Capsule Summary
The PPAR-γ agonist, pioglitazone, was associated with significant side effects and did not improve the primary outcome measure of the Juniper Asthma Quality of Life Questionnaire (AQLQ) score in severe asthmatics. We conclude that no further studies should be performed with pioglitazone for severe asthma.
Keywords: Asthma, Pioglitazone, Peroxisome Proliferator-activated Receptor-γ (PPAR-γ)
To the Editor
The peroxisome proliferator-activated receptor (PPAR) family of nuclear receptor transcription factors regulate metabolism, inflammation, and immunity1. Pharmacological agents that activate PPAR-γ, such as thiazolidinediones (TZD), sensitize cells to insulin and are used to treat diabetes. PPAR-γ is also expressed in the lung where it has anti-inflammatory effects2. Based upon the protective effects of PPAR-γ agonists in murine asthma models2, 3, we performed a clinical trial of the TZD, pioglitazone, to assess whether it might be beneficial for severe asthmatics who are persistently symptomatic despite treatment with high doses of inhaled corticosteroids with or without oral prednisone. The objective of this randomized, double-blind, placebo-controlled, two-period, crossover study was to assess whether the primary outcome measure of the Juniper asthma quality of life questionnaire score (AQLQ) would be significantly higher at the end of the pioglitazone treatment period as compared to placebo in severe asthmatics.
This study of pioglitazone hydrochloride in severe asthma was an investigator-initiated study that was conducted at the NIH Clinical Research Center in Bethesda, Maryland between October 2009 and June 2016. Protocol 09-H-0244 was approved by the NHLBI Institutional Review Board and all participants provided informed consent. Subjects whose baseline asthma status did not change during a 4-week run-in period (e.g., absence of unscheduled health care visits for asthma care and no change in maintenance asthma medications) were randomized to either pioglitazone or matching placebo (see Figure E1 in this article’s Online Repository at www.jacionline.org). Pioglitazone, 30 mg daily, was administered for the initial 2 weeks of the first treatment phase, followed by 45 mg daily for an additional 14 weeks. This was followed by a 4-week washout period. Subjects were assessed for clinical stability during a second 4-week run-in period prior to crossing over to the second treatment phase to receive the other agent. Therefore, each subject received both placebo and study drug at different times during the study. Detailed methods are reported in this article’s Online Repository at www.jacionline.org.
Of the 59 subjects screened for the study, 34 met inclusion criteria for participation and 16 subjects underwent randomization (Figure 1). The study was terminated early due to difficulty in recruiting additional subjects to achieve a sample size of 26 completers. The demographics for the 12 subjects who were enrolled, randomized, and completed the study are shown in Table E1 in this article’s Online Repository at www.jacionline.org. Subjects were predominantly white with elevated body mass index (BMI), moderately severe airflow obstruction, and poor asthma control. All subjects had a history of allergy and were receiving high doses of inhaled corticosteroids. Five subjects were also receiving an inhaled long-acting β2-agonist, 2 subjects were receiving oral prednisone, and 4 subjects were receiving an inhaled long-acting β2-agonist plus oral prednisone. Eleven subjects were receiving a leukotriene-modifying agent, while 5 subjects were receiving omalizumab. Two subjects who were randomized withdrew from the study for personal reasons, while study drug administration was discontinued in two subjects due to adverse events of pedal edema and presumptive angioedema. Details of adverse events are reported in this article’s Online Repository at www.jacionline.org and Table E2.
Figure 1. Flow of subjects through the study showing number of participants screened, randomized, withdrawn, and completed.
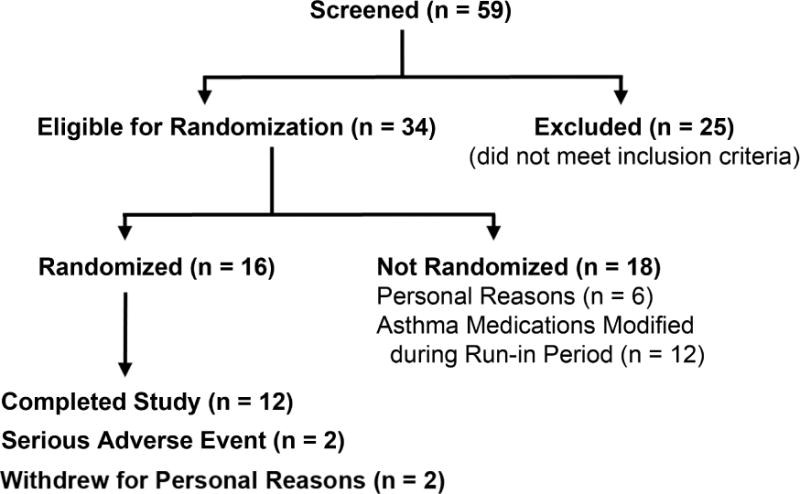
Participants who were excluded did not meet the pre-specified inclusion criteria.
There was no difference regarding the pre-specified primary outcome measure of the Juniper AQLQ score at the end of the pioglitazone as compared to the placebo treatment period (4.7 ± 1.3 vs. 4.6 ± 1.3, p=0.677, Figure 2). Similarly, no difference in the Juniper AQLQ score was observed by period (p=0.456). A similar analysis was performed using a mixed effects method to model correlation within subjects with a random intercept, which yielded the same conclusion. There were also no differences in pre-specified secondary outcome measures regarding asthma symptoms or control (Figure 2), airflow obstruction and inflammation (details are reported in the Online Repository and Figures E2–E4), except for a significantly lower peripheral blood lymphocyte count after the pioglitazone as compared to the placebo treatment periods (2.1 ± 0.8 vs. 2.6 ± 1.1 K/μl, p = 0.002).
Figure 2. Comparison of pioglitazone and placebo treatment periods for the primary end-point of asthma quality of life questionnaire score and secondary end-points of asthma symptoms and control.
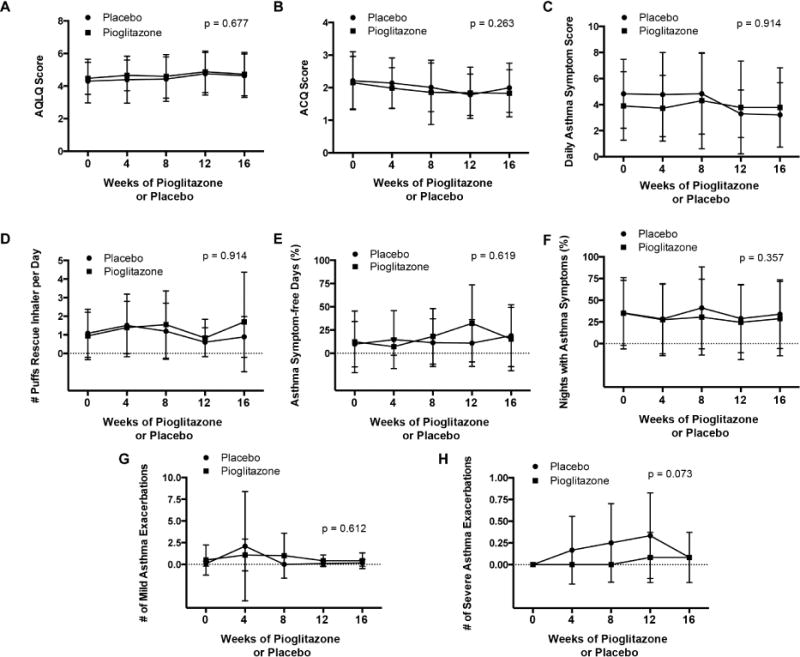
Panel A. Juniper asthma quality of life (AQLQ) score. Panel B. Juniper asthma control questionnaire (ACQ) score. Panel C. Daily asthma symptom score. Panel D. Number of puffs of rescue β2-agonist inhaler per day. Panel E. Asthma symptom-free days (%). Panel F. Nights with asthma symptoms (%). Panel G. Number of mild asthma exacerbations. Panel H. Number of severe asthma exacerbations. Data for daily asthma symptom score, rescue β2-agonist inhaler use, asthma symptom-free days, nights with asthma symptoms are averages for each period based upon the days that subjects reported data. P values are shown for differences between the pioglitazone and placebo treatment periods.
Although several clinical studies generated initial evidence suggesting that the TZD class of PPAR-γ agonist medications might be beneficial for asthma 4, 5, results from subsequent randomized clinical trials have either showed no effect or only a modest improvement in pre-specified primary end-points6–9. Here, we assessed the role of pioglitazone in severe asthma. Our cohort of severe asthmatics was characterized by a history of allergy, obesity, moderately severe airflow obstruction, and elevated FeNO consistent with airway inflammation. We show that treatment with pioglitazone for 4 months was not different than placebo regarding the pre-specified, primary outcome measure of the Juniper AQLQ score. Although our study was terminated prematurely, had we continued until we had achieved 26 completers, we still would have had little chance of obtaining a significant difference between the two treatment arms based upon a conditional power of 0.007. There were also no differences between pioglitazone and placebo regarding pre-specified secondary end-points, except for a reduction in peripheral blood lymphocytes in the pioglitazone treatment arm. We cannot, however, exclude the possibility that a difference between the two treatment arms might have been detected if the duration of the treatment period had been longer.
Two out of 14 subjects experienced severe adverse reactions secondary to pioglitazone. These included significant peripheral edema in one subject and presumptive angioedema in another. Peripheral edema is a recognized adverse effect of pioglitazone therapy that occurs because of fluid retention. Although angioedema is not known to be an adverse effect of pioglitazone, individuals with a known hypersensitivity should not receive this medication. Other potential serious side effects related to pioglitazone, such as congestive heart failure, hypoglycemia, hepatotoxicity, urinary bladder tumors, fractures, or decreased visual acuity due to macular edema did not occur.
Our results suggest that pioglitazone is not safe to use in severe asthma as 14% of subjects experienced pioglitazone-related severe adverse events that necessitated discontinuation of the study medication. Furthermore, although our sample size was small, pioglitazone did not improve the primary end-point of the AQLQ score. Taken collectively with the results from other clinical trials of TZDs, the absence of a positive treatment effect, as well as the potential for significant side effects, suggest that no further clinical trials of the PPAR-γ agonist, pioglitazone, should be performed for severe asthma.
Supplementary Material
Acknowledgments
This research was funded by Division of Intramural Research at the National Heart, Lung, and Blood Institute, NIH, Bethesda, Maryland.
We are most grateful for the participation of the research subjects in this study, as well as their primary physicians. We are also very appreciative of the staffs of the NHLBI Pulmonary Function Laboratory, the NCI Cytopathology Laboratory, Dr. Oleh Hnatiuk, and Dr. Rita Volochayev for their contributions to this study.
Financial Support: Division of Intramural Research at the National Heart, Lung, and Blood Institute, NIH, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–8. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Belvisi MG, Hele DJ, Birrell MA. Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation. Eur J Pharmacol. 2006;533:101–9. doi: 10.1016/j.ejphar.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 3.Ward JE, Tan X. Peroxisome proliferator activated receptor ligands as regulators of airway inflammation and remodelling in chronic lung disease. PPAR Res. 2007;2007:14983. doi: 10.1155/2007/14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinne ST, Feemster LC, Collins BF, Au DH, Perkins M, Bryson CL, et al. Thiazolidinediones and the risk of asthma exacerbation among patients with diabetes: a cohort study. Allergy Asthma Clin Immunol. 2014;10:34. doi: 10.1186/1710-1492-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandhu MS, Dimov V, Sandhu AK, Walters RW, Wichman T, Casale T. The use of the peroxisome proliferator-activated receptors gamma agonist rosiglitazone to treat airway hyperreactivity. Ann Allergy Asthma Immunol. 2012;109:75–7. doi: 10.1016/j.anai.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Spears M, Donnelly I, Jolly L, Brannigan M, Ito K, McSharry C, et al. Bronchodilatory effect of the PPAR-γ agonist rosiglitazone in smokers with asthma. Clin Pharmacol Ther. 2009;86:49–53. doi: 10.1038/clpt.2009.41. [DOI] [PubMed] [Google Scholar]
- 7.Richards DB, Bareille P, Lindo EL, Quinn D, Farrow SN. Treatment with a peroxisomal proliferator activated receptor gamma agonist has a modest effect in the allergen challenge model in asthma: a randomised controlled trial. Respir Med. 2010;104:668–74. doi: 10.1016/j.rmed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JR, Mortimer K, Pang L, Smith KM, Bailey H, Hodgson DB, et al. Evaluation of the PPAR-gamma Agonist Pioglitazone in Mild Asthma: A Double-Blind Randomized Controlled Trial. PLoS One. 2016;11:e0160257. doi: 10.1371/journal.pone.0160257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon AE, Subramanian M, DeSarno M, Black K, Lane L, Holguin F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir Res. 2015;16:143. doi: 10.1186/s12931-015-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


