Abstract
Specialized metabolic enzymes and metabolite diversity evolve through a variety of mechanisms including promiscuity, changes in substrate specificity, modifications of gene expression and gene duplication. For example, gene duplication and substrate binding site changes led to the evolution of the glucosinolate biosynthetic enzyme, AtIPMDH1, from a Leu biosynthetic enzyme. BAHD acyltransferases illustrate how enzymatic promiscuity leads to metabolite diversity. The examples 4-coumarate:CoA ligase and aromatic acid methyltransferases illustrate how promiscuity can potentiate the evolution of these specialized metabolic enzymes.
Introduction
Plant specialized metabolites are lineage-specific compounds, many of which are involved in ecological interactions, such as herbivore defense or pollinator attraction [1,2]. The number of specialized metabolites produced across all plant species is estimated to be in the hundreds of thousands [3]. Specialized metabolic enzymes tend to have lower catalytic efficiency [4] and greater substrate promiscuity [5] than primary metabolic enzymes. This review explores factors involved in enzyme evolution and discusses how these result in metabolite diversity.
We focus on mechanisms that play roles in “potentiation” (Figure 1A); metabolic examples of what was more generally described by Blount et al. [6] as factors that allow for the realization of a new trait. In recent years, enzymatic promiscuity – the ability of an enzyme to catalyze reaction(s) in addition to its primary reaction – has been documented to “potentiate” the evolution of new specialized metabolic activities (Figure 1A) [6]. Substrate promiscuity is documented to play a central role in the evolution of specialized metabolic enzymes (Figure 1B) [5]. Changes in substrate specificity also can result in emergence of novel activities and chemical diversity. Such a shift in substrate specificity can change the primary substrate of an enzyme from an intermediate in an existing biosynthetic pathway to a new substrate, which – in turn – can potentiate novel enzymatic reactions (Figure 1C). Gene duplication and divergence in gene expression patterns or enzyme activities also potentiate the evolution of specialized metabolic enzymes (Figure 1D) [7]. We highlight examples from the past five years in which promiscuity, changes in substrate specificity, gene duplication, and changes in gene expression were shown to play prominent roles in evolution of specialized metabolic enzymes and generation of chemical diversity. These examples illustrate the power of structural analysis – especially in a comparative evolutionary context – to reveal constraints and opportunities to facilitate the modification or engineering of these enzymes.
Figure 1. Mechanisms leading to evolution of chemical diversity and enzymatic novelty.
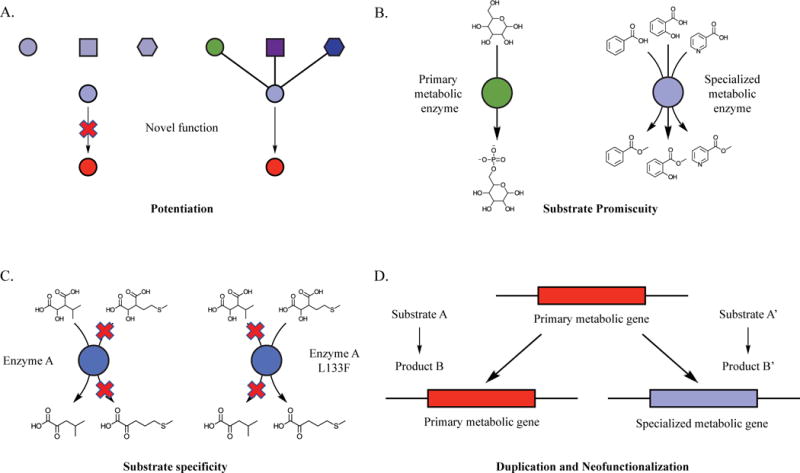
A) Potentiation – The different shapes and colors represent factors involved in the evolution of a novel function or enzymatic activity. These factors enable the realization of novel functions or enzymatic activities. In this example, the green circle represents suitable localization of gene expression, the purple square represents substrate availability in the tissue of interest, and the blue hexagon represents the ability of the enzyme to utilize the substrate [6]. B) Substrate promiscuity – primary metabolic enzymes typically catalyze a specific reaction, while specialized metabolic enzymes tend to be promiscuous and catalyze reactions using multiple substrates. C) Substrate specificity – specific amino acid changes result in alteration of enzyme substrate specificity, resulting in a new enzymatic activity or function. D) Gene duplication and neofunctionalization –a primary metabolic gene is duplicated, facilitating diversification of one isoform into a specialized metabolic function.
Gene duplication and changes in substrate specificity in the evolution of a glucosinolate biosynthetic enzyme
Glucosinolates are a group of structurally diverse, amino-acid derived plant specialized metabolites that mediate interactions between crucifers and insects or pathogens [8]. The biosynthesis of methionine-derived glucosinolates involves a repeated three step elongation process similar to Leu biosynthesis: condensation with acetyl-CoA, isomerization, and oxidative decarboxylation to successively add one carbon units to the aliphatic side chain [9]. The glucosinolate oxidative decarboxylation step is catalyzed by the A. thaliana isopropylmalate dehydrogenase (AtIPMDH1), while two other A. thaliana IPMDH enzymes catalyze the same reaction in Leu biosynthesis; all three enzymes have between 85–93% amino acid identity with each other [10,11]. The Leu biosynthetic substrate (3-isopropylmalate) and glucosinolate substrate (3-(2′-methylthio)-ethylmalate), have the same carboxyl and hydroxyl group configuration but differ in side chain length and composition, suggesting similarities in the binding dynamics between the enzymes and substrates in the two pathways (Figure 2A, side chains are in color).
Figure 2. Structural analysis of AtIPMDH2 and AtHCT.
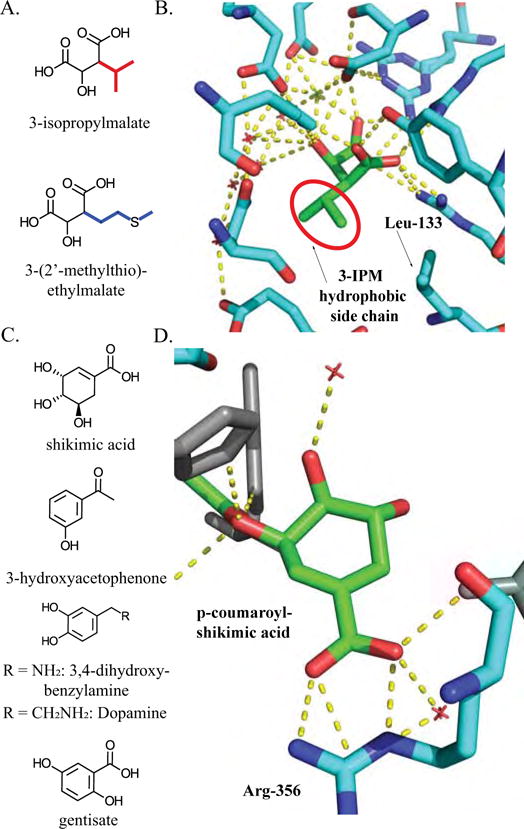
A) Structure of substrates for IPMDH enzyme variants in Arabidopsis — 3-IPM is the leucine biosynthetic intermediate, and 3-(2′-methylthio)-ethylmalate participates in aliphatic glucosinolate biosynthesis. B) Interactions of AtIPMDH2 active site residues with 3-IPM demonstrate extensive interactions with the polar moieties, while not specifically interacting with the 3-IPM hydrophobic side chain. 3-IPM structural backbone is green. Active site residues are cyan. Blue represents nitrogen atoms, and red represents oxygen atoms. Green and red crosses are magnesium and water respectively [PDB ID: 5J32]. The Leu 133 residue responsible for the change in substrate specificity is labeled. C) Substrates used for AtHCT analysis: Shikimic acid is the native substrate; 3-hydroxyacetophenone is a neutral non-native substrate of AtHCT; 3,4-dihydroxybenzylamine and dopamine are substrates that are positively charged under physiological pH. AtHCT has greater in vitro activity with gentisate, a non-native substrate, than with shikimate. D) Three-dimensional representation of Arg-356 interaction with the carboxyl group of p-coumaroyl-shikimic acid substrate in the active site of AtHCT. p-coumaroyl shikimic acid is colored green. Active site residues are colored cyan and red crosses represent water [PDB ID: 5KJU].
The AtIPMDH2 Leu biosynthetic enzyme crystal structure with 3-isopropylmalate (3-IPM) revealed several binding interactions with the polar portion of the substrate [12]. The structure revealed that residues interacting with the polar groups of 3-IPM are conserved between all IPMDH enzymes (Figure 2B) [13]. This, combined with the similarity of the polar groups of 3-IPM and (3-(2′-methylthio)-ethylmalate, suggested that recognition of the side chain is responsible for substrate discrimination. There are no specific substrate-enzyme interactions between the 3-IPM aliphatic isopropyl side chain and the residues in the largely hydrophobic pocket in the active site (Figure 2B) [12,13]. Thus, the differences between the glucosinolate biosynthetic enzyme AtIPMDH1 and Leu IPMDH enzymes presumably are responsible for the difference in substrate specificity.
Sequence alignments of Leu IPMDH enzymes with AtIPMDH1 revealed a key feature that affects the ability of the enzyme to discriminate between Leu and glucosinolate substrates. AtIPMDH1 carries a position 133 Leu:Phe change at a site in the hydrophobic pocket at a site that is invariant in the Leu biosynthetic enzymes from bacteria to plants [10,13]. Reciprocal substitutions of residues at this site led to a decrease in the in vitro catalytic efficiency with the native substrate – e.g. 3-IPM for AtIPMDH2 and AtIPMDH3 and 3-(2′-methylthio)-ethylmalate for AtIPMDH1– and an increase with the non-native substrate [13]. Because the chemical structures of 3-(2′-methylthio)-ethylmalate and 3-IPM differ only by the length and structure of the side chain, this result demonstrates that the substitution of Leu by Phe is sufficient to facilitate the accommodation of the 3-(2′-methylthio)-ethylmalate side chain in the enzyme [12].
This example illustrates many themes found throughout the evolution of specialized metabolic enzymes [7]. A model for the emergence of the glucosinolate IPMDH can be constructed [13], starting with a simple gene duplication that led to the opportunity for ‘recruitment’ of the highly conserved Leu biosynthetic enzyme, IPMDH. This was followed by the L137F change in the amino acid sequence, leading to alteration of substrate specificity. In this model, gene duplication (Figure 1D) was critical for the divergence in structure and function of AtIPMDH1, allowing maintenance of the Leu pathway, while facilitating the incorporation of IPMDH activity into the glucosinolate pathway. The residue change (Figure 1C) facilitated the alteration of substrate specificity resulting in incorporation of the IPMDH activity into the glucosinolate pathway and increased chemical diversity through the production of elongated glucosinolates.
Acyltransferase promiscuity modulates metabolite diversity
Acylsucrose acyltransferases (ASATs) are BAHD (BEAT, AHCT, HCT, and DAT) [14] acyltransferases involved in biosynthesis of protective glandular trichome acylsugars in cultivated tomato and wild relatives [15–17]. The large BAHD family includes enzymes that perform O- and N- acylation of structurally diverse acceptor substrates such as alkaloids, phenylpropanoids, terpenoids and acylsugars [6]. Acylsucroses are produced through consecutive ASAT-mediated conjugation of acyl-CoAs, starting with sucrose to form mono-, di-, tri- and tetra-acylated sucroses [18–20]. These enzymes contribute to intraspecific and interspecific diversity in the profiles of insecticidal acylsugars of tomato and its wild relatives.
The second enzyme of acylsugar biosynthesis in cultivated and wild tomato species (ASAT2) exhibits an interesting pattern of substrate preference for iso- (3-methylbutyrate) and anteiso branched (2-methylbutyrate) C5 as well as nC12 acyl chains [18]. ASAT2 in cultivated tomato shows higher specificity, using aiC5-CoA but not the structurally similar iC5-CoA as a donor substrate. In contrast, the ASAT2 orthologs in other wild tomatoes are promiscuous, using both aiC5-CoA and iC5-CoA. [18]. A combination of primary sequence comparisons and homology modeling of the isozymes using the crystal structure of a distantly related BAHD enzyme led to identification of the amino acid responsible for the difference in promiscuity [18,21]. Specifically, Phe408 restricts and Val408 enables iso-branched C5 acyl chain utilization. This simple change contributes to accumulation of more diverse acylsugars in the wild tomato, Solanum habrochaites LA1777, compared to the cultivated species [22]. This example illustrates that enzymatic promiscuity can arise due to simple changes and facilitate the generation of product diversity in specialized metabolism (Figure 1B).
Hydroxycinnamoyltransferases illustrate how the interface of enzyme and substrate structure shape promiscuity
In addition to sequence changes, structural similarities of the substrates and how they interact with structural elements of the enzymes can also influence enzyme promiscuity. This theme is exemplified by the hydroxycinnamoyltransferases (HCTs), which play a role in phenylpropanoid biosynthesis [23–26]. HCTs are involved in the conjugation of p-coumaroyl CoA to shikimate to form p-coumaroyl shikimate, and can be highly promiscuous [23–26]. For example, the A. thaliana HCT (AtHCT) uses nine substrates besides the native substrate shikimate – some better than shikimate (Figure 2C) – to produce a diversity of products in vitro. [24].
Crystallography and molecular dynamics analysis of AtHCT highlighted two mechanisms by which HCT maintains its in vivo function while being such a promiscuous enzyme [26]. First, comparison of AtHCT apoenzyme:substrate crystal structure and molecular dynamics revealed that this enzyme undergoes a conformational change upon p-coumaroyl-CoA or p-coumaroylshikimate binding: this reduces the volume of the active site and induces changes in the position of residues involved in substrate binding and catalysis [26]. Second, a conserved Arg ‘handle’ in the active site forms electrostatic interactions with the shikimate carboxyl group, orienting the 5-hydroxyl towards the catalytic center (Figure 2D) [26]. An HCT structure from Coleus blumei (CbHCT) was used to corroborate both features [26]. CbHCT was crystallized as a ternary complex with p-coumaroyl-CoA and 3-hydroxyacetophenone (Figure 2C), an uncharged non-native substrate [26]. The CbHCT structure revealed that the active site does not shrink when bound to 3-hydroxyacetophenone [26]. Furthermore, mutation of the Arg to Ala, Asp, or Glu caused a drastic decrease in activity with shikimate [26]. Conversely, those mutations increased activity with positively-charged non-native substrates such as 3,4-dihydroxybenzylamine and dopamine (Figure 2C), demonstrating that the Arg helps discriminate against some non-native substrates [26].
These two structural features play roles in substrate specificity with 3-hydroxyacetophenone or positively-charged substrates, but may not discriminate against substrates that are more structurally similar to shikimate. The Arg residue was shown to form electrostatic interactions with the shikimate carboxyl, discriminating against substrates lacking the carboxyl group [26]. Perhaps more interesting are the promiscuous activities of AtHCT [24]. AtHCT has activity with the non-native substrates gentisate and 3-hydroxyanthranilate, which share the negatively charged carboxyl group with shikimate (Fig 2). We hypothesize that the Arg handle – in combination with the carboxyl moiety in the promiscuous substrates – may facilitate activity with non-native substrates [24]. This is an example of how multiple structural elements of an enzyme – in this case the Arg handle and a dynamic conformation change – help maintain the primary activity despite numerous alternative reactions. These studies also highlight how the interplay of structural similarities of substrates and enzymatic features facilitate promiscuity. Discovery of more mechanisms that enable enzymes to maintain a primary metabolic activity despite being promiscuous will lead to general principles that can guide efforts to engineer specialized metabolic enzymes.
How promiscuity allows 4-coumarate:CoA ligase to catalyze multiple steps in phenylpropanoid biosynthesis
4-coumarate:CoA ligase (4CL) catalyzes multiple steps in the biosynthesis of phenylpropanoids including structural lignins and defensive anti-microbial compounds (Figure 3D). Promiscuity plays a central role in the ability of the enzyme to conjugate at least three different hydroxylated and methylated forms of hydroxycinnamic acids to coenzyme A in multiple plant species (Figure 3A) [27–29]. Two 4CL crystal structures inform our understanding of substrate promiscuity [30,31]. The Populus tomentosa 4CL1 structure revealed a largely hydrophobic hydroxycinnamate binding pocket [30]. Studies of Nicotiana tabacum 4CL2 complexed with feruloyl-adenylate confirmed the existence of this hydrophobic binding pocket, and revealed how it accommodates hydroxycinnamic acids of varying structure [31]. This binding flexibility is mediated by interaction with a tyrosine residue responsible for ring stacking with the substrate phenyl ring (Figure 3B). The enzyme binding pocket accommodating the hydroxycinnamoyl moiety is spacious at one of the meta positions on the phenyl ring and constrained at the other, which accommodates the hydroxy and methoxy groups in caffeic and ferulic acid, respectively (Figure 3C, red arrow shows constrained region and blue arrow identifies the spacious area). A serine residue in the active site hydrogen bonds with the para hydroxyl present in many monolignols (Figure 3B). These combined structural features accommodate diverse modified substrates allowing 4CL to catalyze multiple steps in phenylpropanoid metabolism.
Figure 3. 4-coumarate ligase characteristics that impact product diversity.
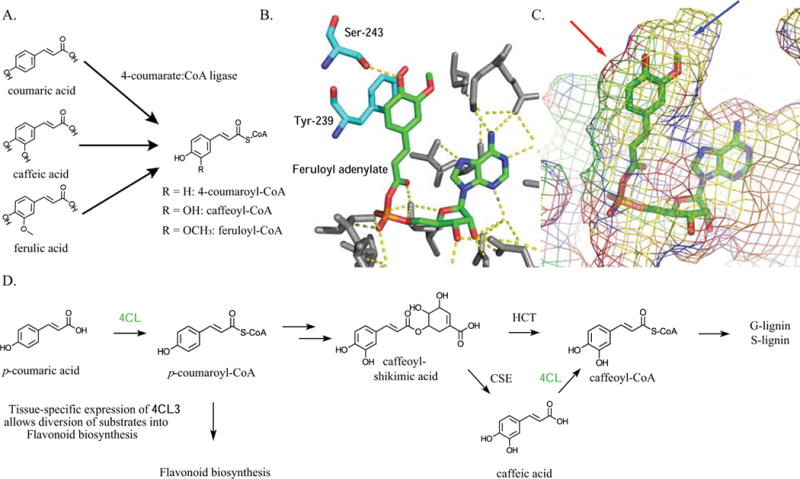
A) At4CL utilizes substrates with hydrogen or hydroxyl and O-methyl substitution at the meta position of the aromatic ring. B) Three-dimensional representation of the active site residue interactions with feruloyl-adenylate from the N. tabacum 4CL2 co-crystal structure. Feruloyl-adenylate backbone is in green, while residues that interact with the feruloyl moiety are cyan. The red color represents oxygen atoms, the blue color represents nitrogen atoms, and the orange represents phosphorus atoms [PDB ID: 5BSV]. C) Mesh model of active site around feruloyl-adenylate. Presences or absence of steric hindrance in the active site allows substitution at one of the meta positions on the ring, but not the other. Red arrow indicates the meta position that is blocked by steric hindrance, while the blue arrow points to position at which substitutions are permitted by the enzyme [PDB ID: 5BSV]. D) Representation of the phenylpropanoid biosynthetic pathway impacted by 4CL activity. 4CL is involved in biosynthetic steps colored green [32].
Four isoforms of 4CL in A. thaliana use multiple hydroxycinnamate substrates in vitro and have distinct – but overlapping – roles [27,32,33]. Recent work suggests that one or more of the At4CL isoforms – in addition to ligating 4-coumaric acid to CoA – catalyzes the formation of caffeoyl-CoA, and this appears to be relevant in vivo, representing a later biosynthetic step towards guaiacyl and syringyl lignin (Figure 3D) [32,34]. The action of 4CL in multiple biosynthetic steps is conferred by the catalytic flexibility provided by promiscuous activities. In addition, At4CL3 is involved in flavonoid biosynthesis, which shares the common intermediate – 4-coumaroyl-CoA – with the lignin pathway, but uses different enzymes to synthesize their downstream products (Figure 3D). Flavonoids and lignins accumulate in different tissues, and differential expression of At4CL3 – along with the downstream flavonoid enzyme genes – diverts metabolites away from the lignin pathway, towards flavonoid biosynthesis [32,35]. 4CL illustrates how a combination of promiscuity, gene duplication, and divergence in gene expression potentiate the diverse roles that 4CL plays in phenylpropanoid biosynthesis.
Promiscuity in Salicylic and Benzoic acid methyltransferase evolution
Salicylic Acid (SA) and Benzoic Acid (BA) methyltransferases provide another example where promiscuity plays a role in enzyme function and evolution. These enzymes are in the SABATH (SAMT, BAMT, and Theobromine synthase) methyltransferase family, involved in the methylation of structurally diverse specialized metabolites [36]. BA and SA differ by the presence of a 2-hydroxyl group on the aromatic ring (Figure 4A), and the enzymes catalyzing methylation of BA and SA are promiscuous [37,38]. The volatile methylated benzenoids are produced in differing ratios across characterized plant groups [39–41], and have ecological roles including insect pollinator attraction [1]. The enzyme responsible for SA methylation in Clarkia breweri is promiscuous, possessing activities with BA, 3-hydroxybenzoic acid, and cinnamic acid substrates (69%, 2%, and 2% of SA activity, respectively) [42].
Figure 4. Promiscuity in SAMTs and BSMTs is facilitated by substrate structural similarity.
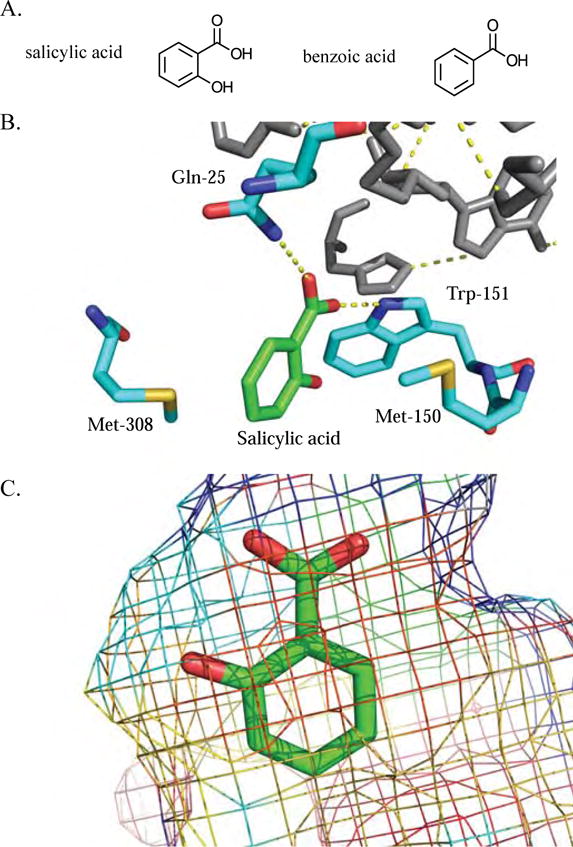
A) SA and BA have structures that differ by a single hydroxyl at the 2 position. B) Three-dimensional representation of C. breweri SAMT active site interactions with SA. Salicylic acid is colored green, while the interacting active site residues are colored cyan. The red color represents oxygen atoms, the blue represent nitrogen atoms, and the yellow represent sulfur atoms [PDB ID: 1M6E]. C) Mesh representation of the C. breweri SAMT active site demonstrating the steric environment around SA [PDB ID: 1M6E].
The structural determinants that facilitate SA carboxyl methyltransferase promiscuity — including those involved in the binding of C. breweri SAMT (CbSAMT) to SA — were explored by X-ray crystallographic analysis of CbSAMT [43]. Methionine residues at positions 150 and 308 form a clamp on both faces of the SA benzyl ring, and the side chain nitrogens of Gln-25 and Trp-151 form hydrogen bonds with the SA carboxylate moiety (Figure 4B) [43]. The SA 2-hydroxyl moiety is not close enough to any active site residues or water molecules to form hydrogen bonds; thus there are no obvious enzyme structural elements that would discriminate between BA and SA [43]. However, the 2-hydroxyl forms an intramolecular hydrogen bond with the carboxylate group of SA, which restricts its movement, potentially influencing SA and BA activities [43]. Finally, the remaining active site residues sterically restrain the SA substrate, allowing for methylation of the carboxylate to occur (Figure 4C) [43]. This example illustrates how similarity of the favored substrate and structural variants can influence promiscuity. These binding site structural elements can accommodate both SA and BA, and this promiscuity appears to have influenced the evolutionary history of a clade of SABATH methyltransferases [44].
A combination of phylogenetic analysis, ancestral sequence reconstruction, and enzyme assays was used to infer that the major activity of the enzyme switched between BA and SA methylation two times during evolution of the clade that includes SAMTs and BA/SA carboxyl methyltransferases from plants in the Apocynaceae and Solanaceae families [44]. A unique feature of this integrative study is that ancestral sequence reconstruction was used to predict and test the enzymatic activity of extinct specialized metabolic enzymes at different nodes in the evolutionary history of this methyltransferase clade [44]. All predicted ancestral enzymes tested possessed some level of activity with both BA and SA [44]. These results led to the hypothesis that two shifts in substrate preference occurred over time, with minor promiscuous activities of ancestral enzymes emerging as the primary activity of descendants [44]. In this example, the promiscuity of these different ancestral enzymes potentiated the shifts in enzymatic activity. This work elegantly demonstrates evolution acting on minor activities of promiscuous enzymes, leading to new major enzyme activities [44].
Conclusion
The examples in this review illustrate multiple ways in which plant specialized metabolic enzymes generate product diversity and metabolic flexibility. Substrate promiscuity, changes in enzyme activity, gene duplication as well as changes in gene expression all provide potentiating environments [6] for the evolution of metabolic novelty. In addition to its importance in understanding evolution of protein form and function, a deeper appreciation of the structural basis of simple changes in substrate specificity (Figure 1C) and how promiscuous enzymes maintain a primary activity despite the ability to utilize one or more alternate substrates should inform protein engineering and synthetic biology approaches.
Highlights.
Promiscuity plays a role in the evolution of specialized metabolic enzymes and metabolic product diversity
Changes in substrate specificity can result in the evolution of novel enzyme activities and pathways
Gene duplications potentiate specialized metabolic enzyme evolution
Substrate structural similarity affects enzyme promiscuity
Acknowledgments
We thank Gaurav Moghe, Pengxiang Fan, and Daniel Lybrand for helpful discussions and suggestions.
Funding
Work on specialized metabolism in the Last laboratory was funded by U.S. National Science Foundation Grants IOS-1025636 and IOS-1546617. B.L. was supported by a Fellowship from the Plant Biotechnology for Health and Sustainability Graduate Training Program supported by the U.S. National Institutes of Health training grant T32–GM110523.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest. This manuscript is not under consideration elsewhere.
References
- 1.Knudsen JT, Tollsten L, Bergström LG. Floral scents—a checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 1993;33:253–280. [Google Scholar]
- 2.Mithöfer A, Boland W. Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol. 2012;63:431–450. doi: 10.1146/annurev-arplant-042110-103854. [DOI] [PubMed] [Google Scholar]
- 3.Dixon RA, Strack D. Phytochemistry meets genome analysis, and beyond. Phytochemistry. 2003;62:815–816. doi: 10.1016/s0031-9422(02)00712-4. [DOI] [PubMed] [Google Scholar]
- 4.Milo R, Last RL. Achieving diversity in the face of constraints: lessons from metabolism. Science. 2012;336:1663–1667. doi: 10.1126/science.1217665. [DOI] [PubMed] [Google Scholar]
- 5.Weng J-K, Philippe RN, Noel JP. The rise of chemodiversity in plants. Science. 2012;336:1667–1670. doi: 10.1126/science.1217411. [DOI] [PubMed] [Google Scholar]
- 6*.Blount ZD, Barrick JE, Davidson CJ, Lenski RE. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature. 2012;489:513–518. doi: 10.1038/nature11514. Summary: A long term evolutionary approach identifies metabolic changes leading to the evolution of aerobic citrate utilization in Escherichia coli. Identifies potentiation, actualization, and refinement as the three stages of the model for citrate utilization evolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moghe GD, Last RL. Something old, something new: conserved enzymes and the evolution of novelty in plant specialized metabolism. Plant Physiol. 2015;169:1512–1523. doi: 10.1104/pp.15.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- 9.Sønderby IE, Geu-Flores F, Halkier BA. Biosynthesis of glucosinolates–gene discovery and beyond. Trends Plant Sci. 2010;15:283–290. doi: 10.1016/j.tplants.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Mawhinney TP, Preuss ML, Schroeder AC, Chen B, Abraham L, Jez JM, Chen S. A redox-active isopropylmalate dehydrogenase functions in the biosynthesis of glucosinolates and leucine in Arabidopsis. Plant J. 2009;60:679–690. doi: 10.1111/j.1365-313X.2009.03990.x. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Chen L, Zhou Y, Mawhinney TP, Chen B, Kang B-H, Hauser BA, Chen S. Functional characterization of Arabidopsis thaliana isopropylmalate dehydrogenases reveals their important roles in gametophyte development. New Phytol. 2011;189:160–175. doi: 10.1111/j.1469-8137.2010.03460.x. [DOI] [PubMed] [Google Scholar]
- 12**.Lee SG, Nwumeh R, Jez JM. Structure and mechanism of isopropylmalate dehydrogenase from Arabidopsis thaliana: Insights on leucine and aliphatic glucosinolate biosynthesis. J Biol Chem. 2016;291:13421–13430. doi: 10.1074/jbc.M116.730358. Summary: Structural analysis of AtIPMDH2 interactions with 3-isopropylmalate. Identifies several residues in the active site responsible for enzymatic activity of AtIPMDH2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Y, Galant A, Pang Q, Strul JM, Balogun SF, Jez JM, Chen S. Structural and functional evolution of isopropylmalate dehydrogenases in the leucine and glucosinolate pathways of Arabidopsis thaliana. J Biol Chem. 2011;286:28794–28801. doi: 10.1074/jbc.M111.262519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St-Pierre B, De Luca V. Evolution of acyltransferase genes. Recent Adv Phytochem. 2000;34:285–315. [Google Scholar]
- 15.Weinhold A, Baldwin IT. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc Natl Acad Sci U S A. 2011;108:7855–7859. doi: 10.1073/pnas.1101306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luu VT, Weinhold A, Ullah C, Dressel S, Schoettner M, Gase K, Gaquerel E, Xu S, Baldwin IT. O-acyl sugars protect a wild tobacco from both native fungal pathogens and a specialist herbivore. Plant Physiol. 2017 doi: 10.1104/pp.16.01904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leckie BM, D’Ambrosio DA, Chappell TM, Halitschke R, De Jong DM, Kessler A, Kennedy GG, Mutschler MA. Differential and synergistic functionality of acylsugars in suppressing oviposition by insect herbivores. PLoS One. 2016;11:1–19. doi: 10.1371/journal.pone.0153345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Fan P, Miller AM, Schilmiller AL, Liu X, Ofner I, Jones AD, Zamir D, Last RL. In vitro reconstruction and analysis of evolutionary variation of the tomato acylsucrose metabolic network. Proc Natl Acad Sci U S A. 2016;113:1–10. doi: 10.1073/pnas.1517930113. Summary: Characterization of the acylsugar biosynthetic network in cultivated and wild tomatoes. Describes use of a phylogenetic framework with homology modeling to identify an active site residue that impacts acyl-CoA substrate promiscuity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilmiller AL, Charbonneau AL, Last RL. Identification of a BAHD acetyltransferase that produces protective acyl sugars in tomato trichomes. Proc Natl Acad Sci U S A. 2012;109:16377–16382. doi: 10.1073/pnas.1207906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schilmiller AL, Moghe GD, Fan P, Ghosh B, Ning J, Jones AD, Last RL. Functionally divergent alleles and duplicated loci encoding an acyltransferase contribute to acylsugar metabolite diversity in Solanum trichomes. Plant Cell. 2015;27:1002–1017. doi: 10.1105/tpc.15.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan P, Moghe GD, Last RL. Comparative biochemistry and in vitro pathway reconstruction as powerful partners in studies of metabolic diversity. Methods Enzymol. 2016;576:1–17. doi: 10.1016/bs.mie.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh B, Westbrook TC, Jones AD. Comparative structural profiling of trichome specialized metabolites in tomato (Solanum lycopersicum) and S. habrochaites: acylsugar profiles revealed by UHPLC/MS and NMR. Metabolomics. 2013;10:496–507. doi: 10.1007/s11306-013-0585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann L, Maury S, Martz F, Geoffroy P, Legrand M. Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J Biol Chem. 2003;278:95–103. doi: 10.1074/jbc.M209362200. [DOI] [PubMed] [Google Scholar]
- 24.Eudes A, Pereira JH, Yogiswara S, Wang G, Teixeira Benites V, Baidoo EEK, Lee TS, Adams PD, Keasling JD, Loqué D. Exploiting the substrate promiscuity of hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase to reduce lignin. Plant Cell Physiol. 2016;57:568–579. doi: 10.1093/pcp/pcw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sander M, Petersen M. Distinct substrate specificities and unusual substrate flexibilities of two hydroxycinnamoyltransferases, rosmarinic acid synthase and hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl-transferase, from Coleus blumei Benth. Planta. 2011;233:1157–1171. doi: 10.1007/s00425-011-1367-2. [DOI] [PubMed] [Google Scholar]
- 26**.Levsh O, Chiang Y-C, Tung CF, Noel JP, Wang Y, Weng J-K. Dynamic conformational states dictate selectivity toward the native substrate in a substrate-permissive acyltransferase. Biochemistry. 2016;55:6314–6326. doi: 10.1021/acs.biochem.6b00887. Summary: Characterization of AtHCT structural elements involved in interactions with shikimate. Illustrates elements of AtHCT and other orthologs that play roles in maintaining catalytic activity with shikimate despite promiscuity of the enzyme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehlting J, Büttner D, Wang Q, Douglas CJ, Somssich IE, Kombrink E. Three 4-coumarate:coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. Plant J. 1999;19:9–20. doi: 10.1046/j.1365-313x.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 28.Hu WJ, Kawaoka A, Tsai CJ, Lung J, Osakabe K, Ebinuma H, Chiang VL. Compartmentalized expression of two structurally and functionally distinct 4- coumarate:CoA ligase genes in aspen (Populus tremuloides) Proc Natl Acad Sci USA. 1998;95:5407–5412. doi: 10.1073/pnas.95.9.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee D, Douglas CJ. Two divergent members of a tobacco 4-coumarate:coenzyme A ligase (4CL) gene family. Plant Physiol. 1996;112:193–205. doi: 10.1104/pp.112.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Gai Y, Yin L, Wang X, Feng C, Feng L, Li D, Jiang X-N, Wang D-C. Crystal structures of a Populus tomentosa 4-coumarate:CoA ligase shed light on its enzymatic mechanisms. Plant Cell. 2010;22:3093–3104. doi: 10.1105/tpc.109.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Li Z, Nair SK. Structural basis for specificity and flexibility in a plant 4-coumarate:CoA ligase. Structure. 2015;23:2032–2042. doi: 10.1016/j.str.2015.08.012. Summary: Describes the structural factors in Nt4CL2 involved in binding interactions with feruloyl-adenylate. Demonstrates that the deletion of a single amino acid results in broadening of the substrate profile. [DOI] [PubMed] [Google Scholar]
- 32*.Li Y, Kim JI, Pysh L, Chapple C. Four isoforms of Arabidopsis 4-coumarate:CoA ligase have overlapping yet distinct roles in phenylpropanoid metabolism. Plant Physiol. 2015;169:2409–2421. doi: 10.1104/pp.15.00838. Summary: Characterization of At4CL isoforms involved in phenylpropanoid metabolism. Uses 4CL isoform mutants to characterize their in vivo roles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamberger B, Hahlbrock K. The 4-coumarate:CoA ligase gene family in Arabidopsis thaliana comprises one rare, sinapate-activating and three commonly occurring isoenzymes. Proc Natl Acad Sci U S A. 2004;101:2209–14. doi: 10.1073/pnas.0307307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanholme R, Cesarino I, Rataj K, Xiao Y, Sundin L, Goeminne G, Kim H, Cross J, Morreel K, Araujo P, et al. Caffeoyl shikimate esterase (CSE) is an enzyme in the lignin biosynthetic pathway in Arabidopsis. Science. 2013;341:1103–1106. doi: 10.1126/science.1241602. [DOI] [PubMed] [Google Scholar]
- 35.Dobritsa A, Geanconteri A, Shrestha J, Carlson A, Kooyers N, Coerper D, Urbanczyk-Wochniak E, Bench BJ, Sumner L, Swanson R, et al. A large-scale genetic screen in Arabidopsis to identify genes involved in pollen exine production. Plant Physiol. 2011;157:947–970. doi: 10.1104/pp.111.179523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao N, Ferrer J-L, Ross J, Guan J, Yang Y, Pichersky E, Noel JP, Chen F. Structural, biochemical, and phylogenetic analyses suggest that indole-3-acetic acid methyltransferase is an evolutionarily ancient member of the SABATH family. Plant Physiol. 2008;146:455–467. doi: 10.1104/pp.107.110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pott MB, Hippauf F, Saschenbrecker S, Chen F, Ross J, Kiefer I, Slusarenko A, Noel JP, Pichersky E, Effmert U, et al. Biochemical and structural characterization of benzenoid carboxyl methyltransferases involved in floral scent production in Stephanotis floribunda and Nicotiana suaveolens. Plant Physiol. 2004;135:1946–1955. doi: 10.1104/pp.104.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hippauf F, Michalsky E, Huang R, Preissner R, Barkman TJ, Piechulla B. Enzymatic, expression and structural divergences among carboxyl O-methyltransferases after gene duplication and speciation in Nicotiana. Plant Mol Biol. 2010;72:311–30. doi: 10.1007/s11103-009-9572-0. [DOI] [PubMed] [Google Scholar]
- 39.Altenburger R, Matile P. Circadian rhythmicity of fragrance emission in flowers of Hoya carnosa R. Br. Planta. 1988;174:248–252. doi: 10.1007/BF00394778. [DOI] [PubMed] [Google Scholar]
- 40.Loughrin JH, Hamilton-Kemp TR, Andersen RA, Hildebrand DF. Headspace compounds from flowers of Nicotiana tabacum and related species. J Agric Food Chem. 1990;38:455–460. [Google Scholar]
- 41.Pott MB, Pichersky E, Piechulla B. Evening specific oscillations of scent emission, SAMT enzyme activity, and SAMT mRNA in flowers of Stephanotis floribunda. J Plant Physiol. 2002;159:925–934. [Google Scholar]
- 42.Ross JR, Nam KH, D’Auria JC, Pichersky E. S-adenosyl-L-methionine:salicylic acid carboxyl methyltransferase, an enzyme involved in floral scent production and plant defense, represents a new class of plant methyltransferases. Arch Biochem Biophys. 1999;367:9–16. doi: 10.1006/abbi.1999.1255. [DOI] [PubMed] [Google Scholar]
- 43*.Zubieta C, Ross JR, Koscheski P, Yang Y, Pichersky E, Noel JP. Structural basis for substrate recognition in the salicylic acid carboxyl methyltransferase family. Plant Cell. 2003;15:1704–1716. doi: 10.1105/tpc.014548. Summary: Structural analysis of Clarkia breweri SAMT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Huang R, Hippauf F, Rohrbeck D, Haustein M, Wenke K, Feike J, Sorrelle N, Piechulla B, Barkman TJ. Enzyme functional evolution through improved catalysis of ancestrally nonpreferred substrates. Proc Natl Acad Sci U S A. 2012;109:2966–2971. doi: 10.1073/pnas.1019605109. Summary: Examines evolution of a clade of salicylic acid and benzoic acid methyltransferases. Focuses on phylogenetic reconstruction of different evolutionary nodes leading to a model for salicylic acid/benzoic acid methyltransferase evolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]


