Abstract
The insula, a ‘cortical hub’ buried within the lateral sulcus, is involved in a number of processes including goal-directed cognition, conscious awareness, autonomic regulation, interoception and somatosensation. While some of these processes are well known in the clinical presentation of migraine (i.e., autonomic and somatosensory alterations), other more complex behaviors in migraine, such as conscious awareness and error detection, are less well described. Since the insula processes and relays afferent inputs from brain areas involved in these functions to areas involved in higher cortical function such as frontal, temporal and parietal regions, it may be implicated as a brain region that translates the signals of altered internal milieu in migraine, along with other chronic pain conditions, through the insula into complex behaviors. Here we review how the insula function and structure is altered in migraine. As a brain region of a number of brain functions, it may serve as a model to study new potential clinical perspectives for migraine treatment.
Keywords: interoception, pain, autonomic function, brain connectivity, salience network, headache, functional networks, sensory processing, emotional processing, brain imaging, fMRI, PET, lateral sulcus
Introduction
The focus of brain systems biology has trended towards integrated networks, which comprise a jigsaw of interacting brain regions. One region, the insula, located bilaterally in the lateral sulcus, plays a complex role in emotion, homeostasis (including error detection), autonomic function, sensation, salience, and awareness (Nieuwenhuys, 2012). It is also known to be involved in specific behaviors related to disease conditions such as migraine. Migraine attacks involve a wide range of sensory, emotional, and cognitive symptomology. It is thus conceivable that the insula may serve as a cortical hub, processing many of the complex sensory and emotional aspects known to be present in the migraine condition.
In this review, we explore known functional components of the insula and their potential role in migraine. Specifically, we first summarize Anatomical Aspects of the Insula in the first section and then briefly outline Functional Divisions of the Insula. Secondly, we discuss The Functional Insula in Migraine, on how the insula that may contribute to some of the observed sensory, physiological, psychological, and cognitive changes in migraine. In the final section, Insula and Migraine Therapy, we review data that support modulation of insula function in therapies used in migraine treatment. The review attempts to provide an overview of insular function in regards to migraine. Clearly, migraine is not “an insula disease”. However, since the insula is involved in complex behaviors associated with the migraine state, both ictally (the period of the migraine attack) and interictally (the period between migraine attacks), the insula could paly an important role in integrating many of the dynamic processes know to be involved in migraine (e.g., sensory, autonomic, cognitive). In addition, while attempting to provide a review of current information regarding this region’s involvement in migraine, it is a brain region involved in other conditions (i.e. fibromyalgia, chronic lower back pain, etc.) and needs to be considered in this context.
Anatomical Aspects of the Insula
The insula is a brain region located within the Sylvian fissure in the fronto-parietal and temporal opercula (Guenot et al., 2004). Figure 1A shows the gross anatomy of the insula. The insula consists of 5 – 7 lobes in humans, which are divided into anterior and posterior portions by the central insular sulcus. Further anatomical divisions have been described elsewhere (Ture et al., 1999). All insular gyri are interconnected, except the anterior and posterior short gyrus (Almashaikhi et al., 2014). Recent studies in humans using tractography (the process of tracing white matter tracts using MRI) reveals that the anterior insula is predominantly connected to limbic and paralimbic brain regions as well as the anterior parts of the inferior frontal gyrus, while the posterior insula is connected with parietal and posterior temporal cortices (Cerliani et al., 2012). These findings correlated with connections have also been reported in anatomical tract tracing studies in primates (reviewed by (Augustine, 1996)). The insula also has various connections with other cortical areas that include the visceral sensory area, visceral motor area, motor association area, vestibular area, as well as subcortical connections to the amygdala (Sah et al., 2003) and thalamus (Mufson and Mesulam, 1984). Accordingly, the insula is highly interconnected throughout the brain, thus providing the underpinnings of its involvement in a wide range of functions. Figure 1B shows horizontal and coronal sections through the insula providing a guide for the relative locations of the activations or gray matter changes shown in Figures 3 and 4.
Figure 1. Structural and Functional Anatomy of the Insula.
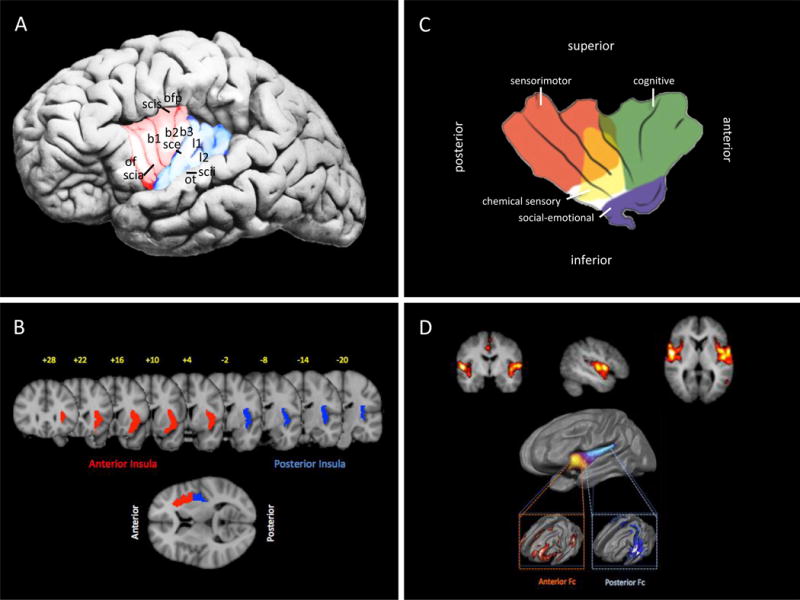
A: Gross Anatomy: Gross anatomy of the insula showing the insular gyri. The shaded red corresponds with the anterior insula, shaded blue with the posterior insula (as shown in Figure 2).
Key: of = operculum orbitofrontale, scia = sulcus circularis insulae pars anterior, b1 = gyrus brevis primus, b2 = gyrus brevis secundus, b3 = gyrus brevis tertius, sce = sulcus centralis insulae, l1 = gyrus longus primus, l2 = gyrus longus secundus, scii = sulcus circularis insulae pars inferior, ot = operculum temporale, scis = sulcus circularis insulae pars superior, ofp = operculum frotoparietale. (Adapted from (Nieuwenhuys, 2012) with permission).
B: MRI of Insula in Humans: The figure was constructed from FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Anterior/posterior insula were obtained from the Harvard-Oxford probabilistic atlas included in the FSL distribution. Anterior/posterior insula probabilistic maps were thresholded at 25% and rendered over the standard MNI152 2-mm anatomical image.
C:Functional Divisions: Functional areas in the insula are shown according to Kurth and colleagues (Kurth et al., 2010). (Adapted from Klein et al. (Klein et al., 2013b) with permission).
D: Functional Connectivity: Top: The insula is involved in the Salience network – which has been defined as a network that includes the insula, anterior cingulate and orbitofrontal cortices with strong connectivity to subcortical and limbic structures (Seeley et al., 2007) and the network has been considered to play a prominent role in pain and analgesia (Borsook et al., 2013b). Bottom: The figure ((Cauda et al., 2011) with permission) shows the two major divisions of the insula, anterior and posterior. The anterior ventral insula is functional connected to the salience detection network, while the dorsal insula is functional connected to a visuomotor integration network.
Figure 3. Migraine and Insula Activation Figures.
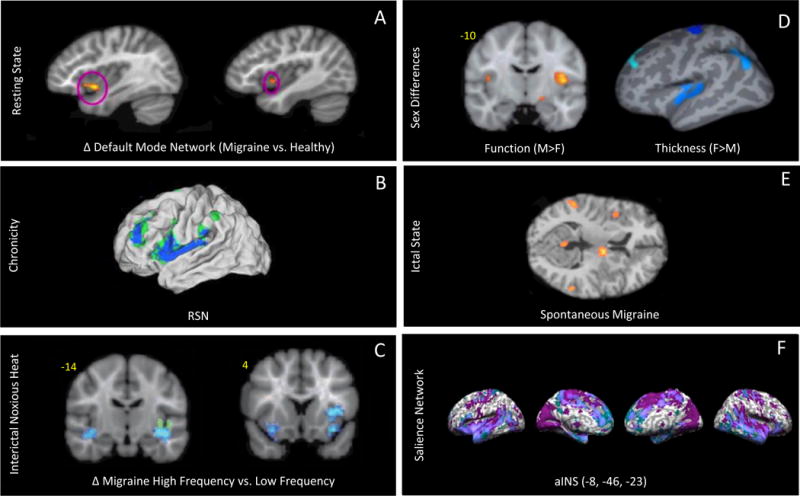
A: Resting State: Group comparison maps showing higher default mode network connectivity in migraine versus healthy controls in the left and right anterior insula. Adapted from (Xue et al., 2012) with permission. (shouldn’t this be discussed somewhere in the paper?)
B:Chronicity: Functional connections with affective pain regions have been shown to differ between patients with chronic migraine (CM) and healthy controls. The connectivity strength of the anterior insula was correlated with the number of years of CM (from (Schwedt et al., 2013) with permission) Remove or move. Not a treatment (should also be discussed in paper??)
C:Interictal Noxious Heat: fMRI contrast analysis of high frequency vs. low frequency migraineurs in response to a +1 °C pain threshold shows significant differences in the contralateral anterior insula in addition and bilateral inferior circular insula. These findings were co-localized with observed structural differences in the inferior circular insula (shown in green). Adapted from (Maleki et al., 2012a) with permission.
D: Sex Differences: Maps illustrating sex differences between male and female migraineurs in the insula. Males have higher fMRI activation in response to painful heat in the insula compared to females, whereas cortical thickness measured by MRI in the insula in females is increased compared to males. Adapted from (Maleki et al., 2012b) with permission.
E: Ictal State: PET measures of spontaneous migraine show right insula activation, adapted from (Afridi et al 2005) with permission
F: Salience Network: The anterior insula (aINS) shows increased connectivity patterns found in migraine subjects that are consistent with the salience network. Adapted from (Hubbard et al al 2014 PMC4399775) with permission.
Figure 4. Implications for Treating Migraine Through Insular Modification.
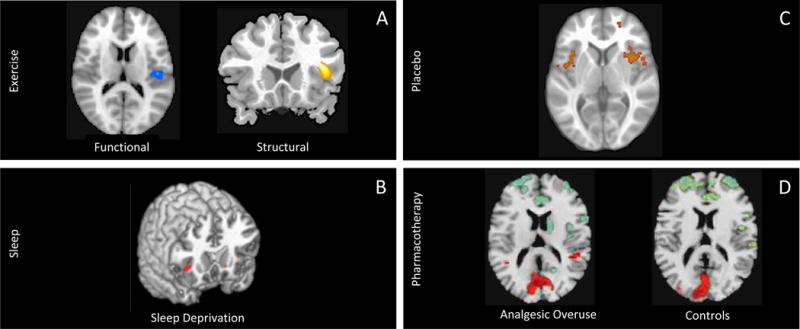
A: Exercise: Left: A single bout of aerobic exercise has been shown to affect insular blood flow (decrease) as assessed with BOLD fMRI using pseudo-continuous arterial spin labeling (pcASL) and to decrease activation by a “go/no-go” task (from (MacIntosh et al., 2014) with permission). Right: Cortical gray matter density in the right anterior insula has been shown to be strongly correlated with aerobic capacity ((Peters et al., 2009) with permission).
B: Sleep Deprivation: Sleep deprivation is associated with reduced activation in right anterior insula (Venkatraman et al., 2011).
C: Placebo: Using fMRI, treatment-resistant chronic back pain patients showed higher activation in the bilateral insula compared to chronic back pain patients who experienced a pain decrease following treatment. Adapted from (Hashmi et al., 2012) with permission.
D:Pharmacotherapy: Using 18F-FDG PET measurements, a significant increase in right insular metabolism has been reported in patents with chronic migraine (CM) and analgesic overuse (AO) compared to both patients with CM without AO and healthy controls (From (Di et al., 2013) with permission)
In addition to the complex connectivity, the neuronal population of the insula varies across the different subregions of the brain structure: from granular neocortex in the posterior-dorsal insula to agranular neocortex in the anterior-ventral insula (Bauernfeind et al., 2013). Specialized neurons, called von Economo Neurons (VENs), have been found in the insula (Allman et al., 2011; Evrard et al., 2012), specifically in the agranular frontoinsular (rostro-ventral) part (Bauernfeind et al., 2013). In humans, VENs are observed in the 36th week of gestation, are rare at birth, and increase in number during the first 8 months after birth (Allman et al., 2011). As they develop, there is a laterality with more found in the right insula (Allman et al., 2010), which shows dominance for a number of functions (see below). It has been therefore suggested that they play a critical role in complex characteristics like self-awareness and social cognition (Critchley and Seth, 2012). Differences in biochemical specificity that have been reported in VENs in humans vs. other hominids may relate to evolutionary development of specific processing such as interoception (Stimpson et al., 2011).
Functional Divisions of the Insula
The insula can also be functionally divided into anterior and posterior parts and exhibit functional divisions involved in somatosensory, autonomic, interoceptive, salience, and cognitive processing (see Figure 1C). The variation in function of the human insula (Cloutman et al., 2012) seems to correlate with structural connectivity noted above (Cerliani et al., 2012). Functionally, the anterior insula shows connections within the middle and inferior temporal cortex and anterior cingulate cortex, and is primarily related to limbic regions, which play a role in emotional modulations (Cauda et al., 2011). Furthermore, the anterior insula may also serve as a hub that integrates interactions with large-scale brain networks (Menon and Uddin, 2010) as it forms an integral part of the salience network (Damoiseaux et al., 2006), thus potentially incorporating behavioral responses with internal or external salient stimuli (Borsook et al., 2013b). The posterior region is more closely connected to the premotor, sensorimotor, supplementary motor and middle-posterior cingulate cortices, indicating a role for the posterior insula in sensorimotor integration (Cauda et al., 2011). Finally, anterior and posterior divisions of the insula are also functionally connected (Cauda et al., 2011) (see Figure 1D). Further details of the functional role of the insula are reviewed elsewhere (Afif and Mertens, 2010; Bauernfeind et al., 2013). It should also be noted that other authors have offered a slightly different version of sub-regional functions of the insula (Cauda et al., 2011; Deen et al., 2011; Kurth et al., 2010).
Although multiple functional features of the insula have been described, an understanding of the details of the functional anatomy of the insula is still elusive. There are some overarching features such as converting physiological states into emotions related to feeling and a sense of being. However, based on surgical, electrophysiological and imaging studies, different anatomical regions may have specific functional domains (Cauda et al., 2014; Dupont et al., 2003). Direct stimulation of the anterior insula in humans elicits very few reportable responses, whereas stimulation of the mid and posterior parts of the insula results in gustatory and somatosensory symptoms (Stephani et al., 2011). Painful sensations have also been reported following stimulation of the posterior insula (Mazzola et al., 2009), with more specificity noted in the upper posterior part of the human insular cortex and right-sided lateralization (Ostrowsky et al., 2002). This latter finding has been confirmed by functional magnetic resonance imaging (fMRI) studies showing that the insula is somatotopically organized (Brooks et al., 2005). Interestingly, the integration of visceral and somatic inputs into more complex outputs from the insula may relate to intra-insular functional connectivity, as significant connectivity between anterior and posterior sub-regions has been observed during neurosurgical evaluation in patients with refractory epilepsy (Almashaikhi et al., 2014).
Figure 2 summarizes afferent and efferent pathways involved in basic functions of the insula (viz., somatosensory, visceroception, cognition, etc.). These connections form the basis for many of the altered functions observed in migraine. Inputs include pain through trigemino-vascular afferents, dizziness through vestibular afferents, and visceral connections that may provide a basis for abdominal symptoms and autonomic changes. Insular outputs to subcortical and brainstem regions that contribute to cortical control of autonomic function, the cingulate cortex contributing to interoceptive responses, and to the frontal lobe that is related to cognitive processing.
Figure 2. Important Afferent and Efferent Insular Pathways in Migraine.
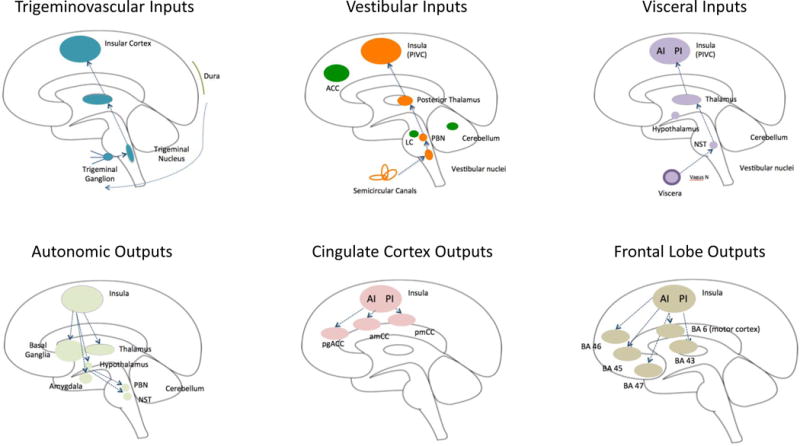
A: Afferent inputs (top row):
Trigeminovascular: The trigeminal system includes afferent inputs from the 3 major divisions of the trigeminal nerve (ophthalmic, maxillary and mandibular) that send their inputs to the trigeminal nuclear complex (Willis and Westlund, 1997). Projections from here are primarily to the thalamus and to the amygdala and hypothalamus through trigeminothalamic (Matsushita et al., 1982), trigemino-parabrachial-amygdala (Bernard and Besson, 1990), and trigeminohypothalamic (Malick and Burstein, 1998) tracts. Projections from these regions to the insula have been described for each primary projection complex (thalamo-insula (Mufson and Mesulam, 1984); hypothalamo-insula (Saper, 2000) and amygdalo-insula (Kevetter and Winans, 1981). As noted in the text some of these connections have been reported in functional imaging studies in migraine patients (amygdala-insular connections (Hadjikhani et al., 2013); see Figure 3B).
Vestibular: Vestibular inputs originate from the semicircular canals in the inner ear and project to the vestibular nuclei in the brainstem. The afferent pathway to the insula includes the parabrachial nuclei, posterior thalamus to the parieto-insula vestibular cortex (Brandt and Dieterich, 1999). These pathways are reviewed elsewhere (Angelaki and Cullen, 2008).
Visceral: Visceral inputs to the insula include pathways from the viscera (e.g., vagus nerve) to the nucleus of the solitary tract (NTS) (McDougal et al., 2011). Projections from the thalamus to the posterior insula relay visceral sensory information (Cechetto, 2014).
B: Efferent outputs (bottom row):
Autonomic: The insula is involved in cortical regulation of autonomic control (Nagai et al., 2010) (Jones, 2011). Efferents from the insula connect with multiple subcortical (basal ganglia, thalamus, and hypothalamus) and brainstem regions (e.g., parabrachial nucleus, nucleus of the solitary tract) (Beissner et al., 2013; Benarroch, 1993).
Cingulate Cortex: Connections between the insula and cingulate cortex are part of a number of networks. It has been postulated that the interactions between the insula and the cingulate are involved in ‘integrating interoceptive information with emotional salience’ (pACC/aMCC) or involved in ‘environmental monitoring, response selection, and skeletomotor body orientation’ (MCC) (Taylor et al., 2009). Pathways that are part of a circuit for processes such as empathy for pain (Engen and Singer, 2013; Singer et al., 2009; Singer et al., 2004) or the initiation of goal-directed behaviors (Devinsky et al., 1995).
Frontal Lobe: Pathways projecting from the insula to the frontal and motor cortex. The anterior insula connects to Brodmann Areas (BA) 45, 46, 47 and 6 (motor cortex), while the posterior insula has connections with 43. These connections are implicated in cognitive processing, speech, memory encoding and recognition (Ramnani and Owen, 2004; Wood and Grafman, 2003).
Key: ACC = Anterior Cingulate Cortex, PIVC = Parieto-Insular Vestibular Cortex; LC = Locus Coeruleus; PBN = Parabrachial Nucleus; AI = Anterior Insula; PI = Posterior Insula; NST = Solitary Nucleus; pgACC = Pregenual Anterior Cingulate Cortex; amCC = Anterior Midcingulate Cortex; pmCC = Posterior Midcingulate Cortex; BA = Brodmann Area.
The Functional Insula in Migraine
Based on its functional role and widespread connectivity with cortical and subcortical brain regions the insula has been proposed to play a significant role in neuropsychiatric disorders (Nagai et al., 2007) including mood and anxiety disorders (Buse et al., 2013; Sheftell and Atlas, 2002), temporal lobe epilepsy (Ostrowsky et al., 2000), craving and drug seeking in addiction (Naqvi et al., 2014), pain (Mazzola et al., 2009; Ostrowsky et al., 2002), and non-motor symptoms of Parkinson’s disease (Christopher et al., 2014). In fact, damage of the insula affects a number of important behaviors and perceptions including individuals’ sense of being, apathy or tiredness (Manes et al., 1999), neglect (Manes et al., 1999), temperature perception (Baier et al., 2014), dysarthria (Baier et al., 2011), auditory agnosia (Bamiou et al., 2003), processing of empathy (Gu et al., 2012), and drug-craving (Garavan, 2010).
As noted above, many of the putative functions ascribed to the insula also appear as symptoms in migraine so studying insular function and dysfunction may lead to further understanding and treatment. Table 1 lists imaging studies on migraine reporting insular changes (see criteria for PubMed search in table key). Migraine is more than a headache. It involves altered sensory, emotional, and cognitive processes (Charles, 2013). In the following sections, we will explore 4 principle domains of altered function (sensory, physiological, psychological and cognitive) in migraine and the putative involvement of the insula in these functions. Examples of insula activation across processes that can induce changes specific to migraine symptoms are those defined for underling functions in other studies including pain (Henderson et al., 2008), autonomic function (Henderson et al., 2012; James et al., 2013), cognition (Wiech et al., 2010), prediction error (Bossaerts, 2010; Preuschoff et al., 2008), and aversive processes such as disgust (Wright et al., 2004), vestibular function (Fasold et al., 2002) and nausea (Napadow et al., 2013).
Table 1.
Migraine Imaging Studies Reporting Changes in Insula Function and Structure
| Migraine type | State of Migraine |
Method | Experimental Paradigm (Stimulation) |
Subjects N (M/F) |
Effect (+/−) |
Laterality (R/L) |
Region (A/P) |
Reference |
|---|---|---|---|---|---|---|---|---|
| Functional Studies | ||||||||
| MWOA | ictal | PET | GTN | 1 (1/0) | + | R+L | P | (Bahra et al., 2001) |
| MWA(2)/MWOA(3) | ictal/interictal | PET | spontaneous | 5 (0/5) | + | R+L | – | (Afridi et al., 2005a) |
| MWOA | Ictal/interictal | PET | GTN | 8 (3/5) | + | R | – | (Maniyar et al., 2014) |
| MWA(8)/MWOA(16) | ictal | PET | GTN | 24 (10/14) | + | R+L | – | (Afridi et al., 2005b) |
| Vestibular | ictal/interictal | PET | spontaneous | 2(0/2) +MC | + | R | P | (Shin et al., 2014) |
| MWOA | ictal | SPECT | magnesium/placebo | 40 (5/35) | + | R | – | (Koseoglu et al., 2008) |
| MWA(4)/MWOA(6) | interictal | fMRI | verbal stimuli | 10(1/9) +MC | + | R+L | A+P | (Eck et al., 2011) |
| MWA(6)/MWOA(14) | ictal/interictal | fMRI | olfactory stimuli | 20 (5/15) | + | R | A+P | (Stankewitz and May, 2011) |
| MWOA | interictal | fMRI | spontaneous | 23(6/17) +MC | + | R+L | A | (Xue et al., 2012) |
| MWA(11)/MWOA(11) | interictal | fMRI | spontaneous | 22(2/20)+C | + | R+L | A | (Hadjikhani et al., 2013) |
| Chronic | interictal | fMRI | spontaneous | 20 (3/17) | + | R+L | A | (Schwedt et al., 2013) |
| MWA (20)/MWOA (10) | ictal/interictal | fMRI | spontaneous/olfactory stimuli | 20 (4/16) | + | R+L | A | (Stankewitz et al., 2013) |
| MWOA | interictal | fMRI | spontaneous | 40 (12/28)+MC | + | L | – | (Zhao et al., 2013) |
| Migraine + allodynia | interictal | fMRI | spontaneous | 38 (6/32) +C | + | R | P | (Schwedt et al., 2014) |
| MWA(26)/MWOA(26) | interictal | fMRI connectivity | spontaneous | 52(18/34) +MC | - (MWA) | R | A | (Niddam 2015 25888585) |
| MWOA | interictal | fMRI connectivity | spontaneous | 15(3/12) +MC | + | R+L | A | (Tso 2015 25663219) |
| Structural Studies | ||||||||
| Episodic | interictal | MRI/fMRI | spontaneous | 22(11/11) +MC | +/− | L | P | (Maleki et al., 2012b) |
| MWOA | interictal | MRI/fMRI | spontaneous | 40(11/29) +MC | + | L | – | (Yuan et al., 2013b) |
| MWOA | interictal | MRI/VBM | spontaneous | 35(3/32)+C | – | R | A | (Schmidt-Wilcke et al., 2008) |
| Drug Effect Studies | ||||||||
| Healthy + Sumatriptan | – | fMRI | soft brush | 12(4/8) | +/− | – | A/P | (Kramer et al., 2007) |
| Healthy + Sumatriptan | – | fMRI | electrical stimuli | 12 (8/4) | + | R | A | (Yuan et al., 2013a) |
| CM + PCA (AO) | interictal | PET | spontaneous | 20(7/13) +MC | + | R | – | (Di et al., 2013) |
| MWOA+ Acupuncture | ictal | PET-CT | electro (EAT) | 30(12/18) +MC | + (EAT) | – | – | (Yang 2012 3480944) |
Key: MWOA- Migraine without Aura, MWA- Migraine with Aura, MC- Matched Controls, GTN- glyceryl trinitrate, pMRI- Perfusion MRI, DWI- Diffusion Weighted Imaging, CM- Chronic Migraine, PCA- Paracetamol Caffeine Aspirin, AO- Analgesic overuse
Search Terms: Literature search of migraine and insula was undertaken using PubMed (http://www.ncbi.nlm.nih.gov/pubmed). Keywords used included: migraine, insula, anti-migraine drugs, gray matter, episodic migraine, and chronic migraine. Additional strategies included manual searches for relevant articles from the selected papers’ reference lists as well as utilization of PubMed’s related articles function.
In Figure 3A demonstrates the functional changes to the insula observed in migraine subjects compared to healthy controls. There is an increase in insular activity that corresponds to the default mode network (DMN) (Xue et al 2012), a network associated with normal brain function while at rest. Figure 3B displays the correlation between the number of years with migraine and increased functional alterations to the resting state network (RSN) (Schwedt et al 2013), a network synonymous with the DMN. The results of these studies indicate that migraine may not only alter insular activity but over time, these repeated attacks continue to continue to increasingly effect insula function.
Sensory Processes in Migraine
Pain
The major sensory symptom of migraine is head pain that is severe and debilitating (Silberstein, 2004). The posterior insula has inputs from the thalamus conveying nociceptive signals to the insular cortex (Craig, 2003; Craig et al., 2000). The insula has been called “a multidimensional integration site for pain” (Brooks and Tracey, 2007). Separation of innocuous (e.g., touch, proprioception, innocuous cold or warmth) and noxious stimuli (heat) has been reported in the human insula, as proprioception activates the contralateral mid-insula, innocuous cooling activates mid- and dorsal posterior insular parts, and pain activates the contralateral posterior insula, indicating that the insula may contribute to sensory-discriminative functions (Mazzola et al., 2012). Most recently, data obtained using intracerebrally recorded nociceptive laser evoked potentials (LEPs) from the full extent of the insula suggests that nociceptive input is first processed in the posterior insula, where it is coded in terms of intensity and anatomical location, after which is conveyed to the anterior insula, where the emotional reaction to pain is processed (Frot et al., 2014). Furthermore, both heat and pinprick stimuli demonstrated contralateral anterior and posterior insula activation (Baumgartner et al., 2010), further supporting the notion that the insula may be involved in sensory integration in pain. In addition, the pain inputs may be further incorporated into complex networks, such as the salience network, through the anterior insula (Wiech et al., 2010). Painful stimulation also leads to an increase in insula to periaqueductal grey functional connectivity (Linnman et al., 2012). Functional connectivity data for anterior and posterior insular regions activated by noxious and non-noxious stimuli have led to further segregation of pain processing, showing that the anterior insula is more strongly functionally connected to affective and cognitive regions, whereas the posterior insula has strong connectivity with sensory-discriminative processing regions (Peltz et al., 2011). The frequent activations of the anterior insula observed in pain fMRI studies may be interpreted as “heightened alertness of either stimulus- or task-driven origin, or both” that integrates internal and external stressors to maintain allostasis (Sterzer and Kleinschmidt, 2010).
Given that the insula appears to be a brain hub or convergence point for afferent inputs (predominantly nociceptive) and emotional processing, one would expect similar activations in pain conditions such as migraine (notwithstanding that other sensory information may flow in parallel). Cortical projections from trigeminovascular neurons in the thalamus have been described to the insula – as well as other cortical areas (Noseda et al., 2011). In patients with insula lesions, acute experimental noxious stimuli produce higher pain intensity ratings and an increased level of responses in the primary somatosensory cortex (S1) compared to healthy controls, suggesting that the insula is involved in “tuning cortical regions to appropriately use previous cognitive information during afferent processing” (Starr et al., 2009). Furthermore, a case of an epileptic focus in the posterior insula has been described, which produced pain (as opposed to inducing post-seizure related headaches) during a seizure and, in addition, stimulation of the same area elicited by pain (Isnard et al., 2011). Mapping of seizure-induced pain in the insula has shown predominantly in the sensory region of the posterior insula (Ostrowsky et al., 2002) supporting the notion that the posterior insula is involved in pain perception. Figure 3C demonstrates how high frequency (HF) migraineurs show decreased activation to painful stimuli compared with low frequency (LF) migraineurs (Maleki et al., 2012a). Additional findings of decreased cortical thickness of the insula in the HF group indicate a dynamic nature of this region with pain. This suggests that insular dysfunction may result form increased migraine frequency (Maleki et al., 2012a). Other insular changes in migraine are illustrated in Figure 3. The dynamic functional and morphological changes in the of the region include integrative process whereby sensory information is transferred to the anterior insula involved in emotional processing of pain (Frot et al., 2014). In this way repetitive migraine attacks may alter emotional processing through regions such as the insula.
Vestibular system
Other sensory alterations in migraine include vestibular dysfunction, which in turn contributes to symptoms of nausea and vomiting. Migraine may present a forme fruste ‘vestibular migraine’ condition (Furman et al., 2013); generally, many migraineurs have symptoms that are vestibular in nature. Small lesions of the insula may present with vertigo (Papacostas et al., 2006) and posterior insula strokes may coincide with both pseudothalamic sensory and vestibular-like syndromes (Cereda et al., 2002). Vestibular neurons in the insula have been found in monkeys (Grusser et al., 1990) that were affected by neck and visual responses. One of the reasons patients with migraine typically obtain relief from laying down may therefore relate to limiting neck and visual inputs that may contribute to vestibular symptoms. Vestibular symptoms, while more common in children and adolescents (Weisleder and Fife, 2001), are present in some 30 – 50% of migraine patients (Stolte et al., 2014).
Visceroception
Both smell and taste are altered in migraine, however, the most common symptoms relate to osmophobia (De Carlo et al., 2010; Kelman, 2004). Described as olfactory aura, burning smells, although uncommon, may precede or come on with the headache of some migraine attacks (Coleman et al., 2011). Osmophobia is frequently present in both pediatric and adult migraineurs (De Carlo et al., 2012; Wang et al., 2012; Zanchin et al., 2005). While around 20% of migraineurs have been reported to be anosmic or hyposmic (Hirsch, 1992), positron emission tomography (PET) studies show hypersensitivity to olfactory stimuli (Demarquay et al., 2008) with activations in a number of brain regions such as the temporal lobe, anterior cingulate, and locus coeruleus, but not the insula. Interictal olfactory hypersensitivity is reported (Marmura et al., 2014; Stankewitz and May, 2011) to be present in about 35% of migraineurs (Demarquay et al., 2006) and seems to be a predictor of olfactory triggers for migraine attacks. In addition, during a migraine attack some migraineurs have olfactory symptoms of microsmia or hyposmia (Marmura et al., 2014). During spontaneous migraine attacks, there is increased activation induced by olfactory stimuli (rose odor) in the amygdala and insula (Stankewitz and May, 2011). The perception of taste is integrated in the anterior insula and in patients with migraine may be involved in the aversive response or disgust to food as a potentially protective mechanism concomitant with symptoms of nausea and vomiting. Afferents from viscera terminate in the granular and dysgranular parts of the insula (Stephani et al., 2011). Taste is represented in the insula in both humans (Small, 2010) and rodents (Sewards and Sewards, 2001). Indeed, the insular cortex is the primary cortical region involved in taste and smell (Maffei et al., 2012), which may be part of a visceral response circuit that regulates food cravings and aversions (de Araujo and Simon, 2009). Interestingly, lesions of the insula are known to affect olfaction in humans (Mak et al., 2005; Stevenson et al., 2013), as well as diminished taste recognition and intensity deficits (Pritchard et al., 1999). Seizure-related emesis (ictus emeticus) (Shuper and Goldberg-Stern, 2004) has been reported in patients with lesions in the insula, suggesting a trigger zone for this process (Fiol et al., 1988) involving the spread of abnormal electrical activity through descending insular or limbic circuits (Shuper and Goldberg-Stern, 2004). Persistent nausea is a marker for severe migraine (Lipton et al., 2013). In addition to symptoms of nausea, abdominal pain may be a correlate of migraine (abdominal migraine) in children and adolescents (Carson et al., 2011). Accordingly, these findings confirm the complexity of visceral pathways in migraine, in which the insula may represent a major brain-processing site.
Physiological Processes in Migraine
The insula is involved in a wide range of physiological processes that are clinically altered in migraineurs, some of which are discussed below.
Autonomic
In migraineurs, alterations in autonomic function have been well documented. Overall, adult patients with migraine exhibit autonomic nervous system hypofunction, as measured in the interictal period (Shechter et al., 2002). Additionally, we have recently shown alterations in hypothalamic functional connectivity in episodic migraineurs compared with healthy controls (Moulton et al., 2014), thus possibly reflecting abnormal autonomic system. Altered hypothalamic function during migraine (Alstadhaug, 2009) may also suggest that the autonomic nervous system can trigger migraine attacks. The insula is one of many cortical regions involved in autonomic control (Cechetto, 2014; Jones, 2011; Nagai et al., 2010). Stimulation of cardiopulmonary afferents produce activation in the anterior insular cortex through a pathway that originates in the nucleus of the solitary tract and synapses in the parabrachial nucleus and the ventroposterior parvocellular nucleus of the thalamus (Cechetto, 2014) (see Figure 2). Autonomic function in the insula in migraine is still to be evaluated, but based on evidence of pain-related activations of the insula and its role in autonomic regulation (Leone et al., 2006), it is highly likely that altered regulation of autonomic function may also involve changes in insular functions (Geraud and Donnet, 2013). Further evidence has been seen in strokes occurring in the insula that result in increased susceptibility to cardio-autonomic dysfunction (Meyer et al., 2004).
Sleep
Sleep disruption is reported in migraine patients (Jennum and Jensen, 2002; Sahota and Dexter, 1990). During rapid eye movement (REM) sleep there is a relative activation in a number of brain areas (limbic and paralimbic) including the insula compared with the awake-state as assessed by PET fluoro-deoxy glucose (FDG) imaging, suggesting that involvement of these regions is part of “the integration of neocortical function with basal forebrain-hypothalamic motivational and reward mechanisms” (Nofzinger et al., 1997). In a follow-up study for slow wave sleep (SWS), the same group reported that whole brain metabolism decreased relative to the awake-state, even after controlling for whole brain decreases, but no changes were observed in the insula (Nofzinger et al., 2002). It has however been recently indicated that slow wave function may correlate with the thickness of the insula (Dube 2015 PMID:25995467). Others have also reported the role of the insula in the sleep-wake cycle including REM and non-REM components (Braun et al., 1997). The potential interaction of the insula and migraine may relate to adaptive and restorative changes, since sleep deprivation alters insula function related to decision-making (Venkatraman et al., 2011) and autonomic function (Konishi et al., 2013; Meerlo et al., 2008), including neural circulatory control (Kato et al., 2000). Given the important role of the insula in autonomic function and that alterations in cerebral blood flow may contribute to the headache phase (Asghar et al., 2011), altered sleep may thus diminish resilience in migraineurs. Figure 4B shows another example of the importance and effect of sleep with a decrease in activation in the insula from sleep deprivation.
Interoception
The role of the insula in interoception was introduced by Craig in the context of pain and sensation relating to mechanisms around ‘how do you feel’ the physiological state of the body (Craig, 2003). While other brain regions may be involved in sense of self (Critchley et al., 2004), the insula plays a key role in connecting these physiological interoceptive processes with feelings (Pollatos et al., 2007). The dorsal posterior insula is involved in interoceptive awareness (Craig, 2003), and more recently, the anterior insula has also been implicated in this process (Zaki et al., 2012). The overall integration of interoception may involve contributions of “discrete modules” (Evrard et al., 2014) and its integration in complex (e.g., salience-related (Borsook et al., 2013b) circuitry and interpretation of sensory inputs to the posterior insula and integration of autonomic control from the anterior insula. Interoception is likely important in migraine since a migraineur’s physiological internal state is different in ictal and interictal states. The physiological state in episodic migraine is manifestly altered in the peri-ictal phase with major sensory and autonomic changes that presumably have effects on interoceptive processing changing ‘feelings, energy, and effort’ and thus the subjective state of being (Craig, 2013).
Sex
Compared with males, females have a higher incidence and prevalence of migraine (Silberstein and Lipton, 1993; Stewart et al., 1994b). Sex differences relate to both brain and behavioral changes. Recently, our group reported structural and functional sex differences in the insula in female vs. male migraineurs (Maleki et al., 2012b). Furthermore, another study by our group shows that the insular cortex does not show normal thinning with age (Maleki et al., 2014 In submission). The latter is of interest since in other clinical disorders such as MDD, patients displayed decreased gray matter volumes in the left dorsal anterior insula (Liu et al., 2014). Figure 3D shows sex differences of insula gray matter volume in male and female migraineurs.
Age
The prevalence of migraine decreases significantly after the age of 65 years in both males and females (Victor et al., 2010). Between the ages of 12 – 30 years, the fiber density decreases between the insula and the frontal and parietal cortices but increases between the insula and the temporal cortex (Dennis et al., 2014). Functional implications also relate to insular connectivity in chronic pain states where brain structure and function shift from being adaptive in younger to being maladaptive in older patients (Ceko et al., 2013). The insula changes reported in aging (Foundas et al., 1998) result in functional alterations in pain processing (Tseng et al., 2013).
Psychological Processes in Migraine
Migraineurs exhibit significant psychiatric comorbidities (Buse et al., 2013; Sheftell and Atlas, 2002). In addition, migraineurs have altered processing in psychological domains such as mood (Marino et al., 2010), tiredness (Raggi et al., 2012), and disgust and/or unpleasantness of environmental stimuli (Demarquay et al., 2006). The insula has been defined as a ‘limbic integration cortex’ and is putatively involved in emotion. Some of these are discussed in more detail below.
Anxiety
As noted in a recent review, the insula may be involved in anxiety regulation (Paulus and Stein, 2006, 2010). Anxiety-prone subjects show increased activity in the anterior insula (Simmons et al., 2011); additionally, this subgroup showed increased insula activation in response to the anticipation of aversive stimuli (Simmons et al., 2006). In migraineurs, anticipation may contribute to enhancing anxiety-related circuits that include the insula and thus ‘drive’ a more anxious phenotype to expect the onset of the next migraine attack. Interestingly, panic attacks have a high comorbidity with migraine (Smitherman et al., 2013; Stewart et al., 1994a). Physical exercise (e.g., yoga) may modulate the nociceptive/pain afferent input and potentially the emotional reactions, such as anxiety, to the insula resulting in a change in insular brain anatomy and connectivity (Villemure et al., 2013). Related to anxiety is the phenomenon of panic attacks that are comorbid with migraine (Smitherman et al., 2013; Stewart et al., 1994a).
Stress and Safety Signals
Migraine is a stressor (Borsook et al., 2012; Radat, 2013). Measures of cortisol in migraine patients were found to be higher than control subjects at most times tested (Ziegler et al., 1979). “Safety signals are learned cues that predict stress-free periods whereas behavioral control is the ability to modify a stressor by behavioral actions” and in this way diminish the effects of stressors (Christianson et al., 2008; Christianson et al., 2011). Part of this stress inducing safety response may be through autonomic regulation (Cechetto, 2014). In tracing studies in rats, there is a convergence of autonomic and limbic function that may underlie the interaction of viscera-somatic inputs and behavioral processes (Saper, 1982). Taken together, the insula may be intimately involved in migraine, possibly through (1) integration of safety signals (Christianson et al., 2011), (2) autonomic function (Mosek et al., 1999; Shechter et al., 2002) (see above), and (3) translation of stress (Bossaerts, 2010) into behavioral outcomes (Craig, 2010). As part of the salience network (Borsook et al., 2013b), the region may help determine the resilience to stressors (i.e., repeated migraine attacks).
Affect and Lateralization
Neuroimaging studies have shown lateralization of affective pain and other processes (Duerden et al., 2013; Mutschler et al., 2012). Right-sided somatosensory lateralization of insula activation is evident from a number of studies in pain (Brooks et al., 2002; Symonds et al., 2006). In a PET study of spontaneous migraine, right-sided activation in the insula was present (Afridi et al., 2005a) (see Figure 3E). Right-sided lateralization has also been observed in cortical thickness in female migraineurs compared to male migraineurs (Maleki et al., 2014 In submission). In patients with major depressive disorder (MDD), insular hypoactivity is reported during homeostatic shifts, suggesting a deficit in the ability to have a normal response to changes in the environment (Strigo et al., 2010). The undulating onset and offset of migraine attacks may show micro episodes that parallel the MDD group, suggesting that mood swings may manifest in altered right insula processing (Coen et al., 2009).
Fatigue
Fatigue impacts some 70 – 84 % of migraine patients; it may last for days after the attack and is the most frequent psychosocial difficulty in these patients (Raggi et al., 2012). As reports of insular damage show, a putative basis for these changes may result from a functional disconnection with brain structures (e.g., frontal lobe, anterior cingulate cortex) involved with willed motor behavior (Manes et al., 1999).
Disgust
Disgust is one of basic emotion (Chapman and Anderson, 2012; Toronchuk and Ellis, 2012) that can be elicited by a wide variety of stimuli, both concrete or abstract in nature (e.g., rotten food, immoral persons) (Suzuki, 2010). Imaging studies on disgust have implicated the role of the insula (Phillips et al., 2004; Sprengelmeyer et al., 2011; Suzuki, 2012; Wicker et al., 2003; Wright et al., 2004). Since pain and disgust have been suggested to share some common themes (Kunz et al., 2013), the insula may therefore be responsible for certain processes in the peri-ictal migraine state (e.g., pain).
Appraisal of Aesthetic Properties
Neuroaesthetic processing may involve the evaluation and appreciation of valence of sensory processes (Brown et al., 2011). Unpleasant feelings can be induced in migraineurs taking triptans (Kramer et al., 2007). In the latter context, an fMRI study reported that following sumatriptan injection, increased activation was observed in the anterior insula and pain-related brain regions to brush stimuli, thus suggesting “sumatriptan could disinhibit nociceptive signaling and make light touch less pleasant” (Kramer et al., 2007). Unmyelinated tactile afferents that are present in hairy skin only project to posterior insular cortex and serve affective aspects of tactile sensation (Liljencrantz et al., 2013). This may be of significance given the recent report that botulinum toxin inhibits mechanoreceptors by altering the neuronal surface expression of high-threshold mechanosensitive ion channels (Burstein et al., 2014).
Odd Perceptions
Migraine attacks may be accompanied by sensory distortions including visual distortions (Huang et al., 2003), synesthesia (Alstadhaug and Benjaminsen, 2010), room tilt (Lopez Dominguez et al., 2007), and the ‘Alice in Wonderland Syndrome’ (Bayen et al., 2012; Ilik and Ilik, 2014). These changes may also include somatoparaphrenia (the sense of ownership towards our own body parts, that usually involve multiple brain lesions) (Gandola et al., 2012), which may be associated with “altered physiological index of perceptual analysis” to pain (Romano et al., 2014). It has been described in migraine patients that involve a diffuse number of structures (Moreira et al., 2010). In an fMRI study, abnormal, bilateral decreased activity is reported in the anterior insula in depressed patients that correlates with abnormal body perception (Wiebking et al., 2010). In patients with left posterior insular lesions, autoscopic abnormalities (visual illusory reduplication of their own body in extrapersonal space) have been described (Heydrich and Blanke, 2013).
Empathy
A circuit for empathy has been defined that includes the anterior insula and anterior midcingulate cortex (Engen and Singer, 2013; Singer et al., 2009). Empathy for pain also includes the anterior insula (Singer et al., 2004). Individuals with greater empathy were found to have more gray matter in the insula which also coincided with greater functional activation within the insula region (Bernhardt et al., 2013). Intriguingly, female episodic migraine patients exhibit more gray matter in the insula than male migraineurs (Maleki et al., 2012b). While the exact functional implications are not known, it has been suggested that ‘hyperempathy’ exists in migraine patients most of whom are female (Wendt, 2010), which is thought to reflect altered interactions with the amygdala, where increased insular connectivity was observed in migraine patients (Hadjikhani et al., 2013).
Cognitive Processes in Migraine
Prediction Error
Prediction error and migraine are reviewed elsewhere (Borsook et al., 2013a). Error awareness is the detection of the conscious and subconscious processing to evaluate physiological signals that are different from a baseline or homeostatic level (Borsook et al., 2013a; Ullsperger et al., 2010). The insula is considered to serve as an important hub in error awareness in a number of neurological conditions (Klein et al., 2013a).
Task Level Control
Because the insula is involved in integrating aspects of sensory-‘ceptive’ information with emotional and motor responses (through the anterior cingulate cortex) (Nelson et al., 2010), the inability to orchestrate ‘task level control’ or attentional control may be compromised. The changes may relate to processes previously reported as “saliency, switching, attention and control” (Menon and Uddin, 2010), which may relate to the inability to exclude information in migraine (Ditchfield et al., 2006; Koppen et al., 2011; Shepherd et al., 2012; Tibber et al., 2014; Wagner et al., 2013). In figure 3F we show how altered insula function is implicated in task error through the consequential effects on the salience network (SN), a network implicated in attention and learning. In fact, errors result in activation of the SN, which is driven by the anterior right insula (Ham et al 2013 23595766).
Insula and Migraine Therapy
Several approaches have been successfully used in routine clinical practice for the treatment of migraine patients, including exercise, stress reduction, pharmacotherapy, placebo, and cognitive behavioral therapy. It is conceivable that these methods mediate their effects through the insula. Below we review evidence on how treatments can affect insular cortex structure and function. It should be noted that while the insula may show activation, it might not mean that the insula mediates treatment directly, but that it may be part of an overall brain response to treatment. Treatment approaches have recently been focusing on altered interoceptive dysfunctions in other conditions such as drug addiction (Paulus et al., 2013). The use of fMRI can elucidate processes involved in functional and structural insula alterations. Figure 4 summarizes the effects on insula activation by a number of approaches used in the treatment of migraine (exercise (Fig 4A), sleep hygiene (Fig 4B), placebo response (Fig 4C)) as well as the effect of analgesic overuse as a result of treatment resulting in insula dysfunction (Fig 4D).
Exercise
Exercise has been considered to help brain health through active modulation of plasticity (Cotman and Berchtold, 2002) including alterations in brain derived neurotrophic factor (BDNF) (Gomez-Pinilla et al., 2008). Aerobic exercise increases cortical gray matter in the right anterior insula (Peters et al., 2009) and inhibits gray matter loss (Gondoh et al., 2009), even in aging population (Colcombe et al., 2006). Studies have suggested that aerobic exercise improves long-term outcomes in migraineurs (Lockett and Campbell, 1992). “Training the Brain” may enhance connectivity within the insula and improve the individual’s pain and emotional processing. Indeed, diminished pain may be observed with enhanced insular health (Starr et al., 2009), though underlying processes are still not understood.
Diminishing Stress
Under stressful conditions there may be increased susceptibility to migraine. The sensory insular cortex is involved in mitigating the effects of stress (Christianson et al., 2008) through safety signals (Christianson et al., 2011). Treatments that target stress may in part contribute to enhanced processing that diminishes stress through cognitive processes but also through training of the insula to limit afferent stress inputs (including pain). Indeed, there is enhanced pain in patients who anticipate pain (in migraineurs because of potentially and temporally ill defined onsets of severe headaches) because negative context may affect immediate and future pain (Phillips et al., 2003; Quartana et al., 2009). Stress may be a major contributor to allostatic load (McEwen and Gianaros, 2011) that may elicit and/or worsen migraine (Borsook et al., 2012). In fact, migraine also affects other well-known brain regions involved in the stress response. For example, decreased functional connectivity has been reported between the hippocampus and the bilateral anterior insula (Maleki et al., 2013).
Hydration
The insula plays a role in interoception – a process of evaluating internal physiological states (see above). Similar to responses observed with migraine patients, dehydration also produces enhanced pain to a cold pressor test with increased activation in brain regions including the insula. With healthy dehydrated subjects, upon rehydration, these effects are diminished (Ogino et al., 2013). In the clinical setting, maintenance of good hydration has long been considered helpful for migraine patients (Ostfeld and Wolff, 1955). Given the importance of the structure in autonomic function, and in particular cardiac function, hydration may have a biological foundation in the interactions of interoceptive processes and migraine.
Sleep
Migraineurs, both chronic and episodic suffer from poor sleep hygiene (Kelman and Rains, 2005; Sancisi et al., 2010; Walters et al., 2014). Figure 4B shows a reduction in activation of the right anterior insula in healthy subjects who have undergone sleep deprivation (Venkatramen 2011) further implicating the role of sleep in insula function, which may also have an effect on migraine attacks. Good sleep hygiene may therefore contribute to a balanced homeostatic and interoceptive state in migraine.
Pharmacotherapy
The main pharmacological treatment modalities for migraine include abortive (e.g., triptans) and preventive treatments (e.g., anti-seizure, antidepressant and potentially botulinum toxin). In a meta-analysis study of fMRI changes produced in emotional activation studies in patients with MDD, antidepressants produced a decrease in insula activation (Delaveau et al., 2011). In healthy subjects, the administration of the antidepressant escitalopram (a highly selective serotonin reuptake inhibitor (SSRI)) resulted in greater activation than overt and covert affective faces and affective words (Henry et al., 2013), suggesting that the mechanism of antidepressants in migraine patients may also modulate insular processing of emotional stimuli. As noted above, triptans produce a decrease of posterior insular cortex activation compared to the saline condition (Kramer et al., 2007). In the context of allodynia in migraine, triptans may be able to diminish the intensity and aversiveness of allodynia (Burstein et al., 2000). There is increasing excitement about the potential introduction of calcitonin gene-related peptide (CGRP) antagonists for migraine. Although most of the focus is on peripheral mechanisms, there are reports in preclinical studies of CGRP immunoreactivity in the insular cortex of rats that increase in response to aversive taste (Peyron et al., 2004). We are unaware of any imaging studies reporting insula changes as a result of specific anti-migraine therapies, whether symptomatic (e.g., nonsteroidals), abortive (e.g., triptans) or preventive (e.g., antidepressants, anti seizure) in nature. However, reports of direct pharmacological effects on insula dysfunction have been noted for paracetamol caffeine aspirin (PCA) powders in chronic migraine patients (Di et al., 2013), amitriptyline in patients with irritable bowel syndrome (IBS) and rectal pain (Morgan et al., 2005) and sumatriptan in healthy subjects (Kramer et al., 2007; Yuan et al., 2013b).
Placebo
Placebo can reduce expectations (Benedetti et al., 2011), diminish pain (Colloca et al., 2013), lessen disgust (Schienle et al., 2013), and enhance treatments (Bingel et al., 2011). The opposite effect, nocebo, may enhance adverse outcomes such as pain (Colloca and Benedetti, 2007). Interestingly, two basic systems are involved – opioidergic (Petrovic et al., 2002) and non-opioidergic mechanisms (Benedetti and Amanzio, 1997). Placebo is a powerful process in migraine (Kam-Hansen et al., 2014). Accumulating data supports the notion that placebos may change the brain (Benedetti et al., 2011). Moreover, the involvement of the insula in the placebo response has been repeatedly demonstrated in imaging studies (Hashmi et al., 2012; Sarinopoulos et al., 2006; Wager et al., 2004). However, it is still unclear how best to harness the placebo effect in the clinic (Jubb and Bensing, 2013). Migraine may be a perfect disease entity to embrace and use placebo in treatments. Figure 4D shows insula activation as a result of the placebo response. Few imaging studies have been performed evaluating the insula’s role in placebo in migraine patients, but such studies should contribute to our understanding on insula function in placebo in migraine patients compared with healthy controls.
Cognitive Behavioral Therapy
Patients with migraine, and specifically chronic migraine headaches, often pose a challenge for practitioners. Especially in patients with a higher frequency of headaches, attention to psychological and behavioral issues become a more important part of the therapeutic approach (Weeks, 2013). Cognitive behavioral therapy (CBT) may be an important and effective tool in the treatment of chronic pain and migraine, particularly when combined with pharmacological treatment. For example, a study in children and adolescents with chronic migraine showed that the use of CBT plus amitriptyline resulted in greater reductions in days with headache and migraine-related disability compared to headache education plus amitriptyline (Powers et al., 2013). CBT can be effective not only for treating chronic migraine, but also for treating comorbid diseases, such as psychiatric disorders and respiratory illness (Carod-Artal, 2014). However, it has also been suggested that individuals with (comorbid) depression and right anterior insula activation could be relatively impaired in their capacity to apply CBT-based therapies (Tegeler et al., 2014). In anxiety, successful CBT resulted in an overall down regulation of initial abnormal hyperactivity in fear-related brain regions such as the insula, amygdala, and anterior cingulate cortex (Lipka et al., 2013). Furthermore, CBT in patients with social anxiety disorder has been shown to reduce insular reactivity to fear-inducing stimuli over time (Klumpp et al., 2013). In summary, there is evidence that CBT can modulate activity in the brain that may be successful in treating migraine. The insula response as measured by brain imaging may provide an objective measure of such interventions (Klumpp et al., 2013).
Conclusions
The insula is involved in a wide range of functions. As such, it provides a cortical hub for the integration of extero- and interoceptive information inputs that can be transposed into higher-level behavioral function – i.e., it links “self-consciousness to the processing and integration of multisensory bodily signals” (Heydrich and Blanke, 2013). Figure 5 summarizes the interactions of putative regional functions of the insula in these functional domains as proposed for normal insula function shown in Figure 1B (Klein et al., 2013a). Accordingly, the insula constitutes an important brain region to study to further elucidate its role and plasticity in migraine and perhaps such an understanding may allow for measures that allow for therapeutic modulation of those processes that are integrated by the insula in the migraine phenotype.
Figure 5. Summary of Putative Insular Alterations in Migraine.
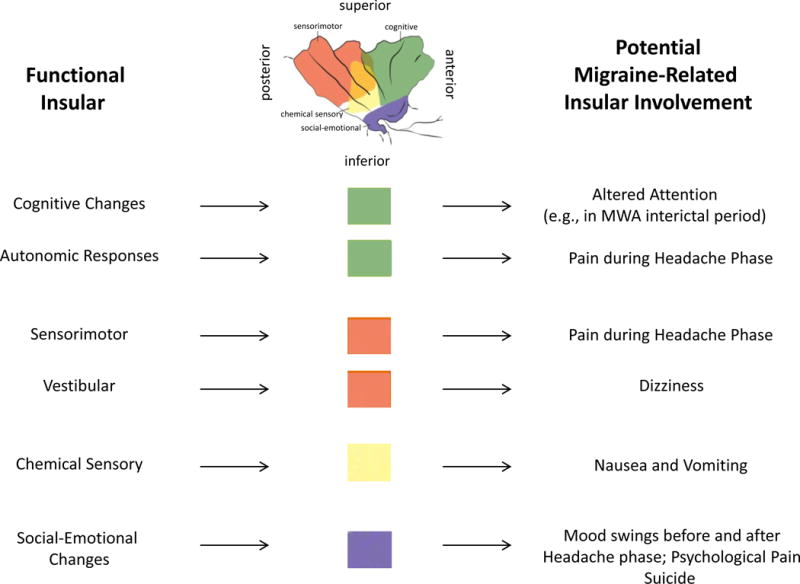
Many of the alterations in function in migraine involve insular processing of afferent and efferent information. The functional regions shown at the top of the figure are from Figure 1 (derived from (Klein et al., 2013b) with permission).
Acknowledgments
This work was supported by NIH R01 (adult ####)
Footnotes
The authors have no conflicts of interest to report.
References
- Afif A, Mertens P. Description of sulcal organization of the insular cortex. Surgical and radiologic anatomy : SRA. 2010;32:491–498. doi: 10.1007/s00276-009-0598-4. [DOI] [PubMed] [Google Scholar]
- Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RS, Goadsby PJ. A positron emission tomographic study in spontaneous migraine. Archives of neurology. 2005a;62:1270–1275. doi: 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain : a journal of neurology. 2005b;128:932–939. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain structure & function. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Park S. The von Economo neurons in apes and humans. American journal of human biology : the official journal of the Human Biology Council. 2011;23:5–21. doi: 10.1002/ajhb.21136. [DOI] [PubMed] [Google Scholar]
- Almashaikhi T, Rheims S, Ostrowsky-Coste K, Montavont A, Jung J, De Bellescize J, Arzimanoglou A, Keo Kosal P, Guenot M, Bertrand O, Ryvlin P. Intrainsular functional connectivity in human. Human brain mapping. 2014;35:2779–2788. doi: 10.1002/hbm.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstadhaug KB. Migraine and the hypothalamus. Cephalalgia : an international journal of headache. 2009;29:809–817. doi: 10.1111/j.1468-2982.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- Alstadhaug KB, Benjaminsen E. Synesthesia and migraine: case report. BMC neurology. 2010;10:121. doi: 10.1186/1471-2377-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annual review of neuroscience. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Annals of neurology. 2011;69:635–645. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain research. Brain research reviews. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–1017. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- Baier B, Zu Eulenburg P, Geber C, Rohde F, Rolke R, Maihofner C, Birklein F, Dieterich M. Insula and sensory insular cortex and somatosensory control in patients with insular stroke. European journal of pain. 2014 doi: 10.1002/j.1532-2149.2014.501.x. [DOI] [PubMed] [Google Scholar]
- Baier B, zu Eulenburg P, Glassl O, Dieterich M. Lesions to the posterior insular cortex cause dysarthria. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2011;18:1429–1431. doi: 10.1111/j.1468-1331.2011.03473.x. [DOI] [PubMed] [Google Scholar]
- Bamiou DE, Musiek FE, Luxon LM. The insula (Island of Reil) and its role in auditory processing. Literature review. Brain research. Brain research reviews. 2003;42:143–154. doi: 10.1016/s0165-0173(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Bauernfeind AL, de Sousa AA, Avasthi T, Dobson SD, Raghanti MA, Lewandowski AH, Zilles K, Semendeferi K, Allman JM, Craig AD, Hof PR, Sherwood CC. A volumetric comparison of the insular cortex and its subregions in primates. Journal of human evolution. 2013;64:263–279. doi: 10.1016/j.jhevol.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner U, Iannetti GD, Zambreanu L, Stoeter P, Treede RD, Tracey I. Multiple somatotopic representations of heat and mechanical pain in the operculo-insular cortex: a high-resolution fMRI study. Journal of neurophysiology. 2010;104:2863–2872. doi: 10.1152/jn.00253.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayen E, Cleret de Langavant L, Fenelon G. The Alice in Wonderland syndrome: an unusual aura in migraine. Revue neurologique. 2012;168:457–459. doi: 10.1016/j.neurol.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clinic proceedings. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M. The neurobiology of placebo analgesia: from endogenous opioids to cholecystokinin. Progress in neurobiology. 1997;52:109–125. doi: 10.1016/s0301-0082(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Carlino E, Pollo A. How placebos change the patient’s brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:339–354. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. Journal of neurophysiology. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Klimecki OM, Leiberg S, Singer T. Structural Covariance Networks of the Dorsal Anterior Insula Predict Females’ Individual Differences in Empathic Responding. Cerebral cortex. 2013 doi: 10.1093/cercor/bht072. [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science translational medicine. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Borsook D, Aasted CM, Burstein R, Becerra L. Migraine Mistakes: Error Awareness. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2013a doi: 10.1177/1073858413503711. [DOI] [PubMed] [Google Scholar]
- Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: the value of salience circuits. Progress in neurobiology. 2013b;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73:219–234. doi: 10.1016/j.neuron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Bossaerts P. Risk and risk prediction error signals in anterior insula. Brain structure & function. 2010;214:645–653. doi: 10.1007/s00429-010-0253-1. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dieterich M. The vestibular cortex. Its locations, functions, and disorders. Annals of the New York Academy of Sciences. 1999;871:293–312. doi: 10.1111/j.1749-6632.1999.tb09193.x. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain : a journal of neurology. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Tracey I. The insula: a multidimensional integration site for pain. Pain. 2007;128:1–2. doi: 10.1016/j.pain.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Brown S, Gao X, Tisdelle L, Eickhoff SB, Liotti M. Naturalizing aesthetics: brain areas for aesthetic appraisal across sensory modalities. Neuroimage. 2011;58:250–258. doi: 10.1016/j.neuroimage.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain : a journal of neurology. 2000;123(Pt 8):1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia : an international journal of headache. 2014 doi: 10.1177/0333102414527648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse DC, Silberstein SD, Manack AN, Papapetropoulos S, Lipton RB. Psychiatric comorbidities of episodic and chronic migraine. Journal of neurology. 2013;260:1960–1969. doi: 10.1007/s00415-012-6725-x. [DOI] [PubMed] [Google Scholar]
- Carod-Artal FJ. Tackling chronic migraine: current perspectives. Journal of pain research. 2014;7:185–194. doi: 10.2147/JPR.S61819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L, Lewis D, Tsou M, McGuire E, Surran B, Miller C, Vu TA. Abdominal migraine: an under-diagnosed cause of recurrent abdominal pain in children. Headache. 2011;51:707–712. doi: 10.1111/j.1526-4610.2011.01855.x. [DOI] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cauda F, Geminiani GC, Vercelli A. Evolutionary appearance of von Economo’s neurons in the mammalian cerebral cortex. Frontiers in human neuroscience. 2014;8:104. doi: 10.3389/fnhum.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF. Cortical control of the autonomic nervous system. Experimental physiology. 2014;99:326–331. doi: 10.1113/expphysiol.2013.075192. [DOI] [PubMed] [Google Scholar]
- Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. Fibromyalgia interacts with age to change the brain. NeuroImage Clinical. 2013;3:249–260. doi: 10.1016/j.nicl.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda C, Ghika J, Maeder P, Bogousslavsky J. Strokes restricted to the insular cortex. Neurology. 2002;59:1950–1955. doi: 10.1212/01.wnl.0000038905.75660.bd. [DOI] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JC, Nanetti L, Crippa A, Gazzola V, D’Arceuil H, Keysers C. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Human brain mapping. 2012;33:2005–2034. doi: 10.1002/hbm.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA, Anderson AK. Understanding disgust. Annals of the New York Academy of Sciences. 2012;1251:62–76. doi: 10.1111/j.1749-6632.2011.06369.x. [DOI] [PubMed] [Google Scholar]
- Charles A. Migraine: a brain state. Current opinion in neurology. 2013;26:235–239. doi: 10.1097/WCO.0b013e32836085f4. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, Baratta MV, Paul ED, Campeau S, Watkins LR, Barth DS, Maier SF. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13703–13711. doi: 10.1523/JNEUROSCI.4270-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Jennings JH, Ragole T, Flyer JG, Benison AM, Barth DS, Watkins LR, Maier SF. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biological psychiatry. 2011;70:458–464. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher L, Koshimori Y, Lang AE, Criaud M, Strafella AP. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain : a journal of neurology. 2014 doi: 10.1093/brain/awu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman LL, Binney RJ, Drakesmith M, Parker GJ, Lambon Ralph MA. The variation of function across the human insula mirrors its patterns of structural connectivity: evidence from in vivo probabilistic tractography. Neuroimage. 2012;59:3514–3521. doi: 10.1016/j.neuroimage.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Coen SJ, Yaguez L, Aziz Q, Mitterschiffthaler MT, Brammer M, Williams SC, Gregory LJ. Negative mood affects brain processing of visceral sensation. Gastroenterology. 2009;137:253–261. 261 e251–252. doi: 10.1053/j.gastro.2009.02.052. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. (Series A, Biological sciences and medical sciences).The journals of gerontology. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Coleman ER, Grosberg BM, Robbins MS. Olfactory hallucinations in primary headache disorders: case series and literature review. Cephalalgia : an international journal of headache. 2011;31:1477–1489. doi: 10.1177/0333102411423315. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Current opinion in anaesthesiology. 2007;20:435–439. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- Colloca L, Klinger R, Flor H, Bingel U. Placebo analgesia: psychological and neurobiological mechanisms. Pain. 2013;154:511–514. doi: 10.1016/j.pain.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current opinion in neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain structure & function. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Craig AD. An interoceptive neuroanatomical perspective on feelings, energy, and effort. The Behavioral and brain sciences. 2013;36:685–686. doi: 10.1017/S0140525X13001489. discussion 707-626. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nature neuroscience. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Critchley H, Seth A. Will studies of macaque insula reveal the neural mechanisms of self-awareness? Neuron. 2012;74:423–426. doi: 10.1016/j.neuron.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Simon SA. The gustatory cortex and multisensory integration. International journal of obesity. 2009;33(Suppl 2):S34–43. doi: 10.1038/ijo.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carlo D, Dal Zotto L, Perissinotto E, Gallo L, Gatta M, Balottin U, Mazzotta G, Moscato D, Raieli V, Rossi LN, Sangermani R, Soriani S, Termine C, Tozzi E, Vecchio A, Zanchin G, Battistella PA. Osmophobia in migraine classification: a multicentre study in juvenile patients. Cephalalgia : an international journal of headache. 2010;30:1486–1494. doi: 10.1177/0333102410362928. [DOI] [PubMed] [Google Scholar]
- De Carlo D, Toldo I, Dal Zotto L, Perissinotto E, Sartori S, Gatta M, Balottin U, Mazzotta G, Moscato D, Raieli V, Rossi LN, Sangermani R, Soriani S, Termine C, Tozzi E, Vecchio A, Zanchin G, Battistella PA. Osmophobia as an early marker of migraine: a follow-up study in juvenile patients. Cephalalgia : an international journal of headache. 2012;32:401–406. doi: 10.1177/0333102412438975. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. Journal of affective disorders. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Demarquay G, Royet JP, Giraud P, Chazot G, Valade D, Ryvlin P. Rating of olfactory judgements in migraine patients. Cephalalgia : an international journal of headache. 2006;26:1123–1130. doi: 10.1111/j.1468-2982.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- Demarquay G, Royet JP, Mick G, Ryvlin P. Olfactory hypersensitivity in migraineurs: a H(2)(15)O-PET study. Cephalalgia : an international journal of headache. 2008;28:1069–1080. doi: 10.1111/j.1468-2982.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, McMahon KL, de Zubicaray GI, Martin NG, Hickie IB, Toga AW, Wright MJ, Thompson PM. Development of insula connectivity between ages 12 and 30 revealed by high angular resolution diffusion imaging. Human brain mapping. 2014;35:1790–1800. doi: 10.1002/hbm.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain : a journal of neurology. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Di W, Shi X, Zhu Y, Tao Y, Qi W, Luo N, Xiao Z, Yi C, Miao J, Zhang A, Zhang X, Fang Y. Overuse of paracetamol caffeine aspirin powders affects cerebral glucose metabolism in chronic migraine patients. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2013;20:655–662. doi: 10.1111/ene.12018. [DOI] [PubMed] [Google Scholar]
- Ditchfield JA, McKendrick AM, Badcock DR. Processing of global form and motion in migraineurs. Vision research. 2006;46:141–148. doi: 10.1016/j.visres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Arsalidou M, Lee M, Taylor MJ. Lateralization of affective processing in the insula. Neuroimage. 2013;78:159–175. doi: 10.1016/j.neuroimage.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: new insights from imaging. Surgical and radiologic anatomy : SRA. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Eck J, Richter M, Straube T, Miltner WH, Weiss T. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain. 2011;152:1104–1113. doi: 10.1016/j.pain.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Engen HG, Singer T. Empathy circuits. Current opinion in neurobiology. 2013;23:275–282. doi: 10.1016/j.conb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Forro T, Logothetis NK. Von Economo neurons in the anterior insula of the macaque monkey. Neuron. 2012;74:482–489. doi: 10.1016/j.neuron.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Logothetis NK, Craig AD. Modular architectonic organization of the insula in the macaque monkey. J Comp Neurol. 2014;522:64–97. doi: 10.1002/cne.23436. [DOI] [PubMed] [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, Wenzel R. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002;17:1384–1393. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Fiol ME, Leppik IE, Mireles R, Maxwell R. Ictus emeticus and the insular cortex. Epilepsy research. 1988;2:127–131. doi: 10.1016/0920-1211(88)90030-7. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Zipin D, Browning CA. Age-related changes of the insular cortex and lateral ventricles: conventional MRI volumetric measures. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 1998;8:216–221. doi: 10.1111/jon199884216. [DOI] [PubMed] [Google Scholar]
- Frank S, Kullmann S, Veit R. Food related processes in the insular cortex. Frontiers in human neuroscience. 2013;7:499. doi: 10.3389/fnhum.2013.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Lee S, Preissl H, Schultes B, Birbaumer N, Veit R. The obese brain athlete: self-regulation of the anterior insula in adiposity. PloS one. 2012;7:e42570. doi: 10.1371/journal.pone.0042570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frot M, Faillenot I, Mauguiere F. Processing of nociceptive input from posterior to anterior insula in humans. Human brain mapping. 2014 doi: 10.1002/hbm.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet neurology. 2013;12:706–715. doi: 10.1016/S1474-4422(13)70107-8. [DOI] [PubMed] [Google Scholar]
- Gandola M, Invernizzi P, Sedda A, Ferre ER, Sterzi R, Sberna M, Paulesu E, Bottini G. An anatomical account of somatoparaphrenia. Cortex; a journal devoted to the study of the nervous system and behavior. 2012;48:1165–1178. doi: 10.1016/j.cortex.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain structure & function. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- Geraud G, Donnet A. Migraine and hypothalamus. Revue neurologique. 2013;169:372–379. doi: 10.1016/j.neurol.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. The European journal of neuroscience. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondoh Y, Sensui H, Kinomura S, Fukuda H, Fujimoto T, Masud M, Nagamatsu T, Tamaki H, Takekura H. Effects of aerobic exercise training on brain structure and psychological well-being in young adults. The Journal of sports medicine and physical fitness. 2009;49:129–135. [PubMed] [Google Scholar]
- Grusser OJ, Pause M, Schreiter U. Vestibular neurones in the parieto-insular cortex of monkeys (Macaca fascicularis): visual and neck receptor responses. The Journal of physiology. 1990;430:559–583. doi: 10.1113/jphysiol.1990.sp018307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, Fan J. Anterior insular cortex is necessary for empathetic pain perception. Brain : a journal of neurology. 2012;135:2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenot M, Isnard J, Sindou M. Surgical anatomy of the insula. Advances and technical standards in neurosurgery. 2004;29:265–288. doi: 10.1007/978-3-7091-0558-0_7. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Ward N, Boshyan J, Napadow V, Maeda Y, Truini A, Caramia F, Tinelli E, Mainero C. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia : an international journal of headache. 2013;33:1264–1268. doi: 10.1177/0333102413490344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ, Apkarian AV. Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain. 2012;153:2393–2402. doi: 10.1016/j.pain.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. Neuroimage. 2008;39:1867–1876. doi: 10.1016/j.neuroimage.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Henderson LA, James C, Macefield VG. Identification of sites of sympathetic outflow during concurrent recordings of sympathetic nerve activity and fMRI. Anat Rec (Hoboken) 2012;295:1396–1403. doi: 10.1002/ar.22513. [DOI] [PubMed] [Google Scholar]
- Henry ME, Lauriat TL, Lowen SB, Churchill JH, Hodgkinson CA, Goldman D, Renshaw PF. Effects of citalopram and escitalopram on fMRI response to affective stimuli in healthy volunteers selected by serotonin transporter genotype. Psychiatry research. 2013;213:217–224. doi: 10.1016/j.pscychresns.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydrich L, Blanke O. Distinct illusory own-body perceptions caused by damage to posterior insula and extrastriate cortex. Brain : a journal of neurology. 2013;136:790–803. doi: 10.1093/brain/aws364. [DOI] [PubMed] [Google Scholar]
- Hirsch AR. Olfaction in migraineurs. Headache. 1992;32:233–236. doi: 10.1111/j.1526-4610.1992.hed3205233.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Cooper TG, Satana B, Kaufman DI, Cao Y. Visual distortion provoked by a stimulus in migraine associated with hyperneuronal activity. Headache. 2003;43:664–671. doi: 10.1046/j.1526-4610.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- Ilik F, Ilik K. Alice in Wonderland syndrome as aura of migraine. Neurocase. 2014;20:474–475. doi: 10.1080/13554794.2013.826676. [DOI] [PubMed] [Google Scholar]
- Isnard J, Magnin M, Jung J, Mauguiere F, Garcia-Larrea L. Does the insula tell our brain that we are in pain? Pain. 2011;152:946–951. doi: 10.1016/j.pain.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Jager HR, Giffin NJ, Goadsby PJ. Diffusion- and perfusion-weighted MR imaging in persistent migrainous visual disturbances. Cephalalgia : an international journal of headache. 2005;25:323–332. doi: 10.1111/j.1468-2982.2004.00858.x. [DOI] [PubMed] [Google Scholar]
- James C, Henderson L, Macefield VG. Real-time imaging of brain areas involved in the generation of spontaneous skin sympathetic nerve activity at rest. Neuroimage. 2013;74:188–194. doi: 10.1016/j.neuroimage.2013.02.030. [DOI] [PubMed] [Google Scholar]
- Jennum P, Jensen R. Sleep and headache. Sleep medicine reviews. 2002;6:471–479. doi: 10.1053/smrv.2001.0223. [DOI] [PubMed] [Google Scholar]
- Jones SE. Imaging for autonomic dysfunction. Cleveland Clinic journal of medicine. 2011;78(Suppl 1):S69–74. doi: 10.3949/ccjm.78.s1.12. [DOI] [PubMed] [Google Scholar]
- Jubb J, Bensing JM. The sweetest pill to swallow: how patient neurobiology can be harnessed to maximise placebo effects. Neuroscience and biobehavioral reviews. 2013;37:2709–2720. doi: 10.1016/j.neubiorev.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, Burstein R. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Science translational medicine. 2014;6:218ra215. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–1175. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- Kelman L. The place of osmophobia and taste abnormalities in migraine classification: a tertiary care study of 1237 patients. Cephalalgia : an international journal of headache. 2004;24:940–946. doi: 10.1111/j.1468-2982.2004.00766.x. [DOI] [PubMed] [Google Scholar]
- Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45:904–910. doi: 10.1111/j.1526-4610.2005.05159.x. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the “olfactory amygdala”. J Comp Neurol. 1981;197:99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Klein TA, Danielmeier C, Ullsperger M. Editorial for E-Book: error awareness-insights from cognitive neuroscience, psychiatry and neurology. Frontiers in human neuroscience. 2013a;7:830. doi: 10.3389/fnhum.2013.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Ullsperger M, Danielmeier C. Error awareness and the insula: links to neurological and psychiatric diseases. Frontiers in human neuroscience. 2013b;7:14. doi: 10.3389/fnhum.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Phan KL. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2013;45:83–91. doi: 10.1016/j.pnpbp.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Takahashi M, Endo N, Numao S, Takagi S, Miyashita M, Midorikawa T, Suzuki K, Sakamoto S. Effects of sleep deprivation on autonomic and endocrine functions throughout the day and on exercise tolerance in the evening. Journal of sports sciences. 2013;31:248–255. doi: 10.1080/02640414.2012.733824. [DOI] [PubMed] [Google Scholar]
- Koppen H, Palm-Meinders I, Kruit M, Lim V, Nugroho A, Westhof I, Terwindt G, van Buchem M, Ferrari M, Hommel B. The impact of a migraine attack and its after-effects on perceptual organization, attention, and working memory. Cephalalgia : an international journal of headache. 2011;31:1419–1427. doi: 10.1177/0333102411417900. [DOI] [PubMed] [Google Scholar]
- Koseoglu E, Talaslioglu A, Gonul AS, Kula M. The effects of magnesium prophylaxis in migraine without aura. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2008;21:101–108. [PubMed] [Google Scholar]
- Kramer HH, Lundblad L, Birklein F, Linde M, Karlsson T, Elam M, Olausson H. Activation of the cortical pain network by soft tactile stimulation after injection of sumatriptan. Pain. 2007;133:72–78. doi: 10.1016/j.pain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Kunz M, Peter J, Huster S, Lautenbacher S. Pain and disgust: the facial signaling of two aversive bodily experiences. PloS one. 2013;8:e83277. doi: 10.1371/journal.pone.0083277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain structure & function. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone M, Proietti Cecchini A, Mea E, Tullo V, Curone M, Bussone G. Neuroimaging and pain: a window on the autonomic nervous system. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2006;27(Suppl 2):S134–137. doi: 10.1007/s10072-006-0588-9. [DOI] [PubMed] [Google Scholar]
- Liljencrantz J, Bjornsdotter M, Morrison I, Bergstrand S, Ceko M, Seminowicz DA, Cole J, Bushnell MC, Olausson H. Altered C-tactile processing in human dynamic tactile allodynia. Pain. 2013;154:227–234. doi: 10.1016/j.pain.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Linnman C, Beucke JC, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2012;153:444–454. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka J, Hoffmann M, Miltner WH, Straube T. Effects of Cognitive-Behavioral Therapy on Brain Responses to Subliminal and Supraliminal Threat and Their Functional Significance in Specific Phobia. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Buse DC, Saiers J, Fanning KM, Serrano D, Reed ML. Frequency and burden of headache-related nausea: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53:93–103. doi: 10.1111/j.1526-4610.2012.02292.x. [DOI] [PubMed] [Google Scholar]
- Liu CH, Jing B, Ma X, Xu PF, Zhang Y, Li F, Wang YP, Tang LR, Wang YJ, Li HY, Wang CY. Voxel-based morphometry study of the insular cortex in female patients with current and remitted depression. Neuroscience. 2014;262:190–199. doi: 10.1016/j.neuroscience.2013.12.058. [DOI] [PubMed] [Google Scholar]
- Lockett DM, Campbell JF. The effects of aerobic exercise on migraine. Headache. 1992;32:50–54. doi: 10.1111/j.1526-4610.1992.hed3201050.x. [DOI] [PubMed] [Google Scholar]
- Lopez Dominguez JM, Rojas-Marcos I, Sanz Fernandez G, Diaz Espejo C. Room tilt illusion: a rare symptom of migraine aura. Neurologia. 2007;22:58–60. [PubMed] [Google Scholar]
- MacIntosh BJ, Crane DE, Sage MD, Rajab AS, Donahue MJ, McIlroy WE, Middleton LE. Impact of a single bout of aerobic exercise on regional brain perfusion and activation responses in healthy young adults. PloS one. 2014;9:e85163. doi: 10.1371/journal.pone.0085163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Haley M, Fontanini A. Neural processing of gustatory information in insular circuits. Current opinion in neurobiology. 2012;22:709–716. doi: 10.1016/j.conb.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak YE, Simmons KB, Gitelman DR, Small DM. Taste and olfactory intensity perception changes following left insular stroke. Behavioral neuroscience. 2005;119:1693–1700. doi: 10.1037/0735-7044.119.6.1693. [DOI] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia : an international journal of headache. 2012a;32:607–620. doi: 10.1177/0333102412445622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain structure & function. 2013;218:903–912. doi: 10.1007/s00429-012-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain : a journal of neurology. 2012b;135:2546–2559. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malick A, Burstein R. Cells of origin of the trigeminohypothalamic tract in the rat. J Comp Neurol. 1998;400:125–144. doi: 10.1002/(sici)1096-9861(19981012)400:1<125::aid-cne9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Manes F, Paradiso S, Springer JA, Lamberty G, Robinson RG. Neglect after right insular cortex infarction. Stroke; a journal of cerebral circulation. 1999;30:946–948. doi: 10.1161/01.str.30.5.946. [DOI] [PubMed] [Google Scholar]
- Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain : a journal of neurology. 2014;137:232–241. doi: 10.1093/brain/awt320. [DOI] [PubMed] [Google Scholar]
- Marino E, Fanny B, Lorenzi C, Pirovano A, Franchini L, Colombo C, Bramanti P, Smeraldi E. Genetic bases of comorbidity between mood disorders and migraine: possible role of serotonin transporter gene. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2010;31:387–391. doi: 10.1007/s10072-009-0183-y. [DOI] [PubMed] [Google Scholar]
- Marmura MJ, Monteith TS, Anjum W, Doty RL, Hegarty SE, Keith SW. Olfactory function in migraine both during and between attacks. Cephalalgia : an international journal of headache. 2014 doi: 10.1177/0333102414527014. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M, Okado N. The cells of origin of the trigeminothalamic, trigeminospinal and trigeminocerebellar projections in the cat. Neuroscience. 1982;7:1439–1454. doi: 10.1016/0306-4522(82)90256-1. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Faillenot I, Barral FG, Mauguiere F, Peyron R. Spatial segregation of somato-sensory and pain activations in the human operculo-insular cortex. Neuroimage. 2012;60:409–418. doi: 10.1016/j.neuroimage.2011.12.072. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Peyron R, Guenot M, Mauguiere F. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain. 2009;146:99–104. doi: 10.1016/j.pain.2009.07.014. [DOI] [PubMed] [Google Scholar]
- McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14037–14045. doi: 10.1523/JNEUROSCI.2855-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual review of medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep medicine reviews. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Strittmatter M, Fischer C, Georg T, Schmitz B. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport. 2004;15:357–361. doi: 10.1097/00001756-200402090-00029. [DOI] [PubMed] [Google Scholar]
- Moreira T, Menetrey A, Carota A. Neurological picture. Cortical oedema: a link between delusional misidentification syndromes and hemiplegic migraine. Journal of neurology, neurosurgery, and psychiatry. 2010;81:52–53. doi: 10.1136/jnnp.2009.178137. [DOI] [PubMed] [Google Scholar]
- Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–607. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosek A, Novak V, Opfer-Gehrking TL, Swanson JW, Low PA. Autonomic dysfunction in migraineurs. Headache. 1999;39:108–117. doi: 10.1046/j.1526-4610.1999.3902108.x. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Becerra L, Johnson A, Burstein R, Borsook D. Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PloS one. 2014;9:e95508. doi: 10.1371/journal.pone.0095508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol. 1984;227:109–120. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neuroscience letters. 2012;520:204–209. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. Journal of the American Society of Hypertension : JASH. 2010;4:174–182. doi: 10.1016/j.jash.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: a review of recent literature. European psychiatry : the journal of the Association of European Psychiatrists. 2007;22:387–394. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Napadow V, Sheehan JD, Kim J, Lacount LT, Park K, Kaptchuk TJ, Rosen BR, Kuo B. The brain circuitry underlying the temporal evolution of nausea in humans. Cerebral cortex. 2013;23:806–813. doi: 10.1093/cercor/bhs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Annals of the New York Academy of Sciences. 2014 doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain structure & function. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R. The insular cortex: a review. Progress in brain research. 2012;195:123–163. doi: 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Miewald JM, Meltzer CC, Price JC, Sembrat RC, Ombao H, Reynolds CF, Monk TH, Hall M, Kupfer DJ, Moore RY. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain : a journal of neurology. 2002;125:1105–1115. doi: 10.1093/brain/awf103. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Wiseman M, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: an FDG PET study. Brain research. 1997;770:192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]
- Noseda R, Jakubowski M, Kainz V, Borsook D, Burstein R. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14204–14217. doi: 10.1523/JNEUROSCI.3285-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino Y, Kakeda T, Nakamura K, Saito S. Dehydration Enhances Pain-Evoked Activation in the Human Brain Compared with Rehydration. Anesthesia and analgesia. 2013 doi: 10.1213/ANE.0b013e3182a9b028. [DOI] [PubMed] [Google Scholar]
- Ostfeld AM, Wolff HG. Studies on headache: headache and hydration. Transactions of the American Neurological Association. 1955:217–219. [PubMed] [Google Scholar]
- Ostrowsky K, Isnard J, Ryvlin P, Guenot M, Fischer C, Mauguiere F. Functional mapping of the insular cortex: clinical implication in temporal lobe epilepsy. Epilepsia. 2000;41:681–686. doi: 10.1111/j.1528-1157.2000.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cerebral cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- Papacostas SS, Myrianthopoulou P, Papathanasiou E. Epileptic seizures followed by nonepileptic manifestations: a video-EEG diagnosis. Electromyography and clinical neurophysiology. 2006;46:323–327. [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain structure & function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL, Haase L. Treatment approaches for interoceptive dysfunctions in drug addiction. Frontiers in psychiatry. 2013;4:137. doi: 10.3389/fpsyt.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz E, Seifert F, DeCol R, Dorfler A, Schwab S, Maihofner C. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage. 2011;54:1324–1335. doi: 10.1016/j.neuroimage.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Peters J, Dauvermann M, Mette C, Platen P, Franke J, Hinrichs T, Daum I. Voxel-based morphometry reveals an association between aerobic capacity and grey matter density in the right anterior insula. Neuroscience. 2009;163:1102–1108. doi: 10.1016/j.neuroscience.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia–imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Peyron R, Schneider F, Faillenot I, Convers P, Barral FG, Garcia-Larrea L, Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Gregory LJ, Cullen S, Coen S, Ng V, Andrew C, Giampietro V, Bullmore E, Zelaya F, Amaro E, Thompson DG, Hobson AR, Williams SC, Brammer M, Aziz Q. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain : a journal of neurology. 2003;126:669–684. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, Young AW, Gray JA. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21:1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Human brain mapping. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SW, Kashikar-Zuck SM, Allen JR, LeCates SL, Slater SK, Zafar M, Kabbouche MA, O’Brien HL, Shenk CE, Rausch JR, Hershey AD. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2013;310:2622–2630. doi: 10.1001/jama.2013.282533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Macaluso DA, Eslinger PJ. Taste perception in patients with insular cortex lesions. Behavioral neuroscience. 1999;113:663–671. [PubMed] [Google Scholar]
- Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert review of neurotherapeutics. 2009;9:745–758. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radat F. Stress and migraine. Revue neurologique. 2013;169:406–412. doi: 10.1016/j.neurol.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Raggi A, Giovannetti AM, Quintas R, D’Amico D, Cieza A, Sabariego C, Bickenbach JE, Leonardi M. A systematic review of the psychosocial difficulties relevant to patients with migraine. The journal of headache and pain. 2012;13:595–606. doi: 10.1007/s10194-012-0482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature reviews. Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Romano D, Gandola M, Bottini G, Maravita A. Arousal responses to noxious stimuli in somatoparaphrenia and anosognosia: clues to body awareness. Brain : a journal of neurology. 2014;137:1213–1223. doi: 10.1093/brain/awu009. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiological reviews. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sahota PK, Dexter JD. Sleep and headache syndromes: a clinical review. Headache. 1990;30:80–84. doi: 10.1111/j.1526-4610.1990.hed3002080.x. [DOI] [PubMed] [Google Scholar]
- Sancisi E, Cevoli S, Vignatelli L, Nicodemo M, Pierangeli G, Zanigni S, Grimaldi D, Cortelli P, Montagna P. Increased prevalence of sleep disorders in chronic headache: a case-control study. Headache. 2010;50:1464–1472. doi: 10.1111/j.1526-4610.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. The Journal of comparative neurology. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- Saper CB. Hypothalamic connections with the cerebral cortex. Progress in brain research. 2000;126:39–48. doi: 10.1016/S0079-6123(00)26005-6. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos I, Dixon GE, Short SJ, Davidson RJ, Nitschke JB. Brain mechanisms of expectation associated with insula and amygdala response to aversive taste: implications for placebo. Brain, behavior, and immunity. 2006;20:120–132. doi: 10.1016/j.bbi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Schienle A, Ubel S, Schongassner F, Ille R, Scharmuller W. Disgust regulation via placebo: an fMRI study. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Ganssbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia : an international journal of headache. 2008;28:1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- Schwedt TJ, Larson-Prior L, Coalson RS, Nolan T, Mar S, Ances BM, Benzinger T, Schlaggar BL. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med. 2014;15:154–165. doi: 10.1111/pme.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedt TJ, Schlaggar BL, Mar S, Nolan T, Coalson RS, Nardos B, Benzinger T, Larson-Prior LJ. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache. 2013;53:737–751. doi: 10.1111/head.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Cortical association areas in the gustatory system. Neuroscience and biobehavioral reviews. 2001;25:395–407. doi: 10.1016/s0149-7634(01)00021-5. [DOI] [PubMed] [Google Scholar]
- Shechter A, Stewart WF, Silberstein SD, Lipton RB. Migraine and autonomic nervous system function: a population-based, case-control study. Neurology. 2002;58:422–427. doi: 10.1212/wnl.58.3.422. [DOI] [PubMed] [Google Scholar]
- Sheftell FD, Atlas SJ. Migraine and psychiatric comorbidity: from theory and hypotheses to clinical application. Headache. 2002;42:934–944. doi: 10.1046/j.1526-4610.2002.02217.x. [DOI] [PubMed] [Google Scholar]
- Shepherd AJ, Beaumont HM, Hine TJ. Motion processing deficits in migraine are related to contrast sensitivity. Cephalalgia : an international journal of headache. 2012;32:554–570. doi: 10.1177/0333102412445222. [DOI] [PubMed] [Google Scholar]
- Shin JH, Kim YK, Kim HJ, Kim JS. Altered brain metabolism in vestibular migraine: comparison of interictal and ictal findings. Cephalalgia : an international journal of headache. 2014;34:58–67. doi: 10.1177/0333102413498940. [DOI] [PubMed] [Google Scholar]
- Shuper A, Goldberg-Stern H. Ictus emeticus (ictal vomiting) Pediatric neurology. 2004;31:283–286. doi: 10.1016/j.pediatrneurol.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Silberstein SD. Migraine. Lancet. 2004;363:381–391. doi: 10.1016/S0140-6736(04)15440-8. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Lipton RB. Epidemiology of migraine. Neuroepidemiology. 1993;12:179–194. doi: 10.1159/000110317. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Human brain mapping. 2011;32:1836–1846. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in cognitive sciences. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Small DM. Taste representation in the human insula. Brain structure & function. 2010;214:551–561. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Smitherman TA, Kolivas ED, Bailey JR. Panic disorder and migraine: comorbidity, mechanisms, and clinical implications. Headache. 2013;53:23–45. doi: 10.1111/head.12004. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Steele JD, Mwangi B, Kumar P, Christmas D, Milders M, Matthews K. The insular cortex and the neuroanatomy of major depression. Journal of affective disorders. 2011;133:120–127. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011;77:476–482. doi: 10.1212/WNL.0b013e318227e4a8. [DOI] [PubMed] [Google Scholar]
- Stankewitz A, Schulz E, May A. Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: an fMRI study. Cephalalgia : an international journal of headache. 2013;33:256–265. doi: 10.1177/0333102412470215. [DOI] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephani C, Fernandez-Baca Vaca G, Maciunas R, Koubeissi M, Luders HO. Functional neuroanatomy of the insular lobe. Brain structure & function. 2011;216:137–149. doi: 10.1007/s00429-010-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain structure & function. 2010;214:611–622. doi: 10.1007/s00429-010-0252-2. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Miller LA, McGrillen K. Perception of odor-induced tastes following insular cortex lesion. Neurocase. 2013 doi: 10.1080/13554794.2013.860175. [DOI] [PubMed] [Google Scholar]
- Stewart W, Breslau N, Keck PE., Jr Comorbidity of migraine and panic disorder. Neurology. 1994a;44:S23–27. [PubMed] [Google Scholar]
- Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology. 1994b;44:S17–23. [PubMed] [Google Scholar]
- Stimpson CD, Tetreault NA, Allman JM, Jacobs B, Butti C, Hof PR, Sherwood CC. Biochemical specificity of von Economo neurons in hominoids. American journal of human biology : the official journal of the Human Biology Council. 2011;23:22–28. doi: 10.1002/ajhb.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolte B, Holle D, Naegel S, Diener HC, Obermann M. Vestibular migraine. Cephalalgia : an international journal of headache. 2014 doi: 10.1177/0333102414535113. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Matthews SC, Simmons AN. Right anterior insula hypoactivity during anticipation of homeostatic shifts in major depressive disorder. Psychosomatic medicine. 2010;72:316–323. doi: 10.1097/PSY.0b013e3181d07873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A. Insula and disgust. Rinsho shinkeigaku = Clinical neurology. 2010;50:1000–1002. doi: 10.5692/clinicalneurol.50.1000. [DOI] [PubMed] [Google Scholar]
- Suzuki A. Emotional functions of the insula. Brain and nerve = Shinkei kenkyu no shinpo. 2012;64:1103–1112. [PubMed] [Google Scholar]
- Symonds LL, Gordon NS, Bixby JC, Mande MM. Right-lateralized pain processing in the human cortex: an FMRI study. Journal of neurophysiology. 2006;95:3823–3830. doi: 10.1152/jn.01162.2005. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human brain mapping. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeler CH, Lee SW, Shaltout HA. Significance of right anterior insula activity for mental health intervention. JAMA psychiatry. 2014;71:336. doi: 10.1001/jamapsychiatry.2013.3507. [DOI] [PubMed] [Google Scholar]
- Tibber MS, Kelly MG, Jansari A, Dakin SC, Shepherd AJ. An inability to exclude visual noise in migraine. Investigative ophthalmology & visual science. 2014;55:2539–2546. doi: 10.1167/iovs.14-13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toronchuk JA, Ellis GF. Affective neuronal selection: the nature of the primordial emotion systems. Frontiers in psychology. 2012;3:589. doi: 10.3389/fpsyg.2012.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng MT, Chiang MC, Yazhuo K, Chao CC, Tseng WY, Hsieh ST. Effect of aging on the cerebral processing of thermal pain in the human brain. Pain. 2013;154:2120–2129. doi: 10.1016/j.pain.2013.06.041. [DOI] [PubMed] [Google Scholar]
- Ture U, Yasargil DC, Al-Mefty O, Yasargil MG. Topographic anatomy of the insular region. Journal of neurosurgery. 1999;90:720–733. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain structure & function. 2010;214:629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Huettel SA, Chuah LY, Payne JW, Chee MW. Sleep deprivation biases the neural mechanisms underlying economic preferences. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:3712–3718. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia : an international journal of headache. 2010;30:1065–1072. doi: 10.1177/0333102409355601. [DOI] [PubMed] [Google Scholar]
- Villemure C, Ceko M, Cotton VA, Bushnell MC. Insular Cortex Mediates Increased Pain Tolerance in Yoga Practitioners. Cerebral cortex. 2013 doi: 10.1093/cercor/bht124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wagner D, Manahilov V, Gordon GE, Loffler G. Global shape processing deficits are amplified by temporal masking in migraine. Investigative ophthalmology & visual science. 2013;54:1160–1168. doi: 10.1167/iovs.12-11242. [DOI] [PubMed] [Google Scholar]
- Walters AB, Hamer JD, Smitherman TA. Sleep disturbance and affective comorbidity among episodic migraineurs. Headache. 2014;54:116–124. doi: 10.1111/head.12168. [DOI] [PubMed] [Google Scholar]
- Wang YF, Fuh JL, Chen SP, Wu JC, Wang SJ. Clinical correlates and diagnostic utility of osmophobia in migraine. Cephalalgia : an international journal of headache. 2012;32:1180–1188. doi: 10.1177/0333102412461401. [DOI] [PubMed] [Google Scholar]
- Weeks RE. Application of behavioral therapies in adult and adolescent patients with chronic migraine. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2013;34(Suppl 1):S11–17. doi: 10.1007/s10072-013-1360-6. [DOI] [PubMed] [Google Scholar]
- Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, Coenen HH, Diener HC. Brain stem activation in spontaneous human migraine attacks. Nature medicine. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Fife TD. Dizziness and headache: a common association in children and adolescents. Journal of child neurology. 2001;16:727–730. doi: 10.1177/088307380101601004. [DOI] [PubMed] [Google Scholar]
- Wendt JS. Stress and migraine - hyperempathy. Headache. 2010;50:675. doi: 10.1111/j.1526-4610.2010.01641.x. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me”. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2010;11:538–549. doi: 10.3109/15622970903563794. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nature reviews. Neuroscience. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Wright P, He G, Shapira NA, Goodman WK, Liu Y. Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport. 2004;15:2347–2351. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]
- Xue T, Yuan K, Zhao L, Yu D, Dong T, Cheng P, von Deneen KM, Qin W, Tian J. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PloS one. 2012;7:e52927. doi: 10.1371/journal.pone.0052927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Zhao L, Cheng P, Yu D, Dong T, Xing L, Bi Y, Yang X, von Deneen KM, Liang F, Gong Q, Qin W, Tian J. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. The journal of pain : official journal of the American Pain Society. 2013a;14:836–844. doi: 10.1016/j.jpain.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Yuan W, Dan L, Netra R, Shaohui M, Chenwang J, Ming Z. A pharmaco-fMRI study on pain networks induced by electrical stimulation after sumatriptan injection. Experimental brain research. 2013b;226:15–24. doi: 10.1007/s00221-013-3405-8. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchin G, Dainese F, Mainardi F, Mampreso E, Perin C, Maggioni F. Osmophobia in primary headaches. The journal of headache and pain. 2005;6:213–215. doi: 10.1007/s10194-005-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Liu J, Dong X, Peng Y, Yuan K, Wu F, Sun J, Gong Q, Qin W, Liang F. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. The journal of headache and pain. 2013;14:85. doi: 10.1186/1129-2377-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DK, Hassanein RS, Kodanaz A, Meek JC. Circadian rhythms of plasma cortisol in migraine. Journal of neurology, neurosurgery, and psychiatry. 1979;42:741–748. doi: 10.1136/jnnp.42.8.741. [DOI] [PMC free article] [PubMed] [Google Scholar]


