Abstract
Background
Immunoglobulin D (IgD) is an enigmatic antibody isotype best known when co-expressed with IgM on naïve B cells. However, elevated soluble IgD (sIgD) and increased IgD+IgM− B cell populations have been described in the human upper respiratory mucosa.
Objective
We assessed whether levels of sIgD and IgD+ B cells are altered in nasal tissue from patients with chronic rhinosinusitis (CRS). We further characterized IgD+ B cell populations and explored clinical and local inflammatory factors associated with tissue sIgD levels.
Methods
sIgD levels were measured by ELISA in nasal tissues, nasal lavages, serum, and supernatants of dissociated nasal tissues. IgD+ cells were identified by immunofluorescence and flow cytometry. Inflammatory mediator levels in tissues were assessed by real-time PCR and multiplex immunoassay. Bacterial cultures from the middle meatus were performed. Underlying medical history and medicine use were obtained from medical records.
Results
sIgD levels and the number of IgD+ cells were significantly increased in uncinate tissue (UT) of CRS without nasal polyps (CRSsNP) compared to control (4-fold, P<.05). IgD+ cells were densely scattered in the periglandular regions of CRSsNP UT. We also found that IgD+CD19+CD38bright plasmablasts were significantly elevated in CRSsNP tissues compared to control (P<.05). Among numerous factors tested, IL-2 levels were increased in CRSsNP UT and were positively correlated with tissue IgD levels. Additionally, the supernatants of IL-2-stimulated dissociated CRSsNP tissue had significantly increased sIgD levels compared to IL-2-stimulated dissociated control tissue ex vivo (P<.05). Tissue from CRS patients with preoperative antibiotic use or those with pathogenic bacteria presence showed higher IgD levels compared to tissue from patients absent these variables (P<.05).
Conclusion
sIgD levels and IgD+CD19+CD38bright plasmablasts were increased in nasal tissue of CRSsNP. IgD levels were associated with increased IL-2 and the presence of pathogenic bacteria. These findings suggest that IgD might contribute to enhance mucosal immunity, inflammation, or respond to bacterial infections in CRS, especially CRSsNP.
Keywords: Chronic rhinosinusitis, IgD, B cells, Interleukin-2, Bacterial infection, Mucosal immunity
Introduction
Chronic rhinosinusitis (CRS) is a heterogeneous clinical condition associated with significant quality of life impairment and persistent local inflammation of the sinonasal mucosa.1–4 CRS is usually subdivided into 2 clinical subgroups: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).5–7 Although CRSsNP is five- to ten-fold more prevalent, it remains poorly characterized compared to CRSwNP. One potential reason is that CRSsNP encompasses a wider variety of CRS subtypes/endotypes with more heterogeneous inflammatory patterns in US-based studies, whereas CRSwNP is more homogeneously characterized by type-2 inflammation in Western populations.8–12 Regardless of subgroup, it is likely that the host immune system plays a prominent role in CRS pathogenesis.1, 13
B cells represent a major component of adaptive immune responses, which participate both in immunity and inflammation through their production of antibodies.14 Five isotypes of antibodies, including immunoglobulins M (IgM), IgD, IgG, IgA, and IgE, are encoded in humans and rodents.15 Of these, IgM, IgG, IgA, and IgE have been described at elevated levels in nasal polyps (NP) from patients with CRSwNP compared to control subjects tissue.16, 17 Despite its discovery over 40 years ago, IgD has remained largely underexplored in inflammatory diseases including CRS.18 IgD is best characterized in transitional and mature naïve B cells where membrane IgD (mIgD) is co-expressed with membrane IgM (mIgM) bearing the same variable region and antibody specificity.19, 20 The translation of mIgD occurs through alternative splicing of a pre-mRNA composed of V(D)J and both heavy-chain Cμ and Cδ exons. Following antigenic stimulation, follicular B cells will transcriptionally down-regulate mIgD in a germinal center reaction and undergo somatic hypermutation (SHM) and class-switch recombination (CSR) of DNA to express IgM, IgG, IgA, and IgE.20, 21
In contrast to mice, in which mIgD expression is restricted to transitional and naïve B cells, human B cells can additionally express mIgD on a unique set of circulating IgM+IgD+CD27+ B cells that share functional characteristics with B cells of the splenic marginal zone.22 Additionally, human B cells are also capable of undergoing class switch recombination through a cryptic Sμ-σ/δ site to only express IgD as well as to produce soluble IgD (sIgD). Interestingly, evidence for this non-canonical class switching is frequently found in the IgD+IgM− plasma cells that have been described in the upper respiratory mucosa (e.g., tonsil and nasal mucosa), but are rarely detected in non-respiratory mucosal sites (e.g. gut and peripheral lymph node).15, 23–25 Studies of tonsil-derived B cells further demonstrated that IgD+IgM− plasma cells have unique features of extensive SHM and predominant use of the lambda light chain (Igλ). Other studies have demonstrated that mIgD interacts directly with two pathogens, Moraxella catarrhalis and Haemophilus influenza.26, 27 Together, these data suggest that IgD-secreting B cells are activated in the human upper airway, but whether they are associated with specific inflammatory conditions like CRS and their precise role in mucosal immunity remains unclear.
In this study, we sought to investigate whether the levels of sIgD and IgD+ B cells are aberrant in nasal tissues from patients with CRS. We further characterized the phenotype of IgD+ B cell populations within nasal tissues, and explored which local factors could affect IgD production in vivo and in vitro. Finally, we assessed clinical parameters associated with IgD in nasal tissues.
Methods
Subjects and sample collection
Control and CRS patients were recruited from the Otolaryngology and Allergy-Immunology Clinics at Northwestern Medicine or the Department of Otolaryngology at Stanford University. Demographic and underlying medical history, and history of antibiotic and corticosteroid use within 4 weeks of sinus surgery were obtained from electronic medical records. Preoperative computed tomography (CT) scans were graded according to methods defined by Lund et al.28 Subject characteristics are included in Table E1. All subjects provided informed consent. The Institutional Review Boards of Northwestern University Feinberg School of Medicine and Stanford University School of Medicine approved this study.
Tissue specimens including uncinate tissue (UT) and NP, nasal lavage fluid, and serum were obtained from subjects and prepared, as previously described.12, 29 Further details are provided in this article’s Online Repository at www.jacionline.org.
ELISA
We measured IgD protein concentrations using the Human IgD ELISA Quantitation set (Bethyl Laboratories, Montgomery, Texas). Additionally, we measured eosinophil cationic protein (ECP) levels using the Mesacup ECP Test (MBL International, Woburn, MA). Tissue concentrations of IgD and ECP were normalized to the total protein concentration measured by the Bicinchoninic acid (BCA) Protein Assay (Thermo Fisher Scientific, Waltham, MA).
Immunofluorescence
Immunofluorescence was performed as described previously.17 Briefly, blocked sections were stained with phycoerythrin-conjugated goat anti-human IgD antibody (Southern Biotech, Birmingham, AL). Further detailed methods are provided in this article’s Online Repository.
Cell isolation and flow cytometry
Cells were isolated from ethmoid sinus tissues (ET) and whole blood samples from the same individuals. Isolated cells were stained for flow cytometric analysis with various B cell surface markers including CD19, CD20, CD27, CD38, CD138, IgM, and IgD. A cocktail of antibodies to CD45, CD3, CD14, CD16, and CD56 was used in a dump channel to exclude non-B cell immunocytes. Furthermore, intracellular IgD and BLIMP-1 staining was performed by using Cytofix/Cytoperm™ Kit (Biosciences, Franklin Lakes, NJ). All conjugated antibodies were individually titrated to maximize specificity while accurately compensating for 12-color, 20- parameter high dimensional fluorescence-activated cell sorting (FACS). The reagent targeting intracellular IgD was conjugated to a fluorophore whose emission is distinct from that of mIgD. For flow cytometric analysis, 1x106 events (minimum) were collected for each sample at the Stanford Shared FACS Facility on a FACS-LSRII.2 (BD Bioscience, San Jose, CA). Data was analyzed by FlowJo software (FlowJo-LLC, Ashland, OR). Further detailed methods and gating strategies (Fig E1) are provided in this article’s Online Repository.
Ex vivo tissue explant culture
Cells were isolated from nasal tissue by using a modified method designed for cutaneous tissue, as previously described.17, 30 Briefly, tissue samples obtained from sinus surgery were placed in 5 mL of RPMI-1640 medium (Life Technologies, Grand Island, NY) supplemented with 5% fetal calf serum (FCS; Atlanta Biologicals, Lawrenceville, GA), 1 mmol/L sodium pyruvate (Life Technologies), 1% penicillin/streptomycin, and 60 U/mL IL-2 (Conditioned-RPMI [C-RPMI]; Life Technologies). Tissue was incubated in C-RPMI for 4 days at 37°C in 5% CO2. Remaining tissue fragments were removed through 70 μm Falcon™ cell strainer (Thermo Fisher Scientific) and supernatant was stored at −20°C until use.
Luminex assay
IL-2, IL-4, IL-10, IFN-γ and IL-21 levels were measured using the Milliplex Map kit (EMD Millipore, Billerica, MA) with a Luminex 200 instrument (Life Technologies) per manufacturer instructions. Tissue concentrations of these mediators were normalized to the total protein concentration measured by the BCA Protein Assay.
Real-Time PCR
mRNA levels of IL-2, IL-21, IL-15, CD40L, and B-cell activating factor (BAFF) in total RNAs isolated from whole nasal tissues were measured using quantitative real-time PCR, as detailed in this article’s Online Repository.
Nasal swab culture
A nasal swab of the middle meatus for bacterial culture was obtained prior to initiating surgery from control subjects and CRS patients. The middle meatus is an anatomic drainage space within the ethmoid sinuses from which tissue samples were also harvested. Cultures obtained from this location reliably reflect the microbiology of the sinus cavities.31 All specimens were obtained with a sterile swab, BBL™ CultureSwab™ (Becton-Dickinson, Franklin Lakes, NJ) and sent immediately to laboratory of the Department of Microbiology at Northwestern Medicine for routine aerobic microbiological cultures. Further details are described in this article’s Online Repository.
Statistical analyses
All data are reported as mean ± standard error of the mean (SEM), unless otherwise noted. Differences between groups were analyzed by using Mann-Whitney U test or Kruskal-Wallis test with Dunn’s correction for multiple comparisons. Correlations were assessed by the Spearman rank correlation. All statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA), and a P value of less than .05 was considered statistically significant.
Results
Levels of sIgD were increased in nasal airway mucosa from patients with CRSsNP
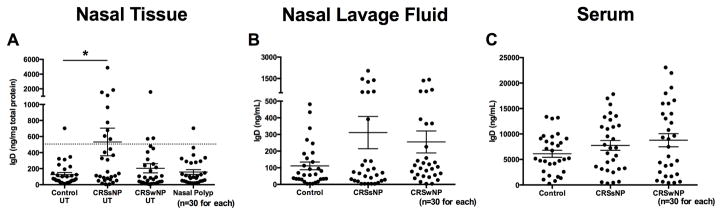
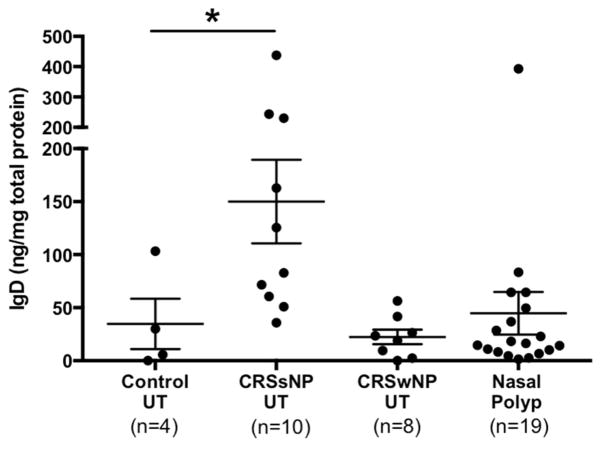
We first assessed the protein levels of sIgD in nasal tissues, nasal lavage, and serum. sIgD levels were significantly elevated in CRSsNP UT (mean=532.3 ng/mg total protein) compared with those in control UT (mean=125.8 ng/mg total protein, P<.05; Fig 1, A), demonstrating a bimodal separation into patients with high IgD levels and those with low IgD levels. Although sIgD levels were not significantly elevated in the nasal lavages of CRSsNP patients, we found a subpopulation showing high IgD levels in both CRSsNP and CRSwNP patient groups (Fig 1, B). Furthermore, there was a significant positive correlation between sIgD levels in patients where we had both tissue and nasal lavage measurement (r =0.30, P =.02, n=55; data not shown). Additionally, there were no significant differences in sIgD levels in serum of CRSsNP compared to control (Fig 1, C).
Figure 1.
Levels of sIgD in nasal tissue of CRSsNP. Protein levels of sIgD in A, nasal tissue, B, nasal lavage fluid, and C, serum were measured by using ELISA. The dotted line indicates the threshold based on the 95th percentile of IgD levels in control UT. The individual patient data points are shown, and solid lines represent means ± SEMs. *P < .05, Kruskal-Wallis test.
IgD+ B cells were increased in nasal airway mucosa of CRS patients
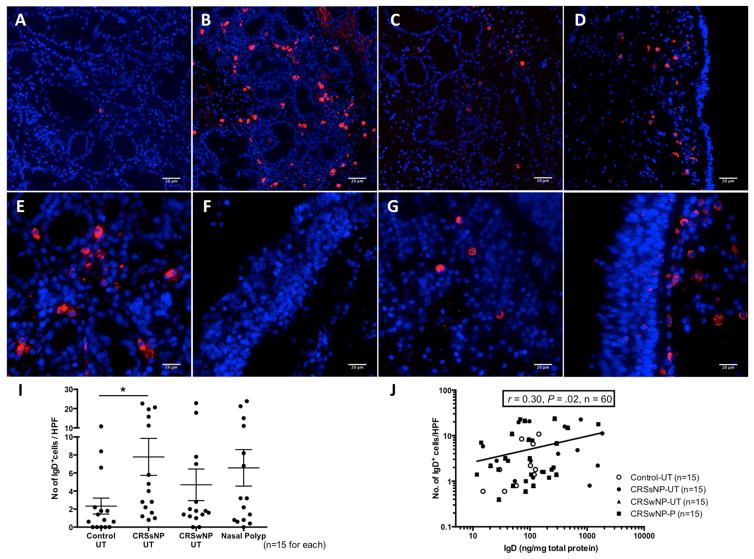
We next sought to determine whether IgD+ B cells were present in nasal tissue by performing immunofluorescence. Within each disease group, 15 surgical samples from the same patients whose tissue homogenates were used for IgD protein analysis were used for immunofluorescene. While rare IgD+ cells were detected in control UT (Fig 2, A), there were samples with abundant IgD+ cells especially in CRSsNP UT (Fig 2, B). IgD+ cells were also observed in UT and NP of CRSwNP, although to a lesser extent (Figs 2, C and D, respectively). Interestingly, IgD+ cells were predominantly located in periglandular areas in CRSsNP UT (Fig 2, E); only 13.3% (n=2) of CRSsNP patients demonstrated the presence of subepithelial IgD+ cells (Fig 2, F). In contrast, IgD+ cells in NP tissues were enriched in the subepithelial region (73.3% of tested CRSwNP patients; Figs 2, G and H). When observed, the IgD+ cells had small nuclei with intense, uniformly stained cytoplasm consistent with plasma cells or plasmablasts. A semiquantitative analysis of the immunofluorescence staining showed that the number of IgD+ cells was increased in nasal tissues from CRS patients but a statistically significant increase was only observed when CRSsNP UT compared with control UT (Fig 2, I; P<.05), although even among CRSsNP patients, a bimodal distribution concordant with our ELISA results was observed. Furthermore, we found that the number of IgD+ cells in the immunofluorescence study was positively correlated with sIgD tissue levels measured by ELISA (r=0.30, P = .02, n=60; Fig 2, J).
Figure 2.
Immunofluorescence analysis of IgD+ cells in nasal tissue by using phycoerythrin-conjugated anti-IgD (red). Representative images of IgD+ cells in UT from A, control, B, CRSsNP, C, CRSwNP, and D, NP from CRSwNP patients (magnification 200x). E, IgD+ cells at the peri-glandular and F, subepithelial area in CRSsNP UT and G, peri-glandular and H, subepithelial area in NP of CRSwNP (magnification 400x). Nuclei were counterstained with 49,6-diamidino-2-phenylindole (blue). I, IgD+ cells in nasal tissue were counted semiquantitatively. J, Correlation between the numbers of IgD+ cells from immunofluorescence assay and IgD protein concentrations in matched nasal tissue. *P < .05. HPF, High-power field.
IgD+ plasmablast populations were exclusively found in nasal airway mucosa from patients with CRS
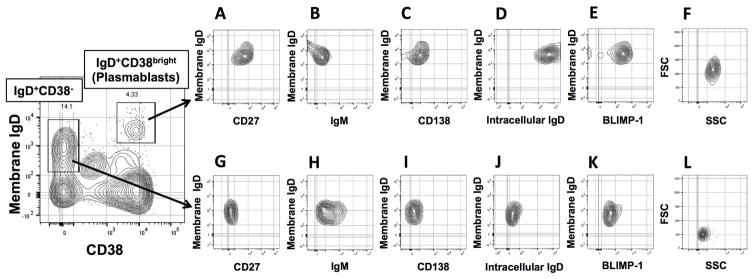
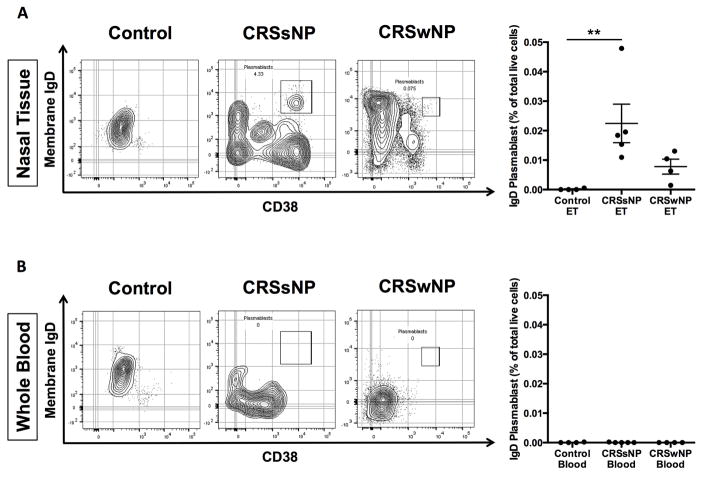
Given that local mucosal, but not systemic, sIgD levels were increased and accumulated IgD+ cells were also found in nasal tissue of CRSsNP, we next sought to characterize the phenotype of these IgD+ B cells using FACS. After performing a sequential FACS gating strategy (Fig E1), CD19+CD20− B cells were segregated into the IgD+ plasmablast population using IgD+ and CD38bright (Fig 3). While CD19+CD20+ B cells in both the peripheral blood and nasal tissues strongly expressed IgM, we found that both CD20 and IgM were absent in IgD+ plasmablasts. As expected for this subpopulation21, 24, IgD+CD38bright plasmablasts had dim expression of CD27, low/negative surface IgM, and did not express CD138 (Figs 3, A–C). Furthermore, we found that only the IgD+CD38bright cell population expressed both intracellular IgD and intracellular BLIMP-1 (Figs 3, D and E versus J and K). Compared to IgD+CD38− cells in the same plot, IgD+CD38bright plasmablast populations had increased forward scatter (FSC) and side scatter profiles (SSC), indicating larger cell size and increased granularity (Fig 3, F versus L). As shown in Fig 4A, the IgD+ plasmablast population (IgD+CD19+CD20−CD38bright) was consistently detected in nasal tissues of both types of CRS, but not control subjects. Consistent with our ELISA and immunofluorescence results, these cells were significantly increased in CRSsNP tissues compared to control (0.02% and 0.00% of total cells, respectively; Fig 4, A; P<.01). No IgD + plasmablasts were detected in the blood of either CRS or control subjects from whom nasal tissue was obtained (Fig 4, B).
Figure 3.
Representative phenotype of IgD+ plasmablasts in nasal tissue as assessed using flow cytometry. Of the CD19+CD20− B cell population, IgD+CD38bright cells (IgD+ plasmablasts) and IgD+CD38− cells (naïve B cells) were subdivided and each population was further analyzed for CD27 (A and G), IgM (B and H), CD138 (C and I), intracellular IgD (D and J), BLIMP-1 (E and K), and forward and side scatter profiles (F and L).
Figure 4.
Representative flow cytometric plots showing the IgD+CD38bright plasmablast population and calculated numbers of IgD+CD38bright cells A, in nasal tissue from control subjects, patients with CRSsNP and those with CRSwNP, and B, in peripheral blood from the same patients. **P < .01, Kruskal-Wallis test. The numbers in flow cytometric plots indicate relative percentage.
IL-2 expression correlated with sIgD levels in nasal airway mucosa in vivo
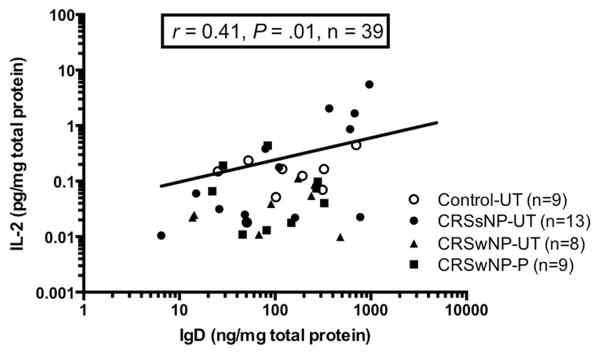
Previous in vitro studies of B cells within tonsil tissues determined that differentiation into IgD-producing B cells could be affected by CD40L, BAFF, or a proliferation-inducing ligand (APRIL) and various combinations of cytokines including IL-2, IL-4, IL-10, IL-15, IL-21, and IFN-γ.21, 32 Therefore, we next sought to evaluate if local levels of these mediators were elevated in CRSsNP and correlated with sIgD levels in nasal tissue. Of the aforementioned molecules, only gene expression levels of IL-2 (Fig E2, A) and IL-21 (data not shown) were significantly elevated in both CRSsNP UT and CRSwNP NP compared with control UT. There was a similar trend in IL-2 protein levels, although not reaching statistical significance (Fig E2, B). Interestingly, we found a significant positive correlation between levels of sIgD and IL-2 proteins in nasal tissues among all patients (r=0.41, P=.01; Fig 5) with similar significant correlations for IL-2 mRNA expression (data not shown). Compared to controls, we could not find significant differences in protein levels of IL-4, IL-21 or IFN-γ, or gene expression of IL-15, CD40L or BAFF in CRS UT. Protein levels of IL-10 and gene expression levels of BAFF were significantly different between CRSwNP NP and control UT (Fig E2, C and D). With the exception of IL-2, protein or gene expression levels of the tested mediators did not correlate with measured IgD protein levels (data not shown).
Figure 5.
Correlation between the levels of IgD and IL-2 in nasal tissue in vivo. Protein levels of IL-2 in tissue were measured by using multiplex immunoassay and IgD were measured by using ELISA. The correlation was assessed by Spearman rank test. *P < .05.
IL-2 enhanced IgD production by CRSsNP nasal airway mucosa
Given that local IL-2 protein levels were associated with IgD levels in vivo, we performed ex vivo analysis to evaluate whether IgD production by nasal tissue following IL-2 stimulation differed among CRS subtypes. To do this, we cultured intact nasal tissue explants from control, CRSsNP and CRSwNP patients in the presence of IL-2 for 4 days, and assessed the protein levels of sIgD in supernatants. sIgD levels released into supernatants of explants from CRSsNP patients were significantly higher compared to those of control subjects (4-fold, P<.05; Fig 6). Taken together, these data suggest that IL-2 levels that were significantly elevated in CRSsNP may prime IgD production.
Figure 6.
Levels of sIgD in ex vivo tissue explant culture. Whole tissue explants were cultured with IL-2 (60U/mL) for 4 days and sIgD levels in supernatants were measured by using ELISA. *P < .05, Kruskal-Wallis test.
Comparison of clinical characteristics of high-IgD versus low-IgD groups
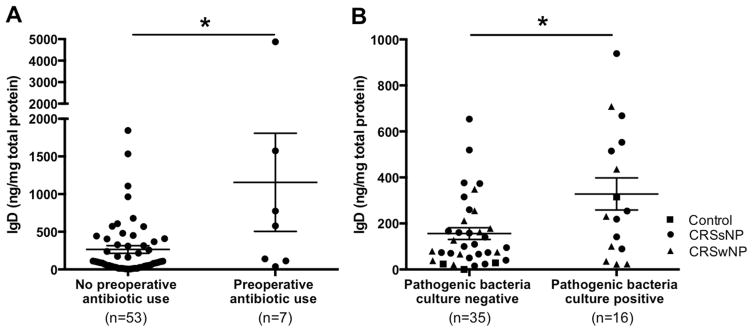
Since sIgD levels and IgD+ cells in nasal tissues appeared bimodally distributed with high-IgD and low-IgD expression (Fig 1, A), we established thresholds for defining high- or low-IgD groups using the 95th percentile of expression of control UT as utilized in prior studies.9, 33 On electronic medical records review, clinical features including medication prescriptions were extracted. As shown in Table 1, patients in the high-IgD group more frequently had CRSsNP compared with the low-IgD cohort (75.0% versus 43.7%, respectively, P=.05). Interestingly, patients in the high-IgD group were more likely to have been prescribed an antibiotic within the 4 weeks prior to sinus surgery than those in the low-IgD patients (33.3% versus 6.3%, respectively, P=.01). The mean tissue IgD level of patients with preoperative antibiotic use was 1156 ng/mg total protein, while that of patients without preoperative antibiotic use was 264.5 ng/mg total protein (P=.04, Fig 7, A). No other clinical parameters were significantly divergent between these 2 groups.
Table 1.
Comparison of clinical features in high-IgD versus low-IgD in nasal tissue from patients with CRS
| High-IgD CRS Patients (n=12) | Low-IgD CRS patients (n=48) | P-value | |
|---|---|---|---|
| IgD (ng/mg total protein) in UT, mean (range) | 1307 (568.7–4875) | 134.1 (5.216–479.8) | <.0001 |
| Prevalence of CRSsNP, % (Ratio of CRSsNP:CRSwNP) | 75.0% (9:3) | 43.7% (21:27) | .05 |
| Purulent rhinorrhea on endoscopy | 0% | 12.5% | 0.33 |
|
| |||
| Medication use (< 4weeks of sinus surgery), % | |||
| Antibiotics | 33.3% | 6.3% | .01 |
| Nasal corticosteroids | 20.0% | 17.2% | .84 |
| Oral corticosteroids | 20.0% | 33.3% | .42 |
|
| |||
| Asthma,% | 41.7% | 37.5% | .79 |
| Atopy,% | 54.5% | 68.3% | .39 |
| ECP (ng/mg total protein), mean (range) | 757.4 (1.53–7682) | 309.9 (14–1694) | .39 |
| Lund-Mackay score28, mean (range) | 10.5 (6–16) | 13.0 (3–24) | .09 |
CRS, chronic rhinosinusitis; UT, uncinate tissue; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; ECP, eosinophil cationic protein
Text in boldface indicates statistically significant differences for that variable between high-IgD CRS patients and low-IgD CRS patients (P < .05).
Figure 7.
Preoperative antibiotic use and presence of pathogenic bacterial infection may influence IgD levels in nasal tissue. A, History of preoperative antibiotic use was obtained from electronic medical records. B, Nasal swab cultures were performed prior to sinus surgery. IgD levels in UT were measured by using ELISA. Dot plots illustrate individual data points, and solid lines represent means ± SEMs. *P < .05.
Elevated IgD levels correlated with pathogenic bacterial infection
Recent research suggests that IgD has the potential to enhance immune surveillance by activating antimicrobial programs.24 However, the contributions of elevated local IgD levels in specific infectious conditions is unknown, although selected studies described an association between chronic infections and increased systemic IgD levels.26, 27, 34 Informed by our findings above that high IgD tissue levels may be linked to recent antibiotic use in CRS patients, we initiated a prospective study to evaluate the association between sinus bacterial infection and tissue IgD levels. Middle meatal swab aerobic cultures in an additional group of 4 control subjects and 47 CRS patients were collected along with UT samples. Speciated pathogenic bacterial growth was found in 31.4% (n=16 including 1 control, 8 CRSsNP, and 7 CRSwNP) of the enrolled patients (n=51). Gram-negative bacteria including Pseudomonas aeruginosa, Escherichia coli and Haemophilus influenza were detected in 3 patients, whereas Gram-positive bacteria, Staphylococcus aureus and Streptococcus pneumonia were detected in the remaining patients (n=13). IgD levels in UT were significantly higher in patients who possessed pathogenic bacterial growth compared with those without bacterial growth (P<.05, Fig 7, B). No significant differences were detected in IgD levels between patients having Gram-positive bacterial growth versus Gram-negative bacterial growth (data not shown).
Discussion
In this study, we presented novel evidence that sIgD levels (Fig 1) and the numbers of IgD+ cells (Fig 2) are significantly increased in nasal tissue of patients with CRSsNP compared with control subjects. Although CRSwNP patients did not have significantly elevated levels of IgD in nasal tissue, there were some patients who also had high levels of sIgD and IgD+ cells. Overall, sIgD levels in CRS tissue appeared to have a bimodal distribution with CRSsNP patients more frequently having high sIgD levels than CRSwNP patients. Unlike prior descriptions that IgD+ plasmablasts exist universally in nasal mucosa,24 they were only increased in nasal mucosa of CRS, particularly CRSsNP, but not in control nasal mucosa or in the blood of either CRS or control patients in this study. Interestingly, the IgD+ cells were concentrated in the periglandular submucosa in CRSsNP, and in the subepithelial area in CRSwNP (Fig 2). The IgD+ cells had a plasma cell-like morphology on immunofluorescence but detailed flow cytometric phenotyping found that most IgD+CD19+CD38bright B cells also expressed intracellular IgD+ and BLIMP-1+ without CD138, thus harboring features of plasmablasts (Fig 3). We next identified the local factors that associated with elevated IgD levels, and found that IL-2 levels correlated with IgD secretion in vivo (Fig 5) with supporting ex vivo results (Fig 6). Furthermore, we showed compelling evidence that preoperative antibiotic use (Table 1 and Fig 7, A), and the presence of pathogenic bacterial infection (Fig 7, B) were associated with higher IgD levels in nasal tissues from CRS patients. Together, these findings suggest that bacterial infection and/or the induced inflammatory response may influence on local IgD production in the setting of CRS. To our knowledge, this is the first in-depth study to identify that local elevation of sIgD and IgD+ plasmablasts is associated with CRS.
In humans, serum IgD is found at relatively lower concentrations than levels of IgG (~100 fold higher), IgA, and IgM, but IgD is present at much higher levels than serum IgE.34 sIgD is also present in various body fluids, including nasal, lacrimal, salivary, mammary, bronchial, and cerebrospinal fluids.15, 21, 35 Notably, it has been shown that greater amounts of sIgD are present in nasal secretions compared to saliva36 or intestinal mucosal fluid, suggesting that IgD may have a unique functional role in upper respiratory mucosal immunity.37 The source of sIgD in the upper airway presumably derives from IgD+CD38bright plasmablasts usually found in secondary lymphoid organs like tonsils and adenoids.23 Our study demonstrated that IgD secretion may also occur directly within inflamed nasal mucosa adjacent to surface epithelium and deeper secretory glands. Consistent with our flow cytometric results, previous studies have described surface markers for these cells to be IgM−IgD+CD38+, although the expression of mature B cell markers like CD20, memory B cell markers like CD27 and mature plasma cell markers like CD138 and BLIMP-1 have been more variably described.23, 24 In the analysis of IgD+ plasmablasts from nasal tissue, we consistently found elevated intracellular IgD and BLIMP-1 but not CD138 expression. Additionally, immunofluorescence and flow cytometry results overlap and indicate that IgD plasmablasts are exclusively found in CRS and are essentially undetectable in control nasal tissues. In CRS, they are present in limited density but most frequently in CRSsNP. This signifies that the immunoregulation of IgD, and its corresponding B cell precursors, are uniquely tailored for the nasal tissue microenvironment in the setting of inflammatory responses found in CRS. The factors that govern this specificity remain unclear at this point.
A significant challenge in dissecting the enigmatic role of IgD in immune physiology has been its inability to activate complement and undescribed Fc receptors on effector cells. Despite its unknown immunomodulatory function, high serum levels of IgD are reported in various diseases including immunodeficiencies, autoimmune diseases and allergic and infectious diseases38–40. Systemic IgD levels are also consistently higher in a group of hereditary periodic fever syndromes that have a number of upper airway manifestations.21, 41 Despite the elevated tissue levels of sIgD, serum sIgD levels were not increased in CRSsNP, indicating the restriction of IgD responses to upper airway sites. Given the periglandular localization of IgD+ cells, we anticipated that IgD levels in nasal secretions would be correspondingly higher in CRSsNP. However, we found that any differences were not statistically significant, despite the fact that IgD levels in nasal lavage significantly correlated with tissue levels. It should be noted that the mechanism by which IgD crosses the airway epithelium remains unknown. Unlike IgM and IgA, whose transport across epithelium is actively regulated by the polymeric immunoglobulin receptor (pIgR), an epithelial receptor for IgD has not been described. It is possible that IgD secretion may only be induced under specific conditions.
In this study, the specificity of the sIgD antibodies found in CRSsNP was not assessed. However, other reports have demonstrated that mIgD interacts directly with two pathogens, Moraxella catarrhalis and Haemophilus influenza, commonly associated with acute rhinosinusitis.26, 42 The effect of Moraxella catarrhalis on B cells is better documented and occurs through a non-immune binding of the bacterial Moraxella IgD-binding protein (MID) located within the mIgD constant region. MID binding to IgD triggers potent mitogenic responses in B cells akin to B cell superantigens like Staphylococcus protein A (SpA).42 Unlike SpA, which only binds to immunoglobulin variable chains expressed on a subset of B cells, IgD is universally expressed by all naïve B-cells, and it is not surprising that in vitro studies demonstrate that MID has stronger superantigenic effects than SpA.42 Indeed, we find that elevated IgD levels are associated with bacterial cultures with known superantigenic effects and active sinus infections being treated with antibiotics. Other groups reported that over 90% of tonsil-derived IgD plasma cells utilize Ig λ and there was unusually high use of specific VH segments reminiscent of a superantigen-driven response.25 Since acute rhinosinusitis and CRS are distinguished only by duration of symptoms (<4 weeks versus >12 weeks of symptoms in CRS43), one can speculate that the superantigen effects of the acute sinusitis pathogens, Moraxella catarrhalis, Haemophilus influenza, and Staphylococcus aureus may be of pathogenic relevance to CRS.
Our findings that sIgD and IgD plasmablasts were most elevated in CRSsNP tissue also stand in partial contrast to our past studies of B-cells in CRS. In previous studies, plasmablasts, B-cell activating cytokines (e.g. BAFF) and immunoglobulins of every isotype except IgD and class-switched autoreactive antibodies have been elevated in NP tissue of CRSwNP.12, 17, 29, 33 In contrast to CRSwNP, CRSsNP is both clinically and molecularly more heterogeneous.44 Thus, given the association of IgD with CRSsNP, we further evaluated the relationships between a variety of locally expressed mediators including CD40L, BAFF, IFN-γ, IL-2, IL-4, IL-10, IL15 and IL-21 and with tissue IgD levels, as these factors had previously been shown to induce differentiation of IgD plasmablasts in vitro.24 We observed that IL-2 levels were significantly elevated in tissue of CRSsNP in concordance with another study.45 Furthermore, these levels were positive correlated with tissue IgD levels in vivo (r =0.41), and ex vivo IL-2-stimulated nasal tissue explants from CRSsNP also produced significantly more sIgD than control tissue explants. The dependence on IL-2 for IgD secretion has previously been reported by Arpin et al46 on tonsil-derived B-cells in vitro, and this study further supports the concept that this mechanism may be relevant in vivo. Since IL-2 is only transiently secreted by T cells in the acute phases of an infection47, and CRS patients with positive bacterial culture or recent antibiotic use had higher levels of IgD in this study, these findings could be interpreted as evidence that sIgD and IgD+ plasmablasts may play an immune role in bacterial exacerbations of CRS. Future in vivo and in vitro studies will be required to investigate the relationship between IgD and bacterial infection.
In summary, increased levels of sIgD and abundant IgD+ plasmablasts are found in nasal tissue in a subpopulation of CRS patients, more commonly among patients with CRSsNP. Mucosal sIgD was associated with high local IL-2 levels and the presence of pathogenic bacteria in the sinonasal microenvironment. These findings suggest that IgD might contribute to enhancing protective mucosal immunity, or by contrast represent a pathologic inflammatory response possibly driven by superantigenic effects. These investigations significantly advance our understanding of IgD and IgD-producing B cells in the setting of sinonasal inflammatory disease.
Supplementary Material
Key Messages.
Soluble IgD levels and IgD+CD19+CD38bright plasmablasts are significantly increased in nasal tissue from patients with CRSsNP.
IL-2 levels are increased in CRSsNP UT and positively correlated with local IgD levels in vivo; IL-2-stimulated cells from dissociated CRSsNP tissue significantly induced sIgD production compared to those from control tissue in vitro.
Pathogenic bacterial infection may promote local IgD secretion in CRS.
Acknowledgments
Funding: This work was supported by NIH grant K23 DC012067 (BKT), Chronic Rhinosinusitis Integrative Studies Program (CRISP) U19 AI106683 (BKT, RCK, KEH, AK, RPS), R01 HL078860, R37 HL068546, R01 HL068546, R01 AI072570 and R01 AI104733, the Triological Society/American College of Surgeons (BKT), the Ernest S. Bazley Foundation, and the Sean N Parker Center for Allergy and Asthma Research at Stanford University (JVN).
List of Abbreviations
- CRS
Chronic rhinosinusitis
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- Ig
Immunoglobulin
- NP
Nasal polyps
- SHM
Somatic hypermutation
- UT
Uncinate tissue
- FACS
Fluorescence-activated cell sorting
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 2.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012:3. 1–298. preceding table of contents. [PubMed] [Google Scholar]
- 3.Ference EH, Stubbs V, Lidder AK, Chandra RK, Conley D, Avila PC, et al. Measurement and comparison of health utility assessments in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5:929–36. doi: 10.1002/alr.21556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomassen P, Van Zele T, Zhang N, Perez-Novo C, Van Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proc Am Thorac Soc. 2011;8:115–20. doi: 10.1513/pats.201005-036RN. [DOI] [PubMed] [Google Scholar]
- 5.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103–15. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–59. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in Chronic Rhinosinusitis: Role in Eosinophilia and Aspirin Exacerbated Respiratory Disease. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson CF, Price CP, Huang JH, Min JY, Suh LA, Shintani-Smith S, et al. A pilot study of symptom profiles from a polyp vs an eosinophilic-based classification of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:500–7. doi: 10.1002/alr.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachert C, Akdis CA. Phenotypes and Emerging Endotypes of Chronic Rhinosinusitis. J Allergy Clin Immunol Pract. 2016;4:621–8. doi: 10.1016/j.jaip.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Min JY, Ocampo CJ, Stevens WW, Price CP, Thompson CF, Homma T, et al. Proton pump inhibitors decrease eotaxin-3/CCL26 expression in patients with chronic rhinosinusitis with nasal polyps: Possible role of the nongastric H,K-ATPase. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206. e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–46. doi: 10.1111/cea.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato A, Hulse KE, Tan BK, Schleimer RP. B-lymphocyte lineage cells and the respiratory system. J Allergy Clin Immunol. 2013;131:933–57. doi: 10.1016/j.jaci.2013.02.023. quiz 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandtzaeg P, Farstad IN, Johansen FE, Morton HC, Norderhaug IN, Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Schryver E, Devuyst L, Derycke L, Dullaers M, Van Zele T, Bachert C, et al. Local immunoglobulin e in the nasal mucosa: clinical implications. Allergy Asthma Immunol Res. 2015;7:321–31. doi: 10.4168/aair.2015.7.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–83. 83 e1–7. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe DS, Fahey JL. A New Class of Human Immunoglobulins. I. A Unique Myeloma Protein. J Exp Med. 1965;121:171–84. doi: 10.1084/jem.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maki R, Roeder W, Traunecker A, Sidman C, Wabl M, Raschke W, et al. The role of DNA rearrangement and alternative RNA processing in the expression of immunoglobulin delta genes. Cell. 1981;24:353–65. doi: 10.1016/0092-8674(81)90325-1. [DOI] [PubMed] [Google Scholar]
- 20.Chen K, Cerutti A. The function and regulation of immunoglobulin D. Curr Opin Immunol. 2011;23:345–52. doi: 10.1016/j.coi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol Rev. 2010;237:160–79. doi: 10.1111/j.1600-065X.2010.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–85. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 23.Arpin C, de Bouteiller O, Razanajaona D, Fugier-Vivier I, Briere F, Banchereau J, et al. The normal counterpart of IgD myeloma cells in germinal center displays extensively mutated IgVH gene, Cmu-Cdelta switch, and lambda light chain expression. J Exp Med. 1998;187:1169–78. doi: 10.1084/jem.187.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–98. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seifert M, Steimle-Grauer SA, Goossens T, Hansmann ML, Brauninger A, Kuppers R. A model for the development of human IgD-only B cells: Genotypic analyses suggest their generation in superantigen driven immune responses. Mol Immunol. 2009;46:630–9. doi: 10.1016/j.molimm.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 26.Singh K, Nordstrom T, Morgelin M, Brant M, Cardell LO, Riesbeck K. Haemophilus influenzae resides in tonsils and uses immunoglobulin D binding as an evasion strategy. J Infect Dis. 2014;209:1418–28. doi: 10.1093/infdis/jit593. [DOI] [PubMed] [Google Scholar]
- 27.Gjorloff Wingren A, Hadzic R, Forsgren A, Riesbeck K. The novel IgD binding protein from Moraxella catarrhalis induces human B lymphocyte activation and Ig secretion in the presence of Th2 cytokines. J Immunol. 2002;168:5582–8. doi: 10.4049/jimmunol.168.11.5582. [DOI] [PubMed] [Google Scholar]
- 28.Lund VJ, Mackay IS. Staging in rhinosinusitis. Rhinology. 1993;107:2. [PubMed] [Google Scholar]
- 29.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. 92 e1–2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benninger MS, Payne SC, Ferguson BJ, Hadley JA, Ahmad N. Endoscopically directed middle meatal cultures versus maxillary sinus taps in acute bacterial maxillary rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2006;134:3–9. doi: 10.1016/j.otohns.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Levan-Petit I, Lelievre E, Barra A, Limosin A, Gombert B, Preud'homme JL, et al. T(h)2 cytokine dependence of IgD production by normal human B cells. Int Immunol. 1999;11:1819–28. doi: 10.1093/intimm/11.11.1819. [DOI] [PubMed] [Google Scholar]
- 33.Tan BK, Klingler AI, Stevens WW, Poposki JA, Peters AT, Suh LA, et al. Heterogenous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7:131–40. doi: 10.1128/cdli.7.2.131-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunnette SL, Gleich GJ, Miller RD, Kyle RA. Measurement of IgD by a double antibody radioimmunoassay: demonstration of an apparent trimodal distribution of IgD levels in normal human sera. J Immunol. 1977;119:1727–31. [PubMed] [Google Scholar]
- 36.Plebani A, Avanzini MA, Massa M, Ugazio AG. An avidin-biotin ELISA for the measurement of serum and secretory IgD. J Immunol Methods. 1984;71:133–40. doi: 10.1016/0022-1759(84)90059-0. [DOI] [PubMed] [Google Scholar]
- 37.Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 38.Lertora JJ, Gomez-Perez FJ, Leslie GA. Structure and biological functions of human IgD. V. Insulin antibodies of the IgD class in sera from some diabetic patients. Int Arch Allergy Appl Immunol. 1975;49:597–606. doi: 10.1159/000231441. [DOI] [PubMed] [Google Scholar]
- 39.Rostenberg I, Penaloza R. Serum IgG and IgD and levels in some infectious and noninfectious diseases. Clin Chim Acta. 1978;85:319–21. doi: 10.1016/0009-8981(78)90310-8. [DOI] [PubMed] [Google Scholar]
- 40.Sirisinha S, Charupatana C, Ramasoota T. Serum immunoglobulins in leprosy patients with different spectra of clinical manifestations. Proc Soc Exp Biol Med. 1972;140:1062–8. doi: 10.3181/00379727-140-36612. [DOI] [PubMed] [Google Scholar]
- 41.Drenth JP, Haagsma CJ, van der Meer JW. Hyperimmunoglobulinemia D and periodic fever syndrome. The clinical spectrum in a series of 50 patients. International Hyper-IgD Study Group. Medicine (Baltimore) 1994;73:133–44. [PubMed] [Google Scholar]
- 42.Jendholm J, Samuelsson M, Cardell LO, Forsgren A, Riesbeck K. Moraxella catarrhalis-dependent tonsillar B cell activation does not lead to apoptosis but to vigorous proliferation resulting in nonspecific IgM production. J Leukoc Biol. 2008;83:1370–8. doi: 10.1189/jlb.1107788. [DOI] [PubMed] [Google Scholar]
- 43.Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–S209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 44.Kato AKA, Stevens WW, Peters AT, Poposki JA, Suh L, et al. Heterogenous Inflammation in Chronic Rhinosinusitis without Nasal Polyps. Journal of Allergy and Clinical Immunology. 2016;137:AB285. [Google Scholar]
- 45.Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope. 2013;123:E72–8. doi: 10.1002/lary.24305. [DOI] [PubMed] [Google Scholar]
- 46.Arpin C, Banchereau J, Liu YJ. Memory B cells are biased towards terminal differentiation: a strategy that may prevent repertoire freezing. J Exp Med. 1997;186:931–40. doi: 10.1084/jem.186.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–90. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.