Abstract
X-ray excited luminescent chemical imaging (XELCI) uses a combination of X-ray excitation to provide high resolution and optical detection to provide chemical sensing. A key application is to detect and study implant-associated infection. The implant is coated with a layer of X-ray scintillators which generate visible near infrared light when irradiated with an X-ray beam. This light first passes through a pH indicator dye-loaded film placed over the scintillator film in order to modulate the luminescence spectrum according to pH. The light then passes through tissue is collected and the spectral ratio measured to determine pH. A focused X-ray beam irradiates a point in the scintillator film, and a pH image is formed point-by-point by scanning the beam across the sample. The sensor and scanning system are described along with preliminary results showing images in rabbit models.
Keywords: X-ray excited optical luminescence, Sensors, Radioluminescence, pH indicators, Biofilm, Implanted Medical Devices
1. Introduction
The demand for implanted medical devices to improve and sustain a high quality of life is increasing as the population continues to age. These devices include prosthetic heart valves, pacemakers, total joint arthroplasty, and orthopedic implants. Despite the medical benefits, many of these devices become infected which leads to patient suffering and high costs from treatments and surgical revisions. For example, approximately 5% of the 2 million fracture fixation surgeries performed annually in the US become infected1, with higher risk for patients with open wounds, debris in the wound, revision of previously infected devices, diabetes, immunosuppressed patients2. Implant associated infections are difficult detect at early stages when the infection is localized, and after full manifestation, treatment usually requires surgical removal of the implant, antibiotic therapy to treat the remaining infection, followed by surgical insertion of a new implant. There is a need for methods to non-invasively detect and study implant associated infection at early stages or during antibiotic treatment.
Bacteria become resistant to antibiotics when they grow in biofilms and loose this resistance if they leave the biofilm. Antibiotic resistance is usually explained through some combination of three principle mechanisms3, 4. First, the antibiotics can be deactivated or diffusion slowed in the top layers of the biofilm which slows ad reduces antibiotic penetration deeper within the biofilm. Second, there are dormant regions in the biofilm due to depletion of oxygen and nutrients by the top layers of the biofilm and inflamed tissue above. These dormant bacteria interact differently with the antibiotics which often interfere with bacterial growth and division. Third, the biofilm has a heterogeneous chemical environment, especially in terms of oxygen and pH, due to formation of acidic products. For example, in vitro studies have imaged pH at the surface as low as 5.0 when the bulk solution 50 μm above the surface was 7.2.5, 6 pH and other chemical changes can affect antibiotic activity, for example, the MIC dose for gentamycin against Staphylococcus aureus was 130 fold higher at pH 5.2 than at pH 7.4.7 Regardless of whether the low pH correlates with dormant regions in the biofilm (mechanism 2) or is itself a cause of the resistance (mechanism 3), it is expected to be an indication of infection. Indeed inflammation and infection have been shown to cause tissue to become acidic8, 9. In addition, researchers have proposed methods to treat bacteria based upon either raising the local pH10, or pH-triggered drug release agents11, 12. However, measuring local acidosis directly above the implant requires a sensor localized on the implant surface.
Previously our group showed proof of concept for imaging the pH drop during in vitro bacterial growth on the sensors and pH restoration during treatment with ciprofloxacin antibiotics. The XELCI was acquired with the sensor sandwiched between two 6 mm thick slices of chicken breast tissue. The method generated high resolution surface-specific pH images, but the images were very slow to acquire because each spectrum was acquired on a microscope spectrometer as the microscope stage was moved13. We also demonstrated a similar time-dependent surface pH measurement using 980 nm excitation of upconversion luminescence, however, spatial resolution is limited by diffuse optical scattering of the excitation beam which has a point spread function fwhm about the tissue depth being imaged through14. Herein we describe a system with much larger travel and sample weight designed to image small animals much more rapidly, using two PMTs to measure luminescence at two wavelengths instead of a microscope coupled spectrometer.
2. XELCI of implanted medical device surface pH
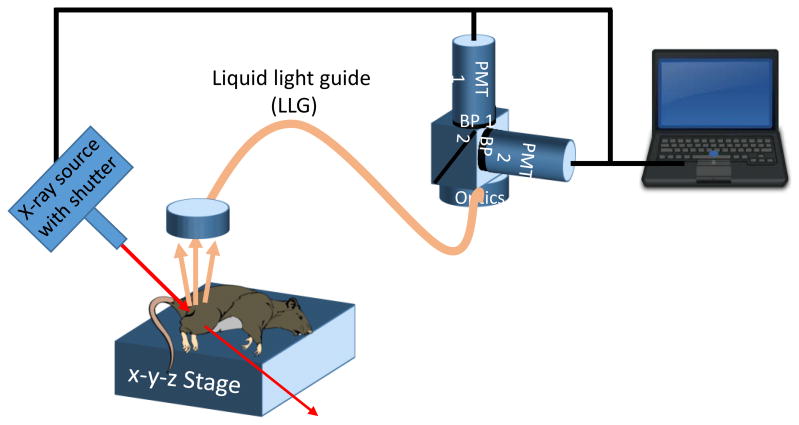
XELCI provides high spatial resolution images of a sensor-coated implant surface, using a combination of x-ray excitation to generate light at a specific spot on the implant surface, and dye-based optical sensors to provide chemical sensitivity. A multilayer sensor film is deposited onto an implanted medical device surface, such as an orthopedic tibial plate (see Figure 1). The first layer consists of x-ray scintillator microparticles such as gadolinium oxysulfide doped with europium. The particles are encapsulated in a strong flexible film such as polydimethylsiloxane (PDMS) for example to help with biocompatibility in addition to preventing destruction of the film due to mechanical forces or other localized analytes. The next layer directly on top of the particle layer is a light modulator with an absorption spectrum which dependent on local analyte concentration being sensed and imaged. In this case, the scintillator is Gd2O2S:Eu, which emits light at 620 nm and 700 nm, and the light modulator is bromocresol green, a pH-sensitive film, which absorbs more 620 nm light at high pH, than at low pH; the 700 nm peak is minimally absorbed regardless of pH, and serves as a spectral reference signal.13 Above the pH sensitive film is tissue which ranges in thickness depending on the site of implantation. In order to image these sensors, sample (or an x-ray and collection optics) are mounted to an x-y linear stage which causes the x-ray spot to scan across the sensor while pH measurements are acquired at each point. Figure 2 shows a schematic of the imaging system, and Figure 3 shows a photograph.
Figure 1. Schematic of radioluminescent sensor.
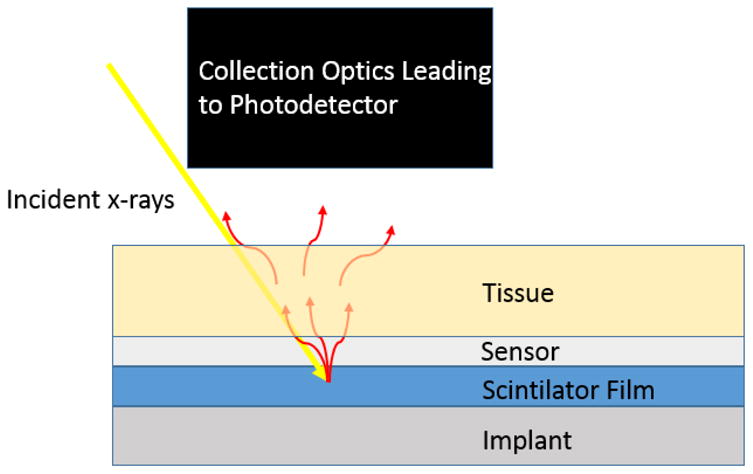
Figure 2. A) Schematic of XELCI scanning system.
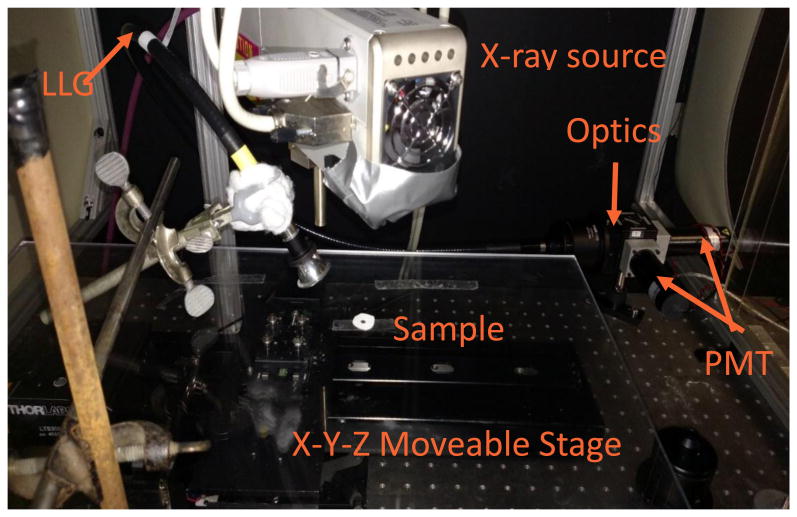
Figure 3.
Photograph of x-ray scanning system.
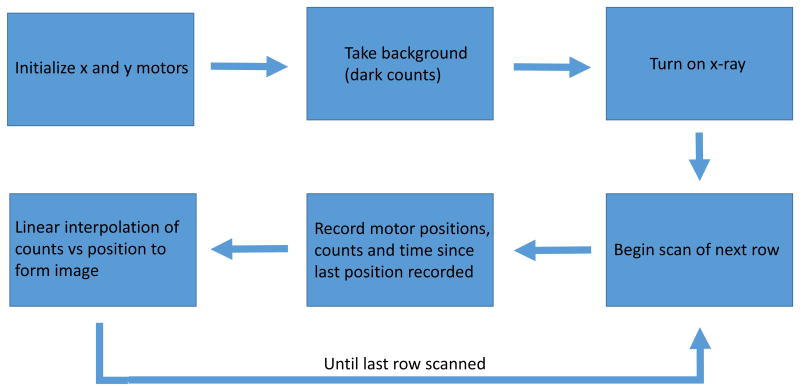
The scanning system can scan the sensors either by meander scanning or raster scanning. The motors can scan continuously or move to discrete locations. It is essential that the motors move consistently for any schema. This means in continuous scanning the speed must be consistent to ensure even exposure times and when moving discretely the motors need to move consistent distances in order to accurately image the sample. Once the particles have been excited, emitted light is modulated by the pH sensitive film. The tissue will then scatter and absorb the emission light from the particles. As tissue thickness increases, light attenuation by scattering and absorption increases. Once the light has escaped the tissue it is collected by an optically reflective plastic cone which directs the light into a liquid light guide that is 7.9 mm in diameter with a field of view of 79°. The collected light is collimated as soon as the end of the guide is reached to ensure proper behavior of the next optical stage which is a 50-50 beamsplitter and two band pass filters to separate the two peaks of interest. The 620 nm and 700 nm light is measured using two photomultiplier tubes (PMTs), each with its own band pass filter. The PMTs are setup in photon counting mode which generates TTL pulses for each detected photon, up to a maximum rate of 80 million pulses/second. A National Instruments c-DAQ 9171 using USB communication then counts the pulses to a saturation point of approximately 20 million pulses per second. Once collected the data is processed, two separate intensity maps are created. From here the final image is formed by taking a ratio of the signal wavelength intensity (620 nm) to the reference wavelength intensity (700 nm). Measuring ratio instead of absolute intensity accounts for variations in thickness and optical collection efficiency
The system is controlled by LabVIEW, a graphic based programming language. A basic flow chart for the control software is shown below. The program first initializes communication with the x- and y-axis motors. These motors are moved to the zero position before scanning. Before beginning the scanning a background is recorded for one second which gives information on dark counts and if any light is present in the area. After this the scan is ready to begin and the x-ray is turned on and the shutter open so that x-rays can generate light in the sample. Starting with the first row of the scan the motor position in the x axis is continuously recorded. At the same time the number of counts for each wavelength of light and time since the previous motor position update is also recorded. Time is recorded to account for any changes in motor speed and thus the exposure time so that each pixel can be normalized to counts/second. The motors scan to the end of the current row at which point the x axis motor returns to the start position and the y axis motor increases its position by a user defined step size. This step size will also determine the size of the pixels in the final image along the x axis. While the motors are moving to the start position of the next row the data is processed by a simple linear interpolation which has to as accurately as possible plot counts vs position. This image is displayed on the front panel in real time so that the user may check to make sure the scan is hitting the target. The user has control over the scan size, speed and start position.
This method provides a low noise, low background and high spatial resolution image in an acceptably long time. For example, a 100 mm × 25 mm scan size with 500 μm step size takes around 15 minutes to complete assuming a 5 mm/s scanning velocity. Higher speeds can be achieved by using larger step size, and in principle faster motors or multiple beams. The resolution of the system is limited to the size of the x-ray beam that excites the particles as well as the thickness of the scintillator and pH sensor films, and the thickness of any opaque well targets. In previous studies, we showed that submillimeter knife-edge resolution is acquired with submillimeter X-ray beams15, 16, and that the resolution can be reduced by using a larger collimated beam13. Here an IFG iMOXS/1-MFR X-ray source (Fischer Technology Inc, Windsor, CT) was used with a slightly focusing polycapillary X-ray optic and a 51 mm working distance. The signal intensity per pixel depends upon the X-ray intensity, scan speed, tissue thickness, and optical collection efficiency. Importantly, as the tissue thickness increases, the intensity of collected light decreases approximately exponentially, with more rapid decay for 620 nm light than 700 nm.17, 18 This decrease in signal intensity results in lower signal to noise ratio, (although longer X-ray exposures can compensate). Greater signal intensity can be acquired using a more intense X-ray beam, larger beam size, more efficient optical collection, or decreasing the tissue thickness by compression19. Although, a portion of the x-ray beam is scattered by the tissue (e.g., a 4 cm scattering mean free path for 25 keV X-rays in soft tissue), the scattered X-rays form a diffuse background orders of magnitude weaker than the focused ballistic photons which allows high resolution imaging through tissue.
This system is subject to many possible sources of error which include shot noise, x-ray fluctuations and motor accuracy. The motors chosen for the x-axis and y-axis respectively for the moveable stage (LS300 and MTS50) each have repeatability to 2 μm or less which is negligible compared to the expected feature sizes on the sensors. Due to the discrete nature of single photon counting, the system will be subject to shot noise. Given that shot noise is proportional to the square root of counts and read noise is constant, a high signal intensity should cause read noise in the DAQ to become negligible. This would cause our system to be “shot noise limited”.
2.1 Target Imaging
In order to test the system a target which consists of black plastic overlaid on top of a film of particles was prepared. The purpose of this target was to block light generated by the excited particles. An image which consists of dim areas where the plastic target lies and bright areas where the plastic target is not shows how complex features can be imaged both with and without tissue over top.
Figure 5 shows XELCI images of a complex target acquired with and without tissue. In the images, dark areas indicate regions where the light intensity was dim and brighter, more yellow regions indicate regions of high intensity. The target in this case is black plastic that forms an “anchor lab”. There are many complex features to note such as the hooks on the anchor, the small hole where a rope would be tied on and the offset cross. The pattern can be easily seen when this sample is imaged without tissue. All of the aforementioned features are easily seen. When the sample is imaged with tissue these features are most certainly more blurry but do appear. The hooks of the anchor especially are much less easily distinguished. This type of imaging shows that complex moiré patterns can be imaged with relative ease and accuracy. Both of these targets show that high resolution images can be produced through tissue with relative ease and through tissue thicknesses comparable to human tibial fracture fixation sites.
Figure 5.
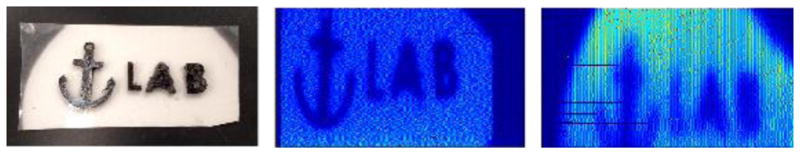
A. Photograph of anchor lab target B. Target mapped with X-ray beam collimated to 500 um. C. Target mapped through 6 mm chicken breast with x ray beam collimated to 1 mm
2.2 pH Imaging
Bacterial biofilm growth on the surface of implanted medical devices are difficult to detect or image. To address this problem a multilayer radioluminescent sensor was made as shown in Figure 6. A pH sensitive dye, bromocresol green, is encapsulated in H+ permeable PEG film. pH is detected due to absorption spectrum changes in the dye. At low pH, a higher portion of the dye is protonated which allows more 620 nm light to pass while at high pH, a larger portion of the dye is deprotonated and absorbs more 620 nm light. Concurrently, the dye only absorbs a small portion of 700 nm light, regardless of pH. The 700 nm light can act as a spectral reference to account for scintillator film inconsistencies, tissue composition changes and detection optics position changes from scan to scan. This mechanism of action is illustrated in Figure 6C.
Figure 6.
A) schematic of pH sensor in vivo placement; B) schematic of sensor layers/design; C) Graph of bromocresol green extinction dependence on pH (left axis) and Gd2O2S:Eu3+ emission spectrum overlaid (right axis).
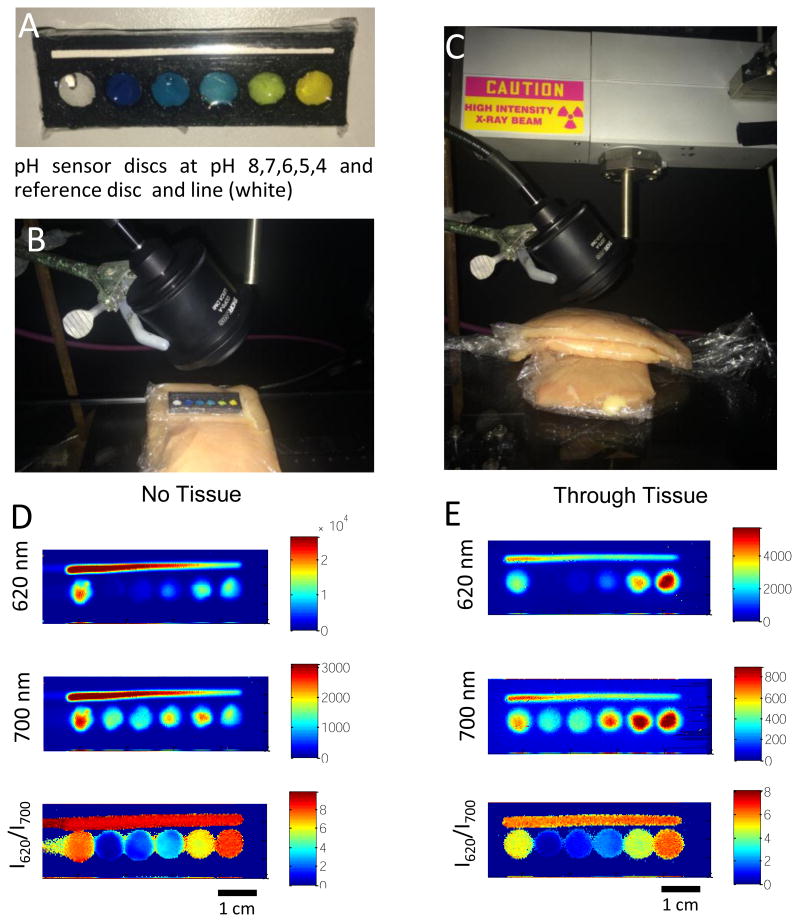
To see whether the sensor can measure pH-dependent spectral ratios through tissue, a target was made with a series of wells, each with a sensor film incubated with a different standard pH buffer solution (pH 8-4) (Figure 7A). In addition, a well and a line with no sensor film were used as reference regions (equivalent to a very low pH with no absorption at 620 nm). The target was placed upon a slice of chicken breast tissue and either imaged from above with no tissue (Figure 7B and D), or a slice of tissue was placed on top of the sensor (Figure 7C). XELCI images were acquired with channels for 620 nm and 700 nm light, and the ratio image was also calculated for a sample without tissue (Figure 7D) or with a 9 mm thick tissue slice (Figure 7E). The ratio image appears bigger because a ratio can be calculated even for a weak signal from a diffuse X-ray spot (although ratios are not calculated for signals less intense than a threshold of 120 counts above background). The images with and without tissue are similar and display similar pH-dependent spectral ratios.
Figure 7.
A) Photograph of the target with 6 wells, a reference well (white) and wells at pH 8, 7, 6, 5, and 4 (left to right). B) Photograph of target on chicken breast tissue with no tissue between target and detector. C) Photograph of target after placing chicken breast over the target. D) pseudo-colored XELCI images at 620 nm, 700 nm, and ratio acquired with no tissue over target. E) pseudo-colored XELCI images of same target acquired with 9 mm of chicken breast over sensor.
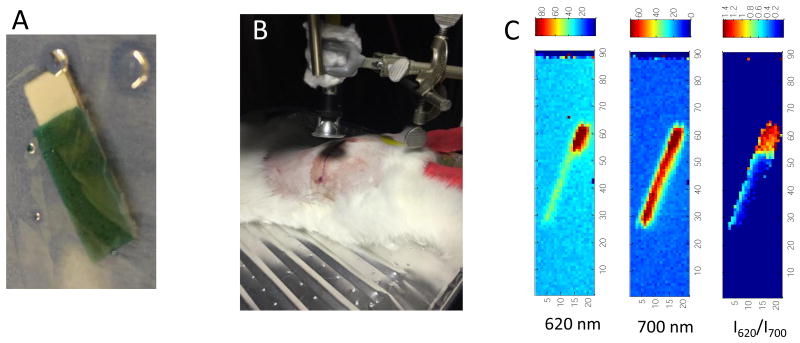
To determine whether the sensors could be used for in vivo imaging, a preliminary pilot study was performed. Sensors were fabricated by gluing a bromocresol green-loaded PEG pH sensor film to a Gd2O2S:Eu scintillator film in PDMS coating a metal implant. The scintillator film was larger than the pH sensor to maintain an uncoated reference region, equivalent to a very low pH region (as shown in Figure 8A). The sensor was then surgically implanted and glued next to the femur of a New Zealand white rabbit, approximately 1 cm beneath the tissue, and the wound was closed and sutured. XELCI measurements were then acquired through tissue (Figure 8B and C) with the animal under anesthesia. The XELCI images (Figure 8C) emit relatively uniformly intense 700 nm light, but the 620 nm light is significantly attenuated in the sensor region and the ratio image is shows distinct sensor and reference regions. This indicates that the sensor is intact and that the sensor region is not highly acidic. Further studies are ongoing to calibrate the sensor and characterize the imaging for detecting infections in vivo.
Figure 8.
A) Photograph of sensor to be implanted, green region corresponds to the pH indicator at neutral pH. B) photo showing setup to image. C) XELCI images of the sensor shown in A for 620nm light, 700 nm light and ratio.
3. Conclusion
Multilayer radioluminescent chemical sensors were used to study pH near an implanted device. A focused x-ray beam is scanned point-by-point and the emitted light is modulated by pH indicator layer. XELCI provides high spatial resolution, low background chemically specific measurements through tissue. Future work will be to more fully characterize sensor performance and use the sensors to monitor local changes in pH during infection and antibiotic treatment.
Figure 4.
Flow chart describing LabVIEW control scheme of x-ray scanning system
Acknowledgments
This research was supported by a grant National Science Foundation (NSF) CAREER award CHE1255535; animal experiments were funded by NIAMS of the National Institutes of Health (NIH) under award number R01 AR070305-01 and NIGMS of NIH under award number 5P20GM103444-07.
References
- 1.Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating Health Care-Associated Infections and Deaths in U.S. Hospitals, 2002. Public Health Reports. 2007;122:160. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mody RM, Zapor M, Hartzell JD, Robben PM, Waterman P, Wood-Morris R, Trotta R, Andersen RC, Wortmann G. Infectious Complications of Damage Control Orthopedics in War Trauma. The Journal of Trauma: Injury, Infection, and Critical Care. 2009;67:758. doi: 10.1097/TA.0b013e3181af6aa6. [DOI] [PubMed] [Google Scholar]
- 3.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 4.Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nature Reviews Microbiology. 2008;6:199. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 5.Hidalgo G, Burns A, Herz E, Hay AG, Houston PL, Wiesner U, Lion LW. Functional Tomographic Fluorescence Imaging of pH Microenvironments in Microbial Biofilms by Use of Silica Nanoparticle Sensors. Appl Environ Microbiol. 2009;75:7426. doi: 10.1128/AEM.01220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vroom JM, De Grauw J, Gerritsen HC, Bradshaw DJ, Marsh PD, Watson K, Birmingham JJ, Allison C. Depth Penetration and Detection of pH Gradients in Biofilms by Two-Photon Excitation Microscopy. Applied and Environmental Microbiology. 1999;65:3502. doi: 10.1128/aem.65.8.3502-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabath L, Toftegaard I. Rapid microassays for clindamycin and gentamicin when present together and the effect of pH and of each on the antibacterial activity of the other. Antimicrobial agents and chemotherapy. 1974;6:54. doi: 10.1128/aac.6.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman RJ, Francis MJ, Duthie RB. Nuclear magnetic resonance studies of experimentally induced delayed fracture union. Clinical orthopaedics and related research. 1987;216:253. [PubMed] [Google Scholar]
- 9.Nelson CL, McLaren AC, McLaren SG, Johnson JW, Smeltzer MS. Is aseptic loosening truly aseptic? Clinical orthopaedics and related research. 2005;437:25. doi: 10.1097/01.blo.0000175715.68624.3d. [DOI] [PubMed] [Google Scholar]
- 10.Lebeaux D, Chauhan A, Létoffé S, Fischer F, Reuse H, Beloin C, Ghigo JM. pH mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms. Journal of Infectious Diseases. 2015;210:1357. doi: 10.1093/infdis/jiu286. [DOI] [PubMed] [Google Scholar]
- 11.Pavlukhina S, Lu Y, Patimetha A, Libera M, Sukhishvili S. Polymer multilayers with pH-triggered release of antibacterial agents. Biomacromolecules. 2010;11:3448. doi: 10.1021/bm100975w. [DOI] [PubMed] [Google Scholar]
- 12.Pichavant L, Amador G, Jacqueline C, Brouillaud B, Héroguez V, Durrieu MC. pH-controlled delivery of gentamicin sulfate from orthopedic devices preventing nosocomial infections. Journal of Controlled Release. 2012;162:373. doi: 10.1016/j.jconrel.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Raval Y, Tzeng TRJ, Anker JN. X-Ray Excited Luminescence Chemical Imaging of Bacterial Growth on Surfaces Implanted in Tissue. Advanced Healthcare Materials. 2015;4:903. doi: 10.1002/adhm.201400685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu K, Tochio K, Kato Y. Improvement of transcutaneous fluorescent images with a depth-dependent point-spread function. Appl Opt. 2005;44:2154. doi: 10.1364/ao.44.002154. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Rogalski MM, Anker JN. Advances in functional X-ray imaging techniques and contrast agents. Physical Chemistry Chemical Physics. 2012;14:13469. doi: 10.1039/c2cp41858d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Patrick AL, Yang Z, VanDerveer DG, Anker JN. High-Resolution Chemical Imaging through Tissue with an X-ray Scintillator Sensor. Anal Chem. 2011;83:5045. doi: 10.1021/ac200054v. [DOI] [PubMed] [Google Scholar]
- 17.Cheong WF, Prahl SA, Welch AJ. A Review of the Optical Properties of Biological Tissues. IEEE Journal of Quantum Electronics. 1990;26:2166. [Google Scholar]
- 18.Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nature Methods. 2010;7:603. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 19.Jiang S, Pogue BW, Paulsen KD. In vivo near-infrared spectral detection of pressure-induced changes in breast tissue. Opt Lett. 2003;28:1212. doi: 10.1364/ol.28.001212. [DOI] [PubMed] [Google Scholar]