ability of apoptin to accumulate in the nuclear matrix and triggering the apoptosis in a number of normal cells have been demonstrated (16). So, it seems that specialized expression of apoptin in tumor cells and inhibition of its expression in normal cells is a favorable goal that needs more consideration. To this end, we used prostate-specific antigen (PSA) regulatory elements (PSES)-pAdenoVator-PSA-Apoptin-IRES-GFP recombinant plasmid carrying the apoptin gene under the control of PSA promoter and PSES for specific expression of apoptin in prostate tumor cells. PSES is a chimeric enhancer composed of Arc3 elements of PSA enhancer and del2 fragment of prostate-specific membrane antigen (PSMA) enhancer. Prostate specificity and potent androgen-independent promoter activity of PSES has been demonstrated in previous studies (17). In our previous study, this plasmid was used for specific expression of N-myc downstream regulated protein (NDRG2) tumor suppressor gene in prostate cancer cell lines (18). We showed that it led to specific expression of NDRG2 in prostate cancer cell lines. The aim of current investigation was to use this recombinant plasmid for the specific expression of apoptin gene in prostate cancer cell line and to evaluate its effects on apoptosis of tumor cells.
Materials and Methods
Cells and cell culture
Human prostatic adenocarcinoma cell line LNCaP cells and human cervical cancer cell line HeLa cells (Pasture Institute, Tehran, Iran) were routinely grown in RPMI 1640 medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 µg/ml streptomycin. LX-2 cells (a normal stellate cell line) were cultured in DMEM medium supplemented with 5% FBS. All cell lines were cultured at 37 °C and 5% CO2.
Construction of plasmids
PSES-pAdenoVator-PSA-IRES-GFP plasmid carrying PSA promoter and PSES chimeric enhancer was used to construct apoptin expressing plasmid (Figure 1). Briefly, CAV DNA was extracted from commercial vaccines (AviPro THYMOVAC™) using a DNA extraction Kit (Cinnagen Inc. Iran), according to the manufacturer’s instructions. PCR amplification of the apoptin (VP3) gene was performed using forward primer: 5´-CTAGTCTAG AATGAACGCTCTCCAAGAAGAT-3´ and reverse primer: 5´-TATATGAGATCTTTACAGTCTTATA CGCCTTTTTGC-3´. The expected product size was 389 bp containing the complete apoptin open reading frame and restriction sites for Xba I and Bgl II (underlined in the respective sequences). PCR-product was purified from the gel using a gel-extraction kit (QIAex II, Qiagen) and was used for cloning. Apoptin fragment was digested with Xba I and Bgl II and cloned into the PSES-pAdenoVator-PSA-IRES-GFP vector using T4 DNA ligase according to the manufacturers’ protocol. Correct construction of the recombinant plasmid was confirmed by colony PCR assay, restriction enzyme analysis (Xba I/Bgl II double digestion), and sequencing. pAdenoVator-CMV-Apoptin-IRES-GFP plasmid in which apoptin expression is under the control of Cytomegalovirus (CMV) promoter was constructed similarly by cloning of apoptin into pAdenoVator-CMV-IRES-GFP plasmid.
Figure 1.
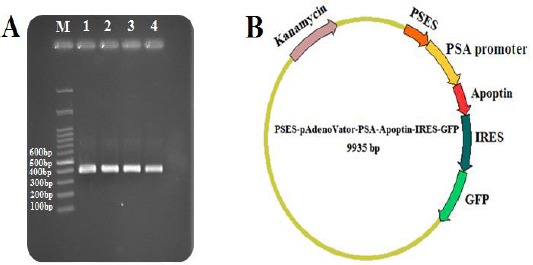
Construction of the PSES-pAdenoVator-PSA-Apoptin-IRES-GFP plasmid. A: PCR amplification of apoptin gene showed expected size (389 bp) bands on the gel (Lane 1-4), M: Molecular weight markers (100 bp). B: Schematic diagram representing PSES-pAdenoVator-PSA-Apoptin-IRES-GFP plasmid. Apoptin and GFP genes expression were conducted under the control of the PSES/PSA specific regulatory elements
Transfection
Cell lines were transfected with PSES-pAdenoVator-PSA-VP3-IRES-GFP, pAdenoVator-CMV-VP3-IRES-GFP, and control plasmids including PSES-pAdenoVator-PSA-IRES-GFP and pAdeno Vator-CMV-IRES-GFP. Transfection was carried out using lipofectamin 3000™ (Invitrogen Inc.), according to the manufacturer’s instructions. Briefly, 100×103 HeLa, and 200×103 LNCaP cells were plated into 6-well plates 48 hr before transfection. When the cells reached to 60% to 80% confluency, 2.5 μg of DNA was added to 125 μl of Opti-MEM medium and 5 μl of P3000 reagent, followed by adding 125 μl of Opti-MEM containing 7.5 μl of Lipofectamine 3000. The mixture was incubated at room temperature for 5 min and then added to the cells. At 72 hr after transfection, the expression of GFP was evaluated using fluorescence microscopy.
Reverse transcription polymerase chain reaction (RT-PCR) and quantitative real-time PCR
The expression of apoptin in different groups was assessed using RT-PCR and real-time PCR. In brief, total RNA was isolated with RNA-X plus reagent (Cinnagen) using instructions provided by the manufacture. RNA concentrations were determined by NanoDrop and cDNA was synthesized using cDNA synthesis kit (Takara, Japan). RT-PCR was performed for apoptin and β-Actin (internal control) using specific primers [Apoptin (230 bp) forward:
5’-CGGATTGGTATCGCTGGAAT-3’; reverse: 5’-GCGGCTGGGAGTAGTGGTAAC-3’; β-Actin (260 bp) forward: 5´-GCCTCGCTGTCCACCTTCCA-3´ reverse:
5´-CTGCTGTCACCTTCACCGTTCCA-3´]. SYBR1 Premix Ex Taq (Takara, Japan) reaction mixtures were prepared according to recommended protocol. Amplification reaction was performed in a 40 cycles of 5 sec at 95 °C for denaturation and 30 sec at 60 °C for the annealing step. The mRNA levels obtained for apoptin were normalized by mRNA level of β-Actin.
SDS-PAGE and western blotting
Expression of apoptin in LNCaP cells transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP was confirmed by western blot analysis using a specific antibody against apoptin. In brief, transfected and non-transfected LNCaP cells were harvested and disrupted using sonication. Proteins concentration of each sample was determined using Bradford method and the samples were then stored at -70 °C until used. The samples mixed with 5x Laemmli buffer and boiled at 100°C for 5 min then loaded onto 13% SDS–PAGE gel, separated by electrophoresis, and then transferred to a PVDF membrane using the semi-dry protocol (Biorad Inc.) at 15 V in 70 min. Immunoblotting was performed with anti - apoptin polyclonal antibody (ab193615; Abcam, Canada). Detection of GAPDH (sc-47724 Santa Cruz) was used as an internal control. A horseradish peroxidase-conjugated anti-rabbit secondary antibody (Sigma, USA) and chemiluminescence substrates (ECL; Amersham BioscienceAB) were used to detect the immuno-labeled bands.
MTT assay
MTT assay was used to investigate the effect of apoptin expression on the viability of LNCaP cells. In brief, 72 hr after transfection, 20 μl of 5 mg/ml of MTT solution (Sigma inc) was added to each well and incubated for 4 hr at 37 °C and 5% CO2. The media containing MTT was discarded. Then, 150 μl of DMSO was added to dissolve the formazan crystals in each well. Absorbance was then measured at 492 nm by ELISA reader instrument.
Flow cytometery
The effect of apoptin expression on the apoptosis of LNCaP cells was assessed by PE annexin V/7-AAD (BD Biosciences, USA) flow cytometric method. In berif, LNCaP cells were harvested at 72 hr post-transfection using 0.25% trypsin solution, centrifuged at 1500 rpm for 5 min and the pellet was resuspended in 500 μl of PBS. The cells were resuspended in annexin V binding buffer, and stained with annexin V and 7-AAD for 15 min at room temperature in the dark. Apoptosis was detected using FACSCalibur flow cytometer and the data were analyzed with flowjo software.
Statistical analysis
SPSS software (Version 15) was applied for analysis of data. Data were presented as mean±SD. One-way analysis of variance (ANOVA) followed by LSD post hoc test was used to determine statistically significant differences between different groups. P<0.05 was considered as significant statistical difference.
Results
Construction of the PSES-pAdenoVator-PSA-Apoptin-IRES-GFP
Apoptin gene (389 bp) was amplified (Figure 1A), and inserted into downstream of PSA promoter to construct PSES-pAdenoVator-PSA-Apoptin-IRES-GFP plasmid (Figure 1B). pAdenoVator-CMV-Apoptin-IRES-GFP was constructed similarly, while apoptin gene was inserted downstream of CMV promoter. The construction of both plasmid was confirmed by colony PCR and sequencing.
Prostate specificity of apoptin expression by PSES-pAdenoVator-PSA-Apoptin-IRES-GFP plasmid
Prostate specificity of PSES/PSA regulatory elements was first confirmed using fluorescence microscopy for the expression of GFP in LNCaP cells transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP and pAdenoVator-CMV-Apoptin-IRES-GFP plasmids. As can be observed in Figure 2, a high level of GFP expression was detected in LNCaP and HeLa cells after transfection with pAdenoVator-CMV-Apoptin-IRES-GFP plasmid (Figure 2A, and B, respectively), while transfection with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP resulted in specific expression of GFP in LNCaP cells (Figure 2C) but not in HeLa cells (Figure 2D). Specific expression of apoptin under control of PSES/PSA regulatory elements in LNCaP cells was also tested using RT-PCR and western blot analyses. RT-PCR results revealed specific expression of apoptin gene (230 bp) under control of PSA promoter and chimeric enhancer. The agarose gel electrophoresis showed a specific band of 230 bp in the PSES-pAdenoVator-PSA-Apoptin-IRES-GFP transfected LNCaP cell lines whereas non-prostatic control cell line (HeLa cells) did not show such band, indicating the specificity of apoptin expression under control of PSA promoter (Figure 3A). To confirm the presence of apoptin protein, protein extracts from the LNCaP cells transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP were subjected to western blot analyses. As can be observed in Figure 3B, apoptin protein (13.5 KDa) is expressed in LNCaP cell that transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP.
Figure 2.
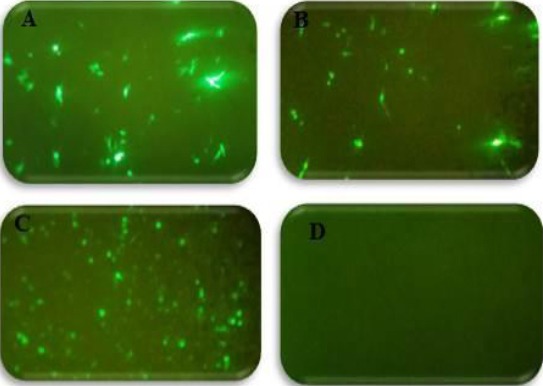
Prostate specificity of PSES/PSA regulatory elements using fluorescence microscopy for the expression of GFP. GFP was expressed in LNCaP cells (A) and HeLa cells (B) transfected with pAdenoVator-CMV-Apoptin-IRES-GFP and also in LNCaP cells transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP (C) while, HeLa cells transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP did not demonstrate GFP expression (D)
Figure 3.
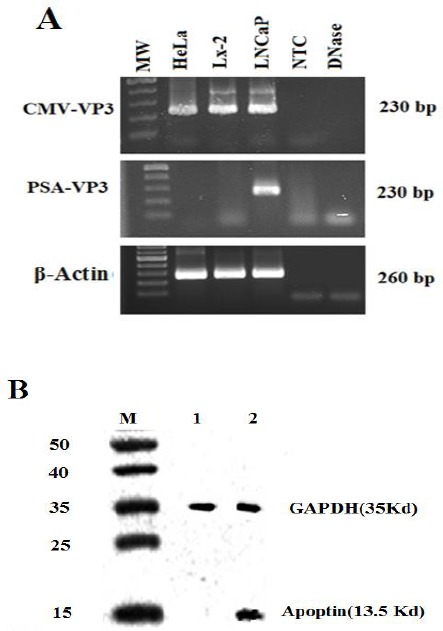
Specific expression of apoptin in LNCaP cells transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP. A: RT-PCR performed with apoptin-specific primers showed that apoptin was expressed in LNCaP cells that transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP, whereas the non-prostatic cell lines (LX-2 and HeLa cell lines) were negative for apoptin expression, indicating by absence of 230 bp band. β-actin expression was used as a reference gene in each sample. B: Western blot showed the presence of apoptin (13.5 KDa) in LNCaP cells transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP (Lane 2) but not in non-transfected cells (Lane 1). GAPDH expression was used as an internal control in each sample. M indicates the protein molecular weight marker (15-50 KDa)
Effects of apoptin expression on cell viability using MTT assay
To investigate the effects of apoptin expression on cell viability, LNCaP cells were transfected with mock plasmid, pAdenoVator-CMV-Apoptin-IRES-GFP (CMV-APO), and PSES-pAdenoVator-PSA-Apoptin-IRES-GFP (PSA-APO) plasmids, and cell viability was determined 72 hr after plasmid transfection using MTT assay. As shown in Figure 4, cell viability was significantly decreased (P<0.001) in the cells transfected with both apoptin containing plasmids compared to control and mock plasmid-transfected cells.
Figure 4.
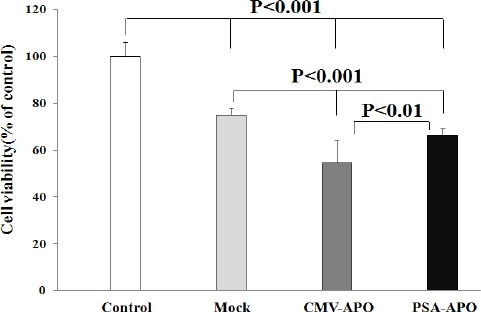
Effects of apoptin expression on cell viability using MTT assay. LNCaP cells were transfected with mock plasmid (mock), pAdenoVator-CMV-Apoptin-IRES-GFP (CMV-APO), and PSES-pAdenoVator-PSA-Apoptin-IRES-GFP (PSA-APO) plasmids and cell viability was determined 72 hr after plasmid transfection using MTT assay. Data were analyzed using one way ANOVA followed by LSD post hoc test
Quantitative analyses of apoptosis by flow cytometry
To quantify the effects of apoptin expression on apoptosis of normal and tumoral cells, LX-2 and HeLa cells were transfected with pAdenoVator-CMV-Apoptin-IRES-GFP or mock plasmids and then apoptosis was measured using annexin V/7AAD flow cytometric quantitative method. As can be observed in Figure 5B, transfection of LX-2 cells with pAdenoVator-CMV-Apoptin-IRES-GFP had no significant effect on cell apoptosis compared to the cells transfected with mock plasmid (Figure 5A). However, the transfection of HeLa cells with pAdenoVator-CMV-Apoptin-IRES-GFP (Figure 5D) significantly increased cell apoptosis compared to the cells transfected with mock plasmid (Figure 5C). Figure 6 compared the effects of apoptin expression on apoptosis of LNCaP cells transfected with mock plasmid (as a control), pAdenoVator-CMV-Apoptin-IRES-GFP, and PSES-pAdenoVator-PSA-Apoptin-IRES-GFP plasmids. As depicted in dot blot flow charts and histogram (Figure 6D), apoptin expression in the cells transfected by both CMV and PSA promoters significantly increased apoptosis compared to the cells transfected by mock plasmid (P<0.001).
Figure 5.
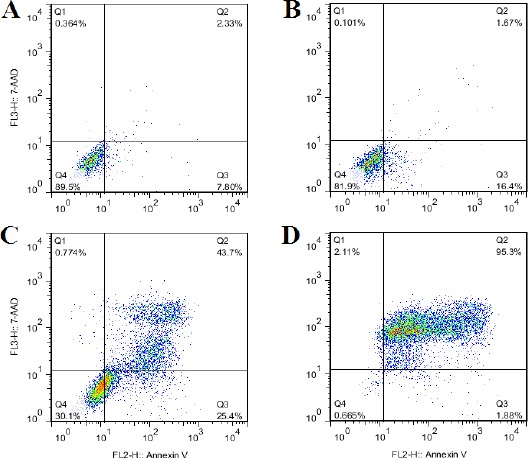
Effects of apoptin expression on apoptosis of LX-2 and HeLa cells transfected with pAdenoVator-CMV-VP3-IRES-GFP or mock plasmids using annexin V/7AAD quantitative flow cytometric analyses. A: LX-2 cells transfected with mock plasmid (pAdenoVator-CMV-IRES-GFP; as a control), B: LX-2 cells transfected with pAdenoVator-CMV-Apoptin-IRES-GFP, C: HeLa cells transfected with mock plasmid (pAdenoVator-CMV-IRES-GFP; as a control), D: HeLa cells transfected with pAdenoVator-CMV-Apoptin-IRES-GFP. Q1, Q2, Q3, and Q4 show necrotic, late apoptotic, early apoptotic, and viable cells, respectively
Figure 6.
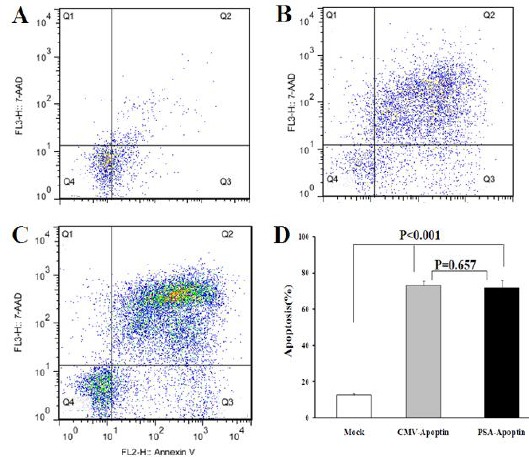
Effects of apoptin expression on the apoptosis of LNCaP cells using quantitative annexin V/7AAD flow cytometric analyses. A: LNCaP cells transfected with mock plasmid (PSES-pAdenoVator-PSA-IRES-GFP; as a control), B: LNCaP cells transfected with pAdenoVator-CMV-Apoptin-IRES-GFP, C: LNCaP cells transfected with PSES-pAdenoVator-PSA-Apoptin-IRES-GFP. The represented data are mean ± SD of at least two independent experiments. Data were analyzed using one way ANOVA followed by LSD post hoc test. Q1, Q2, Q3, and Q4 show necrotic, late apoptotic, early apoptotic, and viable cells, respectively
Discussion
Due to population aging and limitations of current therapies, PCa is continued to be a major cause of cancer-related deaths in men (1). Similar to many kinds of cancers, defects in apoptotic pathways are important characteristics of PCa. Therefore, gene therapeutic modalities that induced apoptosis proposed as an effective therapy for PCa (5). However, safety of such therapeutic modalities still stays an important concern in this context. Apoptin is often known as a tumor specific apoptotic protein, which induces apoptosis in malignant cells independent of their cellular status of P53 and Bcl2. However, some researchers have raised doubts about the tumor specificity of apoptin since the killing effects of this protein has been shown in some normal cells (16), which can hamper its application in cancer gene therapy. One of the strategies commonly applied to restrict the expression of a tumor killing gene in tumor cells and thereby increase its tumor specificity, is to place the expression of the gene under the control of tumor-specific promoters (19). In an effort to confine the expression and effects of apoptin in tumor cells, Qi et al. constructed an adenoviral vector in which the expression of apoptin was controlled by tumor specific human telomerase reverse transcriptase (hTERT) promoter and showed the specific expression and apoptotic effects of apoptin in tumor cells after transfection with this recombinant vector, while the transfection of normal cells did not lead to the expression of apoptin in these cells (20).
PSA and PSMA are among proteins that specifically expressed in prostate cancer cells. The promoters of PSA and PMSA genes displayed prostate cell specificity and used to restrict expression of several genes in prostate cells. Lee et al made an artificial regulatory element (PSES) composed of del2 fragment of PMSA and Arc3 fragment of PSA and checked its prostate tumor specificity (17). They showed that this promoter was able to induce specific expression of GFP in the prostate cell line significantly higher than CMV promoter. We constructed a plasmid composed of PSES and PSA promoter in our previous study and used it for the specific expression of NDRG2 tumor suppressor gene in prostate tumor cells (18). In the current study, we used the same plasmid to express apoptin gene under the control of PSES and PSA promoter. Our results showed the expression of apoptin gene in the LNCaP cells after transfection with this plasmid, while normal and non-prostate tumor cells could not express apoptin under same condition. In contrast, apoptin gene under the control of CMV promoter was expressed in both prostate and non-prostate tumor cells, suggesting tumor specificity of PSES-PSA regulatory elements for LNCaP cells. Furthermore, the results of MTT assay showed that transfection of LNCaP cells with PSA-Apoptin or CMV-Apoptin plasmids reduced the survival of LNCaP cells significantly compared to mock-transfected cells, indicating the effects of apoptin in the reduction of tumor cell survival. 7AAD/annexin V flow cytometric apoptosis assays demonstrated that transfection of LX-2 with the CMV-Apoptin plasmids could not induce apoptosis of these cells, while increased apoptosis of Hela cells significantly. Consistent with the results of MTT assay, flow cytometric apoptosis assays revealed that PSA-Apoptin plasmid was as strong as CMV-Apoptin plasmid and significantly induced apoptosis of LNCaP cells. The exact mechanisms of the apoptotic effects of apoptin still remain unknown. The binding of apoptin into cellular DNA that may lead to breakage of cellular DNA (21), the interaction with anaphase-promoting complex/cyclosome (APC/C) and inhibition of its activity (22), and induction of G2/M arrest are among suggested mechanisms (23).
Conclusion
Taken together, the findings of our study indicated that PSES and PSA regulatory elements caused specific expression of apoptin, which was associated with the reduction of prostate cancer cell line survival through induction of apoptosis. These results suggest the beneficial effects of gene therapy with apoptin under the control of prostate specific elements for prostate cancer therapy. Further in vivo studies are needed to confirm the results of this in vitro study.
Acknowledgment
This manuscript was extracted from the MSc thesis of Vida Mohammadi. It was supported by grant number 94-01-10-9116 from the Vice-Chancellor for Research Affairs of Shiraz University of Medical sciences, Shiraz, Iran. We are also grateful to all staff of Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical sciences, Shiraz, Iran for their technical assistance.
References
- 1.Hassanipour-Azgomi S, Mohammadian-Hafshejani A, Ghoncheh M, Towhidi F, Jamehshorani S, Salehiniya H. Incidence and mortality of prostate cancer and their relationship with the Human Development Index worldwide. Prostate Int. 2016;4:118–124. doi: 10.1016/j.prnil.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merseburger AS, Alcaraz A, von Klot CA. Androgen deprivation therapy as backbone therapy in the management of prostate cancer. Onco Targets Ther. 2016;9:7263–7274. doi: 10.2147/OTT.S117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Marech I, Vacca A, Ranieri G, Gnoni A, Dammacco F. Novel strategies in the treatment of castration-resistant prostate cancer (Review) Int J Oncol. 2012;40:1313–1320. doi: 10.3892/ijo.2012.1364. [DOI] [PubMed] [Google Scholar]
- 5.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;26(30):87–101. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javan B, Shahbazi M. Hypoxia-inducible tumour-specific promoters as a dual-targeting transcriptional regulation system for cancer gene therapy. Ecancermedicalscience. 2017;11:751. doi: 10.3332/ecancer.2017.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong X, Zhao H, Liang S, Zhou D, Zhang W, Yuan L. Gene delivery of apoptin-derived peptide using an adeno associated virus vector inhibits glioma and prolongs animal survival. Biochem Biophys Res Commun. 2017;15(482):506–513. doi: 10.1016/j.bbrc.2016.10.059. [DOI] [PubMed] [Google Scholar]
- 8.Shen Ni L, Allaudin ZN, Mohd Lila MA, Othman AM, Othman FB. Selective apoptosis induction in MCF-7 cell line by truncated minimal functional region of Apoptin. BMC Cancer. 2013;13:488. doi: 10.1186/1471-2407-13-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Liu Y, Wen Z, Li C, Lu H, Tian M, et al. Potent anti-tumor effects of a dual specific oncolytic adenovirus expressing apoptin in vitro and in vivo. Mol Cancer. 2010;9:10. doi: 10.1186/1476-4598-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jangamreddy JR, Panigrahi S, Lotfi K, Yadav M, Maddika S, Tripathi AK, et al. Mapping of apoptin-interaction with BCR-ABL1, and development of apoptin-based targeted therapy. Oncotarget. 2014;5:7198–7211. doi: 10.18632/oncotarget.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nastasie MS, Thissen H, Jans DA, Wagstaff KM. Enhanced tumour cell nuclear targeting in a tumour progression model. BMC Cancer. 2015;15:76–87. doi: 10.1186/s12885-015-1045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backendorf C, Noteborn MH. Apoptin towards safe and efficient anticancer therapies. Adv Exp Med Biol. 2014;818:39–59. doi: 10.1007/978-1-4471-6458-6_3. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Wang H, Ma Z, Fan W, Li Y, Han B, et al. TAT-Apoptin induces apoptosis in the human bladder cancer EJ cell line and regulates Bax, Bcl-2, caspase-3 and survivin expression. Exp Ther Med. 2012;3:1033–1038. doi: 10.3892/etm.2012.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou S, Zhang M, Zhang J, Shen H, Tangsakar E, Wang J. Mechanisms of apoptin-induced cell death. Med oncol. 2012;29:2985–2991. doi: 10.1007/s12032-011-0119-2. [DOI] [PubMed] [Google Scholar]
- 15.He X, Zhang Q, Liu Y, He P. Apoptin induces chromatin condensation in normal cells. Virus genes. 2005:3149–3155. doi: 10.1007/s11262-005-2200-4. [DOI] [PubMed] [Google Scholar]
- 16.Guelen L, Paterson H, Gaken J, Meyers M, Farzaneh F, Tavassoli M. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene. 2004;23:1153–1165. doi: 10.1038/sj.onc.1207224. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Kim HS, Yu R, Lee K, Gardner TA, Jung C, et al. Novel prostate-specific promoter derived from PSA and PSMA enhancers. Mole Ther. 2002;6:415–421. doi: 10.1006/mthe.2002.0682. [DOI] [PubMed] [Google Scholar]
- 18.Zarei MA, Takhshid M, Behbahani AB, Hosseini S, Okhovat M, Dehbidi GRR, et al. Synergistic effects of NDRG2 overexpression and radiotherapy on cell death of human prostate LNCaP cells. J Biomed Physics Eng. 2016;7:257–264. [PMC free article] [PubMed] [Google Scholar]
- 19.Kashkin KN, Chernov IP, Didych DA, Sverdlov ED. Construction of a combinatorial library of chimeric tumor-specific promoters. Biotechniques. 2017;63:107–116. doi: 10.2144/000114586. [DOI] [PubMed] [Google Scholar]
- 20.Qi Y, Guo H, Hu N, He D, Zhang S, Chu Y, et al. Preclinical pharmacology and toxicology study of Ad-hTERT-E1a-Apoptin, a novel dual cancer-specific oncolytic adenovirus. Toxicol appl pharmacol. 2014;15(280):362–369. doi: 10.1016/j.taap.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Leliveld SR, Dame RT, Rohn JL, Noteborn MH, Abrahams JP. Apoptin's functional N- and C-termini independently bind DNA. FEBS lett. 2004;16(557):155–158. doi: 10.1016/s0014-5793(03)01465-0. [DOI] [PubMed] [Google Scholar]
- 22.Kucharski TJ, Gamache I, Gjoerup O, Teodoro JG. DNA damage response signaling triggers nuclear localization of the chicken anemia virus protein apoptin. J virol. 2011;85:12638–12649. doi: 10.1128/JVI.05009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaabane W, Ghavami S, Malecki A, Los MJ. Human gyrovirus-Gpoptin interferes with the cell cycle and induces G2/M arrest prior to apoptosis. Arch immuno ther exp. 2017:464–468. doi: 10.1007/s00005-017-0464-8. [DOI] [PubMed] [Google Scholar]


