Abstract
Purpose
Oral vitamin and mineral supplements reduce the risk of visual loss in age-related macular degeneration (AMD). However, the pathways that mediate this beneficial effect are poorly understood. Macrophages may exert oxidative, inflammatory, and angiogenic effects in the context of AMD. We aim to assess if oral supplements can modulate the macrophage phenotype in this disease.
Methods
Monocytes were isolated from patients with neovascular AMD (nvAMD), cultured, matured to macrophages, and polarized to classical [M1 (stimulated by IFNγ and lipopolysaccharide (LPS))] and alternative [M2 (stimulated with IL-4 and IL-13)] phenotypes. Combinations of antioxidants including lutein+zeaxanthin (1 μM; 0.2 μM), zinc (10 µM), carnosic acid (2 µM), beta-carotene (2 µM), and standardized tomato extract containing lycopene and other tomato phytonutrients were added to the culture media. Levels of anti-inflammatory, antioxidant, and pro-angiogenic gene and protein expression were then evaluated.
Results
Combinations of lutein and carnosic acid with zinc and standardized tomato extract or with beta-carotene yielded an antioxidative, anti-inflammatory, and antiangiogenic effect in M1 and M2 macrophages. These effects manifested in the upregulation of antioxidative genes (HMOX1, SOD1) and the downregulation of pro-angiogenic genes and pro-inflammatory genes (SDF-1, TNF-alpha, IL-6, MCP-1). Lutein monotherapy or a combination of lutein and zinc had less effect on the expression of these genes.
Conclusions
Combinations of supplements can modify the expression of genes and proteins that may be relevant for the involvement of macrophages in the pathogenesis of AMD. Further studies are required to evaluate if the modulation of the macrophage phenotype partially accounts for the beneficial effect of oral supplements in AMD and if modification of the AREDS formula can improve its effect on macrophages.
Introduction
Age-related macular degeneration (AMD) is associated with chronic low-grade systemic and local activation of innate immunity. This inflammatory response includes complement activation and recruitment and activation of mononuclear cells, such as monocytes and their macrophage descendants. The inflammatory response was documented in both the atrophic (dry) stage of AMD (aAMD) and the neovascular stage of the disease (nvAMD).
The involvement of monocytes and macrophages in the pathogenesis of AMD is suggested by several lines of evidence from humans and from experimental rodent models. Among these findings are the histological identification of macrophages in the vicinity of AMD lesions in the retina and choroid [1,2]. We have previously reported on the upregulation of chemokine receptors involved in monocyte recruitment and global pro-inflammatory gene expression patterns in monocytes and peripheral blood mononuclear cells (PBMCs) from nvAMD patients [3-5]. In rodents, macrophages modulate the course of laser-induced choroidal neovascularization, genetically driven retinal degeneration, and photic retinal injury [6-15], further supporting their suggested role in AMD.
Macrophages can polarize in tissue to phenotypes that have a variety of actions. It was previously suggested that classically activated macrophages (M1) have a pro-inflammatory response, while alternatively activated macrophages (M2) may have multiple functions, including a pro-angiogenic effect [16]. In AMD eyes, both M1 and M2 macrophages are present. The actual role of these polarized macrophages in the disease is unclear, and limited data are available on the characteristics of macrophages derived from AMD patients [17]. Among the functions of macrophages that may be important in the context of AMD is a prominent capability to generate oxidative injury [18]. Such injury may result in cell death and accelerated inflammation and angiogenesis. Indeed, oxidative injury is thought to play an important role in the pathogenesis of AMD [19,20].
According to AREDS studies, oral phytonutrient (lutein, zeaxanthin, vitamins C and E) and mineral (zinc, copper) supplements are routinely prescribed once the intermediate stage of AMD is detected. Such supplementation reduces the risk of developing nvAMD and visual loss, but it does not affect the progression of atrophic AMD (aAMD) [21]. While it is thought that antioxidative effects mediate the protective role of such supplementation, there is little data regarding its mechanisms and targets. Furthermore, the magnitude of the therapeutic effect of oral supplements was moderate, with the majority of patients progressing despite supplement therapy.
The involvement of macrophages in AMD that may contribute to oxidative injury in the disease combined with the protective effect of antioxidant supplements against the development of nvAMD may imply that macrophages are one of the mediators of the protective effects of vitamins and minerals in nvAMD. According to this hypothesis, oral supplements may curb the pro-angiogenic effect of macrophages in the context of AMD by modulating their phenotypes. This research was designed to assess this hypothesis and to evaluate if improved macrophage modulation may be possible with a modified supplementation formula. To that end, we have evaluated the effect of a combination of antioxidant supplements on the phenotype of polarized macrophages from nvAMD patients in terms of the expression of genes and proteins that are relevant in the context of the disease.
Methods
Patients
nvAMD patients [n=10, 7 females, 3 males; mean age ± standard error of the mean (SEM): 78.3 ± 2.25 years, range: 65–88 years] were recruited from the retina clinic at the Department of Ophthalmology at Hadassah-Hebrew University Medical Center in Jerusalem. The study was performed on cells isolated from nvAMD patients, as we have previously demonstrated that peripheral blood mononuclear cells (PBMCs), monocytes, and macrophages from AMD patients show altered gene and protein expression and altered function compared with age-matched unaffected individuals [4,5,22,23]. Inclusion criteria for nvAMD patients were age over 55 years, a diagnosis of AMD according to the AREDS criteria [24], and a diagnosis of choroidal neovascularization (CNV) based on a fluorescein angiogram (FA) and optical coherence tomography (OCT). Eyes with neovascular lesions comprised of less than 50% active CNV, sub-retinal hemorrhage greater than 25% of the lesion size, or the presence of other retinal diseases were excluded from the study. Specifically, eyes with any other potential cause for CNV, such as myopia >6 diopters, trauma, or uveitis, were excluded. Also excluded were patients with a major systemic illness, such as cancer, autoimmune disease, congestive heart failure, or uncontrolled diabetes. Approval for all experimental protocols and study involving human subjects were approved by the Local Ethics Committee on Research Involving Human Subjects of the Hadassah Medical Center (File #22–03.08.07). All patients signed informed consent forms that adhered to the tenets of the Declaration of Helsinki before participating in the study.
Macrophage preparation
Blood samples (30 ml) were collected in EDTA tubes (BD Biosciences, San Jose, CA). Monocytes were isolated from the whole blood, differentiated into macrophages (M0), and activated into M1 and M2 phenotypes, as previously described [25-29]. PBMCs were separated using a Histopaque–Ficoll density centrifuge according to the manufacturer’s recommendations (Sigma-Aldrich, Munich, Germany). PBMCs (3*107 cells/cm2) were suspended in Roswell Park Memorial Institute (RPMI) 1640 medium (Biological Industries, Beit Haemek, Israel) and seeded into 6-well plates coated with the amino acid poly-d-lysine, which facilitates the adherence of monocytes. One hour after incubation in a 37 °C and 5% CO2 incubator, cells were washed with PBS, and monocytes were cultured for seven days in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 1% non-essential amino acid (NEAA), 1% glutamine, 1% sodium pyruvate, penicillin-streptomycin (100 units/ml), and 100 ng/ml macrophage colony-stimulating factor (M-CSF, PeproTech, Rocky Hill, NJ). M-CSF was added to the growth medium to induce maturation of the monocytes to macrophages. Polarization of macrophages was achieved by the addition of cytokines as follows: 20 ng/ml IFNγ (PeproTech) and 100 ng/ml lipopolysaccharide (LPS; Sigma-Aldrich) were added on day six to obtain an M1 phenotype. To obtain an M2 phenotype, 50 ng/ml IL-13 (PeproTech) and 20 ng/ml IL-4 (PeproTech) were added on day five of culturing [22,30].
Vitamin and mineral treatment
Following activation, macrophages were incubated with one of four supplement combinations or with a vehicle control. The supplement groups included (final concentrations in parenthesis): G1: lutein+zeaxanthin (Katra, Karnataka, India; 1 μM; 0.2 μM); G2: lutein+zeaxanthin (1 μM; 0.2 μM) and zinc (Navkar, Maharashtra, India; 10 μM); G3: lutein+zeaxanthin (1 μM; 0.2 μM), zinc (10 μM), Lyc-O-Mato (Lycored, Be’er Sheva, Israel; 2 μM; standardized tomato extract containing lycopene [6%] and other tomato phytonutrients such as phytoene, phytofluene, tocopherols, and phytosterols), and carnosic acid (2 μM; added as rosemary extract containing 20% CA; Lycored, Be’er Sheva, Israel); G4: lutein+zeaxanthin (1 μM; 0.2 μM), carnosic acid (2 μM), and beta-carotene (2 μM); and G5: vehicle control. The concentrations of the different compounds in culture were chosen to mimic serum levels obtained following oral supplementation. Supplements were added to the culture media at the time of inducing polarization.
ELISA
Following macrophage polarization and treatment with supplements, supernatant was collected from the macrophage cell cultures and stored at −20 °C. The levels of six proteins were tested using an enzyme-linked immunosorbent assay (ELISA): tumor necrosis factor α (TNF α), C-X-C motif chemokine ligand 12 (SDF1), C-C motif chemokine ligand 2 (MRC1/CCR2), interleukin 8 (IL-8), interleukin 6 (IL-6), and intercellular adhesion molecule (ICAM). The ELISA (PeproTech) was performed according to the manufacturer’s instructions. These proteins were chosen because they were previously implicated in the pathogenesis of AMD. The results were read on 96-well plates using a spectrophotometer (FluoStar BMG LABTECH GmBH, Ortenberg, Germany). The ELISA was performed in duplicate in a volume of 100 ul. Each plate included standard concentration gradients for calibration.
QPCR
RNA was extracted from macrophage cultures using RNA isolation reagent (TriReagent; Sigma-Aldrich) according to the manufacturer’s protocol. The RNA was then treated with DNAase (TURBO DNA-free, Ambion, Austin, TX). The RNA quality and quantity was assessed using a NanoDrop (Thermo Scientific, Waltham, MA) and a bioanalyzer (Agilent, Santa Clara, CA). Reverse transcription of RNA to cDNA was performed using a cDNA kit (High Capacity Reverse Transcription kit; Applied Biosystems, Carlsbad, CA) according to the manufacturer’s protocol. The expression levels of genes associated with macrophage polarization and the response to oxidative injury were then evaluated. The genes tested included tumor necrosis factor α (TNFα; Assay ID # Hs99999043_m1), interleukin 12 (IL-12; Hs01011518_m1), vascular endothelial growth factor (VEGF; Hs00900055_m1; Applied Biosystems), inositol-3-phosphate synthase 1(ISYNA1; Hs01126940_gH), mannose receptor C-type 1 (MRC1; Hs00267207_m1), heme oxygenase 1(HMOX1; Hs01110250_m1), catalase (CAT; Hs00156308_m1), glutathione peroxidase 1(GPX1; Hs00829989_gH), and superoxide dismutase 1(SOD1; Hs00533490_ml). These were evaluated in triplicate using quantitative real-time PCR (QPCR) and the above detailed TaqMan gene expression assays. Fluorescent signals were measured using the StepOnePlus system (Applied Biosystems, Foster City, CA). The expression levels of each gene were compared using hypoxanthine-guanine phosphoribosyl transferase 1 (HPRT1; Hs99999909_m1; Applied Biosystems) [31] as an endogenous control according to the standard 2(ΔΔCT) calculation [32], giving results as a relative quantification (RQ) and fold change ± standard error of the mean (SEM).
Reactive oxygen species (ROS) measurements
Blood samples from three additional patients were collected (n=3, three females, mean age ± standard error of the mean (SEM): 85.6 ± 4.978 years, range: 78–95 years). Monocytes were isolated from the whole blood and activated to macrophages as described above. The macrophages were cultured in 6-well plates for 4 days and were polarized as described. The cells were harvested, re-seeded in adequate 96-well plates, polarized, and treated with the supplements as described above. ROS were measured by staining the cells using the DCFDA cellular ROS detection assay kit (Abcam-ab113851, Cambridge, MA) according to the manufacturer’s instructions and were analyzed using fluorescent microplate measurement (Tecan, Mannedorf, Switzerland).
Statistical analysis
Data was processed using the biostatistical package InStat (GraphPad, San Diego, CA). Values of gene and protein expression over two standard deviations (SDs) from the average were excluded from statistical analysis. Gene and protein expression were summarized using means, SDs, median, and range. Although the data were normally distributed, due to the small sample size the comparisons between groups for gene and protein expression were performed using Mann–Whitney nonparametric tests. Multivariate tests, including Kruskal–Wallis and ANOVA tests, were also applied to compare gene and protein expression across the treatment groups. p<0.05 was considered to be the threshold for statistical significance.
Results
Macrophage activation
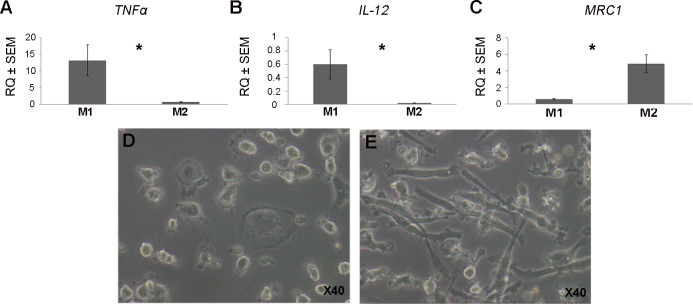
The activation of macrophages was confirmed by microscopy that demonstrated the development of pseudopods in M1 and M2 macrophages. The polarization of cultured macrophages was validated using QPCR for TNFα and IL-12 as markers for M1 polarization and MRC1 for M2 polarization, respectively. Accordingly, M1 macrophages from nvAMD (stimulated with IFNγ and LPS) demonstrated higher IL-12 mRNA levels compared with M2 cells (26.09-fold, p=0.0095; Mann–Whitney test) and a higher TNFα level (21.2-fold, p=0.0016; Mann–Whitney test). M2 macrophages from nvAMD patients (stimulated with IL-13 and IL-4) showed increased mRNA levels of MRC1 compared with M1 cells (13.06-fold, p=0.015; Mann–Whitney test; Figure 1).
Figure 1.
Validation of macrophage polarization. QPCR was done using markers for the phenotype of M1 and M2 macrophages. Panels A and B show expression levels of tumor necrosis factor α (TNF α) and interleukin 12 (IL-12) markers for M1 polarization, while Panel C shows expression levels of mannose receptor C-type 1(MRC1), a marker for M2 polarization. Accordingly, M1 macrophages showed higher mRNA levels of TNFα and IL-12 compared with M2 macrophages (A, B). M2 macrophages demonstrated increased mRNA levels of MRC1 compared with M1 cells (C). *=p<0.01. Panel D indicates M1 macrophage morphology via inverted microscope at 40x magnification, and Panel E indicates M2 macrophage morphology.
Pro-angiogenic, inflammatory, and antioxidant gene and protein expression levels were measured in the cultured M1 and M2 macrophages. Several genes and proteins implicated in AMD were identified in macrophage culture, and some were differentially expressed among macrophage subtypes. For example, QPCR results demonstrated the expression of VEGFa, the major pro-angiogenic cytokine involved in nvAMD, in both macrophage subtypes. VEGFa mRNA levels were increased in M1 macrophages compared to M2 macrophages (14.7-fold, p=0.0012; Mann–Whitney test; Table 1, Table 2). Similarly, protein levels of IL-6 and TNFα were both upregulated in M1 macrophages compared to M2 macrophages (IL-6: 47.3-fold, p=0.0007; TNFα: 3.08-fold, p=0.006; Mann–Whitney test; Table 3, Table 4).
Table 1. QPCR Results for M1 macrophages-group composition.
| Gene name | Mean |
M1 |
|
|
|
|
|---|---|---|---|---|---|---|
| p-value | G1 | G2 | G3 | G4 | G5 | |
| ISYNA1 | mean (SD) |
0.39 (1.67) |
0.43 (0.12) |
1.11 (0.41) |
1.66 (0.35) |
1 (0.59) |
| |
P value * |
0.09 |
0.015 |
0.63 |
0.17 |
|
| MRC1 | mean (SD) |
0.45 (0.47) |
1.1 (0.15) |
1.29 (0.72) |
1.35 (0.17) |
1 (0.58) |
| |
P value |
0.4 |
0.48 |
0.5 |
0.28 |
|
| VEGF | mean (SD) |
1.73 (0.38) |
1.08 (0.65) |
0.57 (0.24) |
0.55 (0.28) |
1 (0.43) |
| |
P value |
0.066 |
0.835 |
0.07 |
0.073 |
|
| SOD1 | mean (SD) |
0.76 (0.25) |
0.94 (0.54) |
3.14 (0.82) |
7.0 (0.94) |
1 (0.42) |
| |
P value |
0.55 |
0.48 |
0.0095 |
0.004 |
|
| HMOX1 | mean (SD) |
0.864 (0.1) |
1.29 (0.63) |
24.144 (7.64) |
18.66 (8.67) |
1 (0.49) |
| |
P value |
0.6 |
0.4 |
0.0061 |
0.0025 |
|
| CAT | mean (SD) |
0.74 (0.28 |
0.79 (0.18) |
1.62 (0.39) |
1.6 (0.95) |
1 (0.18) |
| |
P value |
0.5 |
0.7 |
0.11 |
0.26 |
|
| GPX1 | mean (SD) |
2.99 (1.51) |
1.95 (0.98) |
1.22 (0.89) |
1.33 (0.98) |
1 (0.98) |
| P value | 0.16 | 0.4 | 0.66 | 0.17 |
G1: lutein+ zeaxanthin, G2: lutein+ zeaxanthin and zinc, G3: lutein+ zeaxanthin, zinc, Lycomoto and carnosic acid, G4: lutein+ zeaxanthin, carnosic acid and beta- carotene, G5: vehicle control. Genes analyzed included inositol-3-phosphate synthase 1(ISYNA1), mannose receptor C-type 1(MRC1), vascular endothelial growth factor α (VEGF), superoxide dismutase 1(SOD1), heme oxygenase 1(HMOX1), catalase(CAT) and glutathione peroxidase 1(GPX1). * compared to G5 by Mann–Whitney. Gene expression levels represented in RQ values with the control group transformed to 1 and each subsequent group normalized accordingly.
Table 2. QPCR results for M2 macrophages-group composition.
| Gene name | Mean |
M2 |
|
|
|
|
|---|---|---|---|---|---|---|
| p-value | G1 | G2 | G3 | G4 | G5 | |
| ISYNA1 | mean (SD) |
0.74 (0.52) |
0.44 (0.15) |
0.61(0.65) |
0.29 (0.15) |
1 (0.54) |
| |
P value * |
0.24 |
0.008 |
0.81 |
0.0043 |
|
| MRC1 | mean (SD) |
1.46 (0.17) |
0.84 (0.12) |
0.93 (0.14) |
0.95 (0.26) |
1 (0.36) |
| |
P value |
0.05 |
0.7 |
0.73 |
>0.9999 |
|
| VEGF | mean (SD) |
1.73 (0.78) |
1.2 (0.64) |
2.92 (0.81) |
1.95 (0.59) |
1 (0.0.37) |
| |
P value |
0.08 |
0.62 |
0.0043 |
0.026 |
|
| SOD1 | mean (SD) |
0.72 (0.26) |
0.53 (0.069) |
1.45 (1.25) |
1.27 (1.04) |
1 (0.625) |
| |
P value |
0.73 |
0.14 |
0.45 |
0.83 |
|
| HMOX1 | mean (SD) |
1.13 (0.64) |
0.84 (0.39) |
5.73 (1.08) |
2.94 (0.85) |
1 (0.56) |
| |
P value |
0.8 |
0.7 |
0.0012 |
0.0012 |
|
| CAT | mean (SD) |
1.55 (0.85) |
0.64 (0.41) |
1.58 (0.83) |
0.87 (0.33) |
1 (0.58) |
| |
P value |
0.165 |
0.07 |
0.18 |
0.9 |
|
| GPX1 | mean (SD) |
0.72 (0.4) |
0.31 (0.12) |
0.23 (0.1) |
0.27 (0.05) |
1 (1.34) |
| P value | 0.4 | 0.7 | 0.07 | 0.13 |
G1: lutein+ zeaxanthin, G2: lutein+ zeaxanthin and zinc, G3: lutein+ zeaxanthin, zinc, Lycomoto and carnosic acid, G4: lutein+ zeaxanthin, carnosic acid and beta- carotene, G5: vehicle control. Gene analyzed included inositol-3-phosphate synthase 1(ISYNA1), mannose receptor C-type 1(MRC1), vascular endothelial growth factor α (VEGF), superoxide dismutase 1 (SOD1), heme oxygenase 1 (HMOX1), catalase (CAT) and glutathione peroxidase 1 (GPX1). * compared to G5 by Mann–Whitney. Gene expression levels represented in RQ values with the control group transformed to 1 and each subsequent group normalized accordingly.
Table 3. ELISA results for M1 macrophages-group composition.
| Protein name | Mean |
M1 |
|
|
|
|
|---|---|---|---|---|---|---|
| p-value | G1 | G2 | G3 | G4 | G5 | |
| TNF α | Mean
(SD) |
641.89 (283.4) |
732.59 (181.77) |
483.09
(306.4) |
550.17 (257.8) |
604.207 (291.4) |
| |
P value * |
0.79 |
0.57 |
0.46 |
0.72 |
|
| SDF1 | mean
(SD) |
937.3 (261.57) |
750.79 (190.07) |
852.38 (228.14) |
758.17 (134.5) |
971.51
(117.2) |
| |
P value |
0.95 |
0.02 |
0.23 |
0.006 |
|
| MCP1 | mean
(SD) |
3718.7 (268.34) |
3596.47 (290.3) |
3187.93 (466.08) |
3030.82 (582.9) |
3876.68 (290.2) |
| |
P value |
0.044 |
0.1 |
0.0047 |
0.003 |
|
| IL-8 | Mean
(SD) |
357.29 (123.74) |
383.09 (103.3) |
339.8
(105.8) |
357.78 (70.16) |
349.7
(91.5) |
| |
P value |
0.87 |
0.64 |
0.87 |
0.87 |
|
| IL-6 | Mean
(SD) |
2664.58 (425.1) |
2624.64 (669.9) |
393.41
(136.2) |
859.88 (639.8) |
2395.57 (940.05) |
| |
P value |
0.99 |
0.72 |
0.0006 |
0.007 |
|
| ICAM | mean
(SD) |
476.9
(307.6) |
664.05 (174.64) |
425.68 (235.53) |
553.04 (150.82) |
571.87
(86.98) |
| P value | 0.19 | 0.39 | 0.39 | 0.78 |
G1: lutein+ zeaxanthin, G2: lutein+ zeaxanthin and zinc, G3: lutein+ zeaxanthin, zinc, Lycomoto and carnosic acid, G4: lutein+ zeaxanthin, carnosic acid and beta- carotene, G5: vehicle control. Proteins analyzed included tumor necrosis factor α (TNF α), C-X-C Motif chemokine ligand 12 (SDF1), C-C Motif chemokine ligand 2 (MRC1), interleukin 8 (IL-8), interleukin 6 (IL-6) and intercellular adhesion molecule (ICAM). * compared to G5 by Mann–Whitney. Protein levels represented in values of pg/ml.
Table 4. ELISA results for M2 macrophages-group composition.
| Protein | Mean |
M2 |
|
|
|
|
|---|---|---|---|---|---|---|
| name | p-value | G1 | G2 | G3 | G4 | G5 |
| TNF α | mean (SD) |
136.52 (146.25) |
247.088 (125.47) |
28.157
(49.3) |
9.11
(14.21) |
195.87
(130) |
| |
P value * |
0.45 |
0.32 |
0.022 |
0.014 |
|
| SDF1 | mean (SD) |
813.4 (129.27) |
930.96 (156.94) |
792.39 (195.04) |
904.4 (159.07) |
853.06
162.5) |
| |
P value |
0.62 |
0.8 |
0.45 |
0.53 |
|
| MCP1 | mean (SD) |
3630
(245.7) |
3701.3
(346.9) |
2993.9 (419.9) |
3302.2 (288.5) |
3754.4
(134.2) |
| |
P value |
0.28 |
0.999 |
0.0006 |
0.0023 |
|
| IL-8 | mean (SD) |
351.95 (78.14) |
399.38 (114.72) |
399.06 (100.4) |
408.92 (112.92) |
409.32
(64) |
| |
P value |
0.15 |
0.71 |
0.71 |
0.8 |
|
| IL-6 | mean (SD) |
117.84 (156.19) |
165.75
(161.1) |
51.22
(63.96) |
48.9
(79.63) |
50.64
(63.01) |
| |
P value |
0.73 |
0.18 |
0.99 |
0.94 |
|
| ICAM | mean (SD) |
630.9 (192.86) |
562.8
(237.3) |
638.44 (267.5) |
723.22 (307.22) |
718.85
(253.13) |
| P value | 0.61 | 0.32 | 0.45 | 0.94 |
G1: lutein+zeaxanthin, G2: lutein+zeaxanthin and zinc, G3: lutein+zeaxanthin, zinc, Lycomoto and carnosic acid, G4: lutein+zeaxanthin, carnosic acid and beta-carotene, G5: vehicle control. Proteins analyzed included tumor necrosis factor α (TNF α), C-X-C Motif chemokine ligand 12 (SDF1), C-C Motif chemokine ligand 2 (MRC1), Interleukin 8 (IL-8), Interleukin 6 (IL-6) and intercellular adhesion molecule (ICAM).*compared to G5 by Mann–Whitney. Protein expression represented in values of pg/ml.
Macrophage expression profile following supplement treatment
Supplement treatment was associated with the modulation of both gene and protein expression levels in cultured human macrophages from nvAMD patients (Table 1, Table 2, Table 3, and Table 4). The majority of the effects were demonstrated following treatment with G3 (lutein+zeaxanthin, zinc, Lyc-O-Mato, and carnosic acid) and G4 (lutein+zeaxanthin, carnosic acid, and beta-carotene) supplements. For example, treatment with G3 resulted in an increased level of the antioxidative gene HMOX1 in both M1 (24.1-fold, p=0.0061; Mann–Whitney test) and M2 (5.7-fold, p=0.0012; Mann–Whitney test) macrophages (Table 1, Table 2). Protein expression of the pro-inflammatory gene MCP1 was suppressed in M1 and M2 (1.2-fold, p=0.0047; and 1.25-fold, p=0.0006; Mann–Whitney test, respectively). G3 treatment was also associated with reduced IL-6 in M1 macrophages (6.09-fold, p=0.0006, Mann–Whitney test) and TNFα in M2 macrophages (6.9-fold, p=0.022, Mann–Whitney test; Table 3 and Table 4). There was a trend toward reduced VEGFa mRNA levels in M1 macrophages expressing high levels of VEGF and increased VEGFa levels in M2 cells expressing low levels of VEGF (2.91-fold, p=0.0043; Mann–Whitney test; Table 1, Table 2).
G4 treatment resulted in increased HMOX1 mRNA expression in M1 and M2 macrophages (18.9-fold, p=0.0025 and 2.9-fold, p=0.0012, respectively; Mann–Whitney test) and increased SOD1 mRNA levels in M1 macrophages (7.0-fold, p=0.004; Mann–Whitney test). G4 was also associated with decreased ISYNA1 mRNA levels in M2 macrophages (3.4-fold, p=0.0043; Mann–Whitney test). There was a trend toward reduced VEGFa mRNA levels in M1 macrophages expressing high levels of VEGF and increased VEGFa levels in M2 cells expressing low levels of VEGF (1.96-fold, p=0.026; Mann–Whitney test; Table 1, Table 2). Additionally, G4 resulted in decreased protein levels of IL-6 in M1 macrophages (2.8-fold, p=0.007; Mann–Whitney test), decreased MCP1 protein levels in both M1 (1.3-fold, p=0.003; Mann–Whitney test) and M2 macrophages (1.4-fold, p=0.0023; Mann–Whitney test), and decreased SDF1 protein levels in M1 (1.3-fold, p=0.006; Mann–Whitney test) and decreased TNFα (21.5-fold, p=0.014; Mann–Whitney test) in M2 macrophages (Table 3 and Table 4).
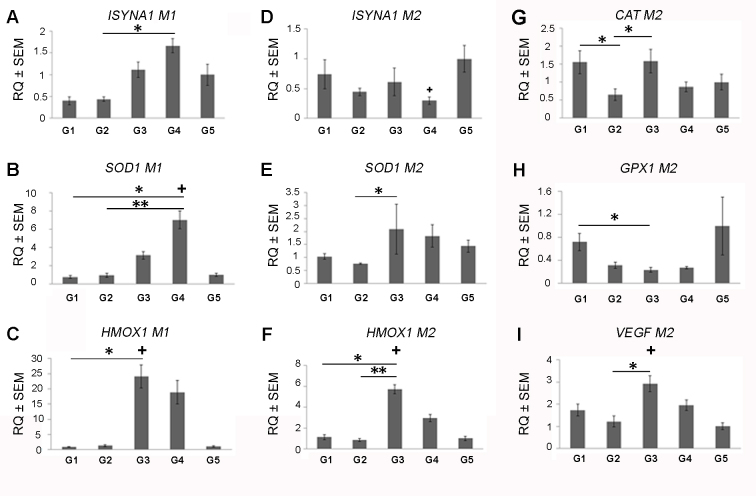
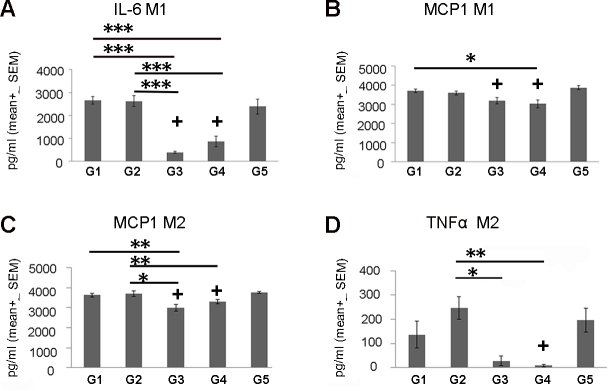
The effect of the different supplement treatment groups was also analyzed using multivariate statistical tests. The results showed that most of the effects were demonstrated following treatment with G3 and with G4 supplements. For example, G3 increased levels of the gene HMOX1 in both M1 and M2 and compared to the G1 group (27.9- fold, p<0.001 and 5.06-fold, p<0.001, respectively; Kruskal–Wallis test; Figure 2C–F). In addition, G3 also increased the expression of the antioxidative genes HMOX1 and SOD1 in M2 macrophages compared with G2 (6.81-fold, p<0.01 and 2.75- fold, p<0.01, respectively; Kruskal–Wallis test; Figure 2E,F). Protein expression of the proinflammatory genes TNFα and MCP1 was suppressed following treatment with G3 compared to G2 in M2 macrophages (8.77-fold, p<0.05 and 1.23-fold, p<0.001, respectively; ANOVA test; Figure 3A,C,D). Treatment with G4 demonstrated increased SOD1 mRNA levels in M1 macrophages compared to both G1 and G2 (1.76-fold, p<0.05 and 2.4-fold, p<0.01, respectively; Kruskal–Wallis test; Figure 2B). Moreover, G4 was associated with decreased MCP1 levels in M1 compared to G1 and in M2 macrophages compared to G2 (1.23- fold, p<0.05 and 1.12-fold, p<0.05, respectively; ANOVA test; Figure 3B,C).
Figure 2.
Gene expression profile of macrophages treated with antioxidant supplements. mRNA expression levels were measured in activated human macrophages treated with the five different experimental groups (G1–G5) using QPCR. A comparison between the different treatment groups was performed using a multivariate and non-parametric analysis (Kruskal–Wallis test). Expression of inositol-3-phosphate synthase 1 (ISYNA1), superoxide dismutase 1 (SOD1), and heme oxygenase 1 (HMOX1) of M1 and M2 are shown in panels A–C and D–F, respectively. Expression of catalase (CAT), glutathione peroxidase 1(GPX1), and vascular endothelial growth factor α (VEGFa) of M2 macrophages are shown in panels G–I, respectively. Gene expression of macrophages treated with supplements was compared to DMSO-treated macrophages from each patient (+=p<0.05) and between experimental groups (*=p<0.05, **=p<0.01, ***=p<0.0001; n=10). The y-axis indicates RQ ± SEM relative to the gene expression of the control group.
Figure 3.
Protein expression profile of macrophages treated with antioxidant supplements. Proteins expression levels were measured in activated human macrophages treated with the five different experimental groups (G1–G5) using ELISA. A comparison between the different treatment groups was performed using a multivariate and parametric analysis (ANOVA test). Expression of interleukin 6 (IL-6) and C-C Motif chemokine ligand 2 (MCP1/CCR2) from M1 and MCP1 and tumor necrosis factor α (TNFα) from M2 are shown in panels A–B and C–D, respectively. Protein expression of macrophages treated with supplements was compared to DMSO-treated macrophages from each patient (+=p<0.05) and between experimental groups (*=p<0.05, **=p<0.01, ***=p<0.0001; n=10). The y-axis indicates RQ ± SEM relative to the gene expression of the control group.
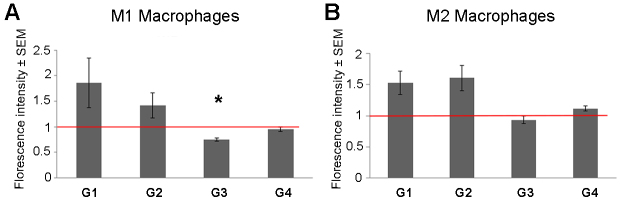
Oxidative stress was measured using ROS analysis. The results showed a decrease in ROS levels following treatment with G3 supplements, which reached statistical significance in M1 macrophages (p=0.017). However, there was a trend toward increased ROS levels following treatment with G1 and G2 supplements (Figure 4A).
Figure 4.
Quantification of reactive oxygen species (ROS) level. Oxidative stress level was compared between treated and control macrophages via ROS measurements. ROS levels in M1 and M2 macrophages are shown in panels A and B, respectively. ROS levels were normalized to the untreated macrophages of the same patient (n=3; *=p<0.05). The y-axis indicates the relative fluorescent intensity ± SEM.
Discussion
This research demonstrates that physiologic levels of antioxidants and minerals may modulate gene and protein expression in cultured human macrophages from nvAMD patients. The expression of genes and proteins that may be important in the context of AMD, including antioxidant genes (HMOX1, SOD1), pro-angiogenic genes (VEGF, SDF1), and pro-inflammatory genes (TNFα, MCP1, IL6), were shown to be regulated. Given the involvement of macrophages in AMD and their suggested capacity to exacerbate oxidative injury, angiogenesis, and inflammation in AMD, such modulation of gene and protein expression may potentially have therapeutic importance in the context of the disease.
Interestingly, the expression patterns that we tested were not markedly affected by lutein+zeaxanthin alone or by the combination of lutein+zeaxanthin and zinc. However, combining these supplements with other phytonutrients yielded a marked effect on the expression levels of several genes and proteins tested. Therefore, a benefit in reducing the pathogenic role of macrophages in AMD may potentially be gained by combining additional supplements with the commonly used AREDS formula.
Combinations of carotenoids and phenolics were previously demonstrated to modulate murine macrophages [33,34]. For example, macrophage gene expression and function were modulated by a combination of lycopene or Lyc-O-Mato and carnosic acid, lutein, and/or beta-carotene in a model of acute peritonitis [33]. Similarly, carnosic acid and carnosol were shown to modulate chemokine production in murine macrophages [34]. Another recent study has suggested that lutein has anti-inflammatory and antioxidant effects in an LPS-activated microglial cell line [35]. Yet, lutein concentrations in that study exceeded the physiologic levels, being 50-fold higher compared with the ones we tested. It was also reported that glutamate-cysteine-ligase expression levels in murine macrophages may be modulated by carotenoids in culture [36]. Our study validates and extends such observations, as mononuclear cells from nvAMD patients are characterized by a pro-inflammatory gene expression signature that is different from age-matched controls [3-5,22] or from rodents. Therefore, combined with our study, these data provide proof-of-concept for the modulation of mononuclear cell gene expression by compounds that may be used as oral supplements in humans.
The beneficial effects of carotenoids and/or phenolic supplements were also demonstrated in vivo in rodent models that recapitulate the features of AMD. For example, lutein and carnosic acid supplementation was associated with the amelioration of oxidative injury in murine models for photic retinal injury [19,37]. Yet, in these in vivo studies it was not evaluated if the modulation of macrophages mediated the protective effects of such supplementation.
Macrophages are characterized by marked heterogeneity in terms of phenotype and effects [38,39]. While macrophage phenotypes are often classified into classical (M1) and alternative (M2) polarization, it is clear that this classification represents an over-simplification of macrophage function [40]. To evaluate if the supplement effects are phenotype-specific, we have tested the two prototype macrophage phenotypes [16,39,41]. Our results show an overall similar antioxidant, anti-inflammatory, and antiangiogenic effect regardless of the specific M1 or M2 macrophage phenotype. Exceptions to this role exist and are associated with variable gene expression levels that characterize the different macrophage phenotypes (i.e., low VEGF expression in M2 versus M1). In that context, it is worth noting that VEGF may be dispensable for the angiogenic role of macrophages in nvAMD [42,43] and that TNFα may mediate such an effect by inducing VEGF expression from the retinal pigment epithelium.
The current study is limited by its cell culture design. Yet, is noteworthy that we have evaluated mononuclear cells isolated from nvAMD patients. This is important as we have previously demonstrated that PBMC, monocytes, and macrophages from nvAMD patients are characterized by a pro-inflammatory gene and protein expression pattern and by an enhanced pro-angiogenic effect in in vitro and in vivo experimental models [4,5,22,23]. Therefore, in vivo validation of these findings is important to support the role of macrophage modulation by oral supplements in ameliorating retinal injury in the context of AMD. We have tested a variety of supplement combinations, but due to the large number of potential supplement combinations and the limited number of cells that may be isolated from a single patient, we have not assessed all possible combinations of the compounds we have tested. Further research is required to identify the ideal supplement combination to modulate the macrophage phenotype in the context of AMD.
In conclusion, compounds that may be administered as oral supplements can modify the phenotype of human macrophages cultured from nvAMD patients. Such phenotype modulation may be of potential benefit in the context of the disease. Future research should identify the preferred compound or compounds for this purpose and assess the validity of this potential therapy in in vivo models for the disease.
Acknowledgments
We thank Dr. Liran Tiosano for his excellent advice and statistical help for the biostatistics portion of this article. This research was funded by a grant from Lycored Inc. The authors declare no other financial or nonfinancial competing interests.
References
- 1.Sarks SH, Van Driel D, Maxwell L, Killingsworth M. Softening of drusen and subretinal neovascularization. Trans Ophthalmol Soc U K. 1980;100:414–22. [PubMed] [Google Scholar]
- 2.Penfold P, Killingsworth M, Sarks S. An ultrastructural study of the role of leucocytes and fibroblasts in the breakdown of Bruch’s membrane. Aust J Ophthalmol. 1984;12:23–31. [PubMed] [Google Scholar]
- 3.Lederman M, Weiss A, Chowers I. Association of neovascular age-related macular degeneration with specific gene expression patterns in peripheral white blood cells. Invest Ophthalmol Vis Sci. 2010;51:53–8. doi: 10.1167/iovs.08-3019. [DOI] [PubMed] [Google Scholar]
- 4.Grunin M, Burstyn-Cohen T, Hagbi-Levi S, Peled A, Chowers I. Chemokine receptor expression in peripheral blood monocytes from patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:5292–300. doi: 10.1167/iovs.11-9165. [DOI] [PubMed] [Google Scholar]
- 5.Grunin M, Hagbi-Levi S. -, Rinsky B, Smith Y, Chowers I. Transcriptome Analysis on Monocytes from Patients with Neovascular Age-Related Macular Degeneration. Sci Rep. 2016;6:29046. doi: 10.1038/srep29046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apte RS. Regulation of angiogenesis by macrophages. Adv Exp Med Biol. 2010;664:15–9. doi: 10.1007/978-1-4419-1399-9_2. [DOI] [PubMed] [Google Scholar]
- 7.Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006;3:e310. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa-Heidmann DG, Caicedo A, Hernandez EP, Csaky KG, Cousins SW. Bone marrow-derived progenitor cells contribute to experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:4914–9. doi: 10.1167/iovs.03-0371. [DOI] [PubMed] [Google Scholar]
- 9.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–92. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, Chowers I, Edwards PA, Baldan A, Parks JS, Ory DS, Apte RS. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013;17:549–61. doi: 10.1016/j.cmet.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsutsumi C, Sonoda KH, Egashira K, Qiao H, Hisatomi T, Nakao S, Ishibashi M, Charo IF, Sakamoto T, Murata T, Ishibashi T. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]
- 13.Zandi S, Nakao S, Chun KH, Fiorina P, Sun D, Arita R, Zhao M, Kim E, Schueller O, Campbell S, Taher M, Melhorn MI, Schering A, Gatti F, Tezza S, Xie F, Vergani A, Yoshida S, Ishikawa K, Yamaguchi M, Sasaki F, Schmidt-Ullrich R, Hata Y, Enaida H, Yuzawa M, Yokomizo T, Kim YB, Sweetnam P, Ishibashi T, Hafezi-Moghadam A. ROCK-Isoform-Specific Polarization of Macrophages Associated with Age-Related Macular Degeneration. Cell Reports. 2015;10:1173–86. doi: 10.1016/j.celrep.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura R, Sene A, Santeford A, Gdoura A, Kubota S, Zapata N, Apte RS. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun. 2015;6:7847. doi: 10.1038/ncomms8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao H, Natoli R, Valter K, Provis JM, Rutar M. Spatiotemporal Cadence of Macrophage Polarisation in a Model of Light-Induced Retinal Degeneration. PLoS One. 2015;10:e0143952. doi: 10.1371/journal.pone.0143952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–42. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 17.Cao X, Shen D, Patel MM, Tuo J, Johnson TM, Olsen TW, Chan CC. Macrophage polarization in the maculae of age-related macular degeneration: A pilot study. Pathol Int. 2011;61:528–35. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E, Feuer W, Huang D, Wen R, Hong L, Wang H, Laird JM, Sene A, Apte RS, Salomon RG, Hollyfield JG, Perez VL. Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int J Inflam. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rezaie T, McKercher SR, Kosaka K, Seki M, Wheeler L, Viswanath V, Chun T, Joshi R, Valencia M, Sasaki S, Tozawa T, Satoh T. Lipton S a. Protective effect of carnosic acid, a pro-electrophilic compound, in models of oxidative stress and light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53:7847–54. doi: 10.1167/iovs.12-10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 21.Eye TA, Study D. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration. JAMA. 2013;309:2005. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 22.Hagbi-Levi S, Grunin M, Jaouni T, Tiosano L, Rinsky B, Elbaz-Hayoun S, Peled A, Chowers I. Pro-Angiogenic Characteristics of Activated Macrophages from Patients with Age-related Macular Degeneration. Neurobiol Aging. 2017 doi: 10.1016/j.neurobiolaging.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Lederman M, Weiss A, Chowers I. Association of neovascular age-related macular degeneration with specific gene expression patterns in peripheral white blood cells. Invest Ophthalmol Vis Sci. 2010;51:53–8. doi: 10.1167/iovs.08-3019. [DOI] [PubMed] [Google Scholar]
- 24.Age-Related Eye Disease Study Research G. The Age-Related Eye Disease Study (AREDS) design implications. AREDS report no. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelinas L, Falkenham A, Oxner A, Sopel M, Legare JF. Highly purified human peripheral blood monocytes produce IL-6 but not TNF{alpha} in response to angiotensin II. J Renin Angiotensin Aldosterone Syst. 2011;12:295–303. doi: 10.1177/1470320310391332. [DOI] [PubMed] [Google Scholar]
- 26.Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARγ Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties. Cell Metab. 2007;6:137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 28.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 2009;28:2114–27. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 30.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Hadad N, Levy R. The synergistic anti-inflammatory effects of lycopene, lutein?? -carotene, and carnosic acid combinations via redox-based inhibition of NF-??B signaling. Vol. 53. Free Radic Biol Med. 2012:1381–91. doi: 10.1016/j.freeradbiomed.2012.07.078. [DOI] [PubMed] [Google Scholar]
- 34.Schwager J, Richard N, Fowler A, Seifert N, Raederstorff D. Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes. Molecules. 2016;21:465. doi: 10.3390/molecules21040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu W, Li Y, Wu Y, Zhang Y, Wang Z, Liu X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-??B inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol Nutr Food Res. 2015;59:1663–73. doi: 10.1002/mnfr.201500109. [DOI] [PubMed] [Google Scholar]
- 36.Akaboshi T, Yamanishi R. Certain carotenoids enhance the intracellular glutathione level in a murine cultured macrophage cell line by inducing glutamate-cysteine-ligase. Mol Nutr Food Res. 2014;58:1291–300. doi: 10.1002/mnfr.201300753. [DOI] [PubMed] [Google Scholar]
- 37.Kamoshita M, Toda E, Osada H, Narimatsu T, Kobayashi S, Tsubota K, Ozawa Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci Rep. 2016;6:30226. doi: 10.1038/srep30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 39.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 40.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Vol. 72. Cell Mol Life Sci. 2015:4111–26. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liyanage SE, Fantin A, Villacampa P, Lange CA, Denti L, Cristante E, Smith AJ, Ali RR, Luhmann UF, Bainbridge JW, Ruhrberg C. Myeloid-Derived Vascular Endothelial Growth Factor and Hypoxia-Inducible Factor Are Dispensable for Ocular Neovascularization-Brief Report. Arterioscler Thromb Vasc Biol. 2016;36:19–24. doi: 10.1161/ATVBAHA.115.306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Han X, Wittchen ES, Hartnett ME. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol Vis. 2016;22:116–28. [PMC free article] [PubMed] [Google Scholar]