Abstract
In diabetic retinopathy, high glucose (HG)-mediated breakdown in cell-cell communication promotes disruption of retinal homeostasis. Several studies indicate that HG condition alters expression of connexin genes and subsequent gap junction intercellular communication (GJIC) in retinal vascular cells and non-vascular cells. A serious consequence of disrupted cell-cell communication is apoptosis and breakdown of the blood-retinal barrier (BRB). More recently, studies suggest adverse effects from HG on retinal Müller cells. This article focuses on HG-mediated changes in connexin expression and GJIC and their subsequent effects on the breakdown of retinal homeostasis, cell death, compromised vascular permeability, and interactions between endothelial cells, pericytes and retinal Müller cells in the pathogenesis of diabetic retinopathy. Additionally, options for rectifying disrupted homeostasis under HG condition associated with diabetic retinopathy are reviewed.
Keywords: connexins, gap junctions, diabetic retinopathy
1. Introduction
Connexins and gap junction intercellular communication in diabetic retinopathy
Gap junction intercellular communication (GJIC) is mediated by docking of connexin hemichannels on two adjacent cells to form conduits that allow communication of electrical signals between cells and the exchange of molecules that are less than 1 kD (Nielsen, Nygaard Axelsen, Sorgen, Verma, Delmar & Holstein-Rathlou, 2012, Wright, Richards & Becker, 2012). GJIC plays a vital role in maintaining tissue homeostasis and regulating cell survival (Dagli & Hernandez-Blazquez, 2007, De Maio, Vega & Contreras, 2002, Li, Mruk & Cheng, 2012, Wei, Xu & Lo, 2004). Currently, twenty-one connexin isoforms have been identified in the human genome (Nielsen et al., 2012), which are characterized on the basis of their molecular weights (Nielsen et al., 2012). These connexins can form homomeric, homotypic, heteromeric and heterotypic gap junctions depending on the type and composition of the connexon subunits involved. Studies have identified different types of connexins that interact with each other in the eye (Vaney & Weiler, 2000). It is also well-known that cell death is a characteristic retinal lesion in early stages of diabetic retinopathy (Chistiakov, 2011). Studies report that connexin-mediated GJIC activities contribute to cell survival by allowing cells to exchange ions, metabolites, secondary messengers, energy molecules, glucose, ATP, and other free radical scavengers though gap junctions (Decrock, Vinken, De Vuyst, Krysko, D’Herde, Vanhaecke, Vandenabeele, Rogiers & Leybaert, 2009). These findings underscore the importance of cell-cell coupling in regulation of cell growth, differentiation, development (Kojima, Nakahama, Ohno-Matsui, Shimada, Mori, Iseki, Sato, Mochizuki & Morita, 2008, Wei et al., 2004) and cell survival (Plotkin, Manolagas & Bellido, 2002). Importantly, connexin expression and subsequent GJIC activity are downregulated by HG condition contributing to retinal vascular cell loss and other retinal lesions associated with diabetic retinopathy (Fernandes, Girao & Pereira, 2004, Li & Roy, 2009, Li, Sato, Haimovici, Okamoto & Roy, 2003, Sato, Haimovici, Kao, Li & Roy, 2002). Taken together, connexins play an integral role in maintaining homeostasis of the retinal microenvironment. Therefore, defining this exact role in the context of diabetic retinopathy is of significant importance.
2. Connexin Structure
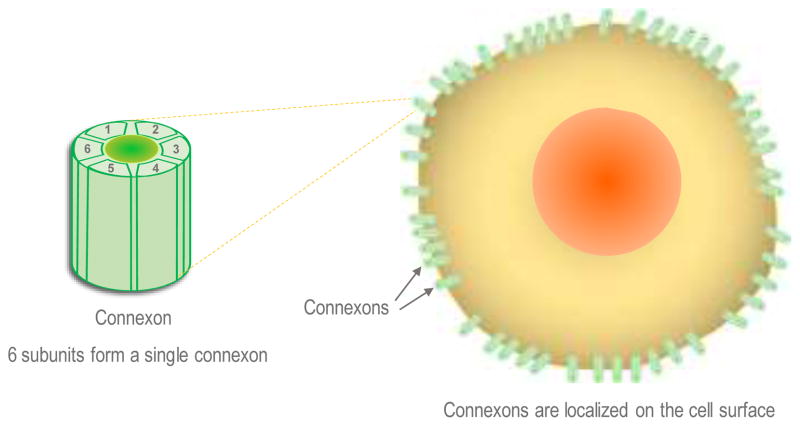
The connexin polypeptides oligomerize into a six-subunit structure known as a connexon (Cottrell & Burt, 2005). These connexons span from the cellular cytoplasmic space through the plasma membrane and out to the extracellular space. The two extracellular loops in Cx43 contain three cysteines, which are required for the docking of two hemichannels (Stains & Civitelli, 2005). The resultant structure created by the two docked hemichannels is a gap junction (Cottrell & Burt, 2005, Stains & Civitelli, 2005), which can be of four types, homomeric, heteromeric, homotypic or heterotypic as described in the next section. Schemes outlining the structure, cellular location, and docking of connexin channels are presented in Figures 1, 2.
Figure 1. Structure and localization of connexons.
Each connexon is a hexameric channel comprised of six homomeric or heteromeric subunits. Connexons are localized on the cell surface; each connexon exhibits a cytoplasmic domain, a transmembrane domain, and an extracellular domain. The extracellular domain from two connexons play a critical role in docking and represent the “gap” junction or the space between the two cells where connexons form a connexin channel.
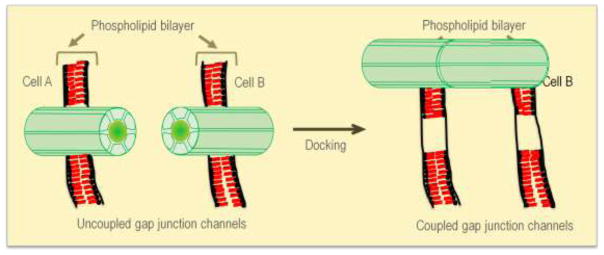
Figure 2. Docking of connexons leads to coupled junctions.
Gap junction intercellular communication is dependent on successful docking of two connexons located on two adjacent cells. When connexons present on the cell surface of one cell dock with connexons present on the cell surface of an adjacent cell it can lead to interlocking of the extracellular domains of each connexon allowing the formation of a gap junction connexin channel between two adjacent cells. These channels allow exchange of small molecules and ions permitting cell to cell communication.
Homotypic and heterotypic gap junctions
Hemichannels consisting of one connexin isoform are referred to as homomeric, while hemichannels formed from multiple connexin isoforms are referred to as heteromeric. The docking of two identical hemichannels results in a homotypic gap junction, whereas the docking of two non-identical hemichannels results in a heterotypic gap junction (Cottrell & Burt, 2005). Both homotypic and heterotypic gap junctions are implicated in disease processes. The physiological properties of heterotypic channels in which the 12 subunits are not the same connexin, differ in their properties of intercellular communication. There are several reports of intercellular channels that are formed from heterotypic connexins including in the retina, glial cells in the CNS, cardiomyocytes, and HeLa cells (Altevogt & Paul, 2004, Guldenagel, Sohl, Plum, Traub, Teubner, Weiler & Willecke, 2000, Martinez, Hayrapetyan, Moreno & Beyer, 2002, Severs, Coppen, Dupont, Yeh, Ko & Matsushita, 2004, Sohl, Guldenagel, Traub & Willecke, 2000). In the retinal vascular cells, Cx43 is abundantly present but Cx37 and Cx40 are also expressed. In different tissues including retina, heterotypic gap junctions have been reported to be present involving Cx37, Cx40, and Cx43.
3. Connexin expression in different retinal cell types
Various types of connexins are expressed in the retina including Cx37 (Guldenagel et al., 2000), Cx40 (Matesic, Tillen & Sitaramayya, 2003), Cx43 (De Maio et al., 2002), and Cx30.2 (Manasson, Tien, Moore, Kumar & Roy, 2013) which are associated with the retinal microvasculature. Aside from these specific vascular connexins, the other types of connexins, which are localized and distributed in different retinal cell types are summarized in Tables 1 and 2. Of the three connexins, Cx37, Cx40, and Cx43 that are present on retinal endothelial cells and pericytes, Cx43 is the most abundant type (Janssen-Bienhold, Dermietzel & Weiler, 1998). Reduced GJIC in vascular cells of the retina have been shown to affect non-vascular cells such as retinal astrocytes and Müller cells. Tracer coupling assays performed in rat retinal astrocytes showed that they are not only coupled to each other but also to Müller cells (Zahs & Newman, 1997). Therefore, reduced GJIC in vascular cells can affect non-vascular cells and thereby compromise BRB homeostasis.
Table 1.
Types of cells present in the retina
| Cell Type | Significance and Retinal Location | References |
|---|---|---|
| Endothelial Cells | Surround the lumen of capillaries; networks concentrated within the superficial, intermediate, and deep plexuses | (Dorrell & Friedlander, 2006) (Hughes, Gardiner, Hu, Baxter, Rosinova & Chan-Ling, 2006) |
| Pericytes | Located abluminally on the outside of capillaries with several processes that extend and wrap around the vessel | (Hughes et al., 2006) (Hughes & Chan-Ling, 2004) (Hamilton, Attwell & Hall, 2010) |
| Müller Cells | Span most of the retinal tissue, extending from the NFL to the ONL, while enclosing many neuronal somata and processes | (Bringmann, Pannicke, Grosche, Francke, Wiedemann, Skatchkov, Osborne & Reichenbach, 2006) (Jadhav, Roesch & Cepko, 2009) (Willbold & Layer, 1998) |
| Astrocytes | Found within the innermost retinal layers, such as the NFL; are in contact with vessels in the superficial plexus as well as extend to the ILM | (Jammalamadaka, Suwannatat, Fisher, Manjunath, Hollerer & Luna, 2015) (Hollander, Makarov, Dreher, van Driel, Chan-Ling & Stone, 1991) |
| Rod Cells | Within the outer retinal photoreceptor layer backed by the pigment epithelium and choroid; the rod pathway involves both the IPL and OPL | (Bloomfield & Dacheux, 2001) (Kolb & Famiglietti, 1974) |
| Cone Cells | Within the outermost retinal layer backed by the pigment epithelium and choroid; cell bodies found within the ONL with axons extending into IPL | (Dacey & Packer, 2003) (Szel, Rohlich, Caffe & van Veen, 1996) |
| Ganglion Cells | Majority of somata constitute the ganglion cell layer with dendrites in the IPL; axons are found in the optic nerve | (Dacey, 1994) (Farah, 2006) (Tian, 1995) (Sernagor, Eglen & Wong, 2001) |
| Bipolar Cells | Cell bodies are found in the INL, axon terminals in IPL, and dendrites in OPL; transmits signals from photoreceptors to ganglion cells | (Eggers & Lukasiewicz, 2011) (Connaughton, 2011) |
| Amacrine Cells | Somata found in the INL but active in IPL; also occupy the ganglion cell layer with ganglions; dendrites and some displaced cells found in IPL | (Kolb, 1997) (Hartveit & Veruki, 2012) |
| Horizontal Cells | Overlay photoreceptor cells in the middle layers of the retina; has dendrites extending into the OPL | (Boije, Shirazi Fard, Edqvist & Hallbook, 2016) (Mariani & Leure-DuPree, 1977) (Poche & Reese, 2009) |
| Retinal Pigment Epithelium Cells | One of the most outer retinal layers just underneath the photoreceptor layer and superior to both Bruch’s membrane and the choroid | (Strauss, 1995) (Bonilha, 2014) |
Table 2.
Differential expression of connexins in retinal cell types affected in diabetic retinopathy
| Cell Type | Types of Connexins Present | Connexins Altered Under High Glucose/Diabetic Conditions | References |
|---|---|---|---|
| Endothelial Cells | Cx37 Cx40 Cx43 Cx30.2 |
Cx43 ↓ | (Fernandes, Girao & Pereira, 2004) (Manasson, Tien, Moore, Kumar & Roy, 2013) (Sato, Haimovici, Kao, Li & Roy, 2002) (Tien, Barrette, Chronopoulos & Roy, 2013) |
| Pericytes | Cx37 Cx40 Cx43 |
Cx43 ↓ | (Durham, Dulmovits, Cronk, Sheets & Herman, 2015) (Li, Sato, Haimovici, Okamoto & Roy, 2003) |
| Müller Cells | Cx43 | Cx43 ↓ | (Ball & McReynolds, 1998) (Bruckner, Grosche, Pannicke, Wiedemann, Reichenbach & Bringmann, 2012) (Muto, Tien, Kim, Sarthy & Roy, 2014) (Zahs & Ceelen, 2006) |
| Astrocytes | Cx26 Cx43 |
Cx26 ↓ Cx43 ↓ |
(Ly, Yee, Vessey, Phipps, Jobling & Fletcher, 2011) (Zahs & Ceelen, 2006) |
| Rod Cells | Cx36 | Unknown | (Deans, Volgyi, Goodenough, Bloomfield & Paul, 2002) (Dedek, Schultz, Pieper, Dirks, Maxeiner, Willecke, Weiler & Janssen-Bienhold, 2006) (Li, Zhang, Blackburn, Wang, Ribelayga & O’Brien, 2013) |
| Cone Cells | Cx36 | Unknown | (Li, Zhang, Blackburn, Wang, Ribelayga & O’Brien, 2013) |
| Ganglion Cells | Cx30.2 Cx36 Cx43 Cx45 |
Unknown | (Hansen, Torborg, Elstrott & Feller, 2005) (Hidaka, Kato & Miyachi, 2002) (Muller, Dedek, Janssen-Bienhold, Meyer, Kreuzberg, Lorenz, Willecke & Weiler, 2010) (Pan, Paul, Bloomfield & Volgyi, 2010) (Schubert, Degen, Willecke, Hormuzdi, Monyer & Weiler, 2005) (Sohl, Guldenagel, Traub & Willecke, 2000) |
| Bipolar Cells | Cx36 Cx45 |
Unknown | (Deans, Volgyi, Goodenough, Bloomfield & Paul, 2002) (Dedek, Schultz, Pieper, Dirks, Maxeiner, Willecke, Weiler & Janssen-Bienhold, 2006) (Hansen, Torborg, Elstrott & Feller, 2005) (Lin, Jakobs & Masland, 2005) (Maxeiner, Dedek, Janssen-Bienhold, Ammermuller, Brune, Kirsch, Pieper, Degen, Kruger, Willecke & Weiler, 2005) |
| Amacrine Cells | Cx36 Cx45 |
Unknown | (Deans, Volgyi, Goodenough, Bloomfield & Paul, 2002) (Dedek, Schultz, Pieper, Dirks, Maxeiner, Willecke, Weiler & Janssen-Bienhold, 2006) (Feigenspan, Teubner, Willecke & Weiler, 2001) (Hansen, Torborg, Elstrott & Feller, 2005) (Maxeiner, Dedek, Janssen-Bienhold, Ammermuller, Brune, Kirsch, Pieper, Degen, Kruger, Willecke & Weiler, 2005) (Mills, O’Brien, Li, O’Brien & Massey, 2001) (Urschel, Hoher, Schubert, Alev, Sohl, Worsdorfer, Asahara, Dermietzel, Weiler & Willecke, 2006) |
| Horizontal Cells | Cx50 Cx57 |
Unknown | (Cha, Kim, Pan, Chun, Massey & Kim, 2012) (Hombach, Janssen-Bienhold, Sohl, Schubert, Bussow, Ott, Weiler & Willecke, 2004) (Huang, Li & He, 2005) (O’Brien, Li, Pan, Keung, O’Brien & Massey, 2006) (Pan, Keung, Kim, Snuggs, Mills, O’Brien & Massey, 2012) |
| Retinal Pigment Epithelium Cells | Cx43 | Cx43 ↓ | (Janssen-Bienhold, Dermietzel & Weiler, 1998) (Losso, Truax & Richard, 2010) (Malfait, Gomez, van Veen, Parys, De Smedt, Vereecke & Himpens, 2001) |
4. Intercellular communication in the retina
Gap junctions and vascular homeostasis
The maintenance of retinal vascular homeostasis is dependent on proper GJIC activity. Studies performed in vitro (Larson, Carson & Haudenschild, 1987) and in vivo (Oku, Kodama, Sakagami & Puro, 2001) have revealed the presence of gap junctions between endothelial cells and pericytes. Several studies have established extensive GJIC in endothelial cells; however, there is limited information related to GJIC and its involvement in pericytes under HG condition (Li et al., 2003). Studies indicate that Cx43 proteins play a critical role in maintaining GJIC activity and vascular function (Liao, Day, Damon & Duling, 2001) especially as conduits for growth-modulating signals in cellular interactions during growth and development (Guthrie & Gilula, 1989, Reaume, de Sousa, Kulkarni, Langille, Zhu, Davies, Juneja, Kidder & Rossant, 1995).
4.1. Communication between retinal vascular cells
In the endothelium of the retinal vasculature as well as the pericytes in the abluminal surface, Cx37, Cx40, and Cx43 are expressed (Zahs & Ceelen, 2006). However, in HG condition, no significant changes in Cx37 or Cx40 protein expression were observed in either human or bovine retinal pericytes (Li et al., 2003). While there are various connexin isoforms, the most predominant connexin isoform expressed in the retina is Cx43 (Evans & Martin, 2002), which plays a primary role in facilitating GJIC in the retinal vasculature. It has been shown that HG condition and diabetes downregulate Cx43 expression in retinal endothelial cells, microvascular endothelial cells, and pericytes, leading to reduced GJIC activity and ultimately retinal endothelial cell and pericyte death, a hallmarks of diabetic retinopathy (Fernandes et al., 2004, Li & Roy, 2009, Li et al., 2003, Sato et al., 2002). Additionally, a study suggests that increased Cx43 degradation (Fernandes et al., 2004) and reduced GJIC may contribute to compromised endothelial cellular function related to BRB breakdown in diabetic retinopathy (Roy, Ha, Trudeau & Beglova, 2010). Our recent study has demonstrated that HG-induced downregulation of Cx43 expression and reduced GJIC may compromise expression levels of tight junction genes, occludin and ZO-1, and thereby contribute to the breakdown of retinal endothelial cell barriers (Tien, Barrette, Chronopoulos & Roy, 2013).
Research from our lab has also shown that HG-induced downregulation of mitochondrial Cx43 protein in retinal endothelial cells reduces Cx43-mediated GJIC activity and may contribute to mitochondrial fragmentation and the release of cytochrome c, indicating a hitherto unknown mechanism for HG-induced apoptosis in diabetic retinopathy (Trudeau, Muto & Roy, 2012). Another study from our laboratory has shown that connexin isotype Cx30.2 is downregulated in HG condition in rat retinal endothelial cells and subsequently reduces GJIC activity. In addition, diabetic rat retinas were also shown to exhibit lower Cx30.2 expression which was concomittant with an increase in the number of acellular capillaries and pericyte loss (Manasson et al., 2013). More recently, we have demonstrated that diabetes-induced Cx43 downregulation promotes vascular cell loss, such as acellular capillaries and pericyte loss, and excess permeability in the diabetic rat retina (Tien, Muto, Barrette, Challyandra & Roy, 2014), suggesting that Cx43 not only regulates cell survival but also facilitates, at least in part, the maintenance of BRB integrity. Furthermore, the retinal microvasculature of streptozotocin (STZ)-induced diabetic rats exhibited reduced cell-cell coupling in pericytes, which was assessed by immunohistochemical analysis of intercellular spread of neurobiotin, a gap junction-permeant tracer molecule (Oku et al., 2001). In contrast, microvessels of insulin-treated diabetic rats showed no significant loss of intercellular communication, suggesting that hyperglycemia alone appears to be a causative factor for loss of GJIC. Overall, these findings emphasize the importance of maintaining connexin-mediated retinal vascular homeostasis in the diabetic retina.
4.2. Communication between retinal vascular cells and glial cells
It has been well established that diabetes alters the retinal vasculature. However, the retina has two major components: vascular and neuronal components. Studies have demostrated that retinal Müller cells, the principal glial cell responsible for maintaining retinal homeostasis, astrocytes, and microglial cells are altered in diabetic retinopathy. Müller cells play a critical role in providing metabolic substrates to neurons, deactivating and facilitating recycling of neurotransmitters, thus enabling the maintenance of ion balance in the retina (Fletcher, Phipps & Wilkinson-Berka, 2005). Retinal Müller cells regulate degradation of glutamate and GABA (Fletcher et al., 2005). Interestingly, Cx43 deficiency was shown to affect the expression of GLAST, which facilitates glutamate transport into Müller cells, thereby participating in promoting apoptosis (Unger, Bette, Zhang, Theis & Engele, 2012). Our research has shown that HG downregulated Cx43 expression in retinal Müller cells and subsequently reduced GJIC activity, leading to Müller cell loss (Muto, Tien, Kim, Sarthy & Roy, 2014). Importantly, HG-induced Cx43 downregulation in retinal Müller cells co-cultured with healthy pericytes led to the impairment of GJIC that ultimately contributed to apoptosis not only in retinal Müller cells but in pericytes as well, emphasizing the importance of cell-cell communication via Cx43 coupling in retinal Müller cell and pericyte survival (Muto et al., 2014). Taken together, the survival of endothelial cells and pericytes in the diabetic retina may be influenced through GJIC activity between the vascular cells and Müller cells.
Connexins in the retinal astrocytes are also influenced by diabetes-induced insult. Retinal astrocytes facilitate retinal neuronal and blood vessel functions. A study indicated that changes in astrocytes due to retinal hypoxia developed earlier than Müller cell gliosis in the pathogenesis of diabetic retinopathy (Ly, Yee, Vessey, Phipps, Jobling & Fletcher, 2011). In addition, 4 weeks of streptozotocin-induced diabetes decreased Cx26 and Cx43 expression in retinal astrocytes, leading to significant astrocyte loss and reduced astrocyte processes (Ly et al., 2011). Interestingly, rat primary astrocytes transfected with Cx43 siRNA and exposed to hydrogen peroxide exhibited a significant increase in the number of apoptotic cells compared to that of cells transfected with scrambled siRNA as control, suggesting that endogenous Cx43 expression confers resistance to hydrogen peroxide-mediated apoptosis (Giardina, Mikami, Goubaeva & Yang, 2007). These findings demonstrate that cell-cell communication between astrocytes is essential for their survival and may play a protective role against diabetes-induced apoptosis.
4.3. Communication between retinal neuronal cells
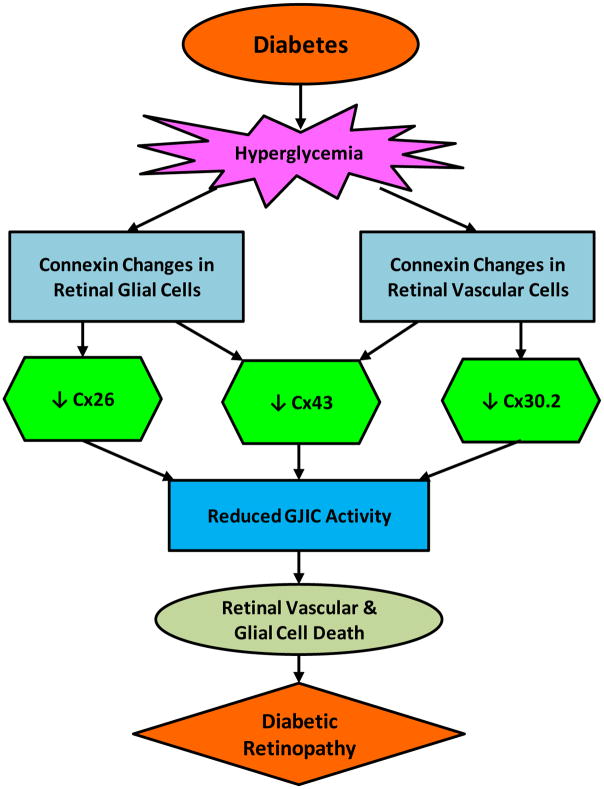
A few studies investigating the interaction of connexins in retinal neuronal cells such as horizontal cells, bipolar cells, amacrine cells, ganglion cells, and photoreceptor cells in diabetic retinopathy are available. However, it is well established that connexins play a key role in retinal neuronal homeostasis. In each of the five retinal neuron types, diverse connexin-mediated electrical synapses have been identified in which gap junctions play a role (Bloomfield & Volgyi, 2009). Interestingly, ganglion cell-amacrine cell coupling is most common for GJIC involving the inner retina (Bloomfield & Volgyi, 2009). Recent research has reported that Cx30.2, a novel gap junction protein in the retinal neurons, facilitates cell-cell coupling between retinal ganglion cell and displaced amacrine cells (Muller, Dedek, Janssen-Bienhold, Meyer, Kreuzberg, Lorenz, Willecke & Weiler, 2010). Although it is unknown whether expression of Cx30.2 is altered in HG condtion in the retinal neuronal cells, it may be plausible since our previous study has shown that HG induces Cx30.2 downregulation in retinal endothelial cells (Manasson et al., 2013). Additionally, a study investigated cell-cell coupling of electrical synapses in ganglion cell subtypes where cellular Cx36 deletion impacted one half of the subtypes and resulted in substantial decrease in most ganglion cell-amacrine cell coupling (Pan, Paul, Bloomfield & Volgyi, 2010). These results indicate that Cx36 and Cx30.2 may be necessary for heterologous coupling and are important for the maintenance of cell-cell communication and exchange of electrical signaling via GJIC between amacrine cells and ganglion cells. Another study showed that cell-cell coupling between retinal amacrine cells is required for synchronized firing of action potentials of retinal ganglion cells (Kenyon & Marshak, 1998), emphasizing the importance of GJIC activity in maintaining homeostasis in retinal neurons. These findings indicate that compromised connexin-mediated cell-cell communication in retinal neuronal activity could contribute to the pathogenesis of diabetic retinopathy. An overview of HG-induced connexin changes in the retinal vasculature and glia in the context of diabetic retinopathy is presented in Figure 3.
Figure 3. High glucose (HG)-induced connexin changes in the retinal vascular and glial cells.
Retinal glial cells undergo Cx26 and Cx43 downregulation whereas retinal vascular cells undergo Cx43 and Cx30.2 downregulation. HG-induced connexin downregulation in the retina leads to a decrease in GJIC activity, promoting cell loss, ultimately contributing to vision loss in diabetic retinopathy.
5. Pannexin and connexin channels
Pannexins form single-membrane channels, and one of their primary functions is apoptotic cell clearance. Connexons are hemichannels present on the cell surface and represent half of the gap junction channel. When two connexons dock, they form an active gap junction connexin channel. Like connexons, pannexins are present on the cell surface but unlike connexons, pannexins do not dock, and are functionally active on their own. A recent study reported decreased level of pannexin 1 in osteocytes exposed to HG condition and in the bone tissue of Akita mice, an animal model of type I diabetes, compared to those of wild-type mice (Seref-Ferlengez, Maung, Schaffler, Spray, Suadicani & Thi, 2016). Currently, it is unknown whether retinal pannexin expression is affected by diabetes. However, recent studies examining the role of purinoceptors in diabetic retinopathy suggest the P2X receptors present in the retinal vascular cells (Kawamura, Sugiyama, Wu, Kobayashi, Yamanishi, Katsumura & Puro, 2003) may play a role in mediating cytokine-induced vascular inflammatory reactions that can degrade the integrity of the BRB (Liou, 2010). A recent study indicated that pannexin 1 level is unchanged in corneas of diabetic subjects compared to those of nondiabetic subjects (Cui, Liu, Qin, Wang & Huang, 2016). Currently, it is unclear whether pannexins play a role in the pathogenesis of diabetic retinopathy.
6. Current strategies for restoration of intercellular communication in diabetes and diabetic retinopathy
Emerging strategies, which can restore and normalize cell-cell communication are currently being investigated. Boldine, an alkaloid antioxidant with anti-inflammatory and hypoglycemic effects, has been shown to prevent HG-induced downregulation of gap junctional communication by improving gap junction-mediated cell coupling as well as increasing the relative level of Cx43 in mesangial cells (Hernandez-Salinas, Vielma, Arismendi, Boric, Saez & Velarde, 2013). In addition, boldine reduced membrane permeability in mesangial cells grown in HG condition with pro-inflammatory cytokines, associated with diabetic nephropathy (Hernandez-Salinas et al., 2013). Studies have shown that insulin treatment in diabetic rats can also prevent downregulation of intercellular communication in retinal pericytes, as determined by increased coupling of neurobiotin, a gap junction-permeant tracer (Oku et al., 2001). Trans-resveratrol, a phytoalexin found in grapes and red wine, was found to upregulate Cx43 expression and showed a protective effect against HG-induced compromised GJIC in retinal pigment epithelial cells (Losso, Truax & Richard, 2010), as determined by scrape-load dye transfer assay. Lycopene, an antioxidant carotenoid pigment, has shown the ability to increase GJIC by upregulating the transcription and expression of Cx43. Further research is necessary to establish the efficacy of these GJIC-restoration therapeutics in diabetic retinopathy patients.
7. Conclusion
In diabetic retinopathy, it is evident that cell-cell communication is compromised and their role in preserving and maintaining retinal homeostasis is undermined. Cx43 reactivity has been detected not only at the interface of vascular cell types but also between non-vascular cell types. This suggests that the HG effect on connexin can impact global interconnectivity between similar cell types in the retina and likely amongst different retinal cell types. Recent observations related to compromised cell-cell communication point not only to issues involving retinal lesions but also to hitherto unknown affects that could compromise BRB. Therefore, HG-mediated changes in Cx43 expression and GJIC activity can have profound effects on retinal cell viability and functionality. Further studies are necessary to better understand molecular mechanisms underlying connexin-mediated intercellular communication and develop strategies to prevent compromised GIIC associated with diabetic retinopathy.
Acknowledgments
This research was supported by NEI, NIH grants R01 EY018218 and EY025528 (SR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. J Neurosci. 2004;24(18):4313–4323. doi: 10.1523/JNEUROSCI.3303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Volgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10(7):495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA. Diabetic retinopathy: pathogenic mechanisms and current treatments. Diabetes Metab Syndr. 2011;5(3):165–172. doi: 10.1016/j.dsx.2012.02.025. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Burt JM. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim Biophys Acta. 2005;1711(2):126–141. doi: 10.1016/j.bbamem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Cui H, Liu Y, Qin L, Wang L, Huang Y. Increased membrane localization of pannexin1 in human corneal synaptosomes causes enhanced stimulated ATP release in chronic diabetes mellitus. Medicine (Baltimore) 2016;95(49):e5084. doi: 10.1097/MD.0000000000005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagli ML, Hernandez-Blazquez FJ. Roles of gap junctions and connexins in non-neoplastic pathological processes in which cell proliferation is involved. J Membr Biol. 2007;218(1–3):79–91. doi: 10.1007/s00232-007-9045-9. [DOI] [PubMed] [Google Scholar]
- De Maio A, Vega VL, Contreras JE. Gap junctions, homeostasis, and injury. J Cell Physiol. 2002;191(3):269–282. doi: 10.1002/jcp.10108. [DOI] [PubMed] [Google Scholar]
- Decrock E, Vinken M, De Vuyst E, Krysko DV, D’Herde K, Vanhaecke T, Vandenabeele P, Rogiers V, Leybaert L. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16(4):524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- Evans WH, Martin PE. Gap junctions: structure and function (Review) Mol Membr Biol. 2002;19(2):121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- Fernandes R, Girao H, Pereira P. High glucose down-regulates intercellular communication in retinal endothelial cells by enhancing degradation of connexin 43 by a proteasome-dependent mechanism. J Biol Chem. 2004;279(26):27219–27224. doi: 10.1074/jbc.M400446200. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Phipps JA, Wilkinson-Berka JL. Dysfunction of retinal neurons and glia during diabetes. Clin Exp Optom. 2005;88(3):132–145. doi: 10.1111/j.1444-0938.2005.tb06686.x. [DOI] [PubMed] [Google Scholar]
- Giardina SF, Mikami M, Goubaeva F, Yang J. Connexin 43 confers resistance to hydrogen peroxide-mediated apoptosis. Biochem Biophys Res Commun. 2007;362(3):747–752. doi: 10.1016/j.bbrc.2007.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldenagel M, Sohl G, Plum A, Traub O, Teubner B, Weiler R, Willecke K. Expression patterns of connexin genes in mouse retina. J Comp Neurol. 2000;425(2):193–201. [PubMed] [Google Scholar]
- Guthrie SC, Gilula NB. Gap junctional communication and development. Trends Neurosci. 1989;12(1):12–16. doi: 10.1016/0166-2236(89)90150-1. [DOI] [PubMed] [Google Scholar]
- Hernandez-Salinas R, Vielma AZ, Arismendi MN, Boric MP, Saez JC, Velarde V. Boldine prevents renal alterations in diabetic rats. J Diabetes Res. 2013;2013:593672. doi: 10.1155/2013/593672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Bienhold U, Dermietzel R, Weiler R. Distribution of connexin43 immunoreactivity in the retinas of different vertebrates. J Comp Neurol. 1998;396(3):310–321. [PubMed] [Google Scholar]
- Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551(Pt 3):787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon GT, Marshak DW. Gap junctions with amacrine cells provide a feedback pathway for ganglion cells within the retina. Proc Biol Sci. 1998;265(1399):919–925. doi: 10.1098/rspb.1998.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima A, Nakahama K, Ohno-Matsui K, Shimada N, Mori K, Iseki S, Sato T, Mochizuki M, Morita I. Connexin 43 contributes to differentiation of retinal pigment epithelial cells via cyclic AMP signaling. Biochem Biophys Res Commun. 2008;366(2):532–538. doi: 10.1016/j.bbrc.2007.11.159. [DOI] [PubMed] [Google Scholar]
- Larson DM, Carson MP, Haudenschild CC. Junctional transfer of small molecules in cultured bovine brain microvascular endothelial cells and pericytes. Microvasc Res. 1987;34(2):184–199. doi: 10.1016/0026-2862(87)90052-5. [DOI] [PubMed] [Google Scholar]
- Li AF, Roy S. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci. 2009;50(3):1400–1407. doi: 10.1167/iovs.07-1519. [DOI] [PubMed] [Google Scholar]
- Li AF, Sato T, Haimovici R, Okamoto T, Roy S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Invest Ophthalmol Vis Sci. 2003;44(12):5376–5382. doi: 10.1167/iovs.03-0360. [DOI] [PubMed] [Google Scholar]
- Li MW, Mruk DD, Cheng CY. Gap junctions and blood-tissue barriers. Adv Exp Med Biol. 2012;763:260–280. doi: 10.1007/978-1-4614-4711-5_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci U S A. 2001;98(17):9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GI. Diabetic retinopathy: Role of inflammation and potential therapies for anti-inflammation. World J Diabetes. 2010;1(1):12–18. doi: 10.4239/wjd.v1.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losso JN, Truax RE, Richard G. trans-resveratrol inhibits hyperglycemia-induced inflammation and connexin downregulation in retinal pigment epithelial cells. J Agric Food Chem. 2010;58(14):8246–8252. doi: 10.1021/jf1012067. [DOI] [PubMed] [Google Scholar]
- Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, Fletcher EL. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci. 2011;52(13):9316–9326. doi: 10.1167/iovs.11-7879. [DOI] [PubMed] [Google Scholar]
- Manasson J, Tien T, Moore C, Kumar NM, Roy S. High glucose-induced downregulation of connexin 30.2 promotes retinal vascular lesions: implications for diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(3):2361–2366. doi: 10.1167/iovs.12-10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AD, Hayrapetyan V, Moreno AP, Beyer EC. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res. 2002;90(10):1100–1107. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- Matesic D, Tillen T, Sitaramayya A. Connexin 40 expression in bovine and rat retinas. Cell Biol Int. 2003;27(2):89–99. doi: 10.1016/s1065-6995(02)00281-0. [DOI] [PubMed] [Google Scholar]
- Muller LP, Dedek K, Janssen-Bienhold U, Meyer A, Kreuzberg MM, Lorenz S, Willecke K, Weiler R. Expression and modulation of connexin 30.2, a novel gap junction protein in the mouse retina. Vis Neurosci. 2010;27(3–4):91–101. doi: 10.1017/S0952523810000131. [DOI] [PubMed] [Google Scholar]
- Muto T, Tien T, Kim D, Sarthy VP, Roy S. High glucose alters cx43 expression and gap junction intercellular communication in retinal muller cells: promotes muller cell and pericyte apoptosis. Invest Ophthalmol Vis Sci. 2014;55(7):4327–4337. doi: 10.1167/iovs.14-14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MS, Nygaard Axelsen L, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. Gap junctions. Compr Physiol. 2012;2(3):1981–2035. doi: 10.1002/cphy.c110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku H, Kodama T, Sakagami K, Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci. 2001;42(8):1915–1920. [PubMed] [Google Scholar]
- Pan F, Paul DL, Bloomfield SA, Volgyi B. Connexin36 is required for gap junctional coupling of most ganglion cell subtypes in the mouse retina. J Comp Neurol. 2010;518(6):911–927. doi: 10.1002/cne.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277(10):8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267(5205):1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Roy S, Ha J, Trudeau K, Beglova E. Vascular basement membrane thickening in diabetic retinopathy. Curr Eye Res. 2010;35(12):1045–1056. doi: 10.3109/02713683.2010.514659. [DOI] [PubMed] [Google Scholar]
- Sato T, Haimovici R, Kao R, Li AF, Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes. 2002;51(5):1565–1571. doi: 10.2337/diabetes.51.5.1565. [DOI] [PubMed] [Google Scholar]
- Seref-Ferlengez Z, Maung S, Schaffler MB, Spray DC, Suadicani SO, Thi MM. P2X7R-Panx1 Complex Impairs Bone Mechanosignaling under High Glucose Levels Associated with Type-1 Diabetes. PLoS One. 2016;11(5):e0155107. doi: 10.1371/journal.pone.0155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T. Gap junction alterations in human cardiac disease. Cardiovasc Res. 2004;62(2):368–377. doi: 10.1016/j.cardiores.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Sohl G, Guldenagel M, Traub O, Willecke K. Connexin expression in the retina. Brain Res Brain Res Rev. 2000;32(1):138–145. doi: 10.1016/s0165-0173(99)00074-0. [DOI] [PubMed] [Google Scholar]
- Stains JP, Civitelli R. Gap junctions in skeletal development and function. Biochim Biophys Acta. 2005;1719(1–2):69–81. doi: 10.1016/j.bbamem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tien T, Barrette KF, Chronopoulos A, Roy S. Effects of high glucose-induced Cx43 downregulation on occludin and ZO-1 expression and tight junction barrier function in retinal endothelial cells. Invest Ophthalmol Vis Sci. 2013;54(10):6518–6525. doi: 10.1167/iovs.13-11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien T, Muto T, Barrette K, Challyandra L, Roy S. Downregulation of Connexin 43 promotes vascular cell loss and excess permeability associated with the development of vascular lesions in the diabetic retina. Mol Vis. 2014;20:732–741. [PMC free article] [PubMed] [Google Scholar]
- Trudeau K, Muto T, Roy S. Downregulation of mitochondrial connexin 43 by high glucose triggers mitochondrial shape change and cytochrome C release in retinal endothelial cells. Invest Ophthalmol Vis Sci. 2012;53(10):6675–6681. doi: 10.1167/iovs.12-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T, Bette S, Zhang J, Theis M, Engele J. Connexin-deficiency affects expression levels of glial glutamate transporters within the cerebrum. Neurosci Lett. 2012;506(1):12–16. doi: 10.1016/j.neulet.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Weiler R. Gap junctions in the eye: evidence for heteromeric, heterotypic and mixed-homotypic interactions. Brain Res Brain Res Rev. 2000;32(1):115–120. doi: 10.1016/s0165-0173(99)00070-3. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- Wright JA, Richards T, Becker DL. Connexins and diabetes. Cardiol Res Pract. 2012;2012:496904. doi: 10.1155/2012/496904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs KR, Ceelen PW. Gap junctional coupling and connexin immunoreactivity in rabbit retinal glia. Vis Neurosci. 2006;23(1):1–10. doi: 10.1017/S0952523806231018. [DOI] [PubMed] [Google Scholar]
- Zahs KR, Newman EA. Asymmetric gap junctional coupling between glial cells in the rat retina. Glia. 1997;20(1):10–22. [PubMed] [Google Scholar]