Abstract
Background and Purpose
Tumors with high expression of excision repair cross complementation 1 (ERCC1) are resistant to platinum-based chemotherapy or chemoradiotherapy. We evaluated the prognostic value of ERCC1 expression in a cohort of laryngeal cancer treated with radiotherapy alone, to determine if expression correlated with clinicopathologic risk factors, local control, or overall survival.
Material and Methods
The study included 123 patients with stages I – II laryngeal squamous cell carcinoma treated to a median dose of 66 Gy using standard fields and fractionation. ERCC1 expression was examined by immunohistochemical analysis of tissue microarrays.
Results
ERCC1 expression did not correlate with standard prognostic factors, local control, or overall survival. At 5 years, local control was 75% vs. 71% (p = 0.78) and overall survival was 68% vs. 54% (p = 0.65), for non-expressors and expressors of ERCC1, respectively. Multivariate analysis identified T stage as the only independent predictor for local control, and T stage and age as predictors for overall survival.
Conclusions
ERCC1 expression did not predict for radiotherapy resistance or worse survival in these laryngeal cancer patients. Though ERCC1 is a marker of resistance to platinum-based chemotherapy and chemoradiation, radiotherapy remains an effective treatment in tumors with high ERCC1 expression.
Keywords: Excision repair cross complementation 1, laryngeal cancer, radiation therapy, tissue microarray, head and neck cancer
Introduction
Laryngeal cancer is the most common cancer of the head and neck [1]. Approximately 60% of patients present with early stage disease (T1-2N0, stage I–II) for which treatment is typically definitive radiotherapy or a larynx conserving surgery. Though not compared in a randomized fashion, these treatment modalities have been shown to offer equivalent local control, overall survival, and larynx preservation [2]. A combined-modality approach is taken for more advanced stage disease, and treatment entails concurrent chemoradiation or total laryngectomy with possible adjuvant therapy. Local recurrence rates at 5 years after definitive radiotherapy are 6–16% and 20–28% for T1 and T2 glottic tumors, respectively, and approximately 25% for T1-2N0 supraglottic tumors [3–7]. Salvage treatment after local failure often involves a total laryngectomy. Therefore, identification of molecular markers predictive of response to radiotherapy in laryngeal cancer would be beneficial in guiding clinical decisions.
Excision repair cross complementation 1 (ERCC1) is emerging as a prognostic marker in both lung cancer as well as cancers of the head and neck. ERCC1 plays a rate-limiting role in the nucleotide excision repair pathway. ERCC1 forms a heterodimer with xeroderma pigmentosum complementation group F (XPF) to form an endonuclease. XPF contains the catalytic domain of the nuclease, whereas ERCC1 is required for DNA binding. The ERCC1-XPF endonuclease cleaves DNA 5′ of helix-distorting lesions, and thus is essential for the repair of platinum-DNA adducts [8]. In addition, ERCC1-XPF functions in homologous recombination [9] and interstrand crosslink repair [10]. Finally, ERCC1-XPF plays a role in double-strand break repair, and has been shown to protect against ionizing radiation in vivo [11].
High levels of ERCC1 mRNA and protein expression have been shown to correlate with resistance to platinum-based chemotherapy or concurrent chemoradiotherapy, as well as inferior progression free survival and overall survival in patients with primary lung cancers or cancers of the head and neck. Handra-Luca et al. demonstrated that high ERCC1 expression was predictive of worse treatment response and disease-specific survival in patients with locally advanced squamous cell carcinomas of the head and neck treated with cisplatin-based induction chemotherapy [12]. Several studies have shown that high levels of ERCC1 expression in locally advanced head and neck tumors predicts for resistance to concurrent chemoradiation [13–15]. ERCC1 expression has also been shown to correlate with worse progression free survival and overall survival in patients with either small cell or non small cell lung cancer treated with platinum-based chemotherapy alone or in combination with radiotherapy [8, 16–24]. Studies have also demonstrated that ERCC expression is a marker for inferior outcomes in esophageal, gastric, colorectal, and ovarian cancer after treatment with platinum-based chemotherapy [8, 25–29].
In addition to the data demonstrating the role of ERCC1 as a marker of resistance to chemoradiation, there is evidence to indicate that ERCC1 expression is also predictive of resistance to radiation alone. Cell lines deficient in ERCC1 are more sensitive to radiation under hypoxic conditions [30]. Moreover, radioresistant lung cancer cell lines demonstrate induction of ERCC1 expression after irradiation, suggesting that high ERCC1 expression correlates with the radioresistant phenotype [31]. Increased ERCC1 expression has also been shown to correlate with radioresistance in a murine xenograft model of tumors derived from cervical carcinoma cells [32]. Finally, single nucleotide polymorphisms in the ERCC1 gene are predictive of response to radiotherapy in patients with early stage squamous cell carcinoma of the head and neck [33]. This work suggests the potential clinical utility of ERCC1 expression as a prognostic marker for response to radiotherapy.
Therefore we evaluated the prognostic value of ERCC1 expression in a cohort of early stage laryngeal cancer treated with radiotherapy alone. Specifically, we determined whether ERCC1 expression correlated with clinicopathologic prognostic factors, local recurrence, or overall survival.
Materials and Methods
Patients Characteristics
Patients with stages I and II squamous cell carcinoma of the glottic and supraglottic larynx treated at the Yale University School of Medicine Department of Therapeutic Radiology between 1975 and 2000 with radiation therapy alone were included in this study. Archived tumor specimens were available for a total of 123 of these patients. Patients received a median daily fraction of 2 Gy (range 1.8–2.55 Gy) to a median dose of 66 Gy (range 49.5–79 Gy) using standard opposed lateral fields with beam energies of 2–6 MeV. Five fractions were delivered per week without planned treatment breaks. 109 of the 123 patients received a total dose of at least 60 Gy, and only 2 patients received more than 70 Gy. Patient charts were reviewed to obtain information regarding clinicopathologic factors, treatment parameters, and outcomes. Median follow-up for the cohort was 4.9 years. A tissue microarray was constructed from the formalin-fixed, paraffin-embedded, pre-treatment biopsies of these patients, as previously described [34]. The primary study endpoints were local control and overall survival.
Immunohistochemistry
Immunohistochemical analysis was performed as previously described on 5 μm thick sections of the tissue microarray using an antibody directed again ERCC1 (ERCC1 Ab-3, clone 8F1, at 1:100, from Thermo Fisher Scientific Inc., Fremont California) [35]. ERCC1 expression was determined by a single pathologist who was blinded to patient outcomes. ERCC1 expression could be effectively analyzed in 90 patients, and therefore further analysis was completed on this subset of cases. The remaining tissue cores were uninterpretable due to tissue loss or lack of sufficient tumor cells. The extent of staining was defined as the percentage of tumor cells with positive staining graded on a scale of 0–3 (0 = 0–10%, 1 = 10–40%, 2 = 40–70%, 3 = 70–100%). The intensity of staining was also graded on a scale of 0–3 (0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining). The h-score was obtained by multiplying the grades of extent and intensity of staining. The median value of all the h-scores was 2, and was used as the cutoff value for defining positive and negative ERCC1 expression. Data analysis was repeated using several alternate definitions of positive ERCC1 expression (greater than 10% of cells staining positive, moderate or greater intensity staining, and strong intensity staining), and results obtained were comparable.
Statistical Analysis
Statistical analysis was performed using Stata Version 11 (StataCorp LP, College Station Texas). The follow-up time and time to recurrence were calculated from the date of diagnosis. ERCC1 expression was compared to standard prognostic variables (age, T stage, tumor subsite, gender, and race) or treatment parameters (total dose, fraction size, and duration of radiotherapy) using t-tests or chi-square tests. Survival estimates were calculated using the Kaplan-Meier product-limit method, and the log-rank test was used to assess for differences. Multivariate analysis using the Cox proportional hazards regression model was completed to assess the independent contribution of variables (age, tumor subsite, T stage, fraction size, total dose, and ERCC1 expression) to survival. A p-value of ≤ 0.05 was considered statistically significant.
Results
Of the 123 patients included in the cohort, 84 (68%) of the cases were T1, and 39 (32%) were T2. Median age at diagnosis was 64 years (range 38–90). The majority of tumors were glottic versus supraglottic (98 versus 25). Patient and treatment characteristics are further summarized in Table 1. With a median follow-up time of 4.9 years, and 8.5 years for those alive, the 5 year local control rate was 74% and the 5-year overall survival rate was 60%.
Table 1.
Patient and Treatment Characteristics
| Characteristics | Entire Cohort (123 pts) | Subset Analyzed (90 pts) |
|---|---|---|
| Follow-up (yrs) | 4.9 | 5.0 |
| Age (yrs) | ||
| Median/Range | 64/38–90 | 63.5/38–90 |
| Sex | ||
| Male | 83 | 62 |
| Female | 14 | 11 |
| Unknown | 26 | 17 |
| Race | ||
| White | 87 | 66 |
| Black | 9 | 6 |
| Unknown | 27 | 18 |
| T Stage | ||
| T1 | 84 | 65 |
| T2 | 39 | 25 |
| Subsite | ||
| Glottic | 98 | 73 |
| Supraglottic | 25 | 17 |
| Total Dose (Gy) | ||
| Mean/Range | 64.4/49.5–79 | 64.1/49.5–70.6 |
| Fraction Size (Gy) | ||
| Mean/Range | 2/1.8–2.55 | 2/1.8–2.55 |
| RT Duration (days) | ||
| Mean/Range | 46.4/27–78 | 45.6/27–68 |
| ERCC1 Expression | ||
| Positive | 36 | 36 |
| Negative | 54 | 54 |
| Data not available | 33 | – |
pts = patients, yrs = years, RT = radiotherapy
ERCC1 expression could be analyzed in 90 patients, and further analysis was completed on this subset of cases. Patient and treatment characteristics for this subset of patients are representative of the entire cohort and detailed in Table 1. Among these patients, the median follow-up time was 5.0 years, and 7.2 years for those alive. The local control rate and overall survival rate was 74% and 63%, respectively. Positive expression, defined as an h-score greater than or equal to 2 (as in previous studies) [13, 14, 17], was detected in 36 (40%) of evaluable cases. ERCC1 expression was predominantly nuclear. Representative positive and negative ERCC1 immunostaining is shown in Figure 1.
Figure 1.
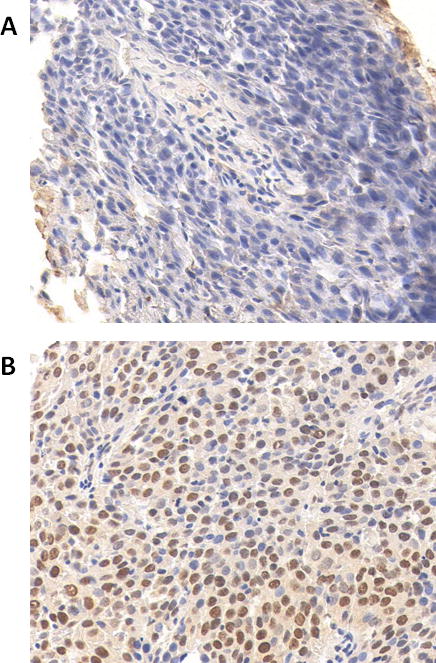
Representative negative (A) and positive (B) ERCC1 immunostaining is shown at 40× magnification.
ERCC1 expression did not significantly correlate with prognostic clinicopathologic factors including age at diagnosis, T stage, tumor subsite, gender, or race (Table 2), on bivariate analysis. Similarly, there was no significant correlation between ERCC1 expression and radiotherapy treatment parameters, such as total dose, fraction size, or RT duration.
Table 2.
Association Between ERCC1 Expression and Prognostic Factors or Treatment Parameters
| ERCC1 Negative | ERCC1 Positive | p-value | |
|---|---|---|---|
|
Follow-Up (mean in years) |
6.85 | 6.05 | 0.750 |
|
Age at Diagnosis (mean in years) |
62.4 | 65.1 | 0.130 |
| T Stage | |||
| T1 | 36 (67%) | 29 (81%) | 0.150 |
| T2 | 18 (33%) | 7 (19%) | |
| Subsite | |||
| Glottic | 43 (80%) | 30 (83%) | 0.660 |
| Supraglottic | 11 (20%) | 6 (17%) | |
| Gender | |||
| Male | 37 (84%) | 25 (86%) | 0.805 |
| Female | 7 (16%) | 4 (14%) | |
| Race | |||
| White | 40 (91%) | 26 (93%) | 0.771 |
| Black | 4 (9%) | 2 (7%) | |
|
Total Dose (mean in Gy) |
64.6 | 63.2 | 0.910 |
|
Fraction Size (mean in Gy) |
2.02 | 2.05 | 0.130 |
|
RT Duration (mean in days) |
47.2 | 43.3 | 0.997 |
RT = radiotherapy
Univariate survival analysis (Table 3) showed that as expected, T stage significantly correlated with local control at 5 years (p=0.0003), and both T stage and age at diagnosis significantly correlated with overall survival at 5 years (p=.0005 and 0.0007). Interestingly, ERCC1 expression did not significantly correlate with local control or overall survival. At 5 years, overall survival for non-expressors of ERCC1 was 68% compared to 54% for ERCC1 expressors (p=0.65). Similarly, local control at 5 years was not significantly different between non-expressors and expressors of ERCC1 (75% vs. 71%, p = 0.78). The Kaplan-Meier survival curves for local control and overall survival stratified by ERCC1 expression are shown in Figure 2A and Figure 2B, respectively. Other prognostic variables and treatment parameters including tumor subsite, gender, race, fraction size, total dose, and RT duration did not correlate with survival outcomes in our analysis (Table 3).
Table 3.
Univariate Survival Analysis
| 5 Yr LC | p-value | 5 Yr OS | p-value | |
|---|---|---|---|---|
| Overall | 74 | 63 | ||
| Age | ||||
| <60 | 76 | 0.5430 | 74 | 0.0007 |
| >60 | 72 | 55 | ||
| T Stage | ||||
| T1 | 83 | 0.0003 | 70 | 0.0005 |
| T2 | 44 | 44 | ||
| Subsite | ||||
| Glottic | 76 | 0.4040 | 64 | 0.4954 |
| Supraglottic | 61 | 57 | ||
| Gender | ||||
| Male | 75 | 0.7976 | 61 | 0.3133 |
| Female | 75 | 72 | ||
| Race | ||||
| White | 77 | 0.1429 | 64 | 0.5117 |
| Black | 50 | 42 | ||
| ERCC1 | ||||
| Negative | 75 | 0.7808 | 68 | 0.6460 |
| Positive | 71 | 54 | ||
| Fraction Size* | 0.523 | 0.921 | ||
| Total Dose* | 0.246 | 0.265 | ||
| RT Duration* | 0.065 | 0.108 |
LC = local control, OS = overall survival, Yr = year, RT = radiotherapy
Analyzed as continuous variables
Figure 2.
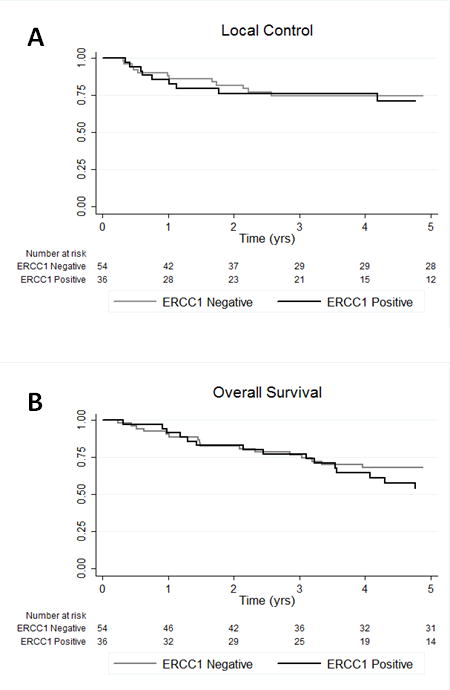
Kaplan-Meier survival curves for local control (A) and overall survival (B) stratified by ERCC1 expression.
Multivariate analysis was performed using the Cox proportional hazards regression model, and is shown in Table 4. T stage was identified as the only independent predictor for local control (hazard ratio 0.19, 95% CI 0.06–0.55, p=0.002). T stage and age were the only independent predictors for overall survival (hazard ratio 0.45, 95% CI 0.22–0.94, p=0.033, and hazard ratio 1.07, 95% CI 1.03–1.11, p=0.000, respectively). On multivariate analysis, ERCC1 expression did not correlate significantly with survival outcomes.
Table 4.
Multivariate Analysis
| Hazard Ratio | 95% CI | p-value | ||
|---|---|---|---|---|
| LC | T Stage | 0.19 | 0.06–0.55 | 0.002 |
| OS | T Stage | 0.45 | 0.22–0.94 | 0.033 |
| Age | 1.07 | 1.03–1.11 | 0.000 |
LC = local control, OS = overall survival
Discussion
ERCC1 has recently been established as a prognostic marker in lung and head and neck tumors, among others [12–29]. Several studies have demonstrated that ERCC1 expression predicts for resistance to cisplatin-based chemoradiation in head and neck cancer patients. Jun et al. reported on a cohort of 60 patients with locally advanced head and neck squamous cell carcinomas treated with platinum-based concurrent chemoradiotherapy, and high ERCC1 expression was an independent predictor for worse survival [13]. Similar studies by Lee et al. and Sun et al. demonstrated that high ERCC1 expression was significantly associated with worse overall survival in patients with locally advanced nasopharyngeal cancer treated with concurrent cisplatin-based chemotherapy with radiation [14, 15]. These results are consistent with the critical function ERCC1 plays in the nucleotide excision pathway, which is required for repair of platinum-DNA adducts.
Experiments in cell culture and animal models have suggested that ERCC1 expression predicts for radioresistance [30–32]. For example, ERCC1 expression is induced in radioresistant lung cancer cell lines [31], and high ERCC1 expression is associated with radioresistance in a murine xenograft tumor model [32]. Clinical studies also suggest a prognostic role for ERCC1 expression in predicting response to radiotherapy, but results are conflicting. Carles et al. reported that a single nucleotide polymorphism (SNP) in the ERCC1 gene predicted for worse outcomes in patients with stage I and II head and neck squamous cell carcinoma treated with radiotherapy alone. Whether the SNP affected ERCC1 expression levels was not reported [33]. A recently published study examined the prognostic value of ERCC1 expression in advanced stage nasopharyngeal cancer patients treated with either radiation alone, or concurrent chemoradiation. High ERCC1 expression was associated with inferior local control in those patients receiving radiotherapy alone. Though intriguing, this result is difficult to interpret in light of the fact that contrary to multiple previous studies, high ERCC1 expression did not correlate with inferior outcomes in those patients treated with concurrent platinum-based chemoradiation [36]. Another recent study demonstrated that ERCC1 overexpression in early stage breast cancer patients treated with breast conserving surgery followed by radiotherapy did not predict for worse survival outcomes, but correlated with favorable clinicopathologic factors [35]. Doll et al. demonstrated that low (rather than high) ERCC1 expression correlated with worse survival in cervical cancer patients treated with radiotherapy alone [37]. These results conflict with reports demonstrating that high ERCC1 predicts for worse outcomes after platinum-based treatment regimens. Yet taken together, these two studies indicate that absent ERCC1 expression is likely a marker of a more aggressive tumor phenotype with impaired DNA damage pathways, while expression of ERCC1 correlates with more favorable tumor biology. Clinical data has clearly established high ERCC1 expression as a marker of resistance to platinum-based chemotherapy or chemoradiotherapy. Yet the role of ERCC1 expression as a potential clinical predictor of response to radiotherapy alone has not yet been defined.
This study evaluated the role of ERCC1 as a prognostic marker for patients with early stage laryngeal cancer treated with definitive radiotherapy. Tissue microarray analysis was used to evaluate ERCC1 expression in a cohort of 123 patients with stages I and II larynx cancer, and outcomes were assessed after radiation therapy. Despite the fact that high ERCC1 expression is an established predictor of resistance to chemoradiation, in our study, ERCC1 expression did not correlate with outcomes. Specifically, ERCC1 expression did not significantly correlate with more aggressive clinicopathologic factors such as more advanced T stage, supraglottic tumor subsite, age at diagnosis, or African-American race. Moreover, survival analysis demonstrated that ERCC1 expression did not predict for worse local control after radiotherapy. Similarly, ERCC1 expression was not a predictor for inferior overall survival in these patients treated with radiation alone. The only significant independent predictor of local recurrence by multivariate analysis was T stage, and the significant independent predictors of overall survival were T stage and age at diagnosis, as expected.
This study relied on immunohistochemistry to detect ERCC1 expression, while other studies have examined gene expression, and therefore study outcomes may have been affected by the method of assessing ERCC1 expression. In addition, it is possible that our results may not be applicable to HPV-positive oropharyngeal cancers, now recognized as a distinct subset of head and neck cancers with unique underlying tumor biology and clinicopathologic risk factors, and associated with an improved response to treatment and favorable outcomes. HPV status was not examined in this cohort, but would not be expected to impact outcomes, as the relationship between HPV and larynx cancer is less well established to date.
While our data demonstrates that ERCC1 expression is not a predictor of radioresistance in early stage laryngeal tumors, it should be considered that these conclusions may not apply in locally advanced tumors in which the hypoxic tumor microenvironment affects radiosensitivity. Cell culture experiments indicate a role for ERCC1 in radioresistance under hypoxic conditions [30]. Therefore while ERCC1 expression is not a marker for resistance to radiotherapy in early stage tumors, it is possible that expression can be prognostic for radioresistance or inferior local control in hypoxic advanced stage tumors, as suggested by the recently published study of advanced stage nasopharyngeal cancer patients [36].
This study indicates that high ERCC1 expression does not predict for radioresistance. Though previous studies have demonstrated that ERCC1 expression is a predictor of resistance to chemoradiation, these tumors are likely resistant to platinum-based chemotherapy rather than chemoradiation per se. Therefore ERCC1 is not of clinical utility in deciding between surgery and radiation as local therapy, but remains a useful marker for platinum-resistance.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
References
- 1.Fremgen AM, Bland KI, McGinnis LS, et al. Clinical highlights from the National Cancer Data Base, 1999. CA Cancer J Clin. 1999;49:145–158. doi: 10.3322/canjclin.49.3.145. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1–T2 glottic carcinomas. Cancer. 2004;100:1786–1792. doi: 10.1002/cncr.20181. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Amdur RJ, Morris CG, Hinerman RW. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001;19:4029–4036. doi: 10.1200/JCO.2001.19.20.4029. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77–82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Spriano G, Antognoni P, Piantanida R, et al. Conservative management of T1-T2N0 supraglottic cancer: a retrospective study. Am J Otolaryngol. 1997;18:299–305. doi: 10.1016/s0196-0709(97)90023-5. [DOI] [PubMed] [Google Scholar]
- 6.Cellai E, Frata P, Magrini SM, et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. I. The case of T1N0 disease. Int J Radiat Oncol Biol Phys. 2005;63:1378–1386. doi: 10.1016/j.ijrobp.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Frata P, Cellai E, Magrini SM, et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. II. The case of T2N0 disease. Int J Radiat Oncol Biol Phys. 2005;63:1387–1394. doi: 10.1016/j.ijrobp.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Gossage L, Madhusudan S. Current status of excision repair cross complementing-group 1 (ERCC1) in cancer. Cancer Treat Rev. 2007;33:565–577. doi: 10.1016/j.ctrv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Adair GM, Rolig RL, Moore-Faver D, Zabelshansky M, Wilson JH, Nairn RS. Role of ERCC1 in removal of long non-homologous tails during targeted homologous recombination. EMBO J. 2000;19:5552–5561. doi: 10.1093/emboj/19.20.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niedernhofer LJ, Odijk H, Budzowska M, et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad A, Robinson AR, Duensing A, et al. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol Cell Biol. 2008;28:5082–5092. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handra-Luca A, Hernandez J, Mountzios G, et al. Excision repair cross complementation group 1 immunohistochemical expression predicts objective response and cancer-specific survival in patients treated by Cisplatin-based induction chemotherapy for locally advanced head and neck squamous cell carcinoma. Clin Cancer Res. 2007;13:3855–3859. doi: 10.1158/1078-0432.CCR-07-0252. [DOI] [PubMed] [Google Scholar]
- 13.Jun HJ, Ahn MJ, Kim HS, et al. ERCC1 expression as a predictive marker of squamous cell carcinoma of the head and neck treated with cisplatin-based concurrent chemoradiation. Br J Cancer. 2008;99:167–172. doi: 10.1038/sj.bjc.6604464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HW, Hwang YH, Han JH, et al. High expression of excision repair cross-complementation group 1 protein predicts poor outcome in patients with nasopharyngeal cancer. Oral Oncol. 2010;46:209–213. doi: 10.1016/j.oraloncology.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Sun JM, Ahn MJ, Park MJ, et al. Expression of excision repair cross-complementation group 1 as predictive marker for nasopharyngeal cancer treated with concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:655–660. doi: 10.1016/j.ijrobp.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 16.Lord RV, Brabender J, Gandara D, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8:2286–2291. [PubMed] [Google Scholar]
- 17.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 18.Ceppi P, Volante M, Novello S, et al. ERCC1 and RRM1 gene expressions but not EGFR are predictive of shorter survival in advanced non-small-cell lung cancer treated with cisplatin and gemcitabine. Ann Oncol. 2006;17:1818–1825. doi: 10.1093/annonc/mdl300. [DOI] [PubMed] [Google Scholar]
- 19.Hwang IG, Ahn MJ, Park BB, et al. ERCC1 expression as a prognostic marker in N2(+) nonsmall-cell lung cancer patients treated with platinum-based neoadjuvant concurrent chemoradiotherapy. Cancer. 2008;113:1379–1386. doi: 10.1002/cncr.23693. [DOI] [PubMed] [Google Scholar]
- 20.Lee HW, Han JH, Kim JH, et al. Expression of excision repair cross-complementation group 1 protein predicts poor outcome in patients with small cell lung cancer. Lung Cancer. 2008;59:95–104. doi: 10.1016/j.lungcan.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Lee HW, Choi YW, Han JH, et al. Expression of excision repair cross-complementation group 1 protein predicts poor outcome in advanced non-small cell lung cancer patients treated with platinum-based doublet chemotherapy. Lung Cancer. 2009;65:377–382. doi: 10.1016/j.lungcan.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Azuma K, Sasada T, Kawahara A, et al. Expression of ERCC1 and class III beta-tubulin in non-small cell lung cancer patients treated with carboplatin and paclitaxel. Lung Cancer. 2009;64:326–333. doi: 10.1016/j.lungcan.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Li ZN, Du YJ, Li XQ, Bao QL, Chen P. Expression of MRP1, BCRP, LRP, and ERCC1 in advanced non-small-cell lung cancer: correlation with response to chemotherapy and survival. Clin Lung Cancer. 2009;10:414–421. doi: 10.3816/CLC.2009.n.078. [DOI] [PubMed] [Google Scholar]
- 24.Kang CH, Jang BG, Kim DW, et al. The prognostic significance of ERCC1, BRCA1, XRCC1, and betaIII-tubulin expression in patients with non-small cell lung cancer treated by platinum- and taxane-based neoadjuvant chemotherapy and surgical resection. Lung Cancer. 2010;68:478–483. doi: 10.1016/j.lungcan.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Warnecke-Eberz U, Metzger R, Miyazono F, et al. High specificity of quantitative excision repair cross-complementing 1 messenger RNA expression for prediction of minor histopathological response to neoadjuvant radiochemotherapy in esophageal cancer. Clin Cancer Res. 2004;10:3794–3799. doi: 10.1158/1078-0432.CCR-03-0079. [DOI] [PubMed] [Google Scholar]
- 26.Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–316. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- 27.Kwon HC, Roh MS, Oh SY, et al. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol. 2007;18:504–509. doi: 10.1093/annonc/mdl430. [DOI] [PubMed] [Google Scholar]
- 28.Shirota Y, Stoehlmacher J, Brabender J, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298–4304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- 29.Dabholkar M, Bostick-Bruton F, Weber C, Bohr VA, Egwuagu C, Reed E. ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J Natl Cancer Inst. 1992;84:1512–1517. doi: 10.1093/jnci/84.19.1512. [DOI] [PubMed] [Google Scholar]
- 30.Murray D, Macann A, Hanson J, Rosenberg E. ERCC1/ERCC4 5′-endonuclease activity as a determinant of hypoxic cell radiosensitivity. Int J Radiat Biol. 1996;69:319–327. doi: 10.1080/095530096145878. [DOI] [PubMed] [Google Scholar]
- 31.Guo WF, Lin RX, Huang J, et al. Identification of differentially expressed genes contributing to radioresistance in lung cancer cells using microarray analysis. Radiat Res. 2005;164:27–35. doi: 10.1667/rr3401. [DOI] [PubMed] [Google Scholar]
- 32.Hampson L, El Hady ES, Moore JV, Kitchener H, Hampson IN. The HPV16 E6 and E7 proteins and the radiation resistance of cervical carcinoma. FASEB J. 2001;15:1445–1447. doi: 10.1096/fj.00-0728fje. [DOI] [PubMed] [Google Scholar]
- 33.Carles J, Monzo M, Amat M, et al. Single-nucleotide polymorphisms in base excision repair, nucleotide excision repair, and double strand break genes as markers for response to radiotherapy in patients with Stage I to II head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66:1022–1030. doi: 10.1016/j.ijrobp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Rewari A, Lu H, Parikh R, Yang Q, Shen Z, Haffty BG. BCCIP as a prognostic marker for radiotherapy of laryngeal cancer. Radiother Oncol. 2009;90:183–188. doi: 10.1016/j.radonc.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goyal S, Parikh RR, Green C, et al. Clinicopathologic significance of excision repair cross-complementation 1 expression in patients treated with breast-conserving surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:679–684. doi: 10.1016/j.ijrobp.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 36.Chan SH, Cheung FM, Ng WT, et al. Can the analysis of ERCC1 expression contribute to individualized therapy in nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys. 2011;79:1414–1420. doi: 10.1016/j.ijrobp.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 37.Doll CM, Prystajecky M, Eliasziw M, et al. Low ERCC1 mRNA and protein expression are associated with worse survival in cervical cancer patients treated with radiation alone. Radiother Oncol. 2010;97:352–359. doi: 10.1016/j.radonc.2010.08.019. [DOI] [PubMed] [Google Scholar]


