Abstract
Objective:
The aim of this work is to show the importance of the depth of myometrium invasion, tumour size and lymphovascular invasion as prognostic factors in dissemination of lymphatic nodes at endometrial carcinoma (CE).
Materials and methods:
In the period from 2010 to 2015 at the University Clinic for Gynecology and Obstetrics in Banja Luka, 221 endometrial cancer surgeries were done (laparatomy 184-83%, laparascopy 37-16,74%). Patients who had uterus bleeding in peri/postmenopause or those whose endometrium thickness was bigger than 5 mm which was established by ultrasound, or those who had in their cavum uteri pathological (PH) diagnosis, underwent fractional curettage (FC) or hysteroscopy in order to obtain pathohistological endometrium diagnosis. Substances which were removed by fractional curettage, biopsy or by surgery were sent to patohystological analysis. We analysed the following factors: age (5 groups), histological grade (G) of tumour, depth of myometrial invasion (DIM), whether it is more or less than 50%, the size of the tumour (if it is bigger or smaller than 2 cm), positive or negative lymphovascular invasion (LVI), positive or negative pelvic lymph nodes (PLN).
Results:
Within histological type the endometrioid type CE 166 (75,11%) was most dominant. Adenocarcinoma of endometrium was present 25 (11,31%), serous CE 11 (4,97%) and clear cell KE 2 (0,90%). Dominant population with CE was over 60 years old 127 (57,46) of female patients. At G3 where DIM was <50% positive PLN were present 2 (3.92%), whereas if DIM was>50%, 6 (26,73%) patients with positive PLN were registred. Tumour size < 2 cm was found with 57 (25,79%) female patients with positive PLN 8 (14,03%), while 164 (74,20%) patients had tumours > 2 cm who had 21 (12,80) PLN metastases. At G1 when tumour was <2 cm, positive PLN had 3 patients (5,88), while when tumour was >2 cm, positive PLN were found at 6 patients (9,69%). At G3 whose size was <2 cm, positive PLN were found at 2 patients (16,66%), but when tumour was >2 cm, PLN metastases were more frequent, 6 (25,00%). Negative LVI was found with 168 patients (76,01%) whose PLN were positive 16 (9,52%), while positive LIV was with 53 patients (23,99%) of whom 14 had PLN metastases (26,41%). At G1 two patients had positive PLN (2,32%) with negative LVI, while with positive LVI, positive PLN were found at 3 patients (11,11%). At G3 having negative LVI positive PLN were found with 6 patients (24,00%), while if LIV was positive, the number of positive PLN were 6 (54,54%).
Conclusions:
With low risk for lymphatic spread (DIM less than 50%, tumour size smaller than 2 cm and lack of LVI at G1 CE) we also encounter low metastasis rate of PLN. Diagnoses of this kind have an aim to lower the number of pelvic lymphadenectomies. With patients who have a high risk of lymphatic spread (myometrium invasion >50%, tumour size > 2cm, LVI present at G2 and G3) metastasis rate of PLN is high, therefore it is necessary to perform pelvic and paraaortic lymphadenectomy which lowers the mortality rate for more than 50% and at the same time patients get an absolute chance of 5-year survival period.
Keywords: Lymphovascular Invasion, Tumor Size, Depth of Myometrial Invasion, Metastases, Pelvic Lymph Node, Endometrial, Carcinoma
1. INTRODUCTION
When we talk about cancer of the uterus body we primarily have in mind endometrial carcinoma (CE), which comprises from 95% to 97% of all uterus body carcinomas. In the central Europe, CE is the third most common carcinoma after breast cancer and colon cancer, and in the first place (41%) among malignant carcinomas of female genital system. CE incidence grows from 2 of 100 000 women per year, younger than 40, to 40-50 of 100 000 women who are in their sixties or seventies. The highest incidence rate is between 50 and 65 years old. Every year 88 000 new cases of this carcinoma is registred in Europe, while 40 000 in North America (1).
CE etiology is unknown, although it is considered that there is a transformation of premalignant phase of intraendometrial neoplasia into CE in a large number of cases (2, 3).
Risk factors for the development of CE are divided into estrogen dependent and estrogen independent factors. Estrogens are considered to be cancerous for endometrium, while gestagens have a protective role. Risk factors for estrogen dependent types of carcinomas are early period, obesity, late menopause at nulliparous, diabetes mellitus, hypertension, infertility. The use of substitutional estrogen therapy increases the CE risk from 2 to 12 times, whereas the use of tamoxifen in breast carcinoma therapy increases the risk of CE from 1,7 to 7,5 times. Around 5% of patients with CE are linked with type II Lynch syndrome. The use of oral contraception lowers the risk for the development of this carcinoma in premenopause and perimenopause. It is most common at younger, slimmer women who do not have risk factors for CE (4). At CE endometrium adenocarcinoma is pathohistologicallydominant from 95–97%, while uterus sarcoma can be found in small proportion, 3–5%. Endometrium adenocarcinoma comprises different subtypeswhere endrometroid adenocarcinoma can be found in 80% of cases, while papillary serous and clear cell endometrium carcinoma can be found at 5% of women. Endometroid adenocarcinoma shows microsatellite instability and mutations PTEN, PIK3CA, K-ras and beta catenin genes and they are common with patients who have inherited non-polyposis colon carcinoma. Serous endometrium carcinomas have p53 mutations and chromosome instability. Clear cell CE has the lack of estrogen and progesterone activity as well as low immunoreactivity for p53 (4, 5). There is no screening method which is specific or sensitive enough to detect CE that can be applied in asymptomatic general population (4). Fractional curettage (FC) is a gold standard in giving a diagnosis for CE, even though it gives 2 %- 6% of false negative results. Hysteroscopy has an advantage in comparison to FC, because due to clear visibility of cavum uteri it is possible to have biopsy or to remove pathological change. Hysteroscopy has 8% of false negative results and there is a possibility of intraperitoneal disseminationof tumour cells through ovaries into abdominal cavity (5). Additional examinations: computerised tomography (CT) – important for discovering extra pelvic pathological processes, magnetic resonance imaging (MRI)– differentially and diagnostically points out to the processes in a cervix, positron emission tomography (PET) are very useful to discover remote metastases (6, 7).
2. AIM
The aim of this work is to emphasise the importance of the depth of myometrium invasion, tumour size and lymphovascular invasion as prognostic factors in dissemination of lymph nodes at CE.
3. PATIENTS AND METHODS
In the period from 2010 to 2015 at University Clinic for Gynecology and Obstetrics in Banja Luka, 221 CE surgeries were done (laparotomy 184; 83,25%, laparoskopy 37; 16,74%). Data from the history of diseases, decisions from consilium, surgery protocol and phatohistological reports were used. Early bleeding in peri/postmenopause as well as already present symptoms enable to diagnose this carcinoma in its early stage in 90% of the cases. Patients who had uterus bleeding in peri/postmenopause or those whose endometrium thickness was bigger than 5 mm which was determined by ultrasound or those who had in their cavum uteri pathological diagnosis, they all underwent fractional curettage (FC) or hysteroscopy in order to obtain pathohistological (PH) endometrium diagnosis. Substances which were removed with FC, biopsy or by surgery (uterus, adnex and pelvic lymph nodes) were sent to PH analysis to the Institute for Pathology of University Clinical Centre in Banja Luka.
In our work we analysed the following:
patients’ age groups (five groups): < 30; 31 – 40; 41 - 50; 51 - 60; over 60;
histological grade of tumour (G);
depth of myometrial invasion (DIM) - if it is more or less than 50%;
tumour size - if it is bigger or smaller than 2 cm;
lymphovascular invasion (LVI) - positive or negative and pelvic lymph nodes (PLN) - positive or negative.
Preoperatively, it was necessary to do gynecological and rectal examination of the patient, to carry out laboratory analyses of blood and urine, EKG, imaging – RTG of lungs, abdominal and pelvic ultrasound, internist and anesthesiologist examination to make sure the patient can cope with the risks of the operation. Additional examinations: computerised tomography (CT) – important for discovering extra pelvic pathological processes, magnetic resonance imaging (MRI)– differentially and diagnostically points out to the processes in a cervix, positron emission tomography (PET) – important to discover remote metastases, colonoscopy and cistoscopy were done in some patients in order to complete stage of disease. Before the surgery it was essential to have the decision of gynecological and oncological consilium in order to perform a surgery according to CE protocol treatment. Two hours before the surgery anticoagulant therapy of low molecular weight heparin was given (Clivarin 0,25), as well as antibiotics (cefalosporinas of II and III generation) half an hour before the surgery. CE treatment was surgical according to the CE treatment protocol which is based on FIGO/TNM classification (hysterectomy, bilateral adnexectomy, pelvic (PL) and paraaortic lymphadenopathy (PAAL).
4. RESULTS
In the period from 2010 to 2015 at the University Clinic for Gynecology and Obstetrics in Banja Luka, 221 CE surgeries were done. Laparotomy was done with 184 patients (83,25%), while laparoscopy with 37 patients (16,74%).
Within the histological type, endometrioid type CE 166 (75,11%) was dominant. Adenocarcinoma of endometrium was present with 25 patients (11,31%), while serous and clear cell CE were present with 11 patients (4,97%), that is, with 2 patients (0,90%), (10).
According to the age of the patients we divided them in five groups.
Table 1.
Age of the patients in our study
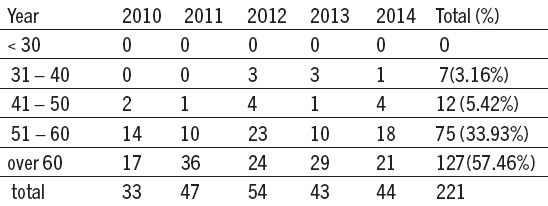
There were 7 patients below 40 (3,16%). In the age group from 51 - 60 CE was registred with 75 patients (33,93%). CE dominant age group was over 60 with 127 patients (57,46%) which matches the statistics CE.
Table 2.
Histological tumour grade of endometrial carcinoma
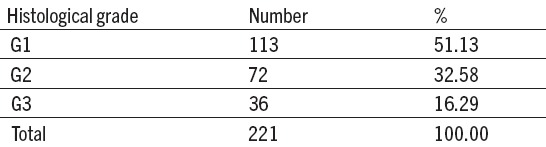
At PH tumour diagnoses, CE was dominant and well differentiated(G1) with 113 patients (51,13%). Medium differentiated (G2) CE was found at 72 patients (32,58%), while slightly differentiated (G3) CE was found with 36 patients (16, 29%).
Table 3.
Depth of myometrium invasion, histological grade and metastases in pelvic lymph nodes at endometrial carcinoma
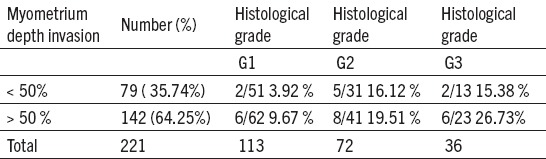
DIM up to 50% was found at 79 patients (35,74%) who had 9 metastases (11,39%), DIM over 50% was registred with 142 patients (64,25%), metastases were present at 20 patients (14,08%).
At G1 CE where DIM was < 50%, positive PLN were observed with 2 patients (3,92%), while if DIM was > 50% positive PLN were found at 6 patients (9,67%). As a differentiation grade of the tumour was worse – G2, PLN results imply that the metastasis grade was bigger. At G3 CE where DIM was <50% there were 2 positive PLN (15,38%), while when DIM was >50% there were 6 positive PLN registred (26,73%).
Tumour size smaller than 2 cm was found with 57 patients (25,79%), positive PLN had 8 patients (14,03). At 164 patients (74,20%) whose tumour was bigger than 2 cm, 21 PLN metastases were registred (12,80%).
Table 4.
Tumour size, histological grade and metastases in pelvic lymph nodes at endometrial carcinoma
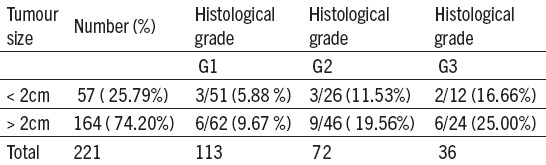
At G1 when a tumour was smaller than 2 cm, positive PLN were found with 3 patients (5,88%), while when it was bigger than 2 cm, positive PLN were observed with 6 (9,67%) patients. At G2 CE both tumour sizes give twice as big number of positive PLN in comparison to G1. At G3 CE whose size is smaller than 2 cm, positive PLN were found with 2 patients (16,66%), but in cases when tumour was bigger than 2 cm, PLN metastases were considerably bigger, found with 6 patients (25,00%).
Table 5.
Lymphovascular invasion, histological grade and metastases in pelvic lymph nodes at endometrialcarcinoma
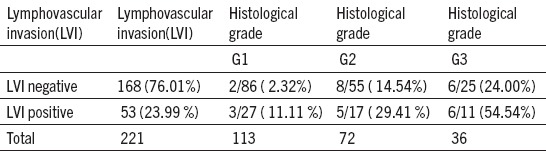
Negative LVI was observed with 168 patients (76,01) who had 16 PLN metastases (9,52%). Positive LVI was found with 53 patients (23,99%) 14 of them had current metastases of PLN (26,41%). At histological grade – G1, with negative LVI, 2 PLN metastases were observed (2,32%). However, with positive LIV, positive PLN were observed with 3 patients (11,11%). Distribution of positive PLN at G2 CE was 3 – 5 times bigger in both LVI records. At G3 which has negative LVI, positive PLN were more frequent with 6 patients (24,00%), while if LVI was positive, the presence of positive PLN was with 6 patients (54,54%). These results show that the significance of LVI as a prognostic factor in PLN metastasis development is bigger if the differentiation grade bigger.
5. DISCUSSION
Along with traditional laparotomy, minimally invasive method - laparoscopy has been more common lately (8). In our study, laparotomy was done with 184 patients (83,25%), while laparoscopy with 37 patients (16,74%). In recently published GOG LAP 2 study, almost the same results have been achieved in terms of survival length and recidive occurence. However, laparoscopy has an advantage in comparison to laparotomy due to shorter stay in hospital, less medicines usage, less pain, less postoperative complications, better quality of life. In both surgical approaches the question of lymphadenectomy role arises in terms of overall survival rate and recidive occurence. PL and PAAL increases 5-year survival in the first stage G3 for 90%, in the third stage for 73% and in the fourth stage for 54%. Lymphadenoctomy is not recommended with patients who have low risk - stage I, (G1 or G2, with mild myometrium invasion < 50%). Lymphadenoctomy is recommended with patients who have medium risk stage I, (G1 or G2 with endometrium invasion > 50%) or with high-medium risk (G3 with endometrium invasion < 50%) (9, 10).
In the early stage of illness it is essential to determine factors which imply to a high recidive risk and those are: histological subtypes, histological stages, myometrium invasion more than 50%, lymphovascular invasion, lymph nodes metastases and tumour size bigger than 2 cm.
In our study, at PH tumour diagnoses, CE was dominant and well differentiated (G1) with 113 patients (51,13%). Medium differentiated (G2) CE was found at 72 patients (32,58%), while slightly differentiated (G3) CE was found with 36 patients (16, 29%). Similar results of differentiation grades can be found in the works of Cetinkaya et al, Han et al (11, 12).
Results of our study shows that DIM up to 50% was found at 79 patients (35,74%) who had 9 metastases (11,39%), DIM over 50% was registred with 142 patients (64,25%), metastases were present at 20 patients (14,08%) which was also stated by other authors in their works, Muallem MZ et al (13). At G1 CE where DIM was < 50%, positive PLN were observed with 2 patients (3,92%), while if DIM was > 50% positive PLN were found at 6 patients (9,67%). As a differentiation grade of the tumour was worse – G2, PLN results imply that the metastasis grade was bigger. At G3 CE where DIM was <50% there were 2 positive PLN (15,38%), while when DIM was >50% there were 6 positive PLN registred (26,73%). Muallem MZ et al. discovered that when at G3 and with DIM >50%, the risk of PLN metastasis development is 3 -5 times bigger, which matches our stated results (13, 14). If the risk rate is low at CE, PLN metastasis rate is low as well and vice versa. This was reported by AlHilli MM and coworkers and Geels YP et al (15, 16).
In our study, tumour size smaller than 2 cm was found with 57 patients (25,79%), positive PLN had 8 patients (14,03). At 164 patients (74,20%) whose tumour was bigger than 2 cm, 21 PLN metastases were registred (12,80%). This result implies that the tumour size is not an independant prognostic factor, as it was pointed out in other researches as well (4, 6, 14, 15).
Results of our study shows that at G1 when a tumour was smaller than 2 cm, positive PLN were found with 3 patients (5,88%), while when it was bigger than 2 cm, positive PLN were observed with 6 (9,67%) patients. At G2 CE both tumour sizes give twice as big number of positive PLN in comparison to G1. At G3 CE whose size is smaller than 2 cm, positive PLN were found with 2 patients (16,66%), but in cases when tumour was bigger than 2 cm, PLN metastases were considerably bigger, found with 6 patients (25,00%). These results confirm that the risk for PLN metastasis development is bigger if the tumour size is bigger and the differentiation level worse. This was stated by Vargas R et al. and Boyrar G et al. in their works (17, 18). The tumour size only is not considered to be an independant prognostic factor, because the risk of metastatic spread of the illnesss is also present with patients who have a small tumour volume, which was pointed out by Oz M and coworkers (4,6, 19).
According to lymphovascular invasion, histological grade and metastases in pelvic lymph nodes at endometrial carcinoma, our study shows that negative LVI was observed with 168 patients (76,01) who had 16 PLN metastases (9,52%). Positive LVI was found with 53 patients (23,99%) 14 of them had current metastases of PLN (26,41%). A lot of authors pointed out in their works that if LVI was present, LGN metastases were present too, which is confirmed by these results (13,14, 15). Lack of LVI and smaller number of metastases discribed Neal SA et al. at G1 and it matches the results from the chart (20). In our study histological grade – G1, with negative LVI, 2 PLN metastases were observed (2,32%). However, with positive LIV, positive PLN were observed with 3 patients (11,11%). Similar results were confirmed by Puljiz M et al. (21). In our study, distribution of positive PLN at G2 CE was 3 – 5 times bigger in both LVI records. At G3 which has negative LVI, positive PLN were more frequent with 6 patients (24,00%), while if LVI was positive, the presence of positive PLN was with 6 patients (54,54%). These results show that the significance of LVI as a prognostic factor in PLN metastasis development is bigger if the differentiation grade bigger. This was confirmed also by other authors in in their works; Reiz and coworkers and Bendiffalah S et al. (22, 23).
6. CONCLUSION
In case of low risk for lymphatic spread (DIM less than 50%, tumour size smaller than 2 cm and lack of LVI at G1 CE), there is a low PLN metastasis rate. These reports serve to lower the number of pelvic lymphadenoctomies which helps to determine more accurate CE stage, but it has no impact on overall 5-year survival period. Patients who have a high risk of lymphatic spread (myometrium invasion more than 50%, tumour size bigger than 2 cm, LVI present at G2 and G3),
The PLN metastasis rate is high, therefore, it is essential to perform pelvic and paraaortic lymphadenoctomy which lowers the death risk (more than 50%), and at the same time increases an absolutechance of 5-year survival period.
Footnotes
• Conflict of interest: none declared.
• Authors contributions: NL, DD, SS and VE performed the examination and participated in treatment of the patient. NL collected the data, analyzed them and wrote the text. SM assisted in writing the text including final editing and critical revision of the scientific content. All authors have read the text and approved the final manuscript.
REFERENCES
- 1.Siegel R, Naishadham D, Junal A. Cancer stastics. 2013. Cancer J Elin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Vergote I, Amant F, Timmerman D. Should we screen for endometrial cancer? Lancet Oncol. 2011;55:12. doi: 10.1016/S1470-2045(10)70280-1. [DOI] [PubMed] [Google Scholar]
- 3.Izetbegovic S, Stojkanovic G, Ribic N, Mehmedbasic E. Features of postmenopausal Uterine Haemorrhage. Med Arh. 2013;67(6):431–4. doi: 10.5455/medarh.2013.67.431-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ðurđević S, Kesić V. Gynecologic onkology. Faculty of Medicine Novi Sad. 2009:177–94. [Google Scholar]
- 5.Breijer MC, Timmermans A, van Doorn HC, Mol BW, Opmeer BC. Diagnostic strategies for postmenopausal bleeding. Obstet Gynecol Int. 2010:850812. doi: 10.1155/2010/850812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lučić N, Ećim-Zlojutro V. Diagnostic protocol and treatment of malignant illnesses of female genital organs. Faculty of Medicine, Banja Luka. 2013:102–33. [Google Scholar]
- 7.Sala E, Rockall A, Kubik-Huch RA. Advances in magnetic resonance imaging of endometrial cancer. Eur Radiol. 2011;21:468–73. doi: 10.1007/s00330-010-2010-5. [DOI] [PubMed] [Google Scholar]
- 8.Hrgovic Z, Marton I. Laparoscopic Hysterectomy and Decision When and Which Surgical Approach Is Indicated? Acta Inform Med. 2011;19(2):114–7. [Google Scholar]
- 9.Fotion S, Trimble EF, Papakonstantinon K, et al. Complete pelvic lymphadenectomy in patients with clinical early, grade I and grade II endometrioid corpus cancer. Anticancer Res. 2009;29(7):2781–5. [PubMed] [Google Scholar]
- 10.Mariani A, Webb MJ, Keeney GL. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;18:1506–19. doi: 10.1067/mob.2000.107335. [DOI] [PubMed] [Google Scholar]
- 11.Cetinkaya K, Atalay F, Bacinoglu A, Dervisoglu H. Tumours. 2016 Aug 3;102(4):422–5. doi: 10.5301/tj.5000497. [DOI] [PubMed] [Google Scholar]
- 12.Han G, Lim D, Leitao Jr, Abu-Rustum NR, Soslow RA. Histological features associated with occult lymph node metastasis in FIGO clinical stage 1, grade I endometrioid carcinoma. Histopathology. 2014 Feb;64(3):389–98. doi: 10.1111/his.12254. [DOI] [PubMed] [Google Scholar]
- 13.Muallem MZ, Sehouli J, Almuheimid J, Richter R, Joukhader R. Risk Factors of Lymph Nodes Matastases by Endometrial Cancer. A Rectospective One - centar Study. Anticancer Res. 2016 Aug;36(8):4219–25. [PubMed] [Google Scholar]
- 14.Cetinkaya K, Atalay F, Bacinogly A. Risk faktors of lymph node metastasis with endometrial carcinoma. Asian Pac J Cancer Prev. 2014;15(15):6353–6. doi: 10.7314/apjcp.2014.15.15.6353. [DOI] [PubMed] [Google Scholar]
- 15.AlHilli MM, Podratz KC, Dowdy SC, et al. Risk-scoring system for the individualized prediction of lymphatic dissemination in patients with endometroid endometrial cancer. Gynecol Oncol. 2013 Oct;131(1):103–8. doi: 10.1016/j.ygyno.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Geels YP, Pijnenborg LM, van den Berg -van Erp SH, Snijders MP, Bulten J, Massuger LF. Absolute depth of myometrial invasion in endometrial cancer is superior to the currently used cut-off value of 50 % Gynecol Oncol. 2013 May;129(2):285–91. doi: 10.1016/j.ygyno.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Vargas R, Rauh-Hain JA, Clemmer J, et al. Tumor size, depth of invasion, and histologic grade as prognostic factors of lymph node involvement in endometrial cancer: a SEER analysis. Gynecol Oncol. 2014 May;133(2):216–20. doi: 10.1016/j.ygyno.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Boyrar G, Salman MC, Gultekin M, et al. Int J. Incidence of lymph node metastasis in surgycally staged FIGO I a G1/G2 endometrial cancer with a tumor size of more than 2cm. Int J Gynecol Cancer. 2017;27(3):486–92. doi: 10.1097/IGC.0000000000000919. [DOI] [PubMed] [Google Scholar]
- 19.Oz M, Korkmaz V, Meydanli MM, Sari ME, Cuylan ZE, Gungor T. Is Tumor Size Really Important for Prediction of Lymphatic Dissemination in Grade 1 Endometrial Carcinoma With Superficial Myometrial Invasion? Int J Gynecol Cancer. 2017 Sep;27(7):1393–98. doi: 10.1097/IGC.0000000000001025. [DOI] [PubMed] [Google Scholar]
- 20.Neal SA, Graybill WS, Garrett- Mayer E, et al. Lymphovascular space invasion in uterine corpus cancer. What is its prognostic significance in the absence of lymph node metastasis. Gynecol Oncol. 2016 Aug;142(2):278–82. doi: 10.1016/j.ygyno.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 21.Puljiz M, Puljiz Ž, Danolović D, et al. Prognostic value of lymphovascular space invasion in endometrial cancer. Med Glas. 2013 Aug;10(2):288–92. [PubMed] [Google Scholar]
- 22.Reis R, Burzawa JK, Tsunoda AT, et al. Lymphovascular Space Invasion Postends Poor Prognostic in Low Risk Endometrial cancer. Int J Gynecol Cancer. 2015 Sep;25(7):1292–9. doi: 10.1097/IGC.0000000000000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bendifallah S, Perrin M, Ouldamer L, et al. Francogyn Study Group Honing the clasiffication of high –risk endometrial cancer with inclusion of lymphovascular space invasion. Surg Oncol. 2017;26(1):1–7. doi: 10.1016/j.suronc.2016.11.001. [DOI] [PubMed] [Google Scholar]


