Capsule Summary
The present study shows a complex relationship between EASI and SCORAD. The results provide support for inclusion of objective-SCORAD and/or SCORAD in addition to EASI in clinical trials.
Keywords: atopic dermatitis, eczema, severity, signs, EASI, SCORAD
To the editor
Atopic dermatitis (AD) manifestations are heterogeneous, with variable lesional severity and extent. A systematic review was performed by Harmonizing Outcome Measures in Eczema1, an international consensus group aimed at standardizing outcome assessments in AD trials, and informed their recommendation for Eczema Area and Severity Index (EASI) be included in the core outcome set, but use of Scoring AD (SCORAD) was also encouraged for clinical trials1. Nevertheless, they were developed independently with different methods and target populations, and fundamentally different approaches. Understanding the gaps and relationship between EASI and SCORAD is imperative for the appropriate design and interpretation of clinical research studies. We hypothesized there are differences between EASI and SCORAD, particularly in patients with localized moderate-severe lesions, as well as xerosis and oozing that are assessed in SCORAD but not EASI.
We performed a prospective, dermatology practice-based, observational study to determine the relationship between EASI and SCORAD. Adolescents and adults (≥13 years) completed the patient-oriented eczema measure (POEM) and were evaluated with a medical history and total body skin examination by a dermatologist (JS). Subjects were enrolled from 9/2014–6/2016. The study was approved by the institutional review boards of Northwestern University and informed consent was waived.
AD was diagnosed using the Hanifin and Rajka diagnostic criteria2. AD severity was assessed using the EASI3, SCORAD, objective-SCORAD (oSCORAD)4, and Patient Oriented Eczema Measure (POEM). Distribution of AD lesions was collected using a standardized checklist.
Data analysis was performed using SAS version 9.4 (SAS Institute). Statistical significance was determined based on two-sided P<0.05. Complete data analysis was performed. EASI, oSCORAD/SCORAD and POEM were not normally distributed. Correlations were performed using Spearman correlations. Mann-Whitney U tests were used to compared oSCORAD/SCORAD scores among patients with low EASI scores with or without xerosis, oozing/weeping or localization of moderate-severe lesions to specific body sites. Additional details of AD assessments are presented in Supplemental Methods.
Linear regression models were constructed with 1. oSCORAD/SCORAD as the dependent variables and EASI as the independent variables and 2. POEM as the dependent variable and oSCORAD/SCORAD and EASI as the independent variables. Based on visual inspection of scatter plots, a nonlinear relationship was examined. Linear and multiple orders of spline functions were tested and retained based on the best statistical fit. A penalized-spline term with one knot was the best fitting model. Inclusion of the penalized-spline in the regression models allowed for a non-linear relationship between variables.
Overall, 388 patients (mean age 41.3±16.8 years; range 13–93 years,) with a total of 678 encounters were included in the study, including 266 females (69.3%), and 247 whites (64.7%) (Table 1).
Table 1.
Subject characteristics (n=388).
| Variable | Value |
|---|---|
| Age (yr) – mean ± std. dev. | 41.3 ± 16.8 |
| Female sex – freq (%) | 266 (69.3%) |
| Race/ethnicity – freq (%) | |
| Caucasian/white | 247 (64.7%) |
| African - American/black | 46 (12.0%) |
| Hispanic | 24 (6.3%) |
| Multiracial/other | 65 (17.2%) |
| Insured – freq (%) | 311 (80.4%) |
EASI and oSCORAD were strongly correlated with each other (rho=0.92; P<0.0001), but only moderately with POEM (rho=0.46 and 0.44; P<0.0001). There were non-linear relationships of EASI with SCORAD (r2linear=0.73, r2nonlinear=0.85) (Figure 1A), oSCORAD (r2linear=0.78, r2nonlinear=0.87) (Figure 1B), and POEM (r2linear=0.14, r2nonlinear=0.33) (Figure 1C), which were significantly better depicted using higher order polynomial functions (P<0.0001) and improvement of model fit (Figure 1A). For EASI≤5, there was a broad range [mean±std. dev.] of oSCORAD (0–48 [13.0±7.9]), SCORAD (0–60 [19.3±10.4]) or POEM (0–28 [7.8±5.8]). Similarly, SCORAD was strongly correlated with POEM (rho=0.56; P<0.0001). For EASI of 5.1–72, there were linear relationships with oSCORAD, SCORAD and POEM. In contrast, oSCORAD (r2linear=0.27, r2nonlinear=0.20) and SCORAD (r2linear=0.29, r2nonlinear=0.30) had linear relationships with POEM with no substantial improvement of model fit, respectively (Figure 1D, E).
Figure 1. Relationship between EASI, oSCORAD, SCORAD and POEM.
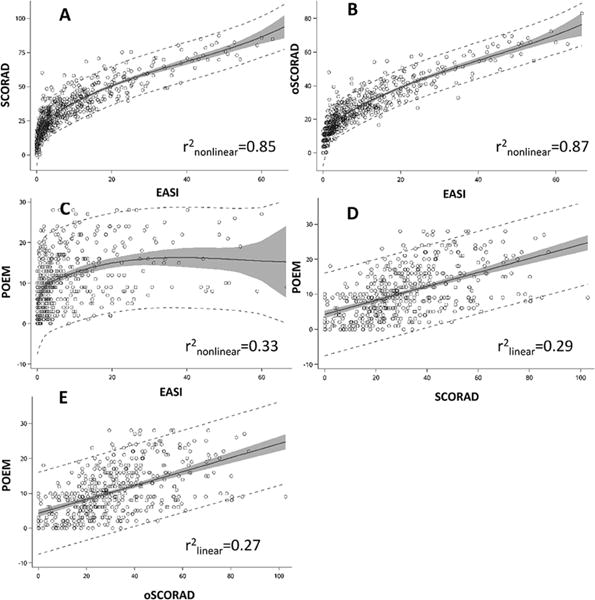
Scatterplots and penalized-splines are plotted for (A) SCORAD vs. EASI, (B) oSCORAD vs. EASI, (C) POEM vs. EASI, (D) POEM vs. SCORAD, and (E) POEM vs. oSCORAD.
Mean BSA was 23.8%±27.6%, EASI was 8.7±11.3, oSCORAD was 22.7±15.0, SCORAD was 31.0±18.2, and POEM was 10.7±7.0.
EASI≤5 was unable to discriminate between severe localized lesions and mild extensive lesions, with broad ranges of scores for erythema (0–3), edema/papulation (0–2), lichenification (0–3), scratching (0–3), oozing/weeping (0–2), xerosis (0–3), and BSA (0–62% [mean±std dev=5.9±7.6]).
Patients with EASI≤5 had significantly higher oSCORAD scores when xerosis was vs. was not present (22.2±9.1 vs. 15.0±10.7; Mann-Whitney U test, P<0.0001), particularly moderate-severe (score of 2–3) vs. mild xerosis (score of 1) was present (26.1±13.1 vs. 21.7±8.3; P=0.04). The presence of oozing/weeping was associated significantly higher oSCORAD (32.1±9.1 vs. 18.4±9.8; P<0.0001). There were no significant differences for the relationship between EASI and oSCORAD/SCORAD across races/ethnicities (white, black, Hispanic, multiracial/other), sex (male, female), age (adolescent, adult) (P≥0.16).
Patients with EASI≤5 had significantly higher oSCORAD when moderate-severe vs. no-mild lesions were localized to the face (29.3±9.5 vs. 18.1±9.9; P<0.0001), eyelids (30.5±11.2 vs. 18.4±9.8; P<0.0001), neck (31.4±10.8 vs. 18.7±10.0; P<0.0001), hands (30.9±9.5 vs. 18.5±10.0; P<0.0001) and feet (34.4±12.9 vs. 19.1±10.3; P=0.02).
The present study shows a complex relationship between EASI and oSCORAD/SCORAD and several limitations of both measures. Xerosis and/or oozing/weeping were associated with significantly higher oSCORAD, but low EASI. This is because xerosis and oozing/weeping are scored in oSCORAD, but not EASI. Patients with low EASI had higher oSCORAD secondary to localized moderate-severe lesions, and higher POEM scores. EASI assesses 4 AD signs and weights them to the BSA affecting 4 sites. oSCORAD/SCORAD assesses 6 AD signs separately from BSA, with representative lesional intensity comprising 76%/61% of the total score. Thus, patients with localized moderate-severe lesions may have high oSCORAD, but low EASI. Selection of a representative lesion in oSCORAD/SCORAD might bias towards reporting more severe disease. However, this does not appear to be the case, since oSCORAD/SCORAD showed a closer relationship with POEM than EASI. EASI and SCORAD were not perfectly correlated with POEM. While each of these are validated AD outcome measures, it may be that no single assessment is adequate for assessing the full severity and/or burden of AD. Of note, the 3 signs most closely associated with patient-reported AD severity (erythema, excoriation and edema/papulation)5 are present in EASI and oSCORAD/SCORAD.
Low EASI scores encompass a heterogeneous group of patients, including some having fairly extensive milder lesions and localized moderate-severe lesions. Studies of mild AD that use low EASI scores as an inclusion criterion may enroll a diverse mixture of patients with different extent and lesional severity. These results shed light on the interpretation of EASI scores in the mild range. Previous interpretability studies found that EASI scores of 0–7.0 encompass almost clear or mild AD6, yet this encompasses a heterogeneous group of patients. In EASI, the lowest surface area category is quite broad at 1–9% and equally weights cases with a 1 cm plaque or 9% of the body site affected. EASI may be a poorer measurer than oSCORAD when assessing patients with more limited disease. Alternatively, it is possible that oSCORAD/SCORAD is a poor measure of mild disease with too broad a range of values. We believe this to be less likely, because unlike EASI, the oSCORAD/SCORAD scores had linear relationships with POEM. Nevertheless, inclusion of xerosis in the oSCORAD/SCORAD might reduce its responsiveness, since xerosis may be present in the absence of active AD lesions. Moreover, assessing xerosis can be challenging in clinical trials, since it varies by the frequency, vehicle and time of last application of emollients/moisturizers.
In conclusion, the present results provide further support for inclusion of oSCORAD and/or SCORAD in addition to EASI in clinical trials. oSCORAD/SCORAD correlated better with POEM than EASI. However, the merits of assessing both EASI and SCORAD in trials has to be weighed against the excess burden of data collection for investigators and patients, as well as difficulties in training investigators in how to assess both scales.
Supplementary Material
Acknowledgments
This research was presented at the Society of Investigative Dermatology 2017 annual meeting in Portland, OR.
JI Silverberg had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: JI Silverberg
Acquisition of Data: JI Silverberg, P Vakharia, R Chopra, R Sacotte, N Patel, S Immaneni, T White, R Kantor, D Hsu
Analysis and interpretation of data: JI Silverberg, P Vakharia, R Chopra, R Sacotte, N Patel, S Immaneni, T White, R Kantor, D Hsu
Drafting of the manuscript: JI Silverberg
Critical revision of the manuscript for important intellectual content: JI Silverberg, P Vakharia, R Chopra, R Sacotte, N Patel, S Immaneni, T White, R Kantor, D Hsu
Statistical analysis: JI Silverberg
Administrative technical or material support: None
Study supervision: None
Financial disclosures: None
Funding Support: This publication was made possible with support from the Agency for Healthcare Research and Quality (AHRQ), grant number K12 HS023011, and the Dermatology Foundation.
Abbreviations used
- AD
atopic dermatitis
- EASI
Eczema Area and Severity Index
- SCORAD
Scoring Atopic Dermatitis
- oSCORAD
Objective component of Scoring Atopic Dermatitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
Funding/Sponsor was involved? No
Design and conduct of the study: Yes__ No_X_
Collection, management, analysis and interpretation of data: Yes__ No_X_
Preparation, review, or approval of the manuscript: Yes__ No_X_
Decision to submit the manuscript for publication: Yes__ No_X_
References
- 1.Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800–7. doi: 10.1016/j.jaci.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 2.Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92:44–7. [Google Scholar]
- 3.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–8. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 4.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10–9. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 5.Charman CR, Venn AJ, Williams H. Measuring atopic eczema severity visually: which variables are most important to patients? Arch Dermatol. 2005;141:1146–51. doi: 10.1001/archderm.141.9.1146. discussion 51. [DOI] [PubMed] [Google Scholar]
- 6.Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353–7. doi: 10.1111/bjd.13662. [DOI] [PubMed] [Google Scholar]
- 7.Spuls PI, Gerbens LA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. POEM a core instrument to measure symptoms in clinical trials: a HOME statement. Br J Dermatol. 2016 doi: 10.1111/bjd.15179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


