Abstract
Objective(s):
Perovskiaabrotanoides Kar., from family Lamiaceae, is a little known medicinal plant growing in various regions of Iran. In the present study, cryptotanshinone (CT), tanshinone 2A (Tan2A), and hydroxycryptotanshinone (HCT) were isolated and purified from the roots of P. abrotanoides. In addition, cytotoxic and apoptotic effects of total root extract (TE) and three purified tanshinones were investigated in human cervical carcinoma (HeLa) and human breast cancer (MCF-7) cell lines.
Materials and Methods:
Alamar Blue® assay was used to determine cell viability. Cell apoptotic rate was detected using propidium iodide staining of DNA fragmentation by flowcytometry (sub-G1 peak). The PARP cleavage, as the sign of apoptosis, was investigated by Western blotting.
Results:
The results revealed that CT, Tan2A, HCT, and TE exhibited significant cytotoxicity in cancer cell lines. All of these compounds caused apoptosis in treated cells and induced sub-G1 peak in the related flowcytometry histograms. HCT was found to have the highest anti-proliferative activity on cancer cells. Western blotting analysis showed cleavage of PARP protein in MCF-7 cells treated with purified tanshinones and TE after 48 hr contact with cells.
Conclusion:
The findings suggest that root extract of P. abrotanoides and purified tanshinones especially Tan2A and HCT have cytotoxic and apoptotic effects against cancer cell lines. So, they may serve as potential cytotoxic agents for future investigations.
Keywords: Apoptosis, Cytotoxicity, HeLa, MCF-7, Perovskiaabrotanoides, Root extract, Tanshinone
Introduction
Medicinal plants, as main resources of pharmaceuticals, are the base of traditional medicine for the majority of the world’s populations. In recent decades, increasing attention has been attracted to the study of plant secondary metabolites and bioactive compounds, in order to achieve drug discovery and development of novel drugs for the treatment of diverse ailments (1, 2). Screening of plants commonly and historically used in traditional medicines is a logical approach to find new sources of phytochemicals and drugs. So, the role of traditional plant products and their biological activities in finding new potent chemicals is crucial (2).
Perovskiaabrotanoides Kar. (Lamiaceae) is one of the medicinal plantsused in Iranian traditional medicine to treat leishmaniasis(3). According to the few scientific investigations carried out on this species, the plant also possesses anti-plasmodial, anti-inflammatory, anti-nociceptive, and antibacterial activities (3, 4).
It has been demonstrated that the roots of this little known medicinal plant are a rich source of lipophilic compounds named tanshinones (3, 5).
Tanshinoneshave attracted attention because of their large variety of biological and pharmacological activities, such as anti-diabetic (6), anti-allergic (7), antioxidative (8, 9), anticancer (10, 11), anti-fibrotic (1), antibacterial (12) and anti-inflammatory properties (13). Recent studies have shown that tanshinones have potent cytotoxic and anti-proliferative effects against various human tumor cell lines including human breast cancer (14, 15), leukemia (11), prostate cancer (16, 17), cervical cancer (10), ovarian cancer (18), and hepatoca-rcinoma cells (19) by inducing apoptosis and cell death.
Cancer, one of the main public health problems, is a leading cause of death all over the world. The World Health Organization (WHO) has predicted that the number of new cancer cases will reach 15 million every year by 2020 (20).
The rising occurrence of cancer cases has resulted in a search for additional preventive and therapeutic modalities (15). Because of some serious problems including population growth, drug side effects, high cost of treatment, and increasing drug resistance, natural products in cancer prevention are still of therapeutic interest (21, 22). Plant derived natural products such as terpenoides have received wide attention in recent years due to their diverse pharmacological activities including cytotoxic and cancer chemopreventive effects (23). Since cancer cells usually exhibit active cell division, a helpful way of finding anticancer drugs is to test whether a compound can selectively kill mitotic cells thus blocking the cell cycle progression (24).
Activation of apoptosis pathways is a key mechanism by which cytotoxic drugs kill tumor cells. Thus, induction of apoptosis in cancer cells has now been considered as an indicator of the cancer treatment response and an important manner to evaluate the clinical effectiveness of many anti-tumor drugs (22, 25). Effective cytotoxic treatments with natural products increase apoptosis in cancer cells, but there are some limitations because of the side effects of these agents in normal cells. Scientific studies should be guided by using natural products for better understanding their modes of action and the ways of application.
This study aimed to investigate in vitro growth inhibition and apoptosis induction by P. abrotanoides Kar. We isolated and purified cryptotanshinone (CT), tanshinone 2A (Tan2A) and hydroxycryptotanshinone (HCT) from the roots of P. abrotanoides. Moreover, cytotoxic and apoptotic activities of the purified tanshinones and the total root extract (TE) against human cervix carcinoma (HeLa), human breast cancer (MCF-7) cell lines and human fibroblasts as normal cells were evaluated.
Materials and Methods
Chemicals and reagents
CT (purity ≥ 98%) and Tan2A(purity ≥ 98%) standards were purchased from Sigma Aldrich. AlamarBlue® from BioSource Invitrogen; doxorubicin (10 mg/5 ml) from Ebewe; Roswell Park Memorial Institute-1640 (RPMI-1640), Dulbecco’s Modified Eagle medium (DMEM) and FCS from Gibco; β-actin and PARP antibodies, anti-rabbit IgG antibody from Cell Signaling Technology; ECL Western blotting detection reagent from Bio-RaD; the fluorescent probe propidium iodide (PI), protease inhibitor cocktail, phosphatase inhibitor cocktail, sodium citrate and Triton X-100 from Sigma; Silica gel (70– 230 mesh) for column chromatography, pre-coated silica gel Gf254 sheets and HPLC grade acetonitrile from Merck; the other solvents as analytical grade from Dr. Mojallali Lab.
Plant material
Roots of P. abrotanoides were collected from wild plants in Razavi Khorasan Province, Iran. The plant was identified by Mr Mohammad Reza Joharchi at the Ferdowsi University of Mashhad. A voucher specimen (no. 39299) was deposited at the Herbarium of Research Center for plant sciences, Ferdowsi University of Mashhad, Iran.
Extraction, isolation and purification of tanshinones
Air-dried and powdered roots (500 g) of P. abrotanoides were extracted with ethyl acetate (3×3 l, 24 hr each) at room temperature (3). The combined extracts were concentrated in a rotary evaporator below 40°C, followed by removal of residual solvent in a freeze dryer. The yield of extraction was about 3.84%. Three grams of extract was subjected to column chromatography over a silica gel column (100×5 cm, 500 g silica gel 70-230 mesh) eluted with a gradient system of petroleum ether (40°C–60°C) :ethyl acetate. Fractions of 250 ml were collected and monitored by TLC as the same mobile phase as column chromatography with different polarity.
The similar obtained fractions were combined according to their TLC profiles.
For further purification, semi-preparative reversed-phase HPLC was performed on a WellchromKnauer system (Herbert Knauer GmbH, Germany) that consisted of a Knauer K-1001 pump, operating at 254 nm, using ACE 5 C18 column (250×21.2 mm, 5 µm) eluted with a gradient solvent system 20-100% MeCN - H2O mixed with 0.05% TFA (v/v) at a flow rate of 10 ml/min. Structure of tanshinones was determined and confirmed by LC-MS, 1H NMR, 13C NMR and DEPT135 methods. LC-DAD-MS analyses were conducted on an Agilent 1200 series HPLC system consisting of an auto-sampler, high-pressure mixing pump, column oven, and DAD detector connected to a Agilent 6410 triple quadruple mass spectrometer. HPLC conditions: Agilent Eclipse XDB -C18 column (4.6 ×100 mm, 3.5 µm), solvent system A: 0.1% formic acid in H2O, B: MeCN; isocratic 20% B for 6 min, 20-95% B over 18 min, 95-100% B over 6 min, 100% B for 6 min, 100-20% B over 6 min; flow rate: 0.5 ml/min; injection volume: 10 µl; column temperature : 25ºC; DAD condition: 254 nm.
ESI-MS conditions: positive ion mode; drying gas (nitrogen) flow rate: 10 L/min; nebulizing gas pressure: 15 psi; source temperature: 300ºC; ion spray voltage: 4 kv; scan range: 100-1000 amu.
NMR experiments were carried out on a Bruker Avance 400 MHz spectrometer in chloroform-d, using TMS as internal standard. The NMR data were processed using MestReC4999 software and compared to those reported in the literature.
TE was obtained by extraction of dried and milled roots of the plant with methanol (1:6 W/V) using the maceration method for 3 days. After every 24 hr, the mixture was filtered and new solvent was added to the plant powder. The combined extracts were concen-trated to dryness under vacuum pressure.
Sample Preparation
Stock solutions of TE and purified tanshinones (CT, Tan2A, and HCT) were prepared by dissolving them in dimethylsulfoxide (DMSO). Different concentrations of TE (1, 5, 25, 50, 100, and 200 µg/ml) and purified tanshinones (1, 5, 10, 25, and 50 µM) were then obtained by diluting stock solutions with media so that the final concentrations of DMSO did not exceed 0.05%. All dilutions were sterilized by filtering through a microfilter (0.2 µm) and prepared fresh before addition to the cells.
Cell Culture
HeLa and MCF-7 cell lines were obtained from Pasteure institute (Tehran, Iran) and respectively cultured in DMEM and RPMI-1640 media with 10% (v/v) fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 90% air and 5% CO2. Periodontal fibroblast cells were obtained according to Tayarani-Najaran et al. (2014) and cultured in DMEM medium in the conditions mentioned above (21).
Cell viability and cytotoxicity assay
The effects of purified tanshinones and TE on HeLa and MCF-7 cells proliferation were determined by AlamarBlue® assay (26). Briefly, cells were cultured overnight at a density of approximately 104 cells/well and then treated with various concentrations of CT, Tan2A, HCT (0-50 µM), and TE (0-200 µg/mL) for 48 hr. AlamarBlue® reagent was added to each well up to 10% of tissue culture medium and incubated for an additional 4 hr at 37°C. The cell viability was determined by measuring the absorbance at 600 nm using Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, USA). Cells without tanshinone treatment which received an equal volume of the solvent, served as control. The cytotoxicity of root extract and purified tanshinones was determined as IC50, which was calculated by Graph Pad prism version 5 software. Doxorubicin was used as a reference drug and positive control for the cytotoxicity evaluation of tanshinones.
PI staining
For the apoptosis assay, hypo-diploid DNA content was assessed by PI staining and flow cytometry. The procedure of Tayarani-Najaran et al. (2010) was employed (27). In brief, HeLa and MCF-7 cells were seeded overnight at a density of approximately 104 cells/well and then treated with different concentrations of CT, Tan2A, HCT, and TE for 48 hr. Floating and adherent cells were collected and incubated at 4°C overnight in the dark with 400 μl of a hypotonic buffer (50 μg/ml PI in 0.1% sodium citrate plus 0.1% Triton X-100). The fluorescence of cells was analyzed using a FACScanflow cytometer (Becton Dickinson, USA). Sub-G1 peaks and apoptosis percentage were analyzed using WinMDI software (ver. 2.8).
Western blot analysis
MCF-7 cells were treated with TE (25, 50, and 100 μg/mL), CT, Tan2A, and HCT (each at 10 and 50 μM) for 48 hr. The cells were harvested and rinsed with cool PBS. The cell pellet was homogenized in a lysis buffer containing 50 mMTris-HCl (pH 7.4), 150 mMNaCl, 1% Triton X-100, 1 mM EDTA, 0.2% SDS, 1% protease inhibitor cocktail, 1% phosphatase inhibitor cocktail, and 1 mMphenylmethylsulfonyl fluoride on ice for 30 min. After centrifugation at 14000 rpm for 15 min at 4°C, the cell supernatants were collected and protein concentration was determined according to Bradford assay and equal amounts of proteins were subjected to 10% SDS-PAGE (W/V). The proteins were transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking with 5% nonfat milk, the blots were subjected to immunoblotting using β-actin and poly (ADP ribose) polymerase (PARP) antibodies as primary antibodies and anti-rabbit IgG, HRP-linked antibody as secondary antibody. PARP cleavage was detected by enhanced chemiluminescence using the ECL Western blotting detection reagent (21).
Statistical analysis
All experiments were carried out in a completely randomized design. Data were subjected to Duncan’s Multiple Range Test. All results were reported as means ± standard error of means (SEM) and P≤0.05 was considered as significant.
Results
Purification and identification of tanshinones from the roots of P. abrotanoides
Ethyl acetate extract of P. abrotanoides roots was fractionated by column chromatography. Semi-preparative HPLC of the active fractions led to purification of three tanshinones including CT, Tan2A, and HCT (purity > 95%) (Figure 1). The structure of HCT was determined by the NMR method. Spectral data of HCT are presented in Table 1. Two other compounds were identified as CT and Tan2A by comparison of their TLC profiles and LC-MS spectra with related standards.
Figure 1.
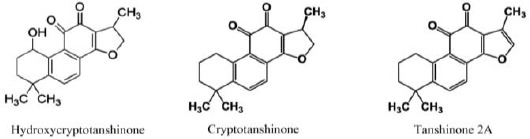
Molecular structures of three tanshinone compounds in Perovskiaabrotanoides roots
Table 1.
400 MHz 1H-NMR and 100 MHz 13C-NMR spectral data for hydroxycryptotanshinone (CDCl3)
| Carbon number | 13C-NMR | Reference* | 1H-NMR | Reference* |
|---|---|---|---|---|
| 1 | 63.4 | (63.38) | 5.06 (m) | 5.04 (br,q), J1,2≈J1,OH≈3.5 Hz |
| 2 | 26.9 | (26.91) | 2.11 (m), 1.91 (m) | 2.11 (m), 1.88(m) |
| 3 | 31. 9 | (31.92) | 1.57(m), 2.16 (m) | 1.55 (m), 2.16 (m) |
| 4 | 35.1 | (35.13) | - | - |
| 5 | 152.1 | (152.11) | - | - |
| 6 | 134.1 | (134.09) | 7.74 (d), J6,7=8.0 Hz | 7.72 (d), J6,7=8.1 Hz |
| 7 | 124.5 | (124.54) | 7.62 (d), J6,7=8.0 Hz | 7.61 (d), J6,7=8.1 Hz |
| 8 | 126. 9 | (126.93) | - | - |
| 9 | 129.8 | (129.83) | - | - |
| 10 | 143.0 | (143.43) | - | - |
| 11 | 186.3 | (186.31) | - | - |
| 12 | 175.4 | (175.37) | - | - |
| 13 | 118.5 | (118.49) | - | - |
| 14 | 170.8 | (170.71) | - | - |
| 15 | 34.5 | (34.59) | 3.62 (m) | 3.61 (m) |
| 16 | 81.8 | (81.81) | 4.42 (dd), 4.94 t | 4.40 (dd), 4.92(t) |
| 17 | 19.1 | (19.13) | 1.39 (d), J15,17=7.2 Hz | 1.37 (d), J15,17=6.8 Hz |
| 18 | 31.1 | (31.19) | 1.42 (s) | 1.40 (s) |
| 19 | 31.5 | (31.57) | 1.27 (s) | 1.26 (s) |
Multiplicity of signals is given in parentheses: s, singlet; d, doublet; t, triplet; q quartet; sp, septet; m, multiplet, br, broad; coupling constants (numeric values) are specified only once at the first occurrence of the coupled resonance
Sairafianpour et al. 2001 (3)
ytotoxicity of purified tanshinones and total extract isolated from the roots of P. abrotanoides
Results of the cytotoxicity assay showed that TE, CT, Tan2A, and HCT decreased cell viability in HeLa and MCF-7 cells in a concentration dependent manner after 48 hr. As indicated in Figure 2, TE severely inhibited cancer cells proliferation. HeLa cells were more sensitive to TE than MCF-7 cells.
Figure 2.
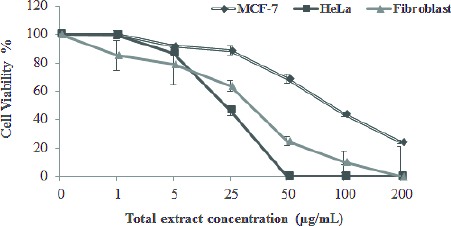
Growth inhibition of cancer cell lines (MCF-7 and HeLa) and normal cells (human fibroblasts) by different concentrations of total root extract of Perovskiaabrotanoides. Cells exposed to increasing levels of root extract showed cytotoxicity. Data are mean ± SEM of three independent experiments, each in triplicates
Among the three purified tanshinones, HCT showed the most potent cytotoxicity against HeLa and MCF-7 cell lines and its IC50 values (17.55 and 16.97 µM for HeLa and MCF-7 cells, respectively) were considerably less than the values obtained for Tan2A and CT (Table 2).
Table 2.
IC50 values for total extract (TE), cryptotanshinone (CT), tanshinone 2A (Tan2A), and hydroxycryptotanshinone (HCT) isolated from the roots of Perovskia abrotanoides in cancer cell lines and normal fibroblast cell.
| Cell line | TE (µg/ml) | CT (µM) | Tan2A (µM) | HCT (µM) |
|---|---|---|---|---|
| IC50 | ||||
| Fibroblast | 28.14 | 68.7 | > 1000 | 84.79 |
| HeLa | 24.83 | 73.18 | 59.53 | 17.55 |
| MCF-7 | 87.69 | 80.00 | 36.27 | 16.97 |
Besides, Tan2A decreased cancer cell viability more than CT (Figure 3). The alamarBlue® results demonstrated that treatment of HCT and Tan2A in the same concentrations had a less cytotoxic effect on human fibroblast cells than cancer cells. In other words, the inhibitory effects of tanshinones, particularly Tan2A, on the normal cells were lower than their effects on the cancer cells. It seemed that MCF-7 cells were more sensitive to Tan2A and HCT treatments than HeLa cells (Figure 3). CT and Tan2A did not show significant cytotoxicity on normal fibroblast cells at the concentrations below 25 µM. The IC50 values of TE, CT, Tan2A, and HCT have been presented in Table 2. Doxorubicin at concentration of 0.92 µM was used as positive control. At this concentration, doxorubicin decreased HeLa and MCF-7 cell viability to 35.3% ± 2.71 and 41.5%±1.89 compared to untreated control cells, respectively (data not shown).
Figure 3.
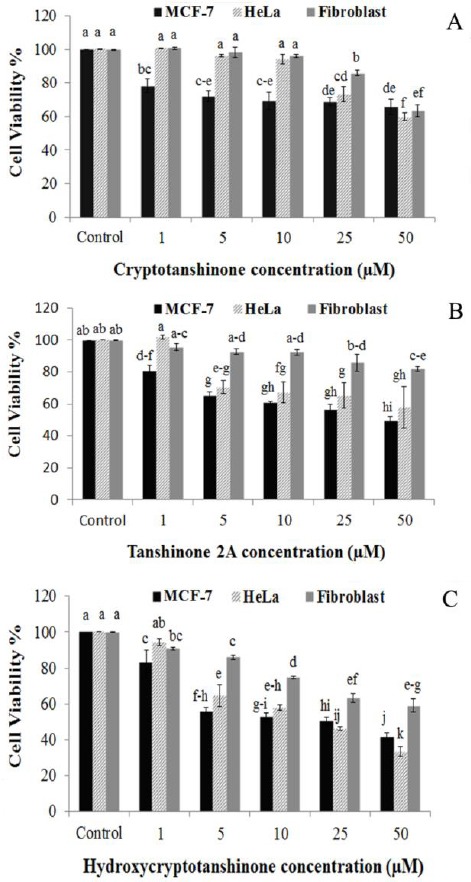
Inhibitory effects of different concentrations of cryptotanshinone (A), tanshinone 2A (B), and hydroxycry-ptotanshinone (C) on HeLa, MCF-7 and fibroblast cell viability after 48 hr. Cancer cell lines were more sensitive to tanshinones treatment than normal fibroblast cells. Data are mean±SEM of three independent experiments, each in triplicates. Bars with different letters indicate significant difference (P≤0.05)
In general, the results suggest that tanshinones may have potent anti-proliferative and cytotoxic activity against cancer cells but limited unfavorable effects on normal cells.
Apoptosis induction by purified tanshinones and total extractisolated from the roots of Perovskiaabrotanoides
The effects of TE and tanshinones on apoptosis induction in HeLa and MCF-7 cells were investigated by PI staining to detect sub-G1 peak resulted from DNA fragmentation. Incubation of cancer cells with various concentrations of TE (5, 25, 50, and 100 µg/ml), CT, Tan2A, and HCT (each at 5, 25, and 50 µM) for 48 hr induced apoptosis and led to accumulation of the sub-G1 region. Untreated control cells did not exhibit sub-G1 peak (Figure 4A). This indicates the role of the apoptosis process in cell death induced by tanshinone compounds.
Figure 4.
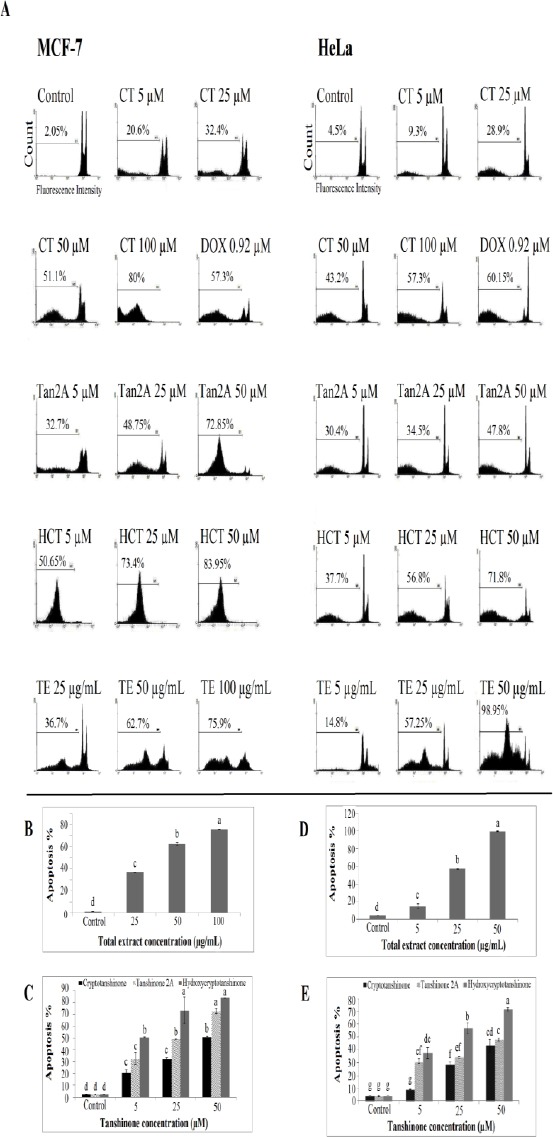
Flow cytometry histogram of apoptosis assay by PI staining method in MCF-7 and HeLa cells treated with different concentrations of isolated tanshinone and total root extract of Perovskiaabrotanoides after 48 hr. Sub-G1 peak as an indicative of apoptotic cells induced in the cells treated with total root extract and tanshinones but not in control cells (A). Effects of different concentrations of total root extract and tanshinones isolated from the roots of P. abrotanoides on apoptosis percentage in MCF-7 (B and C) and HeLa (D and E) cells after 48 hr. Increasing levels of total root extract and tanshinones, increasedthe percentage of apoptosis. Data are representative of at least three independent experiments. Barswith different letters indicate significant difference(P≤ 0.05). CT: Cryptotanshinone; Tan2A: Tanshinone 2A; HCT: hydroxycryptotanshinone; TE: Total extract of roots; DOX: Doxorubicin
TE induced apoptosis more effectively in HeLa cells than MCF-7 ones. At the 50 µg/mL concentration, 62.7% and 98.95% apoptosis occurred in MCF-7 and HeLa cells, respectively (Figures 4B and 4D). After treatment of HeLa and MCF-7 cells with 25 µg/mL TE, the sub-G1 cell populations were respectively 57.2% and 36.7% while the percentage of sub-G1 phase populations in untreated HeLa (4.5%) and MCF-7 cells (1.9%) were crucially decreased.
The presence of tanshinones also led to the significantly higher apoptosis percentage in MCF-7 and HeLa cells (Figures 4C and 4E). Among the three tested tanshinones, HCT was the most powerful compound to induce apoptosis in both cancer cells especially at a dose of 50 µM. In most cases, the rate of apoptosis in Tan2A-treated cells was also significantly higher than those obtained of CT treatments (Figures 4C and 4E). The crude root extract and isolated tanshinones enhanced the level of apoptotic cells in all treatments in a dose-dependent manner.
Effect of purified tanshinones and total extract isolated from the roots of Perovskiaabrotanoides on poly (ADP ribose) polymerase cleavage
Proteolytic cleavage of 116 kDa PARP to 89 kDa fragment is considered as a biochemical hallmark of apoptosis. Western blot analysis showed that PARP protein was cleaved after exposure of MCF-7 cells to different concentrations of TE (25, 50, and 100 µg/ml), CT, Tan2A, and HCT (10 and 50 µM) for 48 hr (Figure 5).
Figure 5.
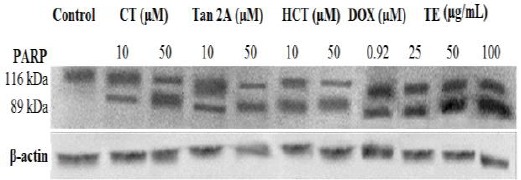
Western blot analysis of PARP of the MCF-7 cells after 48 hr exposure to different concentrations of total extract and tanshinones isolated from the roots of Perovskiaabrotanoides. β-actine was used as loading control. All Western blots were representative of three independent experiments. CT: Cryptotanshinone; Tan2A: Tanshinone 2A; HCT: Hydroxycryptotanshinone; TE: Total extract of roots; DOX: Doxorubicin
The results of Western blot assay together with the results obtained from PI staining and flowcytometry indicated that the crude root extract and isolated tanshinones of P. abrotanoides, at least in part, inhibited the proliferation of cancer cells by inducing apoptosis.
Discussion
The application of medicinal plants to make useful drugs for various diseases and also as a source of effective anticancer agents has attracted recent attention. Due to an increasing rate of mortality associated with cancer and adverse or unwanted side effects of cancer chemotherapy and radiation therapy, discovery of new anticancer natural products and the screening of medicinal plants for this purpose have become the focus of attention (28). It has been proven that biologically active compounds derived from medicinal plants play a key role in integrative cancer treatment and healing (11, 23, 27). Several drugs are used to treat and prevent the development of the tumor, but they are not always effective and usually are accompanied with side effects. Alternative treatments based on plant phytochemicals might be a potential safe candidate to treat cancer (29). Recent studies have shown that tanshinones, which are widely applied to treat cardiovascular diseases, inhibit the proliferation of different cancer cell lines (8, 11, 15, 25).
In this study, the fractionation of root extract of P. abrotanoides by column chromatography and then HPLC analysis of the fractions led to purification of three diterpenoids named cryptotanshinone, tanshinone 2A, and hydroxycryptotanshinon. It should be noted that four tanshinones including cryptotanshinone, hydroxycryptotanshinone, oxocryptotan-shinone, and oxomiltrione were isolated from this source by Sairafianpour et al (3). To the bestof our knowledge, there have been no published reports on the existence of Tan2Ain the roots of P. abrotanoides.
The results demonstrated that TE had cytotoxic effect against HeLa and MCF-7 cells and significantly decreased cell viability. Regarding the presence of CT, Tan2A, and HCT in the roots of P. abrotanoides, some of the biological activities of the plant might be related to these compounds. The cytotoxicity of P. abrotanoides root extract on MCF-7 and WEHI cells has also been reported (30). Other studies have indicated that total extract of the roots of Salvia miltiorrhiza, another rich source of tanshinons, prevented the proliferation of human hepatoma HepG2 (31) and P388 lymphocytic leukemia cells (32). Crude natural extract is usually a complex mixture of various substances which have different physicochemical and pharmacological properties (27). The cytotoxic property of root extract of P. abrotanoides against cancer cells could be attributed to the synergistic effects of tanshinones. The use of total plant extracts in biological assays provides an assessment for the synergistic and/or antagonistic interactions between different components in the crude extract. To better understand the biological activity and decrease possible side effects attributed to the chemical constituents of plant materials, further investigations on the individual pure substances are necessary (33).
The findings of the present study indicate that treatment of HeLa and MCF-7 cells with CT, Tan2A, and HCT significantly inhibited cell proliferation in a dose dependent manner. The effect of tanshinones especially Tan2A and HCT on malignant and normal cells showed a degree of specificity for malignant cells. It has been shown that CT inhibited the proliferation of epidermoid cancer (KB-3-1) (3), urinary bladder cancer (5637), Lung cancer (A-427), and breast cancer (MCF-7) cell lines (34). Similarly, the cytotoxicity, growth inhibition, and apoptosis induction of CT have been demonstrated on DU145 prostate cancer cells (17) and P 388 lymphocytic leukemia cells (32). On PC3 prostate cancer and HepG2 cells, CT caused cell cycle arrest at the S phase and thus inhibited cell proliferation (16, 19). Our results showed the potent cytotoxic activity of Tan2A against malignant cells (IC50 values for HeLa and MCF-7 cell lines were 59.53 and 36.27 µM, respectively). Interestingly, normal fibroblast cells were significantly resistant to cytotoxicity induced by this secondary metabolite (IC50>1000 µM). Tan2A effectively inhibited the proliferation of different cancer cell lines including PC3 prostate cancer (16), HeLa (10), leukemia THP-1 (11), and human breast cancer COC1/DDP (14). Studies carried out on HeLa, PC3, and HepG2 cancer cells revealed that Tan2A affects cell cycle progress and arrests the cells at the G2/M phase (10, 16, 19).
In the present study, treatment of HeLa and MCF-7 cells with HCT induced strong cytotoxicity. The inhibitory effect of HCT on KB-3-1 cancer cells growth has been shown by other researches (3). Mothana et al. (2009) have also reported the potent cytotoxic effect of HCT, isolated from the roots of Merianderabenghalensis, on urinary bladder cancer (5637), lung cancer (A-427) and MCF-7 cell lines (34). According to our results, among three tanshinones studied, HCT was the most powerful cytotoxic compound and its IC50 values were less than 18 µM in both investigated cancer cell lines. Besides, Tan2A had stronger anti-proliferative activity than CT. Consistent with this finding, higher cytotoxicity of Tan2A than CT has also been reported in PC3 (16), HepG2 (19), and P388 cancer cells (32). The lower IC50 value for HCT could suggest this substance as a promising therapeutic candidate to treat human cervix and breast cancers.
Because of apoptosis induction by many anticancer drugs in cancer cells, the apoptosis inducing activity of TE of P. abrotanoides and purified tanshinones were also investigated. The qualitative and quantitative analysis of apoptosis events are considerably important in order to determine and confirm the mechanism of action played by the extract (35). Root extract and three purified tanshinones induced apoptosis in HeLa and MCF-7 cells significantly and increased cell population in the sub-G1 phase. This indicated that apoptosis was involved in the cell death and cytotoxicity of root extract and tanshinones in HeLa and MCF-7 cells. So far no study has been conducted on apoptosis induction by the root extract of P. abrotanoides on cancer cells. The roots of P. abrotanoides contain tanshinone compounds causing apoptosis, thereby revealing its potential cytotoxicity. This cytotoxic activity can be exploited in cancer treatment, leading cancer cells to apoptosis. So, this little known medicinal plant can frequently serves as a source of new drugs with little or no side effects.
The compound HCT was more efficacious in apoptosis induction than Tan2A or CT while the apoptotic activity of CT was less than the other compounds. The results were consistent with the results of alamarBlue® assay. Taken together, according to the results of PI staining and alamarBlue® assays, HCT was the strongest apoptosis inducing agent while the weakest activity belonged to CT. Thus HCT can act as a powerful antiproliferative substance on HeLa and MCF-7 cells. To the best of our knowledge, there is no other report in the literature showing the possible effect of HCT on apoptosis induction in cancer cell lines. The higher apoptosis induction of Tan2A than CT has also been reported in the PC3 cell line (16). Apoptosis is a well-known biological response expressed by cells after suffering DNA damage and is a useful marker for screening compounds for subsequent development as possible anticancer agents (23). Apoptotic cell death is known to be induced by many chemotherapeutic substances routinely used to treat cancer. Normal development of organs is controlled by a balance between cell proliferation and apoptosis (36, 37). On the other hand, apoptosis is a key regulatory of tissue homeostasis, and imbalances between cell death and proliferation may result in tumor formation. The objective of using anticancer agents is to induce apoptosis related signaling pathways and disrupt cell proliferation (23). Thus, induction of apoptosis in cancer cells is considered as a valuable manner to treat cancer. In addition, a wide variety of natural secondary metabolites have been identified to have the potential of apoptosis induction in different cancer cell lines. So, screening of apoptosis inducers from the plants, either as crude extract or as pure compounds isolated from them, is important (36, 37).
PARP is a nuclear enzyme which is involved in DNA repair process and this 113 KDa protein is cleaved to 89 KDa and 24 KDa fragments by Caspase 3 protease (38). Treatment of MCF-7 cells with CT, Tan2A, HCT, and TE caused apoptotic cell death, as indicated by PARP cleavage.
Conclusion
In summary, it can be concluded that root extract of P. abrotanoides and isolated tanshinones (CT, Tan2A, and HCT) can decrease cell viability in MCF-7 and HeLa cell lines in which induction of apoptosis partly could be involved. To our knowledge, induction of apoptosis by the roots of P. abrotanoides and HCT are reported for the first time. So root extract of this species and the secondary metabolites purified from the roots could be considered as promising candidates for further investigations to develop natural anticancer drugs.
Acknowledgment
The authors would like to thank Mr M Malaekeh for his assistance in flowcytometry. This work was supported in part by the Research Affairs of Mashhad University of Medical Sciences, Mashhad, Iran (grant number 900852). The results presented in this paper were part of a student thesis.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Park EJ, Zhao YZ, Kim YC, Sohn DH. PF2401-SF standardized fraction of Salvia miltiorrhiza and its constituents, tanshinone I, tanshinone IIA, and cryptotanshinone, protect primary cultured rat hepatocytes from bile acid-induced apoptosis by inhibiting JNK phosphorylation. Food ChemToxicol. 2007;45:1891–1898. doi: 10.1016/j.fct.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Cragg GM, Newman DJ. Natural products drug discovery in the next millennium. Pharm Biol. 2001;39:8–17. doi: 10.1076/phbi.39.s1.8.0009. [DOI] [PubMed] [Google Scholar]
- 3.Sairafianpour M, Christensen J, Staerk D, Budnik BA, Kharazmi A, Bagherzadeh K, et al. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1,2-quinones from Perovskiaabrotanoides: New source of tanshinones. J Nat Prod. 2001;64:1398–1403. doi: 10.1021/np010032f. [DOI] [PubMed] [Google Scholar]
- 4.Hosseinzadeh H, Amel S. Antinociceptive effects of the aerial parts of Perovskiaabrotanoides extracts in mice. Iran Red Crescent Med J. 2001;4:15–17. [Google Scholar]
- 5.Zaker A, Sykora C, Gossnitzer F, Abrishamchi P, Asili J, Mousavi SH, et al. Effects of some elicitors on tanshinone production in adventitious root cultures of Perovskiaabrotanoides Karel. Ind Crops Prod. 2015;67:97–102. [Google Scholar]
- 6.Kim EJ, Jung SN, Son KH, Kim SR, Ha TY, Park MG, et al. Antidiabetes and antiobesity effect of cryptotanshinone via activation of AMP-activated protein kinase. MolPharmacol. 2007;72:62–72. doi: 10.1124/mol.107.034447. [DOI] [PubMed] [Google Scholar]
- 7.Bai A, Lu N, Gou Y, Fan X. Tanshinone IIA ameliorates trinitrobenzene sulfonic acid (TNBS)-induced murine colitis. Digest Dis Sci. 2008;53:421–428. doi: 10.1007/s10620-007-9863-8. [DOI] [PubMed] [Google Scholar]
- 8.Park EJ, Zhao YZ, Kim YC, Sohn DH. Preventive effects of a purified extract isolated from Salvia miltiorrhiza enriched with tanshinone I, tanshinone IIA and cryptotanshinone on hepatocyte injury in vitro and in vivo. Food ChemToxicol. 2009;47:2742–2748. doi: 10.1016/j.fct.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Cao EH, Liu XQ, Wang JJ, Xu NF. Effect of natural antioxidant tanshinone II-A on DNA damage by lipid peroxidation in liver cells. Free Radic Bio Med. 1996;20:801–806. doi: 10.1016/0891-5849(95)02211-2. [DOI] [PubMed] [Google Scholar]
- 10.Pan TL, Hung YC, Wang PW, Chen ST, Hsu TK, Sintupisut N, et al. Functional proteomic and structural insights into molecular targets related to the growth inhibitory effect of tanshinone IIA on HeLa cells. Proteomics. 2010;10:914–929. doi: 10.1002/pmic.200900178. [DOI] [PubMed] [Google Scholar]
- 11.Liu JJ, Zhang Y, Lin DJ, Zhiao RZ. Tanshinone IIA inhibits leukemia THP-1 cell growth by induction of apoptosis. Oncol Rep. 2009;21:1075–1081. doi: 10.3892/or_00000326. [DOI] [PubMed] [Google Scholar]
- 12.Lee DS, Lee SH, Noh JG, Hong SD. Antibacterial activities of cryptotanshinone and dihydrotanshinone I from medicinal herb Salvia miltiorrhiza Bunge. Biosci Biotechnol Biochem. 1999;63:2236–2239. doi: 10.1271/bbb.63.2236. [DOI] [PubMed] [Google Scholar]
- 13.Fan GW, Gau XM, Wang H, Zhu Y, Zhang J, Hu LM, et al. The anti-inflammatory activities of tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol. 2009;113:275–280. doi: 10.1016/j.jsbmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Su CC, Lin YH. Tanshinone IIA inhibits human breast cancer cells through increased Bax to Bcl-xL ratios. Int J Mol Med. 2008;22:357–361. [PubMed] [Google Scholar]
- 15.Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang J, et al. Potential anticancer activity of tanshinone IIA against human breast cancer. Int J Cancer. 2005;116:799–807. doi: 10.1002/ijc.20880. [DOI] [PubMed] [Google Scholar]
- 16.Gong Y, Li Y, Lu Y, Li L, Abdolmaleky H, Blackburn GL, et al. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int J Cancer. 2011;129:1042–1052. doi: 10.1002/ijc.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park IJ, Kim MJ, Park OJ, Park MG, Choe W, Kang I, et al. Cryptotanshinone sensitizes DU145 prostate cancer cells to Fas (APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulation. Cancer Lett. 2010;298:88–98. doi: 10.1016/j.canlet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Jiao JW, Wen F. Tanshinone IIA acts via p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and lung-resistance protein in cisplatin-resistant ovarian cancer cells. Onco Rep. 2010;25:781–788. doi: 10.3892/or.2010.1107. [DOI] [PubMed] [Google Scholar]
- 19.Lee WY, Chiu LC, Yeung JH. Cytotoxicity of major tanshinones isolated from Danshen (Salvia miltiorrhiza) on HepG2 cells in relation to glutathione perturbation. Food ChemToxicol. 2008;46:328–338. doi: 10.1016/j.fct.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Tavakoli J, Miar S, Majid Zadehzare M, Akbari H. Evaluation of effectiveness of herbal Medication in cancer care: A review study. Iran J Cancer Prev. 2012;5:144–156. [PMC free article] [PubMed] [Google Scholar]
- 21.Tayarani-Najaran Z, Amiri A, Karimi G, Emami SA, Asili J, Mousavi SH. Comparative studies of cytotoxic and apoptotic properties of different extracts and the essential oil of Lavandulaangustifolia on malignant and normal cells. Nutr Cancer. 2014;66:424–434. doi: 10.1080/01635581.2013.878736. [DOI] [PubMed] [Google Scholar]
- 22.Liu JJ, Liu WD, Yang HZ, Zhang Y, Fang ZG, Liu PQ, et al. Inactivation of PI3k/Akt signaling pathway and activation of caspase-3 are involved in tanshinone I-induced apoptosis in myeloid leukemia cells in vitro. Ann Hematol. 2010;89:1089–1097. doi: 10.1007/s00277-010-0996-z. [DOI] [PubMed] [Google Scholar]
- 23.Prajitha V, Thoppil JE. Cytotoxic and apoptotic activities of extract of Amaranthusspinosus L. in Allium cepa and human erythrocytes. Cytotechnology. 2017;69:123–133. doi: 10.1007/s10616-016-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Chan WK, Xu N, Xiao K, Luo H, Luo KQ, et al. Tanshinone IIA, an isolated compound from Salvia miltiorrhiza Bunge, induces apoptosis in HeLa cells through mitotic arrest. Life Sci. 2008;83:394–403. doi: 10.1016/j.lfs.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Nizamutdinova IT, Lee GW, Lee JS, Cho MK, Son KH, Jeon SJ, et al. Tanshinone I suppresses growth and invasion of human breast cancer cells, MDA-MB-231, through regulation of adhesion molecules. Carcinogenesis. 2008;29:1885–1892. doi: 10.1093/carcin/bgn151. [DOI] [PubMed] [Google Scholar]
- 26.Kasaian J, Iranshahi M, Masullo M, Piacente S, Ebrahimi F, Iranshahi M. Sesquiterpene lactones from Ferula oopoda and their cytotoxic properties. J Asian Nat Prod Res. 2014;16:248–253. doi: 10.1080/10286020.2013.866099. [DOI] [PubMed] [Google Scholar]
- 27.Tayarani-Najaran Z, Mousavi SH, Asili J, Emami SA. Growth-inhibitory effect of Scutellarialindbergii in human cancer cell lines. Food ChemToxicol. 2010;48:559–604. doi: 10.1016/j.fct.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 28.Ashwini S, Ezhilarasan D, Anitha R. Cytotoxic effect of Carallumafimbriata against human colon cancer cells. Pharmacogn J. 2017;9:204–207. [Google Scholar]
- 29.Rashid M, Seghatoleslam A, Namavari M, Amiri A, Fahmidehkar MA, Ramezani A, et al. Selective Cytotoxicity and apoptosis-induction of Cyrtopodionscabrum extract against digestive cancer cell lines. Int J Cancer Manag. 2017;10:e8633. [Google Scholar]
- 30.Sahranavard S, Naghibi F, Mosaddegh M, Esmaeili S, Sarkhail P, Taghvaei M, et al. Cytotoxic activities of selected medicinal plants from Iran and phytochemical evaluation of the most potent extract. Res Pharm Sci. 2009;4:133–137. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Shen HM, Ong CN. Salvia miltirrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG2 cells. Cancer Lett. 2000;153:85–93. doi: 10.1016/s0304-3835(00)00391-8. [DOI] [PubMed] [Google Scholar]
- 32.Mosaddik MA. In vitro cytotoxicity of tanshinones isolated from Salvia miltiorrhiza Bunge against P388 lymphocytic leukemia cells. Phytomedicine. 2003;10:682–685. doi: 10.1078/0944-7113-00321. [DOI] [PubMed] [Google Scholar]
- 33.Tayarani-Najaran Z, Makki FS, Alamolhodaei NS, Mojarrab M, Emami SA. Cytotoxic and apoptotic effects of different extracts of Artemisia biennis Willd.onK562 and HL-60 cell lines. Iran J Basic Med Sci. 2017;20:166–171. doi: 10.22038/ijbms.2017.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mothana RA, Jansen R, Gruenert R, Bednarski PJ, Lindequist U. Antimicrobial and cytotoxic abietanediterpenoids from the roots of Merianderabenghalensis (Roxb.) Benth. Pharmazie. 2009;64:613–615. [PubMed] [Google Scholar]
- 35.Nordin ML, Abdul Kadir A, Zakaria ZA, Othman F, Abdullah R, Abdullah MN. Cytotoxicity and apoptosis induction of Ardisiacrispa and its solvent partitions against Mus musculus mammary carcinoma cell line (4T1) Evid Based Complement Alternat Med. 2017;2017:9368079. doi: 10.1155/2017/9368079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taraphdar AK, Roy M, Bhattacharya RK. Natural products as inducers of apoptosis: implication for cancer therapy and prevention. Curr Sci. 2001;80:1387–1396. [Google Scholar]
- 37.Tayarani-Najaran Z, Emami SA, Asili J, Mirzaei A, Mousavi SH. Analyzing cytotoxic and apoptogenic properties of Scutellarialitwinowii root extract on cancer cell lines. Evid Based Complement Alternat Med. 2011;2011:160682. doi: 10.1093/ecam/nep214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon Y, Kim YO, Jeon WK, Park HJ, Sung HJ. Tanshinone IIA isolated from Salvia miltiorrhiza Bunge induced apoptosis in HL60 human premyelocytic leukemia cell line. J Ethnopharmacol. 1999;68:121–127. doi: 10.1016/s0378-8741(99)00059-8. [DOI] [PubMed] [Google Scholar]


