Structured Abstract
Rationale and Objectives
We investigated the feasibility of detecting left ventricular (LV) cardiac magnetic resonance (CMR) strain abnormalities using feature-tracking in patients with pulmonary hypertension (PH).
Materials and Methods
CMR was performed in 16 patients with all groups of PH and in 13 controls. Global and regional peak circumferential strain (%) (which have been shown to be robust by CMR), peak diastolic strain rate (%/s), and dyssynchrony index (ms) were quantified with feature-tracking software. Ventricular function and volumes were calculated from CMR, and right heart pressures measured with catheterization.
Results
LV EF (ejection fraction) was similar in patients (60.2±11.0%) and controls (61.9±4.5%), p=0.150. Global LV peak circumferential strain was significantly different in patients compared to controls, −16.7±2.8% versus −19.9±1.8% respectively (p=0.001). The greatest difference in strain was seen in the LV septum, −11.6±4.3% in patients versus −16.7±4.0% in controls (p<0.001). There was a significant association between septal strain and RV end-diastolic volume index (RVEDVI) (p=0.047) in patients with PH, however no associations with pulmonary artery pressures or RV ejection fraction.
Conclusions
Feature-tracking CMR can detect LV strain abnormalities in patients with PH and preserved or mildly depressed LVEF, with greatest abnormality in the septum. The association between septal strain and RVEDVI suggests that ventricular interdependence may be a mechanism of LV dysfunction in PH. Feature-tracking CMR may be useful for identification of LV dysfunction before LVEF significantly declines in PH patients. The feasibility of detecting LV strain abnormalities in patients with PH shown by this study paves the way for a variety of future investigations into the applications of LV strain in this patient population.
Keywords: cardiac magnetic resonance, feature-tracking, pulmonary hypertension, strain, left ventricular dysfunction
Introduction
Pulmonary hypertension (PH) is a progressive, chronic disease characterized by sustained elevation of resting mean pulmonary artery pressure of 25 mmHg or greater. The World Health Organization (WHO) classifies pulmonary hypertension into five major groups: pulmonary arterial hypertension (PAH), PH due to left heart disease, due to lung disease and/or hypoxia, chronic thromboembolic PH, and PH with unclear multifactorial mechanisms 1. Despite significant progress in medical management of pulmonary arterial hypertension, overall prognosis remains poor, as shown in an analysis of patients with pulmonary arterial hypertension that demonstrated 1-, 3-, 5-, and 7-year survival rates of 85%, 68%, 57%, and 49% 2.
A major limitation of the current management of patients with PH is the requirement for invasive cardiac catheterization to evaluate pulmonary artery pressures in order to risk-stratify patients and evaluate treatment response. The invasive tests performed in patients with PH expose the patients to procedural risks and radiation.
Cardiac magnetic resonance (CMR) imaging allows noninvasive evaluation of cardiac structure and function. A number of clinical studies have established CMR as useful for diagnosis and prognosis in both ischemic and non-ischemic cardiomyopathy as well as in PH 3,4. CMR strain imaging techniques can identify early myocardial dysfunction in patients with preserved ejection fraction, and therefore may be a useful noninvasive tool for evaluation of preclinical myocardial dysfunction 5–8.
Left ventricular dysfunction is known to occur in primary pulmonary hypertension, and is associated with more severe disease and poorer prognosis 9,10. Furthermore, echocardiographic studies have shown abnormal strain in the ventricular septum in children with PH 11. Feature-tracking is a CMR method of myocardial motion quantification that utilizes cine imaging, sequences routinely obtained in most CMR examinations, to follow motion along the myocardial tissue/ventricular cavity border12. Feature-tracking CMR has been validated against CMR tagging and speckle-tracking echocardiography techniques for evaluation of myocardial strain, with good agreement13,14. The ability to obtain strain data from clinically acquired sequences allows application of feature-tracking to patients undergoing CMR for a variety of indications. While recent studies have demonstrated a relationship between disease severity/patient outcomes and right ventricular strain using feature-tracking CMR15, evaluation of left ventricular CMR strain abnormalities in patients with PH is limited. The purpose of this study is to investigate the feasibility of detecting left ventricular CMR strain abnormalities in patients with PH with preserved/mildly depressed left ventricular (LV) function using feature-tracking and to interrogate whether LV strain abnormalities are associated with right ventricular abnormalities and with severity of PH.
Materials and Methods
This analysis included retrospective identification of adult patients with pulmonary hypertension who underwent clinical CMR with short axis cine sequences. Only a small subset of the pulmonary hypertension patients at our institution had clinically performed CMR examinations during the study period, and as such, the etiologies of PH included in our study were heterogeneous. Specifically, sixteen patients with PH confirmed by prior cardiac catheterization who underwent clinical CMR performed at our institution between 2009 and 2014 were retrospectively identified. Patients with all World Health Organization (WHO) groups of PH were included in the study (Table 1). As this was a retrospective pilot study, healthy patients with normal CMRs available from our database were included as the control group. Specifically, thirteen controls including healthy, non-athlete volunteers (n=7) and asymptomatic patients with normal CMR (n=6) referred for imaging due to family history of arrhythmia were also retrospectively identified. The institutional review board approved this study, which is Health Insurance Portability and Accountability Act (HIPAA) compliant. The institutional review board waived the requirement for informed consent.
Table 1.
Demographics, volumetric, and functional parameters in patients with pulmonary hypertension versus controls. Continuous variables expressed as mean ± standard deviation.
| Controls n= 13 | Patients n= 16 | Unadjusted P-value | Age-Adjusted P-value | |
|---|---|---|---|---|
| Age – years | 35.6±12.0 | 52.9±16.5 | 0.004 | - |
| Male (%) | 6/13 (46%) | 5/16 (31%) | 0.429 | 0.989 |
| White (%) | 6/13 (46%) | 10/16 (63%) | 0.397 | 0.173 |
| LVEF (%) | 61.9%±4.5 | 60.2%±11.0 | 0.634 | 0.150 |
| RVEF (%) | 53.3%±5.6 | 41.0%±15.4 | 0.019 | 0.085 |
| LVEDVI ml/m2 | 97.0±36.3 | 73.0±24.9 | 0.057 | 0.571 |
| RVEDVI ml/m2 | 96.6±42.0 | 111.7±42.55 | 0.377 | 0.177 |
| RBBB | - | 5/16 (31%) | - | - |
LVEF denotes left ventricular ejection fraction; RVEF, right ventricular ejection fraction; LVEDVI, left ventricular end-diastolic volume index, RVEDVI, right ventricular end-diastolic volume index; RBBB denotes right bundle branch block.
Image Acquisition
CMR studies were performed with a 1.5 Tesla unit (Achieva, Philips Medical Systems, Best, The Netherlands). The CMR protocols differed according to the clinical scenario leading to the scan but all included steady-state free precession (SSFP) short-axis cine images during breath hold for assessment of ventricular function. Long axis cine and delayed enhancement imaging are not performed for all CMR examinations at our institution, and therefore these sequences were not available for all patients. Controls referred due to family history of arrhythmia as well as healthy volunteers also underwent short axis SSFP cine images. Imaging parameters for the SSFP sequences were: repetition time = 3.0–3.2 milliseconds (ms), echo time = 1.4 ms, flip angle = 70°, field of view (FOV) = 340 mm, number of excitations = 1, slice thickness = 8 mm with no gap and acquisition matrix = 256×144.
Image Analysis
Volumetric analysis was performed on a dedicated workstation (Medis, Sectra, Sweden) by manually outlining the endocardial contour of the left and right ventricles. Left and right ventricular volumes were indexed to body surface area. Left and right ventricular ejection fractions were also calculated. Table 1 shows the demographic characteristics, ventricular ejection fractions, and ventricular volume indices of patients and controls.
Left ventricular strain was quantified on short-axis cine images using feature-tracking/tissue tracking software (CVI42, Circle Cardiovascular Imaging®, Calgary, Canada). The reproducibility of feature tracking CMR analysis with Circle® software has been evaluated in several studies. Schuster et al demonstrated acceptable intra-vendor reproducibility when comparing TomTec Imaging Systems Gmbh® (Unterschleissheim, Germany) and Circle®, with the least inter-vendor variability in global circumferential strain measures16. Morton et al also demonstrated good reproducibility of global and segmental circumferential strain measures using Circle® 17. Global circumferential strain by CMR has been shown to have high agreement with speckle-tracking echocardiography in the literature18,19.
For strain analyses, endocardial and epicardial contours were generated semi-automatically, with subsequent manual correction by a single investigator. Contiguous short axis cine images were evaluated, with the number of cine slices averaging from 8 to 9 depending on patient and cardiac size. Global and regional peak circumferential strains (%) were analyzed, with regional strain measurements obtained from the ventricular septum (American Heart Association segments 2, 3, 8, 9, 14) and lateral wall (American Heart Association segments 5, 6, 11, 12, 16) 20. Circumferential strain measures were selected as they have been shown to have less intra-subject variability compared to longitudinal strain measures using CMR 21. Peak diastolic strain rate in percent per second (%/s) was also calculated. An index of dyssynchrony was calculated consisting of the standard deviation of time to peak circumferential strain, in milliseconds, as previously described 22.
Clinical Parameters
Medical records were reviewed for documentation of WHO group, age, gender, race, electrocardiogram (ECG) abnormalities, echocardiography, and cardiac catheterization parameters. ECG and catheterization records were available for all patients with PH. Median time between CMR and right heart catheterization was 53 days (IQR 7 to 309 days). Echocardiography results were available in 13 patients with PH. ECG and catheterization data were not available for controls.
Statistical Analysis
Continuous parameters were compared between patients with PH and controls using an unpaired Student’s t-test for normally distributed variables, including age, left ventricular ejection fraction, right ventricular ejection fraction, left ventricular end-diastolic volume index, and right ventricular end-diastolic volume index. Proportion of gender and race (white versus non-white) were compared using Chi-square test. Student’s t-test was also used for comparison of global and regional peak circumferential strain, peak diastolic strain rate, and dyssynchrony index between patients and controls. Unadjusted and age-adjusted p-values were reported. Linear regression was performed to evaluate the relationship of global and regional strain to ventricular volume indices and right heart pressures. Logistic regression was performed to evaluate the relationship between the presence of PH and regional strain, controlling for age and gender. Statistical significance was set at p < 0.05.
Results
Demographics
Patients (age 52.9±16.5 years) were significantly older than controls (age 35.6±12.0 years), p=0.004. As a result, other demographic factors and strain analyses were adjusted for patient age. There was no significant difference in the race or gender distribution of patients compared to controls in both adjusted and unadjusted analyses. All groups of PH were represented, with the majority of patients in WHO group 1 (8/16, 50%), WHO group 2 (2/16, 12.5%), or WHO group 5 (4/16, 25%). There was one patient each in WHO groups 3 and 4.
CMR Function and Volumetrics
Normal left ventricular function was defined as those with an LVEF greater than or equal to 50%, mildly depressed LVEF was defined as those with an LVEF from 40–49%, and moderately depressed LVEF was defined as those with an LVEF from 30–39%. LVEF was normal or mildly depressed in the majority of patients with PH (range 43% to 76%), with one PH patient demonstrating a moderately depressed LVEF of 38%. There was no significant difference in LVEF between patients with PH and controls, (60.2±11.0% versus 61.9±4.5% respectively, unadjusted p=0.634 and age-adjusted p=0.150). RVEF was significantly decreased in patients with PH in unadjusted analysis (41.0±15.4% compared to 53.3±5.6%, p=0.019), however not significantly different when adjusted for patient age, p = 0.085. There was no significant difference in left or right ventricular volume indices between the groups in unadjusted and adjusted analyses (Table 1), even though 56% of patients had enlarged right ventricles with a right ventricular end-diastolic volume index (RVEDVI)> 95 ml/m2 23.
Echocardiography
LVEF by echocardiography was ranged from 53% to 75% in patients with PH, mean 66.2±6.5%. Peak pulmonary artery pressure was elevated at echocardiography in patients at 66.1±30.1 mmHg.
Cardiac catheterization
Average mean pulmonary artery and peak pulmonary artery pressures at catheterization were also elevated, 40.8±10.0 mmHg and 64.1±15.7 mmHg respectively, compatible with known diagnoses of PH.
Electrocardiography
Right bundle branch block was present in 5 patients (31.3%) with PH. No controls had a known history of right bundle branch block.
CMR Strain
Strain analysis results are summarized in Table 2. Global left ventricular peak circumferential strain was significantly different in patients with PH compared to controls −16.7±2.8% and −19.9±1.8% respectively, in both unadjusted and age-adjusted analyses (p=0.002 and 0.001). (Figure 1, Figure 2A, Table 2). The difference in circumferential strain was greatest in the septal region, mean peak circumferential strain in the septum of −11.6±4.3% in patients versus −16.7±4.0% in controls, which was statistically significant in both unadjusted and age-adjusted analyses, age-adjusted p<0.001 (Figure 2B, Table 2). In contrast, mean peak circumferential strain in the lateral wall was not significantly different between the groups. Dyssynchrony index was significantly increased in patients compared to controls, however this result did not persist following adjustment for patient age. Regarding diastolic function, there was no significant difference in left ventricular peak diastolic strain rate in controls versus patients with PH (Table 2).
Table 2.
Strain analyses in patients with pulmonary hypertension versus controls.
| Controls n= 13 | Patients n= 16 | Unadjusted P-value | Age-Adjusted P-value | |
|---|---|---|---|---|
| Global strain (%) | −19.9±1.8% | −16.7±2.8% | 0.002 | 0.001 |
| Septal Strain (%) | −16.7±4.0% | −11.6±4.3% | 0.003 | <0.001 |
| Lateral Wall Strain (%) | −22.9±2.2 | −20.5±4.7 | 0.096 | 0.273 |
| Dyssynchrony index (ms) | 61.5±7.9 | 74.4±21.2 | 0.047 | 0.198 |
| Diastolic strain rate (%/s) | 121.0±27.2 | 115.1±42.4 | 0.667 | 0.988 |
Ms denotes milliseconds; s denotes seconds.
Fig. 1.
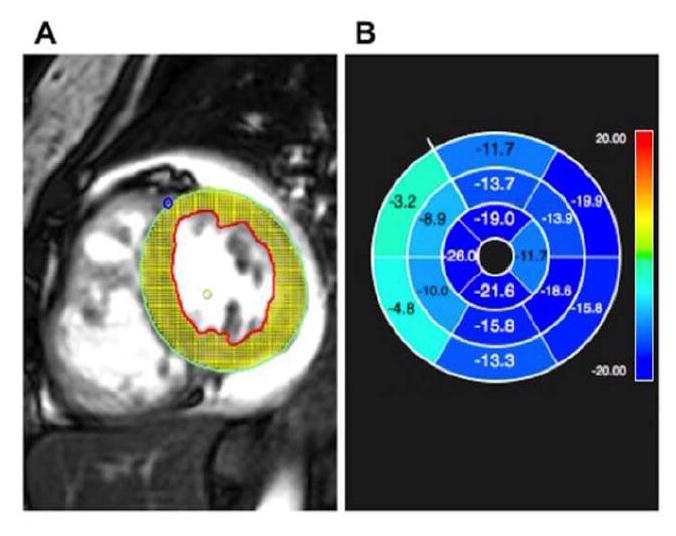
A) Feature-tracking strain analysis software using cardiac magnetic resonance, with representative short axis Steady State Free Precession (SSFP) image demonstrating myocardial tracking
B) Software output demonstrating abnormal peak circumferential strain (light blue/green indicate abnormal strain as indicated by negative numbers closer to zero) in the ventricular septum in a patient with pulmonary hypertension
Fig. 2.
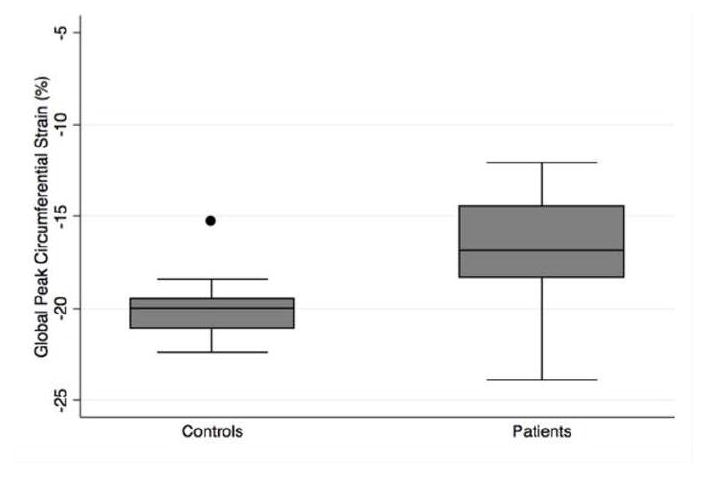
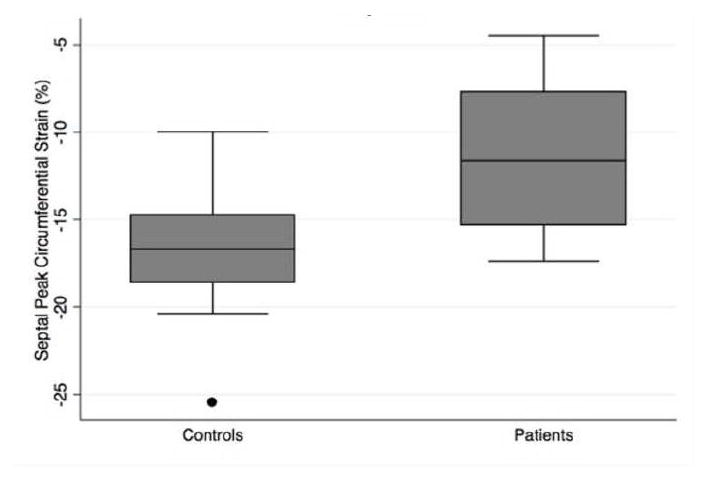
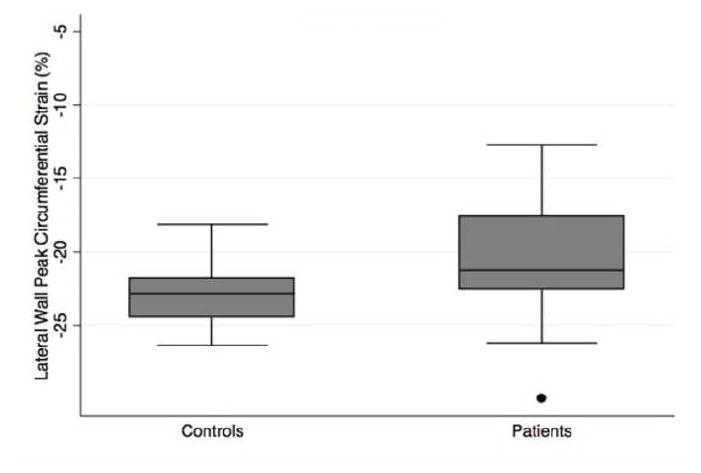
Peak left ventricular circumferential strain (%) in controls compared to patients with pulmonary hypertension, including measures of A) global, B) septal, C) lateral wall strain
Among patients with pulmonary hypertension, there was a statistically significant association between RVEDVI and septal peak circumferential strain (R2 =0.27; p=0.047), with less negative (negative numbers closer to zero) strain associated with larger right ventricular volumes (Figure 3). There was no statistically significant association of septal strain with mean or peak pulmonary artery pressures, left ventricular end-diastolic volume index, or with right ventricular ejection fraction in patients with PH (all p>0.05). Neither global nor lateral wall peak circumferential strains were significantly associated with RVEDVI or the other variables in patients with PH.
Fig. 3.
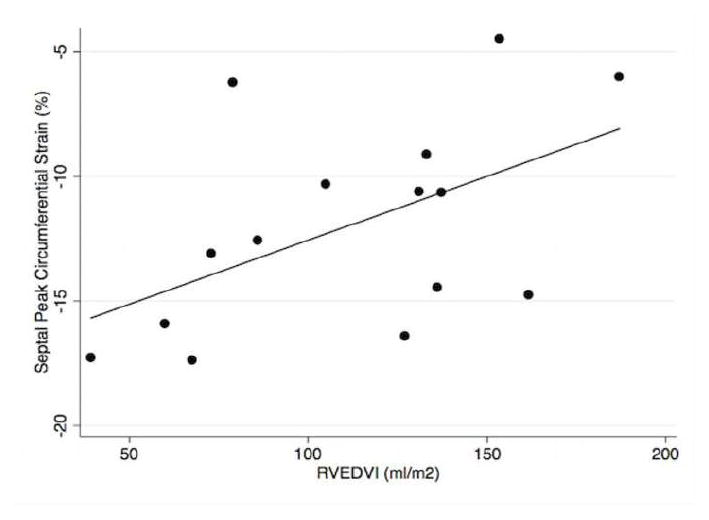
Relationship of ventricular septal peak circumferential strain (%) and right ventricular end-diastolic volume index (RVEDVI) in patients with pulmonary hypertension
Regression Analyses
Age and gender adjusted regression analyses were performed in patients with PH. A logistic regression model demonstrated a significant association between the presence of PH and septal strain as well as global peak circumferential strain, which remained significant after adjusting for patient age and gender (odds ratio = 2.61, p= 0.013). Although a separate regression model with index of dyssynchrony showed a significant association with the presence of PH, the association did not remain significant following adjustment for age and gender.
Discussion
Our analysis of left ventricular strain in patients with pulmonary hypertension compared to controls demonstrates the feasibility of using feature-tracking/tissue-tracking CMR to detect left ventricular dysfunction in patients with known PH before LVEF significantly declines, as demonstrated by the significantly different global peak circumferential strain in these patients compared to controls. The association between PH and CMR strain values remained significant after adjustment for patient age and gender. The strain abnormality in PH patients was not evenly distributed through the left ventricle, but rather was greatest in the ventricular septum. In addition, this septal strain showed a significant linear association with increased right ventricular volume within this cohort of PH patients with mildly increased RV volumes.
The predominant strain impairment of the ventricular septum in PH has also been described using echocardiography 11. Burkett DA et al showed that longitudinal and circumferential strain were abnormal in the septal region of pediatric patients with PH measured by speckle-tracking echocardiography. This preferential septal dysfunction suggests that ventricular interdependence may be a potential etiology for abnormal left ventricular function in patients with PH, as has been suggested by echocardiographic dyssynchrony analyses 24. Similar mechanisms have also been postulated in other diseases primarily involving the right ventricle, such as in patients with pulmonary regurgitation after repair of tetralogy of Fallot and in patients with tricuspid valve disease 8,25,26.
Our study suggests that LV septal strain abnormalities are greater as right ventricular volume increases, but are not influenced by right ventricular ejection fraction or severity of PH in this cohort of patients with preserved/mildly depressed LV function and mildly depressed RV function. The deformity of the septum in the presence of larger right ventricular volumes may be responsible for the early contractile dysfunction not yet leading to a significant decline in LVEF 27–29. Similarly, in patients with tetralogy of Fallot the presence of abnormal septal excursion due to right ventricular volume and pressure overload has been associated with worse LV radial strain at the septum 30.
Our analysis also identified that there was increased dyssynchrony in our PH patient cohort, as indicated by more variability in regional time to peak circumferential strain. However, the patient population was significantly older than our control group, and this relationship did not persist following correction for patient age, suggesting that age alone is the main variable determining dyssynchrony in our patient group. Although right bundle branch block could explain the dyssynchronous contractility, only a minority of the patients presented this ECG abnormality.
The main limitations of this feasibility study are the small sample size, retrospective design, and resultant heterogeneous patient population. Given the small sample size, larger studies will be needed to further investigate our findings. Retrospective review of CMR examinations for various clinical indications was performed in patients with all groups of PH (a benefit of the feature-tracking technique), and therefore patient clinical characteristics were heterogeneous. As patients with all groups of PH were included, it is possible that a subset could display left ventricle strain abnormalities related to primary left ventricular dysfunction rather than due to sequela of pulmonary hypertension. However, we believe that this effect is unlikely to contribute significantly to our results as Group 2 PH patients made up only 2/16 of our cohort. Additionally, left ventricular ejection fraction was preserved or mildly depressed in our cohort, and moderately depressed in only one individual. Because our PH cohort included patients with mild cardiac dysfunction (overall preserved or mildly depressed LVEF, mildly depressed RVEF, and mildly increased RVEDVI compared to normal standards), future analyses are needed to evaluate whether findings are similar in patients with greater impairment of cardiac function. In addition, our patient group was significantly older than controls as a result of our retrospective selection of healthy individuals from our database as the control population, however the global and septal strain differences between patients and controls as well as the relationship between abnormal septal strain and RVEDVI in patients with PH persisted despite adjustment for age. Future investigations could include comparison with age-matched controls.
The feasibility of detecting LV strain abnormalities in patients with PH shown by this study paves the way for a variety of future investigations into the applications of LV strain in PH patients. Subsequent investigations will be needed to evaluate whether LV strain abnormalities are related to PH patient functional decline or outcome in long-term studies. While specific PH treatment medications or treatment duration were not included in this pilot study, these factors could also be evaluated in future analyses to investigate whether treatment type or duration impacts LV strain in PH. Relationship of LV strain to cardiac biomarkers would also be an area of interest to illustrate the impact of strain abnormalities on the myocardium.
Conclusions
In summary, feature-tracking/tissue tracking CMR can detect abnormal left ventricular myocardial strain in patients with pulmonary hypertension and preserved or mildly depressed left ventricular ejection fraction, with greatest involvement of the ventricular septum. Our results show a relationship between septal strain and RVEDVI in patients with PH, suggesting that ventricular interdependence may be a mechanism of abnormal septal strain and LV dysfunction in this patient population. Awareness of these results is important, as quantification of myocardial strain with feature-tracking CMR could be useful for identification of LV systolic dysfunction in patients with known PH before LVEF significantly declines. In the future, further investigation is needed into the relationship between abnormal left ventricular strain by feature-tracking CMR and outcomes in patients with PH, the impact of PH treatment on LV strain, and the biochemical effects of abnormal LV strain in the PH population.
Acknowledgments
Source of Funding
Dr. Kallianos was supported by the National Institutes of Health T32 Training Grant, 2T32EB001631-11.
Dr. Seguro de Carvalho received a grant from the French Federation of Cardiology (Fédération Française de Cardiologie, Toulouse, France) for foreign study placement.
Abbreviations
- CMR
cardiac magnetic resonance
- ECG
electrocardiogram
- EF
ejection fraction
- FOV
field of view
- HIPAA
Health Insurance Portability and Accountability Act
- IQR
interquartile range
- LV
left ventricle
- LVEDVI
left ventricular end diastolic volume index
- LVEF
left ventricular ejection fraction
- m
meter
- ml
milliliters
- ms
millisecond
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- RBBB
right bundle branch block
- RV
right ventricle
- RVEDVI
right ventricular end diastolic volume index
- RVEF
right ventricular ejection fraction
- s
second
- SSPS
steady-state free precession
- WHO
World Health Organization
Footnotes
Conflicts of Interest
For the remaining authors none were declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology. 2013;62(25 Suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 3.Voelkel NF, Quaife RA, Leinwand LA, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114(17):1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 4.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. Journal of the American College of Cardiology. 2011;58(24):2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 5.Kindberg K, Haraldsson H, Sigfridsson A, et al. Myocardial strains from 3D displacement encoded magnetic resonance imaging. BMC medical imaging. 2012;12:9. doi: 10.1186/1471-2342-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kindberg K, Haraldsson H, Sigfridsson A, Sakuma H, Ebbers T, Karlsson M. Temporal 3D Lagrangian strain from 2D slice-followed cine DENSE MRI. Clinical physiology and functional imaging. 2012;32(2):139–144. doi: 10.1111/j.1475-097X.2011.01068.x. [DOI] [PubMed] [Google Scholar]
- 7.Leong DP, De Pasquale CG, Selvanayagam JB. Heart failure with normal ejection fraction: the complementary roles of echocardiography and CMR imaging. JACC Cardiovascular imaging. 2010;3(4):409–420. doi: 10.1016/j.jcmg.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Ordovas KG, Carlsson M, Lease KE, et al. Impaired regional left ventricular strain after repair of tetralogy of Fallot. Journal of magnetic resonance imaging: JMRI. 2012;35(1):79–85. doi: 10.1002/jmri.22686. [DOI] [PubMed] [Google Scholar]
- 9.Brauchlin AE, Soccal PM, Rochat T, Spiliopoulos A, Nicod LP, Trindade PT. Severe left ventricular dysfunction secondary to primary pulmonary hypertension: bridging therapy with bosentan before lung transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2005;24(6):777–780. doi: 10.1016/j.healun.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Pielsticker EJ, Martinez FJ, Rubenfire M. Lung and heart-lung transplant practice patterns in pulmonary hypertension centers. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2001;20(12):1297–1304. doi: 10.1016/s1053-2498(01)00348-5. [DOI] [PubMed] [Google Scholar]
- 11.Burkett DA, Slorach C, Patel SS, et al. Left Ventricular Myocardial Function in Children With Pulmonary Hypertension: Relation to Right Ventricular Performance and Hemodynamics. Circulation Cardiovascular imaging. 2015;8(8) doi: 10.1161/CIRCIMAGING.115.003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hor KN, Baumann R, Pedrizzetti G, et al. Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp. 2011;(48) doi: 10.3791/2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular Magnetic Resonance Myocardial Feature Tracking: Concepts and Clinical Applications. Circulation Cardiovascular imaging. 2016;9(4):e004077. doi: 10.1161/CIRCIMAGING.115.004077. [DOI] [PubMed] [Google Scholar]
- 14.Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016;18(1):51. doi: 10.1186/s12968-016-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Siqueira ME, Pozo E, Fernandes VR, et al. Characterization and clinical significance of right ventricular mechanics in pulmonary hypertension evaluated with cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2016;18(1):39. doi: 10.1186/s12968-016-0258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster A, Stahnke VC, Unterberg-Buchwald C, et al. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: Intervendor agreement and considerations regarding reproducibility. Clin Radiol. 2015;70(9):989–998. doi: 10.1016/j.crad.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson. 2012;14:43. doi: 10.1186/1532-429X-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padiyath A, Gribben P, Abraham JR, et al. Echocardiography and cardiac magnetic resonance-based feature tracking in the assessment of myocardial mechanics in tetralogy of Fallot: an intermodality comparison. Echocardiography. 2013;30(2):203–210. doi: 10.1111/echo.12016. [DOI] [PubMed] [Google Scholar]
- 19.Onishi T, Saha SK, Delgado-Montero A, et al. Global longitudinal strain and global circumferential strain by speckle-tracking echocardiography and feature-tracking cardiac magnetic resonance imaging: comparison with left ventricular ejection fraction. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2015;28(5):587–596. doi: 10.1016/j.echo.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. The international journal of cardiovascular imaging. 2002;18(1):539–542. [PubMed] [Google Scholar]
- 21.Helm RH, Leclercq C, Faris OP, et al. Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: implications for assessing cardiac resynchronization. Circulation. 2005;111(21):2760–2767. doi: 10.1161/CIRCULATIONAHA.104.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen BD, Fernandes VR, Nasir K, et al. Age, increased left ventricular mass, and lower regional myocardial perfusion are related to greater extent of myocardial dyssynchrony in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(10):859–866. doi: 10.1161/CIRCULATIONAHA.108.787408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjaer A, Lebech AM, Hesse B, Petersen CL. Right-sided cardiac function in healthy volunteers measured by first-pass radionuclide ventriculography and gated blood-pool SPECT: comparison with cine MRI. Clinical physiology and functional imaging. 2005;25(6):344–349. doi: 10.1111/j.1475-097X.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 24.Haeck ML, Hoke U, Marsan NA, et al. Impact of right ventricular dyssynchrony on left ventricular performance in patients with pulmonary hypertension. The international journal of cardiovascular imaging. 2014;30(4):713–720. doi: 10.1007/s10554-014-0384-1. [DOI] [PubMed] [Google Scholar]
- 25.Louie EK, Lin SS, Reynertson SI, Brundage BH, Levitsky S, Rich S. Pressure and volume loading of the right ventricle have opposite effects on left ventricular ejection fraction. Circulation. 1995;92(4):819–824. doi: 10.1161/01.cir.92.4.819. [DOI] [PubMed] [Google Scholar]
- 26.Lin SS, Reynertson SI, Louie EK, Levitsky S. Right ventricular volume overload results in depression of left ventricular ejection fraction. Implications for the surgical management of tricuspid valve disease. Circulation. 1994;90(5 Pt 2):II209–213. [PubMed] [Google Scholar]
- 27.Puwanant S, Park M, Popovic ZB, et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation. 2010;121(2):259–266. doi: 10.1161/CIRCULATIONAHA.108.844340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardegree EL, Sachdev A, Fenstad ER, et al. Impaired left ventricular mechanics in pulmonary arterial hypertension: identification of a cohort at high risk. Circulation Heart failure. 2013;6(4):748–755. doi: 10.1161/CIRCHEARTFAILURE.112.000098. [DOI] [PubMed] [Google Scholar]
- 29.Gan C, Lankhaar JW, Marcus JT, et al. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. American journal of physiology Heart and circulatory physiology. 2006;290(4):H1528–1533. doi: 10.1152/ajpheart.01031.2005. [DOI] [PubMed] [Google Scholar]
- 30.Muzzarelli S, Ordovas KG, Cannavale G, Meadows AK, Higgins CB. Tetralogy of Fallot: impact of the excursion of the interventricular septum on left ventricular systolic function and fibrosis after surgical repair. Radiology. 2011;259(2):375–383. doi: 10.1148/radiol.10100895. [DOI] [PubMed] [Google Scholar]


