Abstract
Regenerative medicine employs human mesenchymal stromal cells (MSCs) for their multi-lineage plasticity and their pro-regenerative cytokine secretome. Adipose derived mesenchymal stromal cells (ASCs) are concentrated in fat tissue and the ease of harvest via liposuction makes them a particularly interesting cell source. However, there are various liposuction methods and only few have been assessed regarding their impact on ASC functionality. Here we study the impact of the two most popular ultrasound-assisted liposuction (UAL) devices currently in clinical use VASER (Solta Medical; Hayward, CA, USA) and Lysonix 3000 (Mentor, Santa Barbara, CA, USA) on ASCs yield, viability, osteogenic and adipogenic differentiation capacity and in vivo regenerative performance. After lipoaspirate harvest and processing we sorted for ASCs using Fluorescent Assisted Cell Sorting (FACS) based on an established surface marker profile (CD34+CD31−CD45−). Both UAL samples demonstrated equivalent ASC yield and viability. VASER UAL ACSs showed higher osteogenic and adipogenic marker expression, however still a comparable differentiation capacity was observed. Soft tissue healing and neovascularization were significantly enhanced via both UAL derived ASCs in vivo and there was no significant difference between the cell therapy groups. Taken together, our data suggests that UAL allows safe and efficient harvesting of the mesenchymal stromal cellular fraction of adipose tissue and that cells harvested via this approach are suitable for cell therapy and tissue engineering applications.
Keywords: Adult mesenchymal stromal cells, adipose-derived stromal cells, ASCs, regenerative medicine, cell therapy, ultrasound-assisted liposuction, VASER, Lysonix
INTRODUCTION
Surgeons have been optimizing methods for the removal of fat from different sites in the body for nearly a century. The first documented instance of removal of adipose tissue took place in 1921, when Charles Dujarrier used a uterine curette to remove subcutaneous fat from a patient’s calves and knees, unfortunately resulting in injury of the femoral artery and eventual amputation of the limb [1]. En bloc adipose tissue resection was also attempted, although popularity of the technique was limited by the resultant large scars. Further attempts to remove fat via curettage followed, most notably involving a blunt cannula with suction pioneered by Arpad and Giorgio Fischer in the 1970s [2].
Currently, more than 205,000 surgeries involving liposuction are performed each year in the United States alone, and it is nowadays considered to be a relatively safe procedure. Since the era of modern liposuction, focus has turned to the fine-tuning of the technique to improve factors such as skin retraction, blood loss, and operative time and effort [3]. Along with traditional suction-assisted liposuction (SAL) and other modalities such as power-assisted liposuction (PAL), radiofrequency-assisted Liposuction (RFAL) and laser-assisted liposuction (LAL), the use of ultrasound-assisted liposuction (UAL) has become a major component in many plastic surgery practices today. The basic principle of ultrasound assisted liposuction is to turn electric energy into vibration, causing thermal, cavitational and mechanical effects which lead to fragmentation of fat [4]. The use of ultrasound energy in liposuction was first developed in the late 1980s and 1990s by Scuderi and Zocchi [5], with the rationale that the energy would selectively disintegrate adipose tissue and thereby improve ease of removal with decreased bleeding [6].
Concurrently, both, surgeons and researchers alike have begun to explore the utility of the aspirated adipose tissue, a source which is abundant and otherwise unwanted or discarded. Most prominently, the technique of fat grafting (lipofilling/lipotransfer) has emerged as a method to restore soft tissue deficits [7]. However, the technique is thus far unpredictable in outcomes, often with poor volume retention combined with an incomplete understanding of the underlying physiology of fat grafts and their components. Adipose-derived stromal cells (ASCs), a population of multipotent cells which are found in relatively high proportions within adipose tissue [7], have shown to play a crucial role for the efficacy of lipotransfer. However, ASCs have not only been employed to further augment fat grafts to improve retention [8], they can also be applied to wounds to improve healing [9] and utilized in critical-sized calvarial defects to contribute to bone formation [10]. Evidence is now emerging that harvesting methods may affect the quality and regenerative potential of ASCs in aspirated adipose tissue [3]. In previous reports we have established that SAL lipoaspirates are a functional ASC source when compared to excisional fat and that VASER UAL derived ASCs are not diminished in their regenerative capacities when compared to SAL ASCs [11, 12]. However, it is yet unclear if the two most popular UAL devices differ in their ability to preserve the stromal cellular components of the aspirated tissue and therefore in the resultant regenerative potential. Here we determine the effect of VASER (Solta Medical; Hayward, CA, USA) and Lysonix 3000 (Mentor, Santa Barbara, CA, USA) UAL on ASC regenerative functionality.
METHODS
ASC Harvest and Isolation
Under approval of the Stanford Institutional Review Board (Protocol no. 2188), human lipoaspirate was collected from the abdomen of three adult female patients between the ages of 32–50. No patient had major medical comorbidities. Paired specimens were collected using VASER and Lysonix UAL devices sequentially on adjacent areas of the abdomen.
ASCs were isolated from the lipoaspirate specimens, according to previously described methods [13]. Briefly, lipoaspirates were washed with phosphate-buffered saline (PBS) and digested with 0.075% collagenase type II (Sigma-Aldrich; St. Louis, MO) in M199 (Cellgro; Manassas, VA) for 1 hour at 37°C with gentle agitation. The collagenase was neutralized using an equal volume of complete cell culture medium, consisting of Dulbecco’s modified Eagle’s medium plus GlutaMAX (DMEM; Invitrogen; Carlsbad, CA), 10% fetal bovine serum (FBS; Sigma-Aldrich; St. Louis, MO), and 1% penicillin/streptomycin (ThermoFIsher Scientific; Waltham, MA), and then centrifuged at 1500 rpm at 4°C for 20 minutes (350 rcf/g). The pellet was re-suspended in complete cell culture medium, filtered through a 100 μm cell strainer (Falcon; BD Biosciences, San Jose, CA), and then re-pelleted at 350 rcf/g for 15 minutes. The cell pellet was resuspended in red cell lysis buffer and then centrifuged once more at 350 rcf/g for 15 minutes at room temperature to obtain a stromal vascular fraction (SVF) pellet.
Fluorescence-Activated Cell Sorting
Our group recently demonstrated significant differences in the transcriptional profile between primary ASCs and cultured cells stressing the importance of using primary or very early passage cells in in all translational studies [14]. Therefore, freshly harvested and pelleted SVF cells were directly stained for fluorescence-activated cell sorting (FACS) analysis to identify and isolate the ASC population, defined as the CD45−/CD31−/CD34+ cell fraction. Cells were stained using the mouse anti-human monoclonal antibodies (all BD Biosciences) CD31-PE (Clone WM59; Catalog No. 560983), CD45-PeCy7 (Clone D058-1283, Catalog No. 561294) and CD34-APC (Clone 563, Catalog No.561209) and propidium iodide was used to exclude dead cells. A BD FACSAria (BD Biosciences) machine and FlowJo software (Miltenyi Biotec, San Diego, CA) were used to perform FACS analysis.
In Vitro Cell Viability
Cell Viability of ASCs harvested via VASER and Lysonix was detected with a Vybrant MTT Cell Assay Kit (Invitrogen; Carlsbad, CA) according to manufacturer’s instructions.
In Vitro Osteogenic Differentiation
ASCs harvested using VASER and Lysonix were seeded in standard six-well tissue culture plates in triplicate and cultured in standard medium (DMEM, 10% FBS, 1% penicillin/streptomycin) until wells reached 80% confluency. Osteogenic differentiation medium, consisting of DMEM plus GlutaMAX, 10% FBS, 1% penicillin/streptomycin, 100 μg/mL ascorbic acid, and 10 mM β-glycerophosphate (Sigma-Aldrich), was then applied to the cells and changed every three days. Total RNA was harvested in Trizol (Sigma-Aldrich) prior to osteogenic stimulation (Day 0) for a baseline measurement. After 7 days of culture in osteogenic differentiation medium, total RNA was again harvested. After 14 days, Alizarin Red (Sigma-Aldrich) staining was performed and quantified as previously described [15], to determine mineralization, and total RNA was collected again. Gene expression of runt-related-transcription factor-2 (RUNX2), osteoblastin (OPN) and osteocalcin (OCN) at each time point were determined using quantitative real-time polymase chain reaction (qRT-PCR) analysis (Applied Biosystems Prism 7900HT Sequence Detection System-primers are listed in table 1).
Table 1.
qPCR genes and primer sequences
| Gene Name | Forward primer sequence (5′ to 3′) | Reverse primer sequence (5′ to 3′) |
|---|---|---|
| RUNX2 | ATTCCTGTAGATCCGAGCACC | GCTCACGTCGCTCATTTTGC |
| OPN | TTGCAGCCTTCTCAGCCAA | GGAGGCAAAAGCAATCACTG |
| OCN | CACTCCTCGCCCTATTGGC | CCCTCCTGCTTGGACACAAAG |
| PPAR-γ | CCTATTGACCCAGAAAGCGATT | CATTACGGAGAGATCCACGGA |
| FABP-4 | AGCACCATAACCTTAGATGGGG | CGTGGAAGTGACGCCTTTCA |
| LPL | TCATTCCCGGAGTAGCAGAGT | GGCCACAAGTTTTGGCACC |
runt-related-transcription factor-2 (RUNX2), osteoblastin (OPN), osteocalcin (OCN), peroxisome proliferator-activated receptor γ (PPAR-γ), fatty acid binding protein 4 (FABP4), lipoprotein lipase (LPL)
In Vitro Adipogenic Differentiation
VASER- and Lysonix-harvested ASCs were seeded in six-well tissue culture plates in triplicate, grown to 80% confluency, and exposed to adipogenic differentiation medium, consisting of DMEM plus GlutaMAX, 10% FBS, 1% penicillin/streptomycin, 10 μg/mL insulin (Sigma-Aldrich), 1 μM dexamethasone (Sigma-Aldrich), 0.5 mM methylxanthine (Sigma-Aldrich), and 200 μM indomethacin (Sigma-Aldrich). Total RNA was harvested prior to and after 7 days of culture in adipogenic differentiation medium. After 7 days, Oil Red O (Sigma-Aldrich), staining was also performed. Gene expression levels of peroxisome proliferator-activated receptor γ (PPAR-γ), fatty acid binding protein 4 (FABP4), and lipoprotein lipase (LPL) were determined via qRT-PCR (Applied Biosystems Prism 7900HT Sequence Detection System-primers are listed in table 1).
In Vitro Real-Time Quantitative Polymerase Chain Reaction Analysis
After collection in Trizol, total RNA was isolated using the RNeasy Mini Kit (Qiagen; Valencia, CA) and reverse transcription was performed with TaqMan Reverse Transcription Reagents (Invtrogen), following manufacturer’s instructions. A Prism 7900HT sequence detection system (Applied Biosystems; Foster City, CA) was used to determine osteogenic and adipogenic gene expression, with SYBR Green PCR Master Mix used as the indicator. Expression data were normalized to beta-actin expression levels and then calibrated to Day 0 expression of the VASER sample. Beta-actin was selected from a set of different reference genes that were tested using geNorm in order to select the most stable reference gene for dataset normalization.
Animals
All mice were housed in the Stanford University Veterinary Service Center in accordance with NIH and institution-approved animal care guidelines. All procedures were approved by the Stanford Administrative Panel on Laboratory Animal Care.
Murine Excisional Wound Healing Model
Nude male Crl:CD-1-FOxn1nu mice (Charles River Laboratories, Wilmington, MA, USA http://www.criver.com) between 8 and 12 weeks of age were randomized for treatment to 3 different groups: one received treatment with VASER isolated ASC seeded hydrogels, one with hydrogels seeded with Lysonix isolated ASCs and the control treated with hydrogel scaffolds alone. Pullulan-collagen hydrogel was produced as described before [16]. Fat was aspirated from 3 donors, cells were isolated via FACS (CD45−/CD31−/CD34+ cell fraction) and 5× 105 human ASCs were suspended in 15 μl PBS solution and pipetted onto hydrophobic wax paper. The cells were actively absorbed by capillary, hydrophibic and entropic forces [9]. At the dorsum of each mouse two 6mm thick excisional wounds were created, which were held opened by donut shaped silicone rings fastened with 6–0 nylon sutures for the prevention of wound contraction. The hydrogel was placed in the wound beds followed by covering with an occlusive dressing (Tegaderm; 3M, St. Paul, MN, http://www3m.com). Documentation with digital photography was performed on day 0, 3, 5, 7, 9, 11, 13 and 15. By using ImageJ software (National Institute of Health) the wound area was measured (n=8 wounds by group).
Wound Vascularity Assessment
Vascularity of healed wounds was assessed by immunohistochemical staining for the endothelial cell marker CD31 (n=8 wounds/condition). Briefly, wounds from the excisional model were harvested immediately upon closure and processed for paraffin sectioning. Immunohistochemical staining of seven micron thick paraffin sections (n=5/wound) for CD31 was used to quantify wound vascularity as described previously [16]. After de-paraffinization and washing in PBS, slides were blocked in a humidified chamber for 2 hours. Then the primary antibody (1:100 Rb α CD31, Ab28364; Abcam, Cambridge, U.K., http://www.abcam.com) was incubated overnight at 4°. Secondary staining was performed (1:400 AF547 Gt α Rb; Life Technologies, Grand Island, NY, http://www.lifetechnologies.com). By using DAPI (4′,6-Diamidin-2-phenylindol), nuclei were stained. We used ImageJ software (National Institute of Health) to binarize immunofluorescent images taken with in the same setting. Intensity threshold was set automatically. Pixel-positive area per high power field determined the quantification of CD31 staining [17].
Statistical Analyses
Data are shown as mean ± SEM. Student’s t-tests were used to perform statistical comparisons between groups, with Bonferroni corrections where appropriate for multiple comparisons. A p-value of < 0.05 was considered statistically significant.
RESULTS
VASER and Lysonix Yield Similar Concentrations of ASCs with Comparable Viability
FACS showed no differences in ASC frequency and viability in SVF of both UAL groups (determined by percentage of CD45−/CD31−/CD34+ characterized cells) (Figure 1 A, B). No difference in viability was detected by MTT assay between ASCs harvested via VASER and those harvested via Lysonix (p = 0.34) (Figure 1 C).
Figure 1.
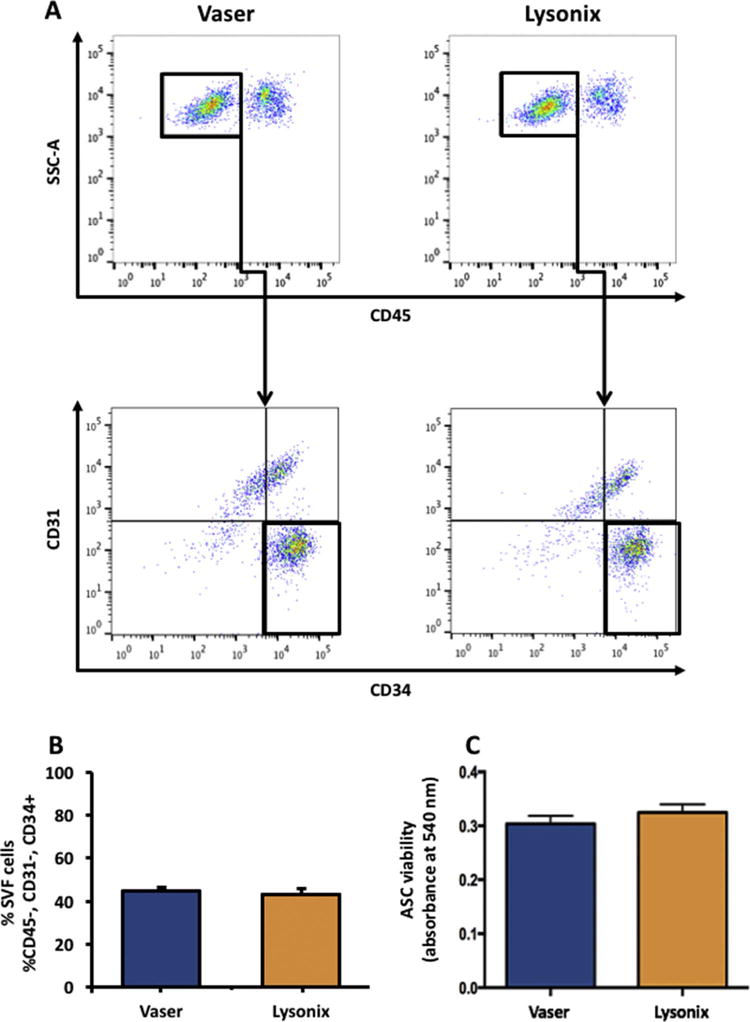
Vaser and Lysonix lipoaspirates yield similar amounts of ASCs with comparable viability. (A): FACS analysis for identification of ASCs (defined as CD45−/CD31−/CD34+) of Vaser and Lysonix derived lipoaspirates; (B): CD45−/CD31−/CD34+ASC-quantification in Vaser and Lysonix derived SVF showed no significant difference in ASC yield across samples (C): MTT assay showed no significant difference in cell viability. n=3; data are given as mean ±SEM. Abbreviations: SVF: Stromal Vascular Fraction; ASC: Adipose derived Stromal Cells.
ASCs Harvested via VASER Have Greater Osteogenic Differentiation Capacity
Under osteogenic induction VASER derived ASCs demonstrated a trend towards greater mineralization, which almost reached significance (Figure 2 A, B). VASER samples further showed higher expression of the osteogenic markers RUNX-2 and OCN. RUNX-2 was significantly higher expressed after 7 days (p < 0.05) and 14 days (p < 0.01) (Figure 2 C), and OCN was more highly expressed after 14 days (p < 0.01) (Figure 2 E). This difference was not seen prior to differentiation at day 0. On the contrary OPN was expressed significantly higher on day 0 in Lysonix harvested samples (Figure 2 D).
Figure 2.
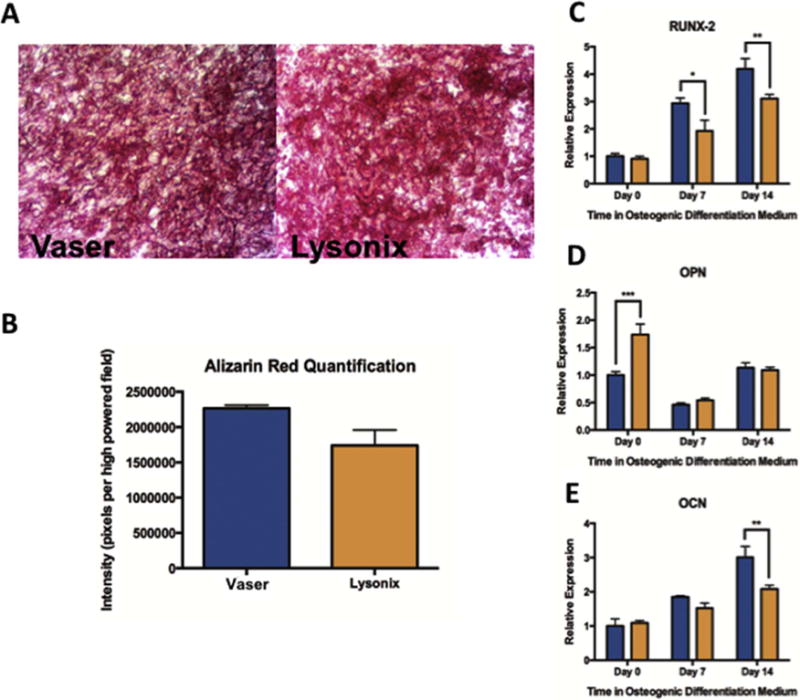
Assessment of Osteogenic Differentiation Capacity of Vaser and Lysonix-derived samples. (A) and (B): Alizarin red staining and quantification showing a trend towards higher mineralization for Vaser samples; (C), (D), and (E): Real-time-PCR quantifying the expression of early (RUNX-2), intermediate (OPN) and late (OCN) osteogenic marker. RUNX-2 showed significantly higher expression in Vaser samples on day 7 and 14 and OCN was significantly higher expressed in Vaser samples on day 14, while OPN was significantly higher on day 0 in samples of Lysonix ASCs. Abbreviations: PCR: polymerase chain reaction, RUNX2: runt related transcription factor 2, OPN: osteopontin, OCN: osteocalcin.
UAL Harvesting Method Has Potential Effects on Adipogenic Differentiation Capacity
ASCs harvested by VASER and subsequently cultured in adipogenic differentiation medium also showed higher expression of key adipogenic markers (Figure 3 A–C). Notably, after 7 days of culture, VASER ASCs displayed significantly higher expression levels of FABP-4 (p < 0.001) and LPL (p < 0.01). At day 7, there was also a trend toward higher PPAR-y expression, although not significant (p = 0.16). However, despite this differential gene expression, ASCs derived from VASER and Lysonix showed similar lipid droplet accumulation as measured by Oil Red O (p = 0.95) (Figure 3 D, E).
Figure 3.
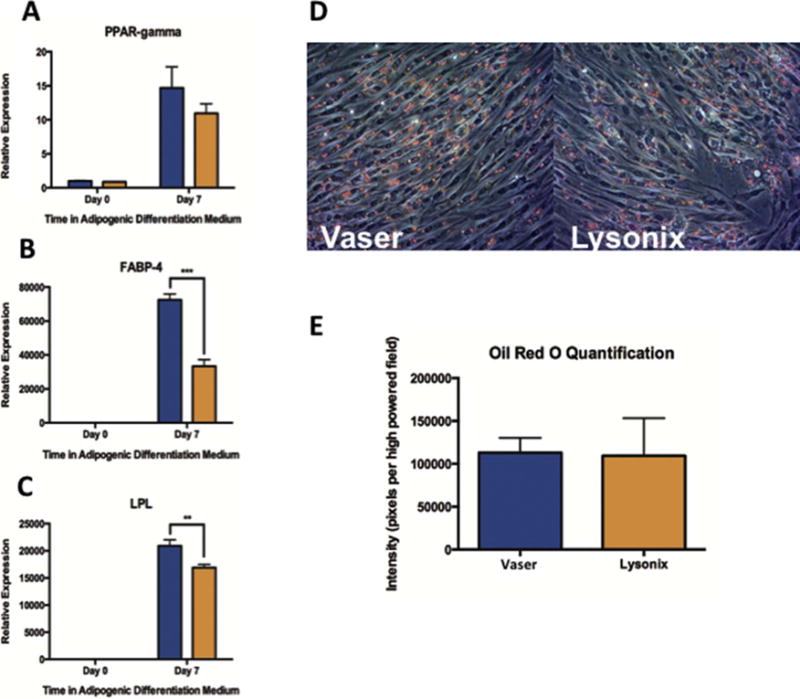
Adipogenic Differentiation Capacity of Vaser and Lysonix derived samples. (A), (B) and (C): Real-time-PCR for quantification of key-adipogenic markers (PPAR-γ, FABP-4 and LPL) showed a higher expression of all markers in Vaser harvested samples at day 7. (D) and (E): Oil Red O staining showed no differences in lipid accumulation. Abbreviations: PPAR-γ: peroxisome proliferator-activated receptor-γ, FABP4: fatty acid binding protein 4, and LPL: lipoprotein lipase.
UAL Derived ASCs Significantly Improve Wound Healing
In vivo murine wound healing showed a significant acceleration of wound closure in both, Vaser and Lysonix hydrogel groups, compared to control (Figure 4 A–C). In the control group, complete wound closure was observed at d= 14,1 – significantly prolonged to mean closure time in UAL groups (d= 11 in Vaser; and d=11,2 in Lysonix; p<0,01) (Figure 4 C). Between the two UAL groups, there was no significant difference regarding healing kinetics or wound closure time.
Figure 4.
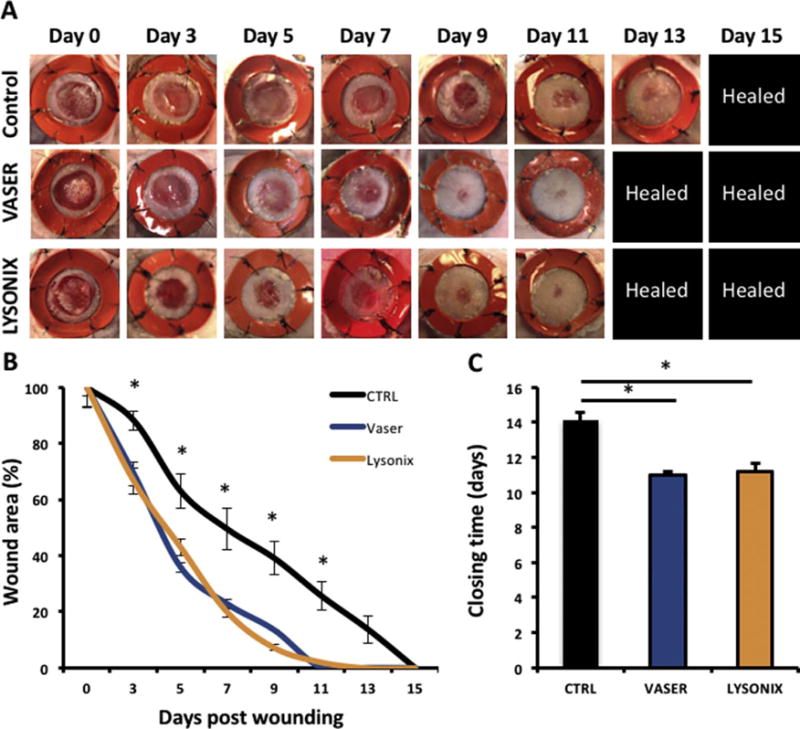
ASCs harvested with either VASER or Lysonix liposuction showed similar ability to enhance murine wound healing. There was a significant enhancement of wound closure compared with control in both cell therapy groups. (A) wound appearance, (B) kinetics of wound size and (C) closure time of CTRL, Vaser and Lysonix groups. n= 8; p< 0.01. All data are given as mean ±SEM. Abbreviations: CTRL: control.
UAL Derived ASCs Significantly Enhance Wound Vascularity
Both UAL groups showed significantly enhanced neovascularization, compared to unseeded hydrogel (Figure 5 A, B). There was no significant difference between Vaser and Lysonix groups regarding the induction of new vessel formation. These data corroborate the regenerative potential of UAL-harvested ASCs.
Figure 5.
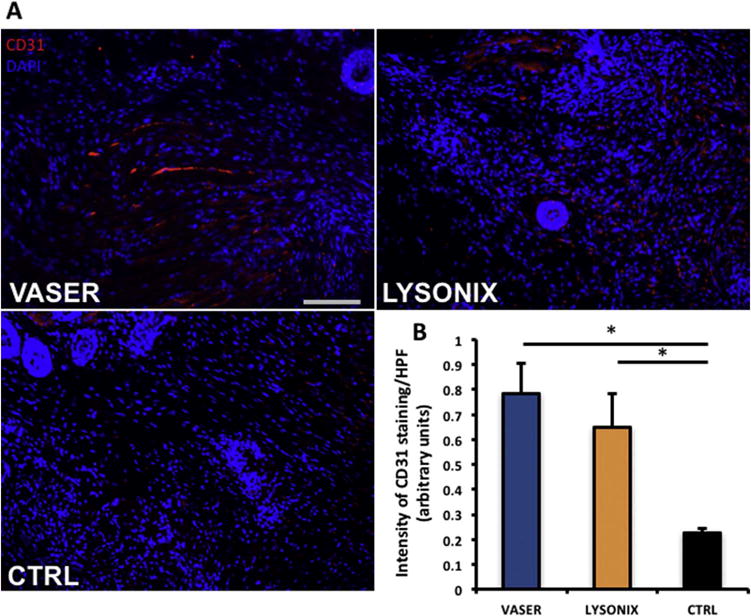
Hydrogels seeded with Vaser and Lysonix derived ASCs resulted in improvement of vascular density of wounds. (A) CD 31 (red) and DAPI (blue) staining were performed and showed increased neovascularization in both ASC seeded hydrogel groups. (B) Quantification showed no significant difference between ASC loaded hydrogels. Scale bar= 100 μm. n=8. Asterisk indicates p≤0,05. All data are mean ± SEM, Abbreviations: CTRL: control; HPF: high power field.
DISCUSSION
ASCs hold great promise in the treatment of tissue defects across the whole body, however, little is known about the effects of specific liposuction methods on these cells within the lipoaspirate. In addition to traditional Suction assisted Liposuction (SAL), Laser assisted Liposuction (LAL), Radio-Frequency Liposuction (RFAL), Power Assisted Liposuction (PAL) and Ultrasound assisted Liposuction (UAL) have recently been developed. UAL devices have undergone several optimizing steps for providing painless and fast liposuction with a focus on minimizing donor site morbidity. After using solid cannulas in first devices, there was a change to perforated cannulas leading to simultaneous emulsification and aspiration [18]. This resulted in a shortening of surgery times. However, these devices were still not sophisticated enough to preserve cellular function. A report from Oedayrajsingh-Varma et al. assessing an early UAL model (Lysonix 2000) concluded that the ultrasound procedure was inferior to surgical isolation via resection and Coleman lipoaspiration for ASC recovery [19]. This device generation was replaced step by step by newer systems again separating the steps of ultrasound deployment and lipoaspiration. Lately the next generation of UAL-devices (i.e. VASER) uses a higher ultrasonic operating frequency (36kHZ). Despite this difference in energy release, we demonstrate here that ultrasound deployed via the two most commonly used UAL devices (VASER and Lysonix) allow the harvest of fully functional ASCs for regenerative medicine. In fact, in line with recent mechanobiology discoveries, mechanical stimulation could be a beneficial impulse for mesenchymal stem cells potentially enhancing their functionality [20] and our data suggests that ultrasonic energy deployed at 36kHZ may be beneficial for osteogenic differentiation of ACSs.
Our group already compared SAL and UAL in a previous study and found no significant difference in ASC viability, adipogenic differentiation capacity and wound healing ability [11]. However, a trend toward higher osteogenic differentiation abilities of VASER derived ASCs observed in our previous study could be confirmed in the present data. The key osteogenic factors Runx-2 and OCN were significantly higher expressed in VASER samples. These findings are strengthened by a study published by Panetta et al. comparing SAL and VASER derived lipoaspirates also showing tendencies of higher expression of Runx-2 and OCN in VASER samples [21]. This osteogenic tendency of VASER ASCs is consistent with published reports on the benefits of ultrasound treatment for bone regeneration and MSC function in fracture healing [22, 23]. Additionally, some effect can be observed in the expression of adipogenic markers and the 36kHZ deployed by the VASER device again seem to be more stimulating than the lower frequency of Lysonix. These phenotypical features of VASER derived ASCs could potentially be leveraged in tissue engineering and treatment of tissue defects. However, whether or not ASCs harvested via a certain method are superior in a particular setting warrants further basic and clinical investigation.
We previously evaluated ASCs harvested by SAL lipoaspiration versus ASCs from excisional adipose tissue and found a significant difference in ASC yield with more cells in excisional fat samples. However, no changes in multilineage differentiation capacity, viability and wound healing were detectable in SAL samples when compared to the minimally manipulated cells derived from excisional fat. Taken together with our previous results, demonstrating the similarity of the regenerative efficacy of SAL and UAL derived ASCs and with the present data, we can conclude, that ultrasonic energy has no harmful effects on the stromal cell fraction of adipose tissue. Furthermore, all modern UAL devices currently in clinical use can be considered as comparable regarding ASC yield, viability and regenerative functionality in vivo.
Liposuction is a dynamic field with continuous advancements and advents of novel devices. Recently invented techniques such as the Tissue Liquefaction Technology (TLT) liposuction, that uses warm saline and low pressure for adipocyte harvesting, may have shown to reduce donor side morbidity, however, the regenerative potential of ASCs harvested via this approach still remains uncertain [24]. In addition to harvesting methods there are multiple other factors influencing the regenerative efficacy of ASCs, some known such as shear stress [25] and lidocaine exposure [26], and some yet to be determined. Ongoing research will help to identify which elements of fat harvesting, processing and application positively or negatively impact ASC functions. This will lead to new techniques and devices optimizing cell health and consequently tissue engineering and cell based therapies.
CONCLUSION
Both investigated UAL devices, Lysonix and VASER liposuction are reliable methods for ASC harvest. Our data suggests that UAL derived ASCs are fully functional for cell-based therapy approaches and tissue engineering. In line with our previous reports this corroborates that ultrasonic energy has no negative effect on the stromal cellular fraction of adipose tissue.
Acknowledgments
Funding for the stem cell research conducted in our laboratory has been provided by the National Institutes of Health (R01-DK074095, R01-AG025016, K08 DE024269), the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine, the Child Health Research Institute at Stanford University, and The Oak Foundation. The authors would like to thank Yujin Park for her assistance with tissue processing and staining, as well as Dr. Dean Vistnes at the Kaplan Cosmetic Surgery Center for lipoaspirate sample collection. Cell sorting was completed at the Stanford Shared FACS Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
All listed authors contributed to the idea generation, design, and completion of this work. DD lead the idea generation and the experimental work. ZNM, AL, EAB, DA, AJW, MSH, GGW and KSH contributed to the experimental work. DD, AL and MMA wrote the manuscript. AFS, HGM, MTL, GCG and DCW guided the idea generation, experimental work and manuscript preparation.
DISCLOSURE OF POTENTIAL CONFLICT OF INTEREST
DD and GCG are listed on the patent “Efficient Stem Cell Delivery Into Biomaterials Using a Novel Capillary Driven Encapsulation Technique” and GCG is listed on the patent “Intelligent Biodegradable Pullulan Regenerative Matrix for Tissue Engineering” assigned to Stanford University. ZNM, AL, MMA, EAB, DA, AJW, MSH, GGW, KSH, AFS, HGM, MTL and DCW have no potential conflicts of interest, affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed herein.
References
- 1.Sterodimas A, et al. Thirtyfour years of liposuction: past, present and future. Eur Rev Med Pharmacol Sci. 2012;16(3):393–406. [PubMed] [Google Scholar]
- 2.Fischer G. Liposculpture: the “correct” history of liposuction. Part I. Dermatologic Surgery. 1990;16(12):1087–1089. doi: 10.1111/j.1524-4725.1990.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 3.Chung MT, et al. Isolation of Human Adipose – Derived Stromal Cells Using Laser – Assisted Liposuction and Their Therapeutic Potential in Regenerative Medicine. Stem cells translational medicine. 2013;2(10):808–817. doi: 10.5966/sctm.2012-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry MG, Davies D. Liposuction: a review of principles and techniques. J Plast Reconstr Aesthet Surg. 2011;64(8):985–92. doi: 10.1016/j.bjps.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Zocchi M. Ultrasonic liposculpturing. Aesthetic plastic surgery. 1992;16(4):287–298. doi: 10.1007/BF01570690. [DOI] [PubMed] [Google Scholar]
- 6.de Souza Pinto EB, et al. Liposuction and VASER. Clin Plast Surg. 2006;33(1):107–15. vii. doi: 10.1016/j.cps.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, et al. Adipose tissue engineering for soft tissue regeneration. T issue Engineering Part B: Reviews. 2010;16(4):413–426. doi: 10.1089/ten.teb.2009.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luan A, et al. Cell – Assisted Lipotransfer Improves Volume Retention in Irradiated Recipient Sites and Rescues Radiation – Induced Skin Changes. Stem cells. 2016 doi: 10.1002/stem.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg RK, et al. Capillary Force Seeding of Hydrogels for Adipose – Derived Stem Cell Delivery in Wounds. Stem cells translational medicine. 2014;3(9):1079–1089. doi: 10.5966/sctm.2014-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walmsley GG, et al. A System for the Surveillance of Stem Cell Fate and Function. Stem Cell and Translational Investigation. 2016;3 [Google Scholar]
- 11.Duscher D, et al. Ultrasound-assisted liposuction does not compromise the regenerative potential of adipose-derived stem cells. Stem cells translational medicine. 2016;5(2):248–257. doi: 10.5966/sctm.2015-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duscher D, et al. Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells. Journal of translational medicine. 2016;14(1):126. doi: 10.1186/s12967-016-0881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto D, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue engineering. 2006;12(12):3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 14.Januszyk M, et al. Evaluating the Effect of Cell Culture on Gene Expression in Primary Tissue Samples Using Microfluidic-Based Single Cell Transcriptional Analysis. Microarrays (Basel) 2015;4(4):540–50. doi: 10.3390/microarrays4040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garza RM, et al. Studies in fat grafting: Part IV. Adipose-derived stromal cell gene expression in cell-assisted lipotransfer. Plastic and reconstructive surgery. 2015;135(4):1045. doi: 10.1097/PRS.0000000000001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rustad KC, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33(1):80–90. doi: 10.1016/j.biomaterials.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duscher D, et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Scientific reports. 2014;4 doi: 10.1038/srep07144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graf R, et al. Ultrasound-assisted liposuction: an analysis of 348 cases. Aesthetic plastic surgery. 2003;27(2):146–153. doi: 10.1007/s00266-002-1516-x. [DOI] [PubMed] [Google Scholar]
- 19.Oedayrajsingh-Varma MJ, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8(2):166–77. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 20.Meier EM, et al. Mechanical Stimulation Increases Knee Meniscus Gene RNA-level Expression in Adipose-derived Stromal Cells. Plast Reconstr Surg Glob Open. 2016;4(9):e864. doi: 10.1097/GOX.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panetta NJ, et al. Tissue harvest by means of suction-assisted or third-generation ultrasound-assisted Upoaspiration has no effect on osteogenic potential of human adipose-derived stromal cells. Plastic and reconstructive surgery. 2009;124(1):65–73. doi: 10.1097/PRS.0b013e3181ab10cd. [DOI] [PubMed] [Google Scholar]
- 22.Wei FY, et al. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS One. 2014;9(9):e106722. doi: 10.1371/journal.pone.0106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung YJ, et al. Focused low-intensity pulsed ultrasound enhances bone regeneration in rat calvarial bone defect through enhancement of cell proliferation. Ultrasound Med Biol. 2015;41(4):999–1007. doi: 10.1016/j.ultrasmedbio.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Dolen U, et al. Fat grafting with tissue liquefaction technology as an adjunct to breast reconstruction. Aesthetic Plastic Surgery. 2016;40(6):854–862. doi: 10.1007/s00266-016-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang H, Tate MLK. Structure-function relationships in the stem cell’s mechanical world B: emergent anisotropy of the cytoskeleton correlates to volume and shape changing stress exposure. Molecular & cellular biomechanics: MCB. 2011;8(4):297. [PMC free article] [PubMed] [Google Scholar]
- 26.Girard A, Festy F, Roche R. Local anesthetics: use and effects in autologous fat grafting. Surg Curr Res. 2013;3:142. [Google Scholar]


