Abstract
Introduction:
Atrial fibrillation (AF) is the most common form of cardiac arrhythmia in clinical practice and its prevalence increases with age. Patients who develop AF also have cardiovascular risk factors, structural heart disease, and comorbidities, all of which can increase mortality. AF causes a significant economic burden with the increasing trend in AF prevalence and hospitalizations.
Research Objectives:
The objective of our study is to evaluate the impact of the most common known risk factors on the incidence of atrial fibrillation as an important precursor of cardiac and cerebrovascular morbidity and mortality among our patients in Bosnia and Herzegovina during median follow up period (September 2006 - September 2016). The other objective is to estimate the CHA2DS2-VASc score among our patients based on clinical parameters.
Patients and methods:
This study includes 2352 ambulant and hospitalized patients with atrial fibrillation. All patients underwent clinical evaluation which includes thorough assessment for potential risk factors and concomitant conditions in order to determine which of them represent the most common among examinees with atrial fibrillation.
Results:
The results show that male gender has slightly more incidence of AF. Obesity and overweight with BMI ≥ 27, cigarettes smoking and sedentary life style are almost present in patients with AF. Arterial hypertension, coronary artery disease, diabetes mellitus, chronic obstructive pulmonary disease, chronic renal dysfunction, structural and valvular heart disease and peripheral vascular disease are the most common comorbidities among our patients. The mean CHA2DS2-VASc score was 3.2±1.4 and the mean HAS-BLED score was 2.1±1.2.
Conclusion:
Atrial fibrillation is the most common sustained cardiac rhythm disorder. The study shows that obesity, alcohol consumption, smoking cigarettes and dyslipidemia can be considered as triggers and predisposing factors for appearance of AF. Arterial hypertension, coronary artery disease, chronic obstructive pulmonary disease, diabetes mellitus, Peripheral vascular disease and chronic kidney disease are playing important role in developing of AF.
Keywords: Atrial fibrillation, Risk factors, Stroke, Myocardial infarction, Sudden death, Heart failure
1. INTRODUCTION
Atrial fibrillation (AF) is the most common form of cardiac arrhythmia in clinical practice and its prevalence increases with age. By year 2030, 14–17 million AF patients are anticipated in the European Union. Patients who develop AF also have cardiovascular risk factors, structural heart disease, and comorbidities, all of which can increase mortality. Atrial fibrillation is a major medical and public health problem owing to its increasing prevalence and strong association with morbidity and mortality. AF causes a significant economic burden which has grown in the past decades and is expected to grow even further in the upcoming period with the increasing trend in AF prevalence and hospitalizations. Therefore, an adequate treatment strategy is warranted.
2. RESEARCH OBJECTIVES
The first objective of our study is to evaluate the impact of the most common known risk factors and other comorbidities on the incidence of atrial fibrillation as an important precursor of cardiac and cerebrovascular morbidity and mortality among our patients in Bosnia and Herzegovina. The second objective is to estimate the CHA2DS2-VASc and HAS-BLED score among our patients.
3. PATIENTS AND METHODS
This study includes 2352 ambulant and hospitalized patients with atrial fibrillation (AF) who were enrolled during median 9.7 ± 1.8 follow up period (September 2006 until September 2016). A complete medical history was taken, all patients underwent clinical evaluation which includes thorough assessment for concomitant conditions. The AF was documented by 12-lead ECG or ambulatory ECG Holter recording in order to establish the diagnosis of AF. TTE or TEE Echocardiography was performed to assess left atrial diameter and volume, as well as quantitative and qualitative evaluation of left ventricle parameters, heart structure and hemodynamic relevant parameters. In this study we evaluate the potential risk factors for atrial in order to determine which of the mentioned risk factors are the most common among examinees with atrial fibrillation. Individualized risk stratification was performed using the CHA2DS2-VASc. In our study we estimate CHA2DS2-VASc and HAS-BLED score among our patients in order to evaluate the use of oral anticoagulation therapy among our patients and the risk of bleeding.
4. RESULTS
During the follow up period, we analyzed and follow up 2352 patients with ECG documented different type of atrial fibrillation in order to assess the impact of common risk factors and comorbidities on appearance of atrial fibrillation. The demographic data, risk factors, clinical and comorbidity characteristics for all patients are shown on Table 1. The echocardiographic data and characteristics for all patients are shown on Table 2. The pattern of atrial fibrillation is shown on Table 3. The pharmacological therapy and antiarrhythmic drugs are shown on Table 4.
Table 1.
Baseline demographics and clinical characteristics
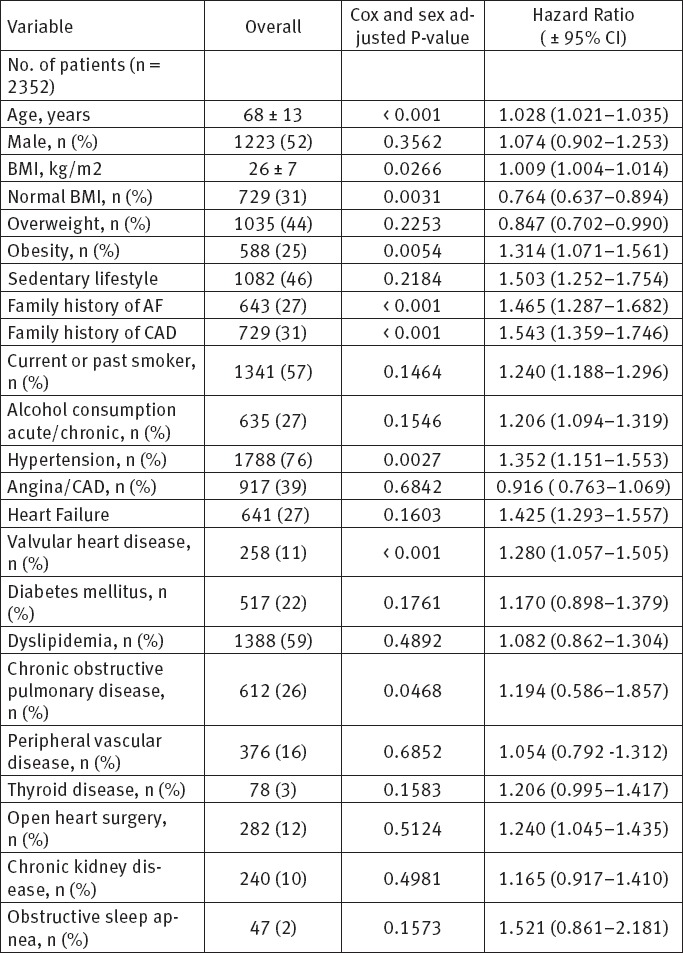
Table 2.
Echocardiographic parameters
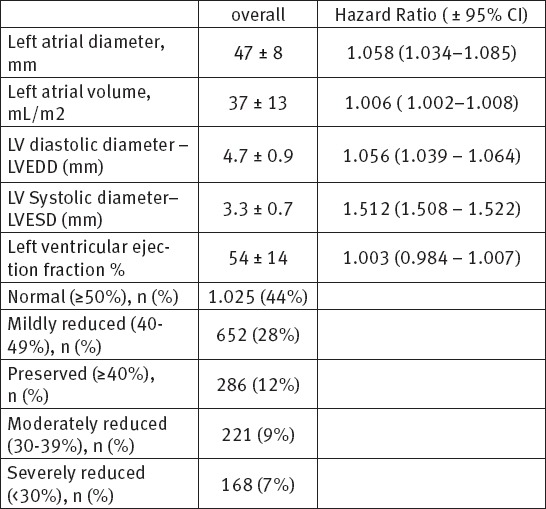
Table 3.
Patterns of atrial fibrillation n (%)
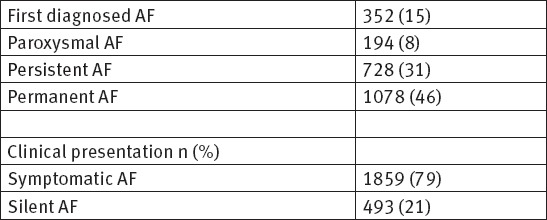
Table 4.
Pharmacological therapy n (%)
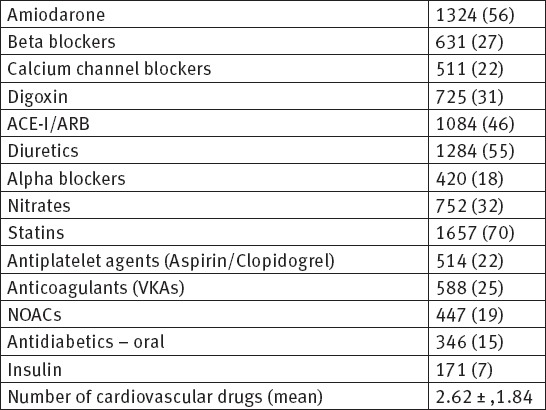
In our study among 2352 patients, AF was reported to be first detected in 352, paroxysmal in 194, persistent in 728, and permanent in 1078 patients. Concomitant diseases were present in 84% of all patients. As many as 79% of patients were symptomatic during the follow up period. Oral anticoagulation drugs (VKAs and NOACs) were prescribed to 44.2% of the patients with AF. Antiplatelet agents were prescribed in 22% of patients. A rhythm control strategy was applied in 63% of currently symptomatic patients and in 47% of patients who never experienced symptoms according to the ESC guidelines. In this study, the baseline age of patients was (68 ± 13). The results show that male gender has slightly more incidence of AF. Obesity and overweight with BMI ≥ 27, cigarettes smoking and sedentary life style are almost present in patients with AF. Arterial hypertension, coronary artery disease, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, valvular heart disease and peripheral vascular disease are the most common comorbidities among our patients and they are playing important role in developing of AF.
The number of used drugs in our patient population indicates that patients with AF should receive intensified treatment to reduce their risk of cardiovascular complications. The mean CHA2DS2-VASc score was 3.2±1.4 with adjusted stroke rate 2.7% per year, the mean HAS-BLED score was 2.1±1.2. Our study shows that the majority of patients with atrial fibrillation have mild to moderate enlargement of left atrium, 47% have mitral valve annulus calcification and more than half of patients have mildly-moderately reduced LV EF. The quality of patients life with AF is clear impaired in more than 67%.
5. DISCUSSION
Atrial fibrillation is the most common arrhythmia in the general population, with a prevalence of 1.5-2%, which increases with age. In addition, it occurs more frequently in males, with a male to female ratio of 1.2:1. By 2030, 14-17 million AF patients are anticipated in the European Union, with 120 000–215 000 newly diagnosed patients per year. Given that AF is associated with significant morbidity and mortality, this increasing number of individuals with AF will have major public health implications (1-6). AF is frequently associated with cardiac disease and comorbidities. The most common comorbidities are hypertension (67–76%), heart failure (22–42%), diabetes (20–24%), obesity (20–35%), chronic pulmonary disease (10–18%), thyroid dysfunction (8–11%), renal failure (11–22%) stroke/transient ischemic attack (9–16%), and neuropsychiatric disturbances (19%).(5, 6). In our study, the mean age of the patients was 68 ± 13 years, the males represent 52% of patients with AF. The published studies also have reported a predominance of males (generally around 60%), the mean age of the patients in most reports is between 65 and 70 years, which is comparable to the mean age of our population (7, 8).
Obesity is a growing epidemic with its global prevalence doubling over the past 34 years. Based on World Health Organization global estimates, in 2014 >1.9 billion adults were overweight. In Europe and North America, >60% of adults are overweight. A recent meta-analysis estimates a 3.5–5.3% excess risk of AF for every one unit of BMI increase. Our study shows that overweight and obesity has become a major risk factors for AF. Body mass index (BMI) is the most commonly used parameter to determine categories of ‘overweight’ and ‘obese’. Overweight was detected in 44% of patients [HR 0.847 (CI 0.702–0.990)], and Obesity in 25% [HR 1.314 (CI 1.071–1.561)]. Population studies by Want et al, Frost et al, Tedrow et al, Huxley et al, Karasoy et al, Knuiman et al, Tsang et al and Sandhu et al. shows an association of obesity with mitral fibrillation (9,10).
In our study 59% of patients had dyslipidemia, the majority with high triglycerides and low HDLc [HR 1.082 (CI 0.862 - 1.3049)], which means that dyslipidemia was associated with a higher risk of AF. Alvaro Alonso et al. found in 2 large community-based cohorts, high triglycerides and low HDLc were associated with a higher risk of AF after accounting for relevant clinical risk factors and biomarkers. Results were similar in both MESA and FHS data and robust in several sensitivity analyses. In these 2 community-based cohorts, high-density lipoprotein cholesterol and triglycerides but not low-density lipoprotein cholesterol or total cholesterol were associated with the risk of AF, accounting for other cardio metabolic risk factors. Dyslipidemia is a major contributor to development of atherosclerosis and coronary disease (11,12).
Hypertension has been recognized as the principal and most common risk factor responsible for death and disability of non-communicable diseases worldwide. In our study 1788 (76%) of patients (177 had hypertension [HR 1.352 (CI 1.151–1.553)]. This is in line with the literature which shows prevalence’s ranging from 52 to 90% (13). Hypertension is a main contributor to AF risk and has been identified as such by multiple investigators (15). In the Multi-Ethnic Study of Atherosclerosis, sustained pre-hypertension (SBP 120–129 mmHg) and hypertension (SBP ≥130 mmHg) conferred a 1.8- and 2.6-fold increase in the risk of AF, respectively (14, 15). In our study 47 patients (2%) had obstructive sleep apnea all of them with BMI ≥ 29.4 [HR 1.521 (CI 0.861 – 2.181)]. As published earlier, obstructive sleep apnea is commonly associated with obesity and has a prevalence of 40-50% in the AF population (16).
Pallisgaard JL et al. found in their study that diabetes is an independent risk factor for developing atrial fibrillation/flutter, most pronounced in young diabetes patients (17). In our study the prevalence of diabetic patients was 22%, [HR 1.170 (CI 0.898 – 1.379)]. In the early 1990s, the Framingham study indicated DM to be an independent risk factor for AF with OR of 1.4 for men and 1.6 for women after 38 years follow up. In the analysis of 41436 residents in Japan, the prevalence of DM in AF patients is higher than in controls (20% vs 12%). In conclusion, the association between AF and DM has been proved both in epidemiology and experimental studies (18). Atrial fibrillation is also common in the diabetic population, with AF risk linked to diabetes duration and glycemic control (19, 20).
In our study 46% patients had sedentary life style [HR 1.503 (CI 1.252–1.754)]. The prospective cohort study of Morseth B et al. Conclude that leisure time physical activity was associated with AF in a J-shaped pattern. Moderate physical activity was associated with a reduced risk of AF. Low RHR was a risk factor for AF. The results support the hypothesis that moderate and vigorous physical activity may affect AF risk via different pathophysiological mechanisms (21, 22).
Heeringa J et al. (23) examined the association between cigarette smoking and risk of atrial fibrillation. The results shows that current and former smoking of cigarettes are associated with increased risk of atrial fibrillation. No differences were found between men and women (22). In our study 57% of patients with AF were past or current smokers, [HR 1.240 (CI 1.188–1.296)], which support the results that smoking was associated with the incidence of AF (23), with more than a two-fold increased risk of AF attributed to current smoking (Chamberlain AM et al. - ARIC) study (24).
In our study 27% patients with AF were acute/chronic alcohol consumers, [HR 1.206 (CI 1.094–1.319)] (25). In a prospective cohort study, they studied the association between self-reported alcohol use and incident atrial fibrillation among 16 415 women and men enrolled in the Copenhagen City Heart Study. The conclusion was that heavy alcohol consumption is associated with a higher risk of atrial fibrillation (24). Kodama S et al. had published the meta-analysis of fourteen eligible studies in order to summarize the estimated risk of atrial fibrillation (AF) related to alcohol consumption. The results of meta-analysis suggest that not consuming alcohol is most favorable in terms of AF risk reduction (25).
In our study, family history of CAD was present in 31% of patients with AF, [HR 1.543 (CI 1.359–1.746)], also CAD was found in 39% of patients [HR 0.916 (CI 0.763–1.069)], which is in consistent with the results of published studies. Violi F et al. searched MEDLINE via PubMed and Cochrane database between 1965 and 2015. The main conclusion was that AF patients had a significant residual risk of MI despite anticoagulant treatment (26).
In our study, family history of atrial fibrillation was noted in 27% of patients had family history of AF, [HR 1.465 (CI 1.287–1.682)]. The Framingham Heart Study has indicated that besides hypertension and diabetes, familial history of AF was also an independent risk factor in an individual. More recently - GWAS meta-analysis for atrial fibrillation has identified six new risk loci. Overall, it is increasingly clear that a genetic predisposition contributes to the risk of developing AF (27-32).
In our study, 26% of patients with AF had COPD,[HR 1.194 (CI 0.586–1.857)]. Chronic obstructive pulmonary disease (COPD) is independently associated with atrial fibrillation (AF). Decreased oxygenation, hypercapnia, pulmonary hypertension, diastolic dysfunction, oxidative stress, inflammation, changes in atrial size by altered respiratory physiology, increased arrhythmogenicity (33, 34). In a large-scale, retrospective, case-control studies, patients with COPD had a 4.41 times higher risk of AF (95% CI 4.00-4.87) and COPD is present in 10-15% of patients with AF (27-29).
In our study, 16% of patients with AF had peripheral arterial disease (PAD), [HR 1.054 (CI 0.792–1.312)]. PAD shares several risk factors with atrial fibrillation (AF), and persons with PAD have an increased risk of stroke (30). Griffin WF et al. examined the relationship between PAD and AF in 5143 participants (85% white, 43% male) in the Cardiovascular Health Study (CHS), a longitudinal, observational study of adults aged 65 years and older and conclude that the presence of PAD should alert practitioners to the increased risk of AF (31).
In our study, 10% of patients with AF had chronic kidney disease (CKD), [HR 0.4981 (CI 0.917–1.410)]. Chronic kidney disease (CKD) is associated with the risk of multiple life-threatening complications. Also, atrial fibrillation (AF) is common in this group of patients (32). Alonso et al. conclude that in the large population-based study (ARIC), reduced kidney function and presence of albuminuria were strongly associated with the incidence of AF independently of other risk factors (33). Odutayo A et al. made systematic review and meta-analysis for 104 eligible cohort studies, and the results shows that atrial fibrillation is associated with an increased risk of death and an increased risk of cardiovascular and renal disease (34).
The assessment of cardiac chamber sizes and function, the atrial contribution to left ventricular filling, the pericardium, and valvular function by echocardiography may be helpful in determining the conditions associated with AF, the risk for recurrent AF following cardioversion, and the hemodynamic benefit of maintaining sinus rhythm. Also, identification of patients at increased risk for thromboembolic complications of AF before cardioversion and in patients with chronic AF (35, 36).
In our study, the most prescribed and used drugs for rhythm control were Amiodarone 56%, followed by digitalis 31% and beta-blockers 27%. The same drugs were used in other published studies (37). Lafuente C et al. performed a systematic review to determine the effect of long-term treatment with those drugs on death, embolisms, adverse effects, and atrial fibrillation recurrence. Forty-four trials were included, with a total of 11 322 patients. They conclude that Class IA, IC, and III drugs are effective in maintaining sinus rhythm but increase adverse effects, and class IA drugs may increase mortality (38).
In our study oral anticoagulation drugs (VKAs and NOACs) were prescribed to 44.2% of the patients with AF. Antiplatelet agents were prescribed in 22% of patients. In our study the mean CHA2DS2-VASc score was 3.2±1.4 with adjusted stroke rate 2.7% per year, the mean HAS-BLED score was 2.1±1.2. In our population, prescription of anticoagulants was low both in patients without an indication of receiving them for AF (10%) and in higher-risk patients with a CHA2DS2-VASc score ≥ 2. In the reviewed literature, the prescription of oral anticoagulation on hospital discharge was also lower in patients with paroxysmal vs. permanent AF (51 vs. 80%, 55 vs. 74%, 78 vs. 91%) (39-42).
Wodchis WP et al. after systematic review and for previously published studies reporting the costs for AF patients, and the study conclude AF-related medical costs are high, reflecting resource-intensive and long-term treatments including anticoagulation treatment. These costs, accompanied with increasing prevalence, justify increased attention to the management of patients with AF (43). In other systematic review by Wolowacz S.E. et al. for the economic burden of AF, hospitalizations consistently represented the major cost driver. In the USA, AF hospitalizations alone cost $6.65 billion in 2005. Costs and hospitalizations attributable to AF have increased markedly over recent decades and are expected to increase in future due to ageing populations (44,45).
6. CONCLUSION
The incidence of AF has increased progressively in the last decades, relating closely to the aging of the population and increasing prevalence of risk factors such as hypertension, obesity, dyslipidemia, cigarettes smoking, diabetes mellitus, heart disease, chronic obstructive pulmonary disease and sleep apnea. Atrial fibrillation should be considered as a manifestation of hypertensive heart disease. AF is known to have a significant impact on healthcare costs and loss of productivity. Effective treatment of patients with atrial fibrillation includes not only rate control, rhythm control, and prevention of stroke, but also management of cardiovascular risk factors and concomitant diseases. OAC therapy can prevent the majority of ischemic strokes in AF patients and can prolong life. A multidisciplinary AF team approach is highly recommended.
Footnotes
• Author Contribution: All authors participated in each step of article and gave final approvement for submission of revised version.
• Conflict of interest: none declared.
REFERENCES
- 1.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.Masic I, Dilic M, Raljevic E, Vulic D, Mott D. Trends in Cardiovascular Diseases in Bosnia and Herzegovina and Perspectives with Heart Score Programme. Med Arh. 2010;64(5):260–3. doi: 10.5455/medarh.2010.64.260-263. [DOI] [PubMed] [Google Scholar]
- 3.Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular diseases in Europe: epidemiological update 2016. European Heart Journal. 2016;37:3232–45. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 4.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen TN, Hilmer SN, Cumming RG. Review of epidemiology and management of atrial fibrillation in developing countries. Int J Cardiol. 2013;167:2412–20. doi: 10.1016/j.ijcard.2013.01.184. [DOI] [PubMed] [Google Scholar]
- 6.Massimo Zoni-Berisso, Fabrizio Lercari, Tiziana Carazza, Stefano Domenicucci. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, Van Gilst WH, Van Gelder IC, Rienstra M. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the netherlands. J Am Coll Cardiol. 2015 Sep 1;66(9):1000–7. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 8.Le Heuzey J-Y, Breithardt G, Camm J, Crijns H, Dorian P, Kowey PR. The RecordAF study: design, baseline data, and profile of patients according to chosen treatment strategy for atrial fibrillation. Am J Cardiol. 2010;105(5):687–93. doi: 10.1016/j.amjcard.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Nalliah CJ, Sanders P, Kottkamp H, Kalman M.J. The role of obesity in atrial fibrillation. European Heart Journal. 2016;37:1565–72. doi: 10.1093/eurheartj/ehv486. [DOI] [PubMed] [Google Scholar]
- 10.Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29(18):2227–33. doi: 10.1093/eurheartj/ehn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso A, Yin X, Roetker NS, Magnani JW, Kronmal RA, Ellinor PT, Chen LY, Lubitz SA, McClelland RL, McManus DD, Soliman EZ, Huxley RR, Nazarian S, Szklo M, Heckbert SR, Benjamin EJ. Blood Lipids and the Incidence of Atrial Fibrillation: The Multi-Ethnic Study of Atherosclerosis and the Framingham Heart Study. J Am Heart Assoc. 2014;7(3(5)):e001211. doi: 10.1161/JAHA.114.001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eduardo Dytz Almeida, Raphael Boesche Guimarães, Laura Siga Stephan, Alexandre Kreling Medeiros, Katia Foltz, Roberto Tofani Santanna, Leonardo Martins Pires, Marcelo Lapa Kruse, Gustavo Glotz de Lima, Tiago Luiz Luz Leiria. Clinical Differences between Subtypes of Atrial Fibrillation and Flutter: Cross-Sectional Registry of 407 Patients. Arq Bras Cardiol. 2015;105(1):3–10. doi: 10.5935/abc.20150049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Neal WT, Soliman EZ, Qureshi W, Alonso A, Heckbert SR, Herrington D. Sustained pre-hypertensive blood pressure and incident atrial fibrillation: the multi-ethnic study of atherosclerosis. J Am Soc Hypertens. 2015;9:191–6. doi: 10.1016/j.jash.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory YH. Lip, Antonio Coca. Hypertension and cardiac arrhythmias. A consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE) European Heart Journal. 2017;38(4):223–5. [Google Scholar]
- 15.Naser N, Dzubur A, Durak A, Kulic M, Naser NU. Blood Pressure Control in Hypertensive Patients, Cardiovascular Risk Profile and the Prevalence of Masked Uncontrolled Hypertension (MUCH) Med Arch. 2016 Jul 27;70(4):274–9. doi: 10.5455/medarh.2016.70.274-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662–9. doi: 10.1093/eurheartj/ehn214. [DOI] [PubMed] [Google Scholar]
- 17.Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, Kramer DB, Zimetbaum PJ, Buxton AE, Josephson ME, Anter E. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300–5. doi: 10.1016/j.jacc.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Pallisgaard JL, Schjerning AM, Lindhardt TB, Procida K, Hansen ML, Torp-Pedersen C, Gislason GH. Risk of atrial fibrillation in diabetes mellitus: A nationwide cohort study. Eur J Prev Cardiol. 2016;23(6):621–7. doi: 10.1177/2047487315599892. [DOI] [PubMed] [Google Scholar]
- 19.Yihong Sun, Dayi Hu. The link between diabetes and atrial fibrillation: cause or correlation? J Cardiovasc Dis Res. 2010 Jan-Mar;1(1):10–11. doi: 10.4103/0975-3583.59978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL, Page RL, Heckbert SR. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853–8. doi: 10.1007/s11606-010-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR, Soliman EZ, Pankow JS, Selvin E. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the atherosclerosis risk in communities study. Heart. 2012;98:133–8. doi: 10.1136/heartjnl-2011-300503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morseth B, Graff-Iversen S, Jacobsen BK, Jørgensen L, Nyrnes A, Thelle DS, Vestergaard P, Løchen ML. Physical activity, resting heart rate, and atrial fibrillation: the Tromsø Study. Eur Heart J. 2016;1(37(29)):2307–13. doi: 10.1093/eurheartj/ehw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156(6):1163–9. doi: 10.1016/j.ahj.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, Eberly LE, Alonso A. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm. 2011;8(8):1160–6. doi: 10.1016/j.hrthm.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Grønbæk M. Alcohol Consumption and Risk of Atrial Fibrillation in Men and Women. The Copenhagen City Heart Study. Circulation. 2005;112:1736–42. doi: 10.1161/CIRCULATIONAHA.105.547844. [DOI] [PubMed] [Google Scholar]
- 26.Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, Anasako Y, Nishigaki Y, Yachi Y, Iida KT, Ohashi Y, Yamada N, Sone H. Alcohol Consumption and Risk of Atrial Fibrillation: A Meta-Analysis. J Am Coll Cardiol. 2011;57(4):427–36. doi: 10.1016/j.jacc.2010.08.641. [DOI] [PubMed] [Google Scholar]
- 27.Violi F, Soliman ZE, Pignatelli P, Pastori D. Atrial Fibrillation and Myocardial Infarction: A Systematic Review and Appraisal of Pathophysiologic Mechanisms. Journal of the American Heart Association. 2016;5:e003347. doi: 10.1161/JAHA.116.003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah V, Desai T, Agrawal A. The Association between Chronic Obstructive Pulmonary Disease (COPD) and Atrial Fibrillation: A Review. Chron Obstruct Pulmon Dis. 2016;1:2. [Google Scholar]
- 29.Goudis CA. Chronic obstructive pulmonary disease and atrial fibrillation: An unknown relationship. J Cardiol. 2017;69(5):699–705. doi: 10.1016/j.jjcc.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira C, Providência R, Ferreira JM, Gonçalves ML. Atrial Fibrillation and Non-cardiovascular Diseases: A Systematic Review. Arq Bras Cardiol. 2015;105(5):519–26. doi: 10.5935/abc.20150142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neal WT, Efird JT, Nazarian S, Alonso A, Heckbert SR, Soliman EZ. Peripheral arterial disease and risk of atrial fibrillation and stroke: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;17(3(6)):e001270. doi: 10.1161/JAHA.114.001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffin WF, Salahuddin T, O'Neal WT, Soliman EZ. Peripheral arterial disease is associated with an increased risk of atrial fibrillation in the elderly. Europace. 2016;18(6):794–8. doi: 10.1093/europace/euv369. [DOI] [PubMed] [Google Scholar]
- 33.Franczyk B, Gluba-Brzózka A, Ciałkowska-Rysz A, Banach M, Rysz J. The Problem of Atrial Fibrillation in Patients with Chronic Kidney Disease. Curr Vasc Pharmacol. 2016;14(3):260–65. doi: 10.2174/1570161114666160115130836. [DOI] [PubMed] [Google Scholar]
- 34.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J. Chronic Kidney Disease Is Associated With the Incidence of Atrial Fibrillation. The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;28(123(25)):2946–53. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;6(354):i4482. doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 36.Tae-Seok Kim, Ho-Joong Youn. Role of Echocardiography in Atrial Fibrillation. J Cardiovasc Ultrasound. 2011;19(2):51–61. doi: 10.4250/jcu.2011.19.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 38.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. European Heart Survey Investigators Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26(22):2422–34. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 39.Lafuente-Lafuente C, Mouly S, Longás-Tejero MA, Mahé I, Bergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med. 2006 Apr 10;166(7):719–28. doi: 10.1001/archinte.166.7.719. [DOI] [PubMed] [Google Scholar]
- 40.Xiuli Xu, Choumi T, Alida, Bo Yu. Administration of Antiarrhythmic Drugs to Maintain Sinus Rhythm After Catheter Ablation for Atrial Fibrillation: A Meta-Analysis. Cardiovascular Therapeutics. 2015;33:242–6. doi: 10.1111/1755-5922.12133. [DOI] [PubMed] [Google Scholar]
- 41.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 42.Lip GY, Laroche C, Ioachim PM, Rasmussen LH, Vitali-Serdoz L, Petrescu L, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry) Eur Heart J. 2014;1435(47):3365–76. doi: 10.1093/eurheartj/ehu374. [DOI] [PubMed] [Google Scholar]
- 43.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–51. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda JG, Van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace. 2011;13:1375–85. doi: 10.1093/europace/eur194. [DOI] [PubMed] [Google Scholar]
- 45.Masic I, Rahimic M, Dilic M, Kadribasic R, Toromanovic S. Socio-medical Characteristics of Coronary Disease in Bosnia and Herzegovina and the World. Mater Sociomed. 2011;23(3):171–83. doi: 10.5455/msm.2011.23.171-183. [DOI] [PMC free article] [PubMed] [Google Scholar]


