Abstract
In recent years, advances in optical coherence tomography (OCT) techniques have increased our understanding of diabetic retinopathy, an important microvascular complication of diabetes. OCT angiography is a non-invasive method that visualizes the retinal vasculature by detecting motion contrast from flowing blood. Visible-light OCT shows promise as a novel technique for quantifying retinal hypoxia by measuring the retinal oxygen delivery and metabolic rates. In this article, we discuss recent insights provided by these techniques into the vascular pathophysiology of diabetic retinopathy. The next milestones for these modalities are large multicenter studies to establish consensus on the most reliable and consistent outcome parameters to study diabetic retinopathy.
Keywords: Retina, Imaging, Diabetic retinopathy, OCT, OCT angiography, Visible-light OCT
1.1 Basics of Optical Coherence Tomography Angiography
Optical coherence tomography angiography (OCTA) is a relatively recent technique that provides three-dimensional (3D) imaging of the retinal and choroidal vasculature. While conventional OCT excels at capturing static 3D structural information within the retina, the image contrast from blood vessel walls alone is not sufficient to provide meaningful angiograms. On the other hand, OCTA exploits the movement of blood cells to generate angiograms with exquisite image contrast.
In order to extract information about blood cell movement, OCTA systems acquire two or more consecutive B-scans at the same location in the retina (Figure 1). Recent improvements in OCT data acquisition speeds have allowed complex scanning protocols to be implemented within a few seconds, leading to the feasibility of clinical OCTA (Gao, et al., 2016). Algorithms were then developed to extract motion contrast from the variable scattering of moving blood cells. For example, red blood cells scatter differently depending on their motion, orientation, or shape (Srinivasan, Chan & Lam, 2012). OCTA algorithms remove OCT signals with unchanged scattering from static tissue and preserve the OCT signals associated with motion in order to produce the 3D OCTA volume and en face projection (Figure 1(F)).
Figure 1. Scanning Patterns Used in OCT and OCTA.
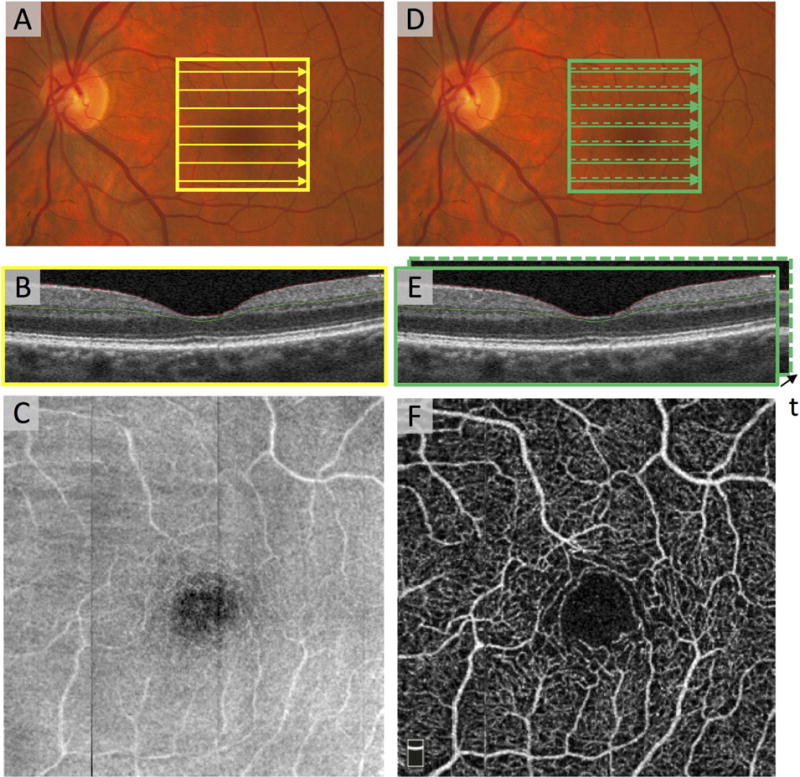
(A) Fundus photograph of the macula and optic nerve head in a healthy eye. The solid yellow box denotes 3×3 mm2, which was imaged by OCT. The yellow arrows depict the path that the illumination beam takes as it raster scans. The scanning density along the y direction is sparse for illustration purposes. (B) The OCT B-scan corresponding to arrow across the fovea in (A). (C) The en face OCT acquired from the region in (A). (D) The solid green box indicates a region on the macula imaged with OCTA. The green solid arrow and green dashed arrow indicate the first and second B-scan locations for OCTA. (E) The two co-localized B-scans are separated in time. (F) Co-localized B-scans are fed into an OCTA algorithm to produce en face OCTA image of the region.
Raw data from OCT A-scans is complex-valued, therefore OCTA algorithms can compare the amplitude, the phase, or both between B-scans (Zhang, et al., 2015a). The optical microangiography (OMAG) algorithm compares both amplitude and phase differences between consecutive B-scans (An, Shen & Wang, 2011, Wang, et al., 2010., while split-spectrum amplitude-decorrelation (SSADA) uses only amplitude. The SSADA algorithm also splits the OCT spectrum into 11 sub-bands, thereby improving the signal to noise ratio but decreasing axial resolution of the final image (Gao, et al., 2015).
Patients were recruited for this study in the Department of Ophthalmology at Northwestern University in Chicago, Illinois between June 15, 2015 and December 9, 2016. This study was approved by the Institutional Review Board of Northwestern University, followed the tenets of the Declaration of Helsinki and was performed in accordance with the Health Insurance Portability and Accountability Act regulations. Written informed consent was obtained from all participants.
1.2 Comparison of Fluorescein and OCT Angiography
Fluorescein angiography (FA) is the current “gold standard” for evaluating the vasculature in diabetic retinopathy (DR). This procedure requires intravenous dye injection which can lead to adverse reactions (Kwiterovich, et al., 1991). OCTA is a noninvasive, label-free technique that has expanded our understanding of the microvascular changes in DR and could potentially reduce the need for FA. Many of the common vascular features of DR are clearly visualized in OCTA (Choi, et al., 2016, Ishibazawa, et al., 2015) (Figure 2). Yet, most OCTA studies utilize a 3 × 3 mm2 (~7°) scanning area centered on the macula, compared the much wider field (50°, 120°, and 200°) possible with more conventional imaging modalities (Figure 3).
Figure 2. Common Features of Diabetic Retinopathy on OCTA.
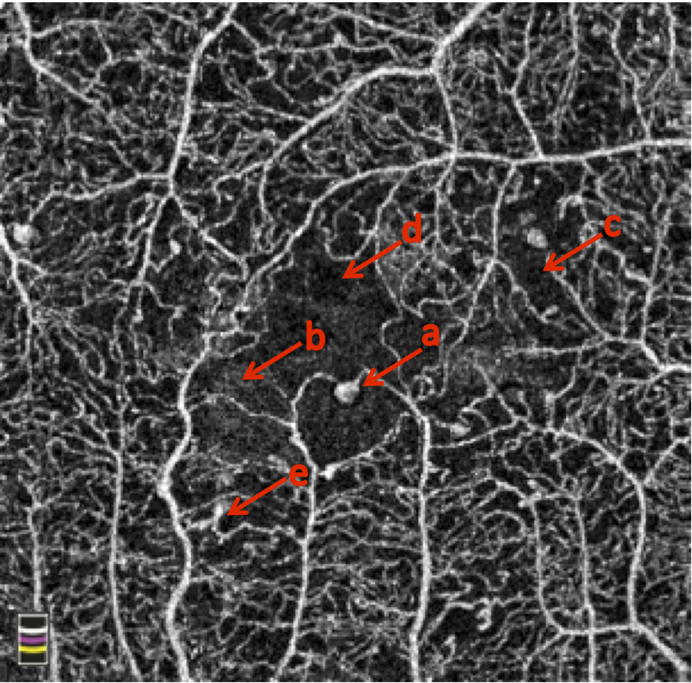
(a) microaneurysms, (b) enlarged foveal avascular zone, (c) non-perfusion, (d) edema, (e) abnormal vascular loops.
Figure 3. Field of View Limitations in OCTA Shown in Two Eyes with Proliferative Diabetic Retinopathy.
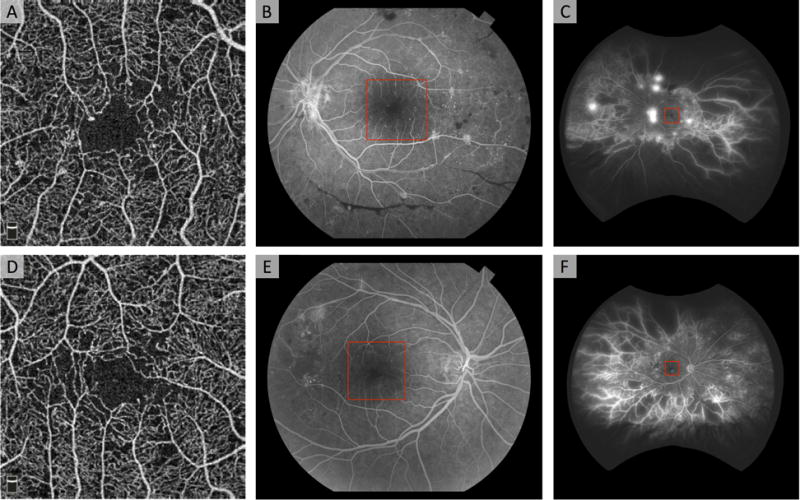
(A, D) En face 3 × 3 mm2 OCTA image of the superficial capillary plexus reveals mild diabetic changes including enlarged foveal avascular zone, areas of non-perfusion, and microaneurysms. (B, E) Fluorescein angiography obtained with fundus camera (50° field of view) reveals hemorrhages and neovascularization (NV) in B, and non-perfusion and NV in E. (C, F) Fluorescein angiography obtained with Optos wide-field imaging (200° field of view) reveals extensive peripheral non-perfusion and NV.
Fluorescein angiography is interpreted by examining how the image changes over time as dye passes through the retinal blood vessels. The dynamic features of blood (i.e., dye leakage, dye staining) are used to identify retinal lesions. In DR, hypofluorescent non-perfusion and hyperfluorescent microaneurysms (MAs) are typically best seen in the early phases of the angiogram. Diabetic macular edema (DME) and neovascularization (NV) start as hyperfluorescent areas that leak in the later phases (Group, 1991a, Group, 1991c, Group, 1987). While dye leakage provides useful information, it can blur or occlude the boundaries of capillary dropout or NV. In contrast, while OCTA does not visualize the dynamic features of blood, the images are not occluded by leakage, and therefore OCTA more clearly delineates the boundaries of capillary loss and the detailed structure of NV (Couturier, et al., 2015, Hwang, et al., 2015).
While FA provides little depth-resolved information, OCTA can produce en face angiograms of the specific capillary plexuses from the 3D OCT volume. Most commercial OCTA software divides the retinal vasculature into two plexuses: superficial capillary plexus (SCP) and deep capillary plexus (DCP). However, it is important to note that anatomically, the retinal vasculature in the macula consists of three distinct plexuses – the SCP, the middle capillary plexus (MCP), and the DCP (Campbell, et al., 2017, Park, Soetikno & Fawzi, 2016). Figure 4 shows an example of the commonly segmented SCP and DCP on OCTA compared with FA in a patient with DR. Note that many of the same vessels are seen in each of the three angiograms due to limitations of OCTA in separating layers in the axial direction, as well as projection artifacts.
Figure 4. OCTA and Fluorescein Angiography (FA) of Diabetic Retinopathy.
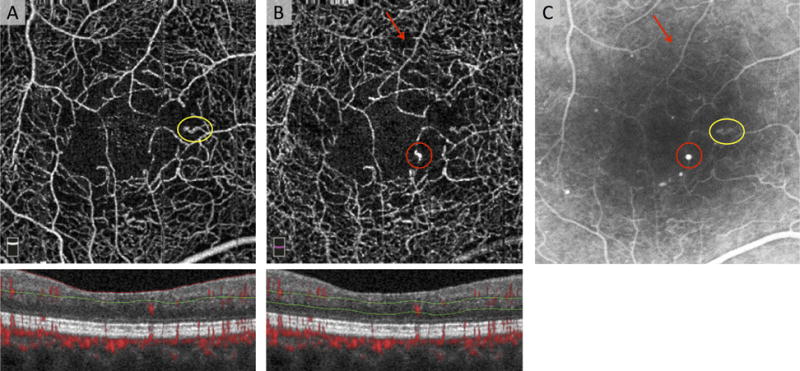
OCTA of the (A) superficial capillary plexus (SCP), and (B) deep capillary plexus (DCP) with cross-sectional OCTA below including red flow overlay, and red and green segmentation boundaries. Note that many of the vessels in the SCP (A) are also seen in the DCP (B) due to projection artifact. (C) Corresponding FA. The yellow circle on in C corresponds to an abnormal, dilated capillary loop that is seen in the SCP (A). This loop is also seen in the DCP, but this is likely due to projection artifact. The red circle in C corresponds to a microaneurysm that is only visible in the DCP (B). The red arrow points to an area of capillary non-perfusion that is better delineated with OCTA, but is not appreciated as clearly on FA due to dye leakage.
1.3.1 Quantifying Macular Vessel Density on OCT Angiography
Since OCTA is not obscured by dye leakage, macular vessel density measurements from OCTA may be more practical and precise than those from FA. Several methods to quantify vessel density with automated algorithms have been described (Chu, et al., 2016). The major steps in these algorithms are shown in Figure 5. First, the 2D en face projection (Figure 5(A)) is enhanced and then thresholded through a variety of techniques, which generates a binary vascular map (Kim, et al., 2016, Reif, et al., 2012). After binarization, vessel centerlines are defined by skeletonization (Agemy, et al., 2015) as shown in green in Figure 5(C).
Figure 5. Quantifying Vessel Density in OCTA Images.
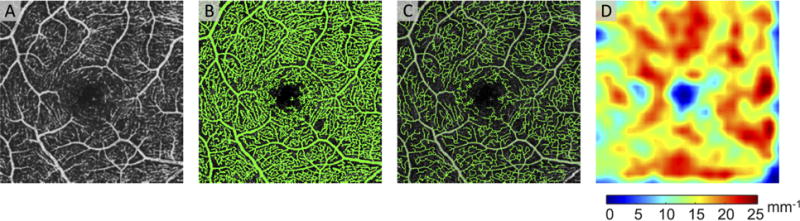
(A) An example of an en face 3×3 mm2 region centered on the healthy human fovea. (B) A thresholded image shows the binarized vasculature in green. (C) After skeletonization, the vessel centerlines are determined. The vessel centerlines are shown as green lines overlayed on top of the en face OCTA. (D) The vessel density is calculated from the vessel skeleton.
One approach to quantifying the number of vessels within an area of retina, which we refer to as “vessel density” in this review, is to measure from the binarized image as a percentage of vessel pixels (Shahlaee, et al., 2016). In another approach, which we refer to as “skeletonized vessel density”, the vessel skeleton map is used to calculate vessel density as the length of vessel skeleton (mm−1) (Figure 5(D)). In the first approach, large vessels count more towards the vessel density metric, while in the second approach, large vessels and small capillaries carry the same weight. This difference can lead to discordances in vessel density outcomes.
1.3.2 Clinical Applications of Quantitative Macular Vessel Density
Significant differences for numerous OCTA variables have been reported in eyes with various stages of DR compared to healthy controls (Agemy et al., 2015, Al-Sheikh, et al., 2016, Choi et al., 2016, Kim et al., 2016, Samara, et al., 2016, Zahid, et al., 2016). Generally, vessel density values gradually become lower from healthy controls to patients with diabetes mellitus without DR to non-proliferative (NPDR) to proliferative (PDR), but results vary based on the methods used to obtain the vessel density value. Fractal dimensional analysis, which measures the complexity of vascular patterns, is also significantly reduced in patients with DR compared to healthy controls (Zahid et al., 2016). Figure 6 shows an example of the capillary changes associated with increasing DR severity for each of the three plexuses.
Figure 6. Parafoveal Vessel Density Decreases and Foveal Avascular Zone (FAZ) Area Increases with Worsening Diabetic Retinopathy (DR) Severity.
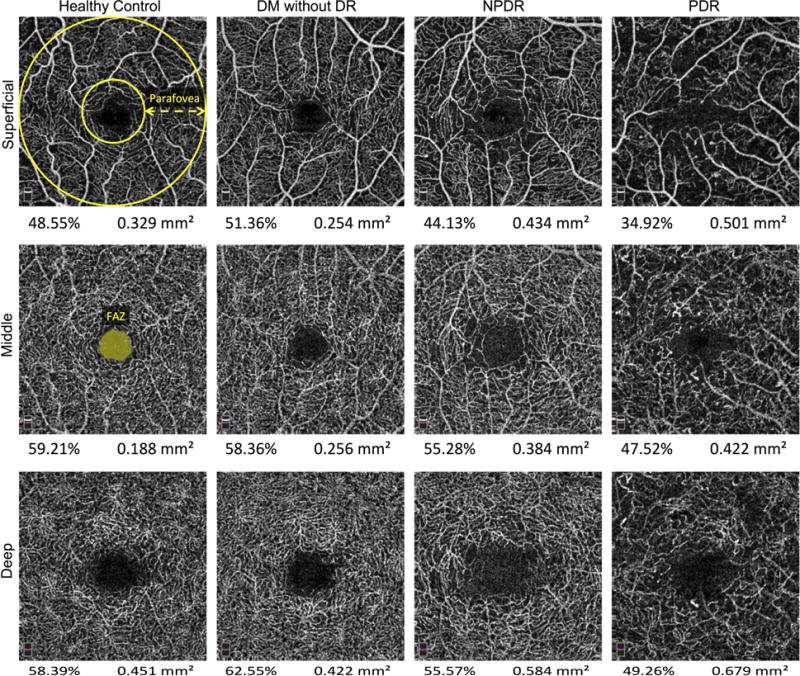
En face OCTA of the superficial (SCP, Top Row), middle (MCP, Middle Row), and deep capillary plexus (DCP, Bottom Row) of a healthy patient (Left Column), a patient with diabetes mellitus (DM) without DR (Left Middle Column), a patient with nonproliferative (NPDR, Right Middle Column), and a patient with proliferative (PDR, Right Column). The parafovea is defined as the percent area occupied by vessels between the two yellow circles overlaid on the healthy patient SCP. The FAZ area can be automatically or manually calculated and is defined as the avascular area of the central fovea, reported in mm2 (seen on the healthy patient MCP). The vessel density and FAZ area are seen below each angiogram.
Table 1 also highlights some significant correlations reported between DR severity and macular vessel density, as well as other OCTA-based parameters (i.e., skeletonized vessel density, fractal dimension, vessel diameter index or average vessel caliber, foveal avascular zone size) (Agemy et al., 2015, Bhanushali et al., 2016, Kim et al., 2016, Lin et al., 2016). Larger population studies are needed to understand the role of OCTA in distinguishing the clinical stages of DR. However, the research community must first reach a consensus on the best approach for calculating vessel density and other OCTA parameters to overcome the wide discordance between studies.
Table 1.
Quantitative OCTA Studies of Capillary Abnormalities in Diabetic Retinopathy
| Reference | Eyes/pts DM Eyes/pts HC | DR Severity (no. of eyes) | Vessel Density Lower in DR vs Control | Trend with DR Severity | FAZ Larger in DR vs Control | Projection Artifact Removal | Segmentation, Density Type, Methods | Device |
|---|---|---|---|---|---|---|---|---|
| (Agemy et al., 2015). | 55 of 34 21 of 12 |
11 mild NPDR, 9 moderate NPDR, 9 severe NPDR, 26 PDR | SCP and DCP (P<0.05) | (P<0.05) | NA | Not performed | Skeletonized vessel density | RTVue, SD-OCTA (840 nm) |
|
|
||||||||
| (Al-Sheikh et al., 2016). | 28 of 18 40 of 22 |
10 mild NPDR, 10 moderate NPDR, 2 severe NPDR, 6 PDR, (13 had DME) | SCP (P<0.001), DCP (P=0.028) | NA | SCP (P=0.003), DCP (P<0.001) | Not performed | Vessel density, DCP segmented to included only the inner nuclear layer | Topcon SS-OCTA (1050 nm) |
|
|
||||||||
| (Bhanushali, et al., 2016). | 209 of 122 60 of 31 |
35 mild NPDR, 95 moderate NPDR, 57 severe NPDR, 22 PDR, Controls were younger with higher female percentage | SCP and DCP (P<0.001) | None | SCP and DCP (P=0.001) | Not performed | Fractal = distance b/t large and small vessels, Vessel density; Gender and age difference not corrected for | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (de Carlo, et al., 2015). | 61 of 39 28 of 22 |
61 NoDR | NA | NA | Full thickness “DM NoDR” vs “Control” (P=0.04) | Not performed | FAZ area, FAZ remodeling, Non-perfusion | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (Di, et al., 2016). | 113 of 65 85 of 62 |
53 NoDR, 45 NPDR, 15 PDR | NA | NA | Full thickness “DM NoDR” vs “Control” (P=0.04) | Not performed | FAZ: area, vertical radius (VR), and horizontal radius (HR) | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (Freiberg, et al., 2015a). | 29 of 15 25 of 22 |
18 NPDR, 4 pre-PDR, 13 PDR | NA | NA | MFD SCP (P=0.008), DCP (P<0.001) | Not performed | Maximum FAZ diameter (MFD) | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (Hwang, et al., 2016a). | 12 of 12 12 of 12 |
2 mild NPDR, 1 moderate NPDR, 9 PDR | NA | Total avascular area detects DR with 100% specificity (AROC = 1) | FAZ area detects DR with 50% specificity (AROC = 0.77) | Not performed | Automated avascular quantification, Area under receiver operating curve (AROC), 6×6 | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (Hwang, et al., 2016b). | 47 of 29 29 of 15 |
11 mild to moderate NPDR, 13 severe NPDR, 23 PDR | 100% sensitivity and specificity detect DR with PR-OCTA of all 3 plexuses | NA | NA | PR-OCTA | Sensitivity and Specificity of detecting DR better with PR-OCTA, Custom segmentation | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (Ishibazawa et al., 2015). | 47 of 25 0 controls |
11 mild NPDR, 13 moderate NPDR, 12 severe NPDR, 11 PDR | SCP ischemia was greater than DCP ischemia (P=0.018) | NA | NA | Not performed | Manually traced ischemic area | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (Kim et al., 2016). | 84 of 50 14 of 8 |
32 mild NPDR, 16 severe NPDR, 36 PDR | Less parameters significantly different between sup-groups in DCP compared to SCP. | Parameters: VD, SD, FD, VDI correlated with DR severity – most were (p<0.05) | NA | Not performed | Vessel density (VD), Skeletonized vessel density (SD), Fractal dimension (FD), Vessel diameter index (VDI) | Cirrus SD-OCTA (840 nm) |
|
|
||||||||
| (Lin, et al., 2016). | 51 of 33 0 controls |
17 NoDR to mild NPDR, 21 moderate to severe NPDR, 13 PDR | NA | Full Thickness: PI decrease with DR severity (p<0.001 to 0.862) | NA | Not performed | Perfusion index (PI) = % coverage of vessels (vessel density) | Cirrus SD-OCTA (840 nm) |
|
|
||||||||
| (Salz, et al., 2016). | 43 of 30 11 of 6 |
13 NoDR, 11 mild NPDR, 6 moderate NPDR, 5 severe NPDR, 8 PDR | More MAs in DCP than SCP. Full Thickness PIA: Higher in DM vs Control (P<0.001), PDR vs NoDR (P<0.05), and PDR vs NPDR (P<0.05) | NA | DM vs Control (P<0.001), PDR vs NoDR (P<0.05), and PDR vs NPDR (P<0.05) | Not performed | FAZ area, Perifoveal intercapillary area (PIA) | Prototype SS-OCTA (1060 nm) |
|
|
||||||||
| (Samara et al., 2016). | 84 of 55 34 of 27 |
32 mild NPDR, 31 moderate to severe NPDR, 21 PDR, (No DME) | SCP (P<0.001), DCP (P<0.001) | VLD, VAD, FAZ correlated with VA for SCP & DCP (p<0.001) | SCP (p<0.01), DCP (P<0.001) | Not performed | FAZ area, Vessel area density (VAD), Vessel length density (VLD) | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (Schottenhamml, et al., 2016). | 21 of 21 5 of 5 |
7 NoDR, 9 NPDR, 5 PDR | NA | Full Thickness: General intercapillary area increased with DR severity | Less difference between groups when FAZ was included in analysis | Not performed | Mean largest 10 and 20 intercapillary areas; Vesselness filter; No statistics | Prototype SS-OCTA (1050 nm) |
|
|
||||||||
| (Takase, et al., 2015). | 44 eyes 19 eyes |
24 NoDR, 17 mild NPDR, 3 moderate NPDR | NA | NA | All DM vs controls: SCP (P<0.01), DCP (P<0.01), NoDR vs Control (P<0.01) | Not performed | Manually traced FAZ area | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (Zahid et al., 2016). | 13 of 8 56 of 29 |
5 mild NPDR, 8 PDR (No DME), Controls were younger | SCP (P<0.05), DCP (P<0.05), plus fractal | NA | NA | Not performed | Fractal dimensional analysis, Vessel density; Age difference not corrected for | RTVue SD-OCTA (840 nm) |
|
|
||||||||
| (Zhang, et al., 2016b). | 13 of 13 13 of 13 |
13 mild NPDR, Controls were younger | Segmented angiograms distinguish better, SCP: EAA (P<0.001) and TAA (P=0.007); DCP: EAA (P<0.001) and TAA (P=0.022) |
NA | NA | PR-OCTA | Total (TAA) and Extrafoveal avascular area (EAA), Vesselness filter; Age difference not corrected for | RTVue SD-OCTA (840 nm) |
Definitions: AROC = Area under receiver operating curve; DCP = Deep capillary plexus; DM = Diabetes mellitus; DME = Diabetic macular edema; DR = Diabetic retinopathy; EAA = Extrafoveal avascular area; FAZ = Foveal avascular zone; FD= Fractal dimension; HC = Healthy controls; HR = Horizontal radius of the foveal avascular zone; MA = Microaneurysm; MCP = Middle capillary plexus; MFD = Maximum foveal avascular zone diameter; NA = Not applicable (parameter not measured in the study); NoDR = Diabetes mellitus without diabetic retinopathy; NPDR = Non-proliferative diabetic retinopathy; PDR = Proliferative diabetic retinopathy; PI = Perfusion index (vessel density); PIA = Perifoveal intercapillary area; PR-OCTA = Projection-resolved optical coherence tomography angiography; Pts = Patients; SCP = Superficial capillary plexus; SD = Skeletonized vessel density; SD-OCTA = Spectral-domain optical coherence tomography angiography; Sig. = statistically significant; SS-OCTA = Swept-source optical coherence tomography angiography; TAA = Total avascular area; VA = Visual acuity; VAD = Vessel area density; VD = Vessel density; VDI = Vessel diameter index; VLD = Vessel length density; Vesselness filter = Enhances vessels by correcting for fluctuations in structural OCT signal intensity and by suppressing background noise; VR = Vertical radius of the foveal avascular zone.
1.4 Foveal Avascular Zone on OCT Angiography
Enlargement of the foveal avascular zone (FAZ), a common feature of DR, results from the occlusion of retinal capillaries and loss of precapillary arterioles near the fovea. These features have been extensively reported using FA (Arend, et al., 1994, Bertram, et al., 1991, Bresnick, et al., 1984), including in patients with diabetes without clinical DR (Arend, et al., 1991). FA images can be acquired through the use of a digital camera, scanning laser ophthalmoscope, as well as video angiography (Freeman, et al., 1998, Wolf, et al., 1989). FAZ enlargement in FA is associated with worse visual acuity due to the functional consequences of ischemia near the fovea (Arend, et al., 1995, Bresnick et al., 1984). Similarly, a significant correlation between FAZ area and visual acuity in patients with DR was shown using OCTA (Balaratnasingam, et al., 2016). OCTA studies of the FAZ in diabetic eyes have shown high-contrast images of the foveal capillaries, beyond what can be appreciated in FA (Al-Sheikh et al., 2016, Bhanushali et al., 2016, Choi et al., 2016, de Carlo et al., 2015, Di et al., 2016, Freiberg, et al., 2015b, Samara et al., 2016, Takase et al., 2015).
While there is a significant correlation between FAZ size on OCTA versus FA (Cennamo, et al., 2016), OCTA may be a more suitable technique than conventional FA for evaluating the FAZ because 1) OCTA images are not occluded by dye leakage, and 2) the non-invasive nature of OCTA allows earlier and more frequent imaging for following disease progression. Numerous OCTA studies show that patients with diabetes without clinical DR have a significantly enlarged FAZ compared to healthy controls (de Carlo et al., 2015, Di et al., 2016, Takase et al., 2015). The non-invasive nature of OCTA allows routine imaging of the retinal vasculature and FAZ in patients with or without clinical DR, even when FA is not indicated. This could provide valuable information about early and subtle microvascular changes around the fovea in patients with diabetes. Note that segmenting the retina using OCTA to measure the FAZ in each of the plexuses may not be ideal for obtaining scientific or clinical information, since the three macular capillary plexuses merge at the FAZ (Snodderly, Weinhaus & Choi, 1992).
1.5 Ischemia on OCT Angiography
The extent of diabetic macular ischemia (DMI) in FA provides important clinical and prognostic information regarding disease severity and progression (Group, 1991a, Group, 1991b). Recently, an OCTA-based grading scale for DMI showed moderate agreement with the grading results of DMI in FA, suggesting the suitability of OCTA in quantifying DMI and following DR progression (Bradley, et al., 2016).
Several OCTA parameters have been used to quantify macular ischemia on OCTA (i.e., perifoveal intercapillary area, total avascular area, extrafoveal avascular area) (Table 1). Automated algorithms quantifying DMI from full retinal thickness angiograms have shown the ability to distinguish DR from healthy controls (Hwang et al., 2016a), as well as trends of avascular area increasing with disease severity (Schottenhamml et al., 2016). Demonstrating the usefulness of OCTA in segmenting the individual capillary plexuses, studies showed that grading DMI in the three distinct plexuses (SCP, MCP, and DCP) had a higher sensitivity and specificity for determining DR versus healthy controls, as well as determining DR stage, compared to grading full retinal thickness angiograms (Zhang et al., 2016b, Hwang et al., 2016b). These results are likely due to the projection artifacts present in the full thickness angiograms, which can occlude the full extent of non-perfusion. Larger studies that distinguish the three networks may show important results, as they each serve different physiological functions and may be affected differently in DR (Park, Soetikno & Fawzi, 2016).
1.6 Diabetic Macular Edema and Microaneurysms
Diabetic macular edema (DME), the leading cause of vision loss in patients with DR, is visualized in en face OCTA as areas completely devoid of flow signal with smooth borders that do not follow the surrounding vessels (de Carlo, et al., 2016b, Matsunaga, et al., 2015). Eyes with DR and DME have increased FAZ and reduced vessel density in OCTA compared to those with DR without DME (Di et al., 2016, Kim et al., 2016). In OCTA, cystic spaces are surrounded by capillary non-perfusion, which show no evidence of reperfusion after resolution of DME (Mané, et al., 2016), suggesting that DME might preferentially develop in areas of ischemia.
Microaneurysms can originate in any of the three capillary plexuses (Park, Soetikno & Fawzi, 2016). The slower and more turbulent blood flow below the motion sensitivity threshold of OCTA inside MAs could explain why OCTA is unable to visualize the full extent of MAs seen on conventional FA (Couturier et al., 2015, Miwa, et al., 2016, Salz et al., 2016, Ploner, et al., 2016). A technique called variable interscan time analysis (VISTA) may help to overcome this shortcoming by adjusting the time between consecutive B-scans (Choi, et al., 2015).
Poor responders to anti-vascular endothelial growth factor (VEGF) for DME had, reportedly, more MAs and a larger FAZ area in the DCP compared to good responders (Lee, et al., 2016). Another study found a greater proportion of MAs in the DCP in areas of edema compared to surrounding areas, as well as a significant correlation between macular volume and MA density of the DCP (Hasegawa, et al., 2016). These studies suggest the possibility that MAs in the DCP contribute to the pathogenesis of DME and response to therapy.
1.7 Vascular Flow Compared With Retinal and Vascular Structure
An advantage of OCTA over FA is that OCTA provides both flow (en face OCTA) and structure (en face structural OCT, cross-sectional OCT) information within the same imaging modality. Utilizing this advantage, one study reported an association between DCP capillary non-perfusion on en face OCTA and photoreceptor disruption on cross-sectional OCT eyes with DR (Scarinci, Nesper & Fawzi, 2016). This observation is important in understanding the mechanism of visual compromise associated with macular ischemia in DR. In a comparison between en face OCTA and en face structural OCT in DR, some blood vessels in ischemic areas were seen on en face structural OCT, but not visualized with OCTA or FA (Miwa et al., 2016). These vessels, which do not demonstrate flow on OCTA but are still seen structural imaging, may have flow velocities below the OCTA detection threshold (Figure S3).
1.8 Neovascularization
While FA is currently a better method than OCTA for detecting NV due the much wider field of view, dye leakage on FA can obscure the morphology, so the borders of retinal NV may be better delineated on OCTA (Savastano, et al., 2016). Studies show that NV lesions are similar in shape and size in OCTA compared to FA (Yu, et al., 2016). Furthermore, quantitative evaluation of NV is possible on OCTA, which could be an objective way to follow patients and evaluate treatment response (Ishibazawa et al., 2015). A series of 12 eyes with preretinal NV showed NV in OCTA was located adjacent to non-perfusion (11 eyes, 92%) and intraretinal microvascular abnormalities (IRMA; 6 eyes, 50%) (de Carlo, et al., 2016a). While considered high-risk characteristics in NPDR, it remains debatable whether IRMA directly evolves into NV, and longitudinal OCTA studies could help determine if IRMA develops into NV over time (Cogan & Kuwabara, 1963, Imesch, Bindley & Wallow, 1997).
1.9 Limitations and Prospects of OCTA
OCTA suffers from artifacts related to the image acquisition technique and post-processing of en face images (Figure S5). Since OCTA obtains images by detecting motion contrast, any movement of the patient or the device during acquisition results in artifactual decorrelation (Figure S5(E)). Motion correction algorithms are applied to the raw data to improve image registration. For example, the Angiovue device uses motion correction technology (MCT), whereby two sequentially acquired OCTA volumes are registered to one another and merged to reduce motion artifacts (Kraus, et al., 2012). Eye tracking algorithms in conjunction with a pupil camera or scanning laser ophthalmoscope can also be used to detect and compensate for changes in eye fixation (Zhang, et al., 2015b). Interestingly, motion correction can also introduce new artifacts, especially from the volume merging step (Figure S5;D,F,G,H).
Another major limitation of OCTA is “projection artifact” or “decorrelation tails” (Zhang, Zhang & Wang, 2015). An example B-scan with projection artifacts from a healthy human eye shows flow signal from more superficial layers projected onto the deeper retinal layers in Figure S5(B). The projection artifacts are more prominent on retinal layers with high OCT reflectivity, such as the retinal pigment epithelium (RPE) (Zhang, Zhang & Wang, 2015). Recognition of projection artifacts is an important prerequisite for accurate interpretation of OCTA images.
A simple way to remove projection artifacts is to subtract superficial en face OCTA images from deeper en face OCTA images (Figure S6). The MCP in Figure S6(B) shows many projection artifacts, especially from the larger superficial blood vessels from the SCP (Figure S6(A)). A mask is applied to the SCP in the subtraction approach (Figure S6(C)), which is then multiplied by a weighting factor α, which prevents excessive subtraction of vasculature from the SCP. The weighted SCP mask is then subtracted from the underlying MCP resulting in Figure S6(D). The disadvantage of artifact removal via subtraction is that it can often leave the angiograms with a disconnected appearance. Algorithms such as projection-resolved OCTA (PR-OCTA) seek to remove projection artifacts, while simultaneously preserving vessel connectedness and image quality (Hwang et al., 2016b, Zhang, et al., 2016a, Zhang et al., 2016b).
The OCTA processing algorithm does not provide quantitative blood flow velocity. In other words, the perceived brightness of an OCTA voxel cannot be directly translated to flow speed. Demonstrating this statement, ex vivo blood flow phantom experiments have shown that the relationship between SSADA algorithm decorrelation and velocity is nonlinear and approximately sigmoidal. The decorrelation value is correlated to velocity in a linear fashion only within a certain range, after which the decorrelation value becomes saturated. Moreover, the relationship between decorrelation values and flow speed depends on many factors, including the scanning speed (inter-scan time of repeated B-scans at the same location), the blood vessel diameter, and the blood flow velocity (Su, et al., 2016, Tokayer, et al., 2013). Therefore, current OCTA technology cannot accurately quantify blood flow speed and conclusions about flow speed should be withheld.
Despite the aforementioned pitfalls of OCTA, the future of OCTA for the imaging of DR appears promising. New light sources, such as swept-source OCT, may further improve imaging speed. This, in turn, would facilitate OCTA scans with larger fields of view (Choi, et al., 2013). The development of improved angiography algorithms, may further improve the signal-to-noise ratio and the image quality. The VISTA method adds semi-quantitative information about blood flow velocity and, therefore, may be useful in quantifying flow changes in diabetic retinopathy (Choi et al., 2015). Improved algorithms that correct for focal OCT signal attenuation will improve vessel connectivity and contrast (Zhang et al., 2016b). Lastly, improved image processing methods, such as vesselness filters, are being developed to improve the connectivity of blood vessels on OCTA images (Camino, et al., 2016).
2.1 Basics of Visible-Light Optical Coherence Tomography
Retinal vascular abnormalities are believed to result in retinal tissue hypoxia in DR (Cai & Boulton, 2002, Wangsa-Wirawan & Linsenmeier, 2003). Retinal neuronal hypoxia is thought to upregulate growth factors, such as VEGF, which have been implicated in the pathogenesis of later stage DR. While OCTA yields novel insights into the structural changes of the diabetic retinal vasculature, it cannot directly probe the degree of retinal hypoxia in DR. Visible-light OCT (vis-OCT) aims to provide a comprehensive assessment of tissue function by measuring the retinal oxygen delivery rate (DO2) and retinal oxygen metabolic rate (MRO2), while retaining most of the capabilities of OCTA. These measurements are considered the “gold standard” metrics for assessing hypoxia in living tissue. Imaging the DO2 and MRO2 would quantify the level of hypoxia in the diabetic retina in vivo.
In order to establish the retinal oxygen delivery and metabolic rates, two different values must be measured within the retinal circulation. The first is the total blood flow entering and leaving the retina, and the second is the hemoglobin oxygen saturation within the retinal vessels. Current imaging modalities quantify either one of the two metrics at a time, but a technique that quantifies both metrics simultaneously has not yet been shown in the living human eye. Structural imaging modalities such as structural OCT, OCTA, and fundus photography provide neither of the two key parameters. Multi-wavelength photography and scanning laser ophthalmoscopy (SLO) can provide oxygen saturation alone (Wangsa-Wirawan & Linsenmeier, 2003), while Doppler-OCT, laser Doppler flowmetry, and adaptive-optics SLO can measure blood velocity alone (Pournaras & Riva, 2012, Zhong, et al., 2008). While these techniques can be combined to measure the retinal oxygen metabolism (Werkmeister, et al., 2015), vis-OCT can quantify both retinal blood flow and oxygen saturation in a single imaging device. With such capabilities, vis-OCT can measure the DO2 and MRO2, lending important insights into the functional state of retinal tissue.
While measurements of blood flow using OCT have been well studied, hemoglobin oxygen saturation measurements using vis-OCT have only recently been developed. When light interacts with a mixture of oxygenated and deoxygenated hemoglobin, it is attenuated to a degree dependent on the ratio of oxygenated and deoxygenated hemoglobin, known as the hemoglobin oxygen saturation (sO2). Therefore, similar to other forms of oximetry, vis-OCT oximetry aims to extract the wavelength-dependent attenuation of light as it passes through the blood vessel. Because oxygenated and deoxygenated hemoglobin have a much higher absorption in the visible spectrum, vis-OCT uses a probing light within the visible spectral range, enabling accurate oxygen measurements with OCT (Chen, et al., 2015). Yi et al. were the first to report retinal oximetry in rodents using vis-OCT (Yi, et al., 2013).
The process of extracting sO2 using vis-OCT is shown in Figure 7. A 3D vis-OCT image of a healthy rat fundus is acquired and reconstructed in a similar fashion to conventional OCT, as discussed previously. Figure 7(A) shows an example of an en face vis-OCT image from a healthy rat retina. The full-spectrum B-scan shown in Figure 7(B) shows several vessels in cross-section and corresponds to the white dashed line on Figure 7(A). The B-scan is labeled ‘full-spectrum’ because it represents light scattering and absorption from the full bandwidth of the probing light. In this case, the vis-OCT probing light spectrum extends from 523 nm to 604 nm, and all the information from these wavelengths is contained in the full-spectrum B-scan. To obtain information from smaller bands of light, the full-spectrum B-scan was divided into fourteen split-spectrum B-scans by a process called ‘spectrum splitting’. Figure 7(C) shows the fourteen split-spectrum B-scans for a portion of the full-spectrum B-scan, delineated by the white dashed box in Figure 7(B). The colored borders around each split-spectrum B-scan correspond to the wavelength of light being scattered from the retina. To extract oxygen saturation, the OCT signal at the bottom of each of the circles labeled in Figure 7(C) is measured. These measurements correspond to light that has both passed through the vessel and scattered back. Fitting the measurements to a model of OCT light attenuation yielded the final sO2 value. The sO2 values determined for each vessel were color-coded on the en face vis-OCT image using a pseudo-color map. Figure 7(D) shows an example of the final sO2 map for a normal rat retina. Highly oxygenated arteries were red (95% to 100%), while deoxygenated veins ranged from blue to orange (50% to 85%).
Figure 7. Retinal Oximetry with vis-OCT.
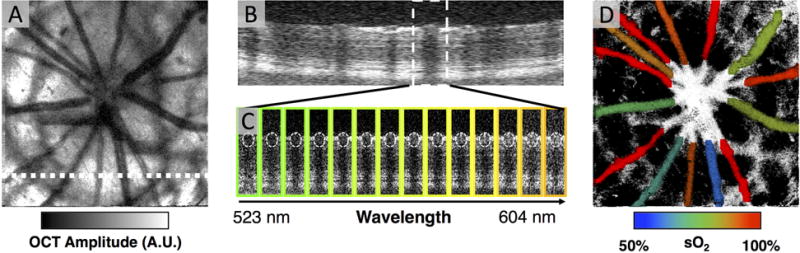
(A) An en face vis-OCT image from a healthy rat eye. A.U.: arbitrary units. (B) A full-spectrum vis-OCT B-scan from the white dashed line in (A). (C) For the vessel within the white dashed box in (B), fourteen split-spectrum B-scans were generated by ‘splitting the spectrum.’ The border around each split-spectrum B-scan represents the approximate color corresponding to the wavelengths split from the full-spectrum. The wavelengths ranged from 523 nm to 604 nm (D) After fitting the OCT signals at the bottom of white dashed circles in (C), the oxygen saturation of hemoglobin was found. The vessel in (C) had an oxygen saturation of 0.5, which indicates that it was a vein.
In addition to retinal oximetry, vis-OCT can also measure the volumetric blood flow rate, using established Doppler OCT methods (Wang, et al., 2007). To measure the volumetric blood flow rate (in μL/min), the blood vessel diameter (in μm) and blood flow velocity (in mm/s) must be measured. The vessel diameter can be measured from the vessel walls visible on the OCT B-scans. The blood flow velocity, on the other hand, is measured from the Doppler phase shift imparted by moving red blood cells on the phase of the OCT signal. Similar to the situation in OCTA, detecting these Doppler shifts requires observing differences in the OCT signal at the same location, but at different points in time. The dual-circle Doppler OCT is one method to obtain volumetric blood flow rate, which allows estimation of the Doppler angle (Wang et al., 2007, Wang, et al., 2008).
An example illustrating the process for dual-ring Doppler vis-OCT in the rat eye is shown in Figure S7. Figure S7(A) depicts an en face vis-OCT image of a healthy rat retina. In the dual-ring Doppler OCT method, there are two key features that define the scanning pattern. First, two circles are scanned around the optic nerve head, as illustrated by the white solid circles in Figure S7(A). These two circular scans are needed to establish an intermediate metric called the Doppler angle. The Doppler angle is the angle between the scanning illumination beam and the norm of the blood vessel central axis. Second, the individual circular scans must have high scanning density. A high scanning density underlies the assumption that adjacent A-lines of the B-scan are approximately at the same location. Therefore, two adjacent A-lines can be treated as if they are at the same location, and the phase difference between the two A-lines is then related to the blood flow velocity. The Doppler angle and the Doppler phase shift are the two key parameters needed to establish absolute blood flow speed.
Figure S7(B) shows the high density circular B-scan from the inner circular scanning path in Figure S7(A). A magnified image of an artery-vein pair in Figure S7(B) is shown in Figure S7(C). As depicted by the yellow arrows in Figure S7(C), the diameter was measured across the vessel in the axial direction. After correction for bulk-motion and phase unwrapping, the phase difference between adjacent A-lines in the high density B-scan was proportional to the blood flow speed (White, et al., 2003). To improve the quality of the measurements, the phase differences were obtained at multiple time points in the cardiac cycle to yield an average blood flow speed. Using the corrected phase differences and the Doppler angle, the absolute blood flow velocity within the vessel can be determined and overlaid on the B-scan, as exemplified by Figure S7(D). If we define that an artery has a positive velocity, the vein has a negative velocity, consistent with direction of blood flow in relation to the heart.
To calculate the DO2 and MRO2, the retinal oximetry and blood flow measurements from vis-OCT are combined. According to the Fick principle, the MRO2 is equal to the product of the arterial-venous sO2 difference and the volumetric blood flow rate. Since vis-OCT can measure both parameters, the MRO2 can be established using vis-OCT alone. Because the DO2 and MRO2 provide absolute measures of the metabolism of oxygen in the retinal tissue, vis-OCT provides improved insights into the functional state of retinal tissue, above and beyond structural imaging.
2.2 Validation of Metabolic Imaging with vis-OCT
The procedure for retinal oximetry and blood flow measurements with vis-OCT has been demonstrated and validated in rodent models (Chen, Yi & Zhang, 2015, Soetikno, et al., 2015, Yi, et al., 2015b, Yi et al., 2013). The MRO2 of the inner retina was measured using vis-OCT during progressive systemic hypoxic challenge, where the inhaled oxygen content was progressively decreased (Yi et al., 2013). The measured vis-OCT sO2 values showed high correlation with changes in inhaled oxygen content and the peripheral oxygenation, as measured by a pulse oximeter, suggesting that vis-OCT measurements were accurate in vivo. In the same study, dual-ring Doppler vis-OCT was used calculate vessel diameter, blood flow velocity, and volumetric blood flow rate in rodents. A high scanning rate was used for the blood flow measurements, such that the pulsatile flow within the arterioles was observable (min: 0.3 μl/min; peak: 1.2 μl/min). Interestingly, under conditions of systemic hypoxia, the oxygen delivered from the inner retinal circulation increased. Based on models of oxygen diffusion in the retina, it was hypothesized that inner retinal oxygen delivery rate increased as a result of retinal vascular auto-regulation in an attempt to compensate for decreased oxygen delivery from the choroidal circulation, which is not auto-regulated under conditions of systemic hypoxia.
2.3 Vis-OCT in Animal Models of DR
Most rodent models of DR only replicate the early stages of DR, but not the later proliferative stages (Robinson, et al., 2012). However, the oxygen-induced retinopathy (OIR) model in rodents is one of the few which exhibits a proliferative retinopathy (Jo, et al., 2013). Originally developed as a model of retinopathy of prematurity, the OIR model has also been used to investigate proliferative retinopathy, as a surrogate of PDR (Penn, Tolman & Henry, 1994). Starting at birth, rat pups are placed into an oxygen-controlled chamber, and are exposed to alternating 50% oxygenation (hyperoxia) and 10% oxygenation (hypoxia) every 24 hrs. On postnatal day 14, the rat pups are returned to room air (21% oxygenation). By postnatal day 18, the retinal vasculature exhibits vaso-attenuation and areas of peripheral NV, which simulate proliferative stages of DR. Interestingly, by postnatal day 31, these retinal vascular features disappear, and the retinal vasculature appears to normalize in the rat OIR model. The pathophysiology of spontaneous normalization of the vasculature has not been elucidated.
Vis-OCT has measured the sO2 and volumetric blood flow on postnatal day 18 in the OIR model. Interestingly, both the DO2 and MRO2 were significantly decreased in rats with OIR compared to healthy age-matched control rats. Surprisingly, the DO2 and MRO2 were decreased to the same degree, suggesting that the oxygen delivered was sufficient to meet the metabolic demands of the retina. To explain this unexpected result, we identified significantly decreased retinal thickness in rats with OIR compared to controls. We hypothesized that significant retinal thinning (and neuronal loss) could account for the decreased metabolic demand of the retina. The decreased metabolic demand likely occurred in response to severe perinatal retinal hypoxia in developing rats under OIR, with dramatic neuronal pruning, which decreased the stimulus for angiogenesis. These results provide a potential explanation for the normalization of the vasculature by postnatal day 30. Further longitudinal studies, using vis-OCT in diabetic models and the OIR model, promise to further improve our understanding of the role of hypoxia in the pathogenesis of DR and other ischemic retinopathies.
2.4 Vis-OCT in Humans
Although studies in animal models provide us with new insights, the ultimate goal of vis-OCT research is to develop vis-OCT systems that are capable of measuring oxygenation and blood flow in the human eye. Such a device would report the oxygen delivery and metabolic rates as relevant metrics, which could be useful for following DR progression over time. Structural imaging with research vis-OCT systems have already been demonstrated and measurements of sO2 in humans have been performed (Chen, et al., 2017). Yi et al. first reported structural imaging with a human vis-OCT system (Yi, et al., 2015a). Figure 8 shows an example from the prototype vis-OCT system. To capture the metabolic rate of oxygen for the entire retina, or the macula, requires imaging the major arteries and veins, entering and exiting the respective regions. Demonstrating the feasibility of imaging these major vessels, Figure 8(A) and Figure 8(B) show vis-OCT en face images of the optic nerve head and the macula, respectively, in a healthy 23-year-old volunteer. The field of view was approximately 6° by 6°.
Figure 8. Structural Imaging of the Human Macula with vis-OCT.
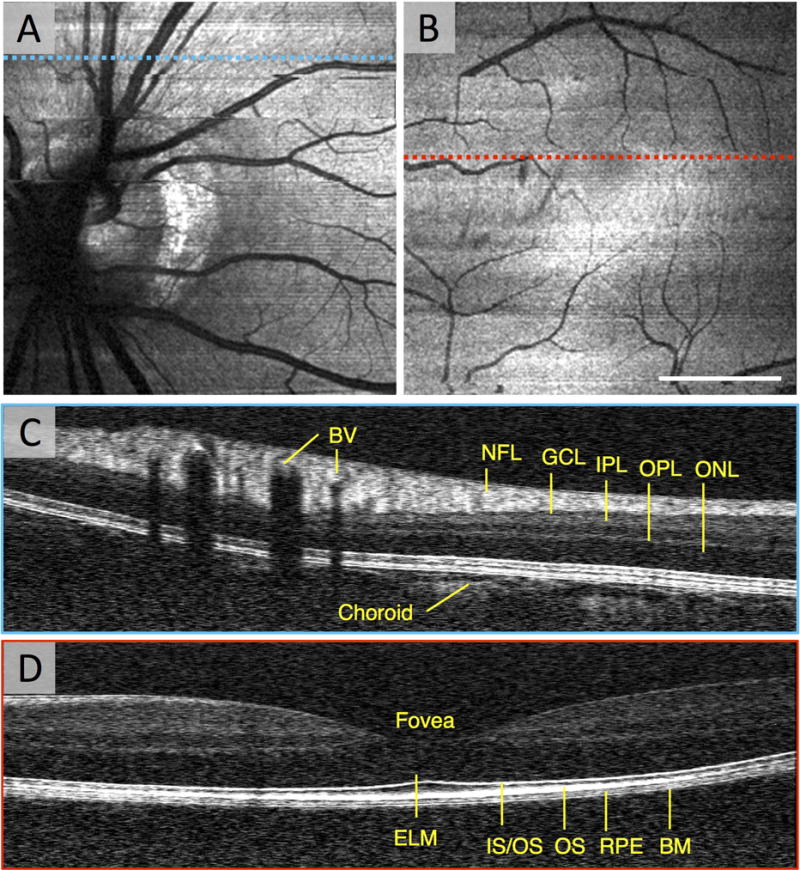
(A) An en face vis-OCT image of the optic nerve head in a healthy 23 year old volunteer. (B) An en face vis-OCT image of the macula in the same volunteer. (C) The vis-OCT B-scan of some major vessels superior to the optic nerve head corresponding to the blue dashed line in (A). (D) The vis-OCT B-scan of the fovea corresponding to the red dashed line in (B). BV: blood vessel. NFL: nerve fiber layer. GCL: ganglion cell layer. IPL: inner plexiform layer. OPL: outer plexiform layer. ONL: outer nuclear layer. ELM: external limiting membrane. IS/OS: inner segment/outer segment junction. OS: outer segments. RPE: retina pigment epithelium. BM: Bruch’s membrane. Scale bar: 2 degrees.
Because vis-OCT uses shorter wavelengths than near-infrared OCT, the axial resolution of vis-OCT is much higher than near-infrared OCT. The axial resolution of the human vis-OCT system was measured to be 0.97 μm in air, while commercial near-infrared systems typically have axial resolutions of 5–7 μm. A vis-OCT B-scan corresponding to the blue dashed line in Figure 8(A) is shown in Figure 8(C). The higher axial resolution is especially demonstrated by the ability to resolve greater details of the outer retinal layers. Because hemoglobin strongly attenuates visible light, a major difference between vis-OCT and near infrared-OCT images is the presence of sharply demarcated and intensely hypo-reflective shadows beneath the blood vessels in vis-OCT. Although this may seem like an undesirable artifact, the shadows are related to the attenuation of the OCT signal, which is exploited to perform retinal oximetry. Another notable difference is that only the superficial layers of the choroid are apparent on the vis-OCT B-scan, presumably because the RPE strongly attenuates the illumination in the visible range.
Chen et al. were first to demonstrate retinal oximetry in humans using vis-OCT (Chen et al., 2017). In order to calculate the sO2 in human eyes, the authors applied a similar approach to that described in rodents. However, because vis-OCT currently has a lower signal-to-noise ratio in humans than in rodents, a more sophisticated approach for extracting the OCT signal passing through the vessel was developed. More specifically, the statistical distribution of the noise in OCT was used to better estimate the OCT signal. Several arteries and veins near the optic nerve head in healthy volunteers were measured. Arteries near the optics nerve head ranged from 93% to 98% oxygenation, while veins ranged from 76% to 79%. Going forward, further improvements in the oximetry algorithms and vis-OCT hardware will facilitate sO2 measurements in larger patient cohorts. Furthermore, combining Doppler vis-OCT with retinal oximetry is expected to facilitate MRO2 measurements in the human eye in vivo.
2.5 Future of vis-OCT
Due to safety concerns with visible light, the laser power permitted for vis-OCT is significantly lower compared to that of near infrared OCT. Nevertheless, the feasibility of quality vis-OCT imaging has been demonstrated with a laser power at the pupil of 226 μW (Yi et al., 2015a). This power was less than ten times the ANSI maximum permissible exposure for two hours of continuous exposure. For comparison, fundus autofluorescence systems based on SLO also operate in the visible wavelength range at 488 nm, and the vis-OCT power measurement was lower than commercial SLO autofluorescence systems (~270 μW). Despite the limited photon budget, errors in sO2 calculation related to low signal-to-noise situations can be minimized by using algorithms based on OCT statistics (Chen et al., 2017). In addition to minimizing the illumination power, safety measures have been built into prototype vis-OCT systems to specifically prevent a stationary beam on the retinal surface. These measures include automatic shutter mechanisms, which block the illumination immediately after scanning, and deflect the illumination beam when not in use.
Before vis-OCT becomes clinically applicable, there are several challenges that need to be addressed. First, there is the question of whether the vis-OCT beam itself will affect the MRO2. Studies of retinal flicker have found maximum neuronal metabolic responses to flicker at ~10 Hz (Riva, Logean & Falsini, 2005), which is far slower than the frame rate for a typical vis-OCT imaging session, suggesting that this may be insignificant. Second, because of the visible illumination, eye movement artifacts are more common in vis-OCT. Eye tracking and motion correction techniques will likely be necessary to achieve high quality clinical imaging. Finally, absolute measures of the MRO2 with vis-OCT are currently limited to the inner retinal circulation. Relative measurements of choroidal sO2 have been demonstrated in rodents (Chen, Yi & Zhang, 2015), but measurements of high-speed choroidal flow would be challenging for current OCT techniques.
Compared to OCTA, using vis-OCT to investigate human DR is at an earlier phase of technology development. Nevertheless, vis-OCT has already provided important functional information regarding retinal oxygen metabolic changes in animal models and human subjects. An even greater hope is that vis-OCT might prove useful in patient care, by providing functional metrics of the metabolic state of the retina. Vis-OCT can be combined with OCTA technology to obtain oxygen saturation maps over larger areas of the retina (Chen, Yi & Zhang, 2015, Shah, et al., 2016, Yi, et al., 2014). In addition, vis-OCT could also be combined with conventional near infrared-OCT to facilitate patient imaging or to compare the two imaging techniques (Chen, et al., 2016). Ultimately, the combination of higher axial resolution, oxygen saturation measurements, and blood flow measurements, all available in vis-OCT, make it an exciting new technology for investigating DR.
Supplementary Material
En face OCTA allows visualization of the superficial (A), middle (B), and deep (C) capillary plexuses. The extent of non-perfusion and degree of vascular abnormalities differ between the capillary plexuses on en face OCTA. The red and green lines on cross-sectional OCTA are the segmentation boundaries for each layer.
En face OCTA of the (A) superficial (SCP) and (B) deep capillary plexus (DCP) with segmentation boundaries on the cross-sectional OCT below. The yellow line in B shows the location of the cross-sectional OCT. The green line shows an area of rarefied DCP capillaries on OCTA corresponding to reduced reflectivity of the inner / outer segment junction on OCT.
(A) OCTA of the superficial capillary plexus (SCP). (B) OCT of the SCP. (C and D) Enlarged insets from the yellow box in A and B, respectively. The arrows point to blood vessels that appear on structural OCT, but not on OCTA. These may represent vessels with no flow or with flow rates below the OCTA threshold.
(A) OCTA of the optic nerve head reveals abnormal NV vasculature in the upper right quadrant surrounded by normal appearing vasculature. The red segmentation boundaries are seen on the cross-sectional OCT below. FA of the corresponding location shows dye leakage and less distinct NV morphology in the early phase (B) and more leakage and indistinct boundaries in the late phase (C).
(A) Media opacity. (B) Projection artifacts, the top arrow on cross-sectional OCT points to actual location of flow inside the vessel, while the bottom arrow points to the flow projected onto the retinal pigment epithelium. Red lines on cross-sectional OCT are segmentation boundaries for the en face image. (C) Segmentation error caused by epiretinal membrane (arrow). Red and green lines on cross-sectional OCT show the segmentation boundaries following the membrane. (D) Stretch artifact (arrow) from software correction of eye motion. (E, F) Eye motion artifacts before (E) and after (F) motion correction technology (MCT). (G) ‘Quilting’ artifact from motion and MCT correction. (H) ‘Vessel doubling’ artifact from motion and MCT correction.
(A) An example en face OCTA image of the superficial capillary plexus. (B) An en face OCTA image of the middle capillary plexus with many projection artifacts resembling vessels from (A). (C) A thresholded image of A is multiplied with A and a weighting factor. (D) After subtraction of the major superficial retinal vessels, the middle capillary plexus is now free of projection artifact.
(A) An en face vis-OCT image from a healthy rat eye. The white solid circles indicate the scanning paths for the dual-circle Doppler vis-OCT method. The arrow indicates a clockwise scanning direction. (B) The vis-OCT circular B-scan corresponding to the inner circular scan path in (A). (C) A zoomed view of an artery-vein pair corresponding to the solid yellow box in (B). Vessel diameter was measured using the high axial resolution of OCT, as indicated by the yellow arrows. (D) Doppler shifts on the OCT signal were used to recover the blood flow speed within the artery and vein respectively. The artery had a positive velocity, while the vein had a negative velocity, consistent with the direction of flow in these respective vessels.
Acknowledgments
This work was supported in part by National Institutes of Health Grants DP3DK108248, R01EY026078, T32GM008152 (Northwestern MSTP), F30EY026472, and research instrument support by Optovue, Inc. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
HFZ has financial interest in Opticent Inc., which did not support this work.
Abbreviations
- DCP
Deep capillary plexus
- DM
Diabetes mellitus
- DME
Diabetic macular edema
- DMI
Diabetic macular ischemia
- DO2
Retinal oxygen delivery rate
- DR
Diabetic retinopathy
- EAA
Extrafoveal avascular area
- FA
Fluorescein angiography
- FAZ
Foveal avascular zone
- FD
Fractal dimension
- HC
Healthy controls
- HR
Horizontal radius of the foveal avascular zone
- IRMA
Intraretinal microvascular abnormalities
- MA
Microaneurysms
- MCP
Middle capillary plexus
- MCT
Motion correction technology
- MFD
Maximum foveal avascular zone diameter
- MRO2
Retinal oxygen metabolic rate
- NoDR
Diabetes mellitus without diabetic retinopathy
- NPDR
Non-proliferative diabetic retinopathy
- NV
Neovascularization
- OCTA
Optical coherence tomography angiography
- OIR
Oxygen-induced retinopathy
- OMAG
Optical microangiography
- PDR
Proliferative diabetic retinopathy
- PI
Perfusion index (vessel density)
- PIA
Perifoveal intercapillary area
- PR-OCTA
Projection-resolved optical coherence tomography angiography
- RPE
Retinal pigment epithelium
- SCP
Superficial capillary plexus
- SD
Skeletonized vessel density
- SD-OCT
Spectral domain optical coherence tomography
- sO2
Hemoglobin oxygen saturation
- SSADA
Split-spectrum amplitude-decorrelation angiography
- SS-OCTA
Swept-source optical coherence tomography angiography
- TAA
Total avascular area
- VA
Visual acuity
- VAD
Vessel area density
- VEGF
Vascular endothelial growth factor
- VD
Vessel density
- VDI
Vessel diameter index
- Vis-OCT
Visible-light OCT
- VISTA
variable interscan time analysis
- VLD
Vessel length density
- VR
Vertical radius of the foveal avascular zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflicting relationship exists for all other authors.
Proprietary Interest: The authors have no proprietary interest in the subject of this manuscript.
References
- Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG, Gentile RC, Hsiao YS, Zhou Q, Ko T. Retinal Vascular Perfusion Density Mapping Using Optical Coherence Tomography Angiography in Normals and Diabetic Retinopathy Patients. Retina. 2015;35(11):2353–2363. doi: 10.1097/IAE.0000000000000862. [DOI] [PubMed] [Google Scholar]
- Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-Source Oct Angiography Imaging of the Foveal Avascular Zone and Macular Capillary Network Density in Diabetic Retinopathyoct-Angiography in Diabetic Retinopathy. Investigative Ophthalmology & Visual Science. 2016;57(8):3907–3913. doi: 10.1167/iovs.16-19570. [DOI] [PubMed] [Google Scholar]
- An L, Shen TT, Wang RK. Using Ultrahigh Sensitive Optical Microangiography to Achieve Comprehensive Depth Resolved Microvasculature Mapping for Human Retina. Journal of Biomedical Optics. 2011;16(10) doi: 10.1117/1.3642638. 106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend O, Wolf S, Harris A, Reim M. The Relationship of Macular Microcirculation to Visual Acuity in Diabetic Patients. Archives of Ophthalmology. 1995;113(5):610–614. doi: 10.1001/archopht.1995.01100050078034. [DOI] [PubMed] [Google Scholar]
- Arend O, Wolf S, Jung F, Bertram B, Pöstgens H, Toonen H, Reim M. Retinal Microcirculation in Patients with Diabetes Mellitus: Dynamic and Morphological Analysis of Perifoveal Capillary Network. British Journal of Ophthalmology. 1991;75(9):514–518. doi: 10.1136/bjo.75.9.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend O, Wolf S, Remky A, Sponsel WE, Harris A, Bertram B, Reim M. Perifoveal Microcirculation with Non-Insulin-Dependent Diabetes Mellitus. Graefe’s Archive for Clinical and Experimental Ophthalmology. 1994;232(4):225–231. doi: 10.1007/BF00184010. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Inoue M, Ahn S, McCann J, Dhrami-Gavazi E, Yannuzzi LA, Freund KB. Visual Acuity Is Correlated with the Area of the Foveal Avascular Zone in Diabetic Retinopathy and Retinal Vein Occlusion. Ophthalmology. 2016;123(11):2352–2367. doi: 10.1016/j.ophtha.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Bertram B, Wolf S, Fiehöfer S, Schulte K, Arend O, Reim M. Retinal Circulation Times in Diabetes Mellitus Type 1. British Journal of Ophthalmology. 1991;75(8):462–465. doi: 10.1136/bjo.75.8.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanushali D, Anegondi N, Gadde SG, Srinivasan P, Chidambara L, Yadav NK, Roy AS. Linking Retinal Microvasculature Features with Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiographyretinal Vasculature Changes in Diabetic Retinopathy. Investigative Ophthalmology & Visual Science. 2016;57(9):OCT519–OCT525. doi: 10.1167/iovs.15-18901. [DOI] [PubMed] [Google Scholar]
- Bradley PD, Sim DA, Keane PA, Cardoso J, Agrawal R, Tufail A, Egan CA. The Evaluation of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiographyocta in Diabetic Retinopathy. Investigative Ophthalmology & Visual Science. 2016;57(2):626–631. doi: 10.1167/iovs.15-18034. [DOI] [PubMed] [Google Scholar]
- Bresnick GH, Condit R, Syrjala S, Palta M, Groo A, Korth K. Abnormalities of the Foveal Avascular Zone in Diabetic Retinopathy. Archives of Ophthalmology. 1984;102(9):1286–1293. doi: 10.1001/archopht.1984.01040031036019. [DOI] [PubMed] [Google Scholar]
- Cai J, Boulton M. The Pathogenesis of Diabetic Retinopathy: Old Concepts and New Questions. Eye. 2002;16(3):242–260. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- Camino A, Zhang M, Dongye C, Pechauer AD, Hwang TS, Bailey ST, Lujan B, Wilson DJ, Huang D, Jia Y. Automated Registration and Enhanced Processing of Clinical Optical Coherence Tomography Angiography. Quantitative Imaging in Medicine and Surgery. 2016;6(4):391. doi: 10.21037/qims.2016.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Zhang M, Hwang T, Bailey S, Wilson D, Jia Y, Huang D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Scientific Reports. 2017;7 doi: 10.1038/srep42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cennamo G, Romano MR, Nicoletti G, Velotti N, Crecchio G. Optical Coherence Tomography Angiography Versus Fluorescein Angiography in the Diagnosis of Ischaemic Diabetic Maculopathy. Acta Ophthalmologica. 2016 doi: 10.1111/aos.13159. [DOI] [PubMed] [Google Scholar]
- Chen S, Shu X, Nesper PL, Liu W, Fawzi AA, Zhang HF. Retinal Oximetry in Humans Using Visible-Light Optical Coherence Tomography [Invited] Biomedical Optics Express. 2017;8(3):1415–1429. doi: 10.1364/BOE.8.001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Shu X, Yi J, Fawzi A, Zhang HF. Dual-Band Optical Coherence Tomography Using a Single Supercontinuum Laser Source. Journal of Biomedical Optics. 2016;21(6):066013–066013. doi: 10.1117/1.JBO.21.6.066013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yi J, Liu W, Backman V, Zhang HF. Monte Carlo Investigation of Optical Coherence Tomography Retinal Oximetry. IEEE Transactions on Biomedical Engineering. 2015;62(9):2308–2315. doi: 10.1109/TBME.2015.2424689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yi J, Zhang HF. Measuring Oxygen Saturation in Retinal and Choroidal Circulations in Rats Using Visible Light Optical Coherence Tomography Angiography. Biomedical Optics Express. 2015;6(8):2840–2853. doi: 10.1364/BOE.6.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Mohler KJ, Potsaid B, Lu CD, Liu JJ, Jayaraman V, Cable AE, Duker JS, Huber R, Fujimoto JG. Choriocapillaris and Choroidal Microvasculature Imaging with Ultrahigh Speed Oct Angiography. PloS One. 2013;8(12):e81499. doi: 10.1371/journal.pone.0081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Moult EM, Waheed NK, Adhi M, Lee B, Lu CD, Talisa E, Jayaraman V, Rosenfeld PJ, Duker JS. Ultrahigh-Speed, Swept-Source Optical Coherence Tomography Angiography in Nonexudative Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology. 2015;122(12):2532–2544. doi: 10.1016/j.ophtha.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Waheed NK, Moult EM, Adhi M, Lee B, De Carlo T, Jayaraman V, Baumal CR, Duker JS, Fujimoto JG. Ultrahigh Speed Swept Source Optical Coherence Tomography Angiography of Retinal and Choriocapillaris Alterations in Diabetic Patients with and without Retinopathy. Retina. 2016 doi: 10.1097/IAE.0000000000001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan DG, Kuwabara T. Capillary Shunts in the Pathogenesis of Diabetic Retinopathy. Diabetes. 1963;12(4):293–300. doi: 10.2337/diab.12.4.293. [DOI] [PubMed] [Google Scholar]
- Couturier A, Mané V, Bonnin S, Erginay A, Massin P, Gaudric A, Tadayoni R. Capillary Plexus Anomalies in Diabetic Retinopathy on Optical Coherence Tomography Angiography. Retina. 2015;35(11):2384–2391. doi: 10.1097/IAE.0000000000000859. [DOI] [PubMed] [Google Scholar]
- de Carlo TE, Bonini Filho MA, Baumal CR, Reichel E, Rogers A, Witkin AJ, Duker JS, Waheed NK. Evaluation of Preretinal Neovascularization in Proliferative Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Ophthalmic Surgery, Lasers and Imaging Retina. 2016a;47(2):115–119. doi: 10.3928/23258160-20160126-03. [DOI] [PubMed] [Google Scholar]
- de Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA, Baumal CR, Crawford C, Reichel E, Witkin AJ, Duker JS, Waheed NK. Detection of Microvascular Changes in Eyes of Patients with Diabetes but Not Clinical Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Retina. 2015;35(11):2364–2370. doi: 10.1097/IAE.0000000000000882. [DOI] [PubMed] [Google Scholar]
- de Carlo TE, Chin AT, Joseph T, Baumal CR, Witkin AJ, Duker JS, Waheed NK. Distinguishing Diabetic Macular Edema from Capillary Nonperfusion Using Optical Coherence Tomography Angiography. Ophthalmic Surgery, Lasers and Imaging Retina. 2016b;47(2):108–114. doi: 10.3928/23258160-20160126-02. [DOI] [PubMed] [Google Scholar]
- Di G, Weihong Y, Xiao Z, Zhikun Y, Xuan Z, Yi Q, Fangtian D. A Morphological Study of the Foveal Avascular Zone in Patients with Diabetes Mellitus Using Optical Coherence Tomography Angiography. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2016;254(5):873–879. doi: 10.1007/s00417-015-3143-7. [DOI] [PubMed] [Google Scholar]
- Freeman WR, Bartsch DU, Mueller AJ, Banker AS, Weinreb RN. Simultaneous Indocyanine Green and Fluorescein Angiography Using a Confocal Scanning Laser Ophthalmoscope. Archives of Ophthalmology. 1998;116(4):455–463. doi: 10.1001/archopht.116.4.455. [DOI] [PubMed] [Google Scholar]
- Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S. Optical Coherence Tomography Angiography of the Foveal Avascular Zone in Diabetic Retinopathy. Graefe’s Archive for Clinical and Experimental Ophthalmology= Albrecht von Graefes Archiv fur Klinische und Experimentelle Ophthalmologie. 2015a doi: 10.1007/s00417-015-3148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S. Optical Coherence Tomography Angiography of the Foveal Avascular Zone in Diabetic Retinopathy. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2015b:1–8. doi: 10.1007/s00417-015-3148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Jia Y, Zhang M, Su JP, Liu G, Hwang TS, Bailey ST, Huang D. Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016 Oct;57(9):27–36. doi: 10.1167/iovs.15-19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Liu G, Huang D, Jia Y. Optimization of the Split-Spectrum Amplitude-Decorrelation Angiography Algorithm on a Spectral Optical Coherence Tomography System. Optics letters. 2015;40(10):2305–2308. doi: 10.1364/OL.40.002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, E.T.D.R.S.R. Classification of Diabetic Retinopathy from Fluorescein Angiograms: Etdrs Report Number 11. Ophthalmology. 1991a;98(5):807–822. [PubMed] [Google Scholar]
- Group, E.T.D.R.S.R. Fluorescein Angiographic Risk Factors for Progression of Diabetic Retinopathy: Etdrs Report Number 13. Ophthalmology. 1991b;98(5):834–840. [PubMed] [Google Scholar]
- Group, E.T.D.R.S.R. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—an Extension of the Modified Airlie House Classification: Etdrs Report Number 10. Ophthalmology. 1991c;98(5):786–806. [PubMed] [Google Scholar]
- Group, T.D.C.a.C.T.R. Color Photography Vs Fluorescein Angiography in the Detection of Diabetic Retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1987;105:1344–1351. doi: 10.1001/archopht.1987.01060100046022. [DOI] [PubMed] [Google Scholar]
- Hasegawa N, Nozaki M, Takase N, Yoshida M, Ogura Y. New Insights into Microaneurysms in the Deep Capillary Plexus Detected by Optical Coherence Tomography Angiography in Diabetic Macular Edemamas in Deep Capillary Plexus Detected by Octa in Dme. Investigative Ophthalmology & Visual Science. 2016;57(9):OCT348–OCT355. doi: 10.1167/iovs.15-18782. [DOI] [PubMed] [Google Scholar]
- Hwang TS, Gao SS, Liu L, Lauer AK, Bailey ST, Flaxel CJ, Wilson DJ, Huang D, Jia Y. Automated Quantification of Capillary Nonperfusion Using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmology. 2016a;134(4):367–373. doi: 10.1001/jamaophthalmol.2015.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TS, Jia Y, Gao SS, Bailey ST, Lauer AK, Flaxel CJ, Wilson DJ, Huang D. Optical Coherence Tomography Angiography Features of Diabetic Retinopathy. Retina (Philadelphia, Pa) 2015;35(11):2371. doi: 10.1097/IAE.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TS, Zhang M, Bhavsar K, Zhang X, Campbell JP, Lin P, Bailey ST, Flaxel CJ, Lauer AK, Wilson DJ. Visualization of 3 Distinct Retinal Plexuses by Projection-Resolved Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmology. 2016b doi: 10.1001/jamaophthalmol.2016.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imesch PD, Bindley CD, Wallow IH. Clinicopathologic Correlation of Intraretinal Microvascular Abnormalities. Retina. 1997;17(4):321–329. doi: 10.1097/00006982-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, Yokota H, Yoshida A. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. American Journal of Ophthalmology. 2015;160(1):35–44. e31. doi: 10.1016/j.ajo.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Jo DH, Cho CS, Kim JH, Jun HO, Kim JH. Animal Models of Diabetic Retinopathy: Doors to Investigate Pathogenesis and Potential Therapeutics. Journal of Biomedical Science. 2013;20(1):1. doi: 10.1186/1423-0127-20-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying Microvascular Density and Morphology in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography Angiography. Investigative Ophthalmology & Visual Science. 2016;57(9):OCT362–OCT370. doi: 10.1167/iovs.15-18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Potsaid B, Mayer MA, Bock R, Baumann B, Liu JJ, Hornegger J, Fujimoto JG. Motion Correction in Optical Coherence Tomography Volumes on a Per a-Scan Basis Using Orthogonal Scan Patterns. Biomedical Optics Express. 2012;3(6):1182–1199. doi: 10.1364/BOE.3.001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiterovich KA, Maguire MG, Murphy RP, Schachat AP, Bressler NM, Bressler SB, Fine SL. Frequency of Adverse Systemic Reactions after Fluorescein Angiography: Results of a Prospective Study. Ophthalmology. 1991;98(7):1139–1142. doi: 10.1016/s0161-6420(91)32165-1. [DOI] [PubMed] [Google Scholar]
- Lee J, Moon BG, Cho AR, Yoon YH. Optical Coherence Tomography Angiography of Dme and Its Association with Anti-Vegf Treatment Response. Ophthalmology. 2016;123(11):2368–2375. doi: 10.1016/j.ophtha.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Lin AD, Lee AY, Zhang Q, Rezaei KA, Kinyoun J, Wang RK, Lee CS. Association between Oct-Based Microangiography Perfusion Indices and Diabetic Retinopathy Severity. British Journal of Ophthalmology. 2016 doi: 10.1136/bjophthalmol-2016-309514. bjophthalmol-2016-309514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mané V, Dupas B, Gaudric A, Bonnin S, Pedinielli A, Bousquet E, Erginay A, Tadayoni R, Couturier A. Correlation between Cystoid Spaces in Chronic Diabetic Macular Edema and Capillary Nonperfusion Detected by Optical Coherence Tomography Angiography. Retina. 2016 doi: 10.1097/IAE.0000000000001289. [DOI] [PubMed] [Google Scholar]
- Matsunaga DR, Jack JY, De Koo LO, Ameri H, Puliafito CA, Kashani AH. Optical Coherence Tomography Angiography of Diabetic Retinopathy in Human Subjects. Ophthalmic Surgery, Lasers and Imaging Retina. 2015;46(8):796–805. doi: 10.3928/23258160-20150909-03. [DOI] [PubMed] [Google Scholar]
- Miwa Y, Murakami T, Suzuma K, Uji A, Yoshitake S, Fujimoto M, Yoshitake T, Tamura Y, Yoshimura N. Relationship between Functional and Structural Changes in Diabetic Vessels in Optical Coherence Tomography Angiography. Scientific Reports. 2016;6 doi: 10.1038/srep29064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Soetikno BT, Fawzi AA. Characterization of the Middle Capillary Plexus Using Optical Coherence Tomography Angiography in Healthy and Diabetic Eyes. Retina (Philadelphia, Pa) 2016 doi: 10.1097/IAE.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JS, Tolman BL, Henry MM. Oxygen-Induced Retinopathy in the Rat: Relationship of Retinal Nonperfusion to Subsequent Neovascularization. Investigative Ophthalmology & Visual Science. 1994;35(9):3429–3435. [PubMed] [Google Scholar]
- Ploner SB, Moult EM, Choi W, Waheed NK, Lee B, Novais EA, Cole ED, Potsaid B, Husvogt L, Schottenhamml J. Toward Quantitative Optical Coherence Tomography Angiography: Visualizing Blood Flow Speeds in Ocular Pathology Using Variable Interscan Time Analysis. Retina. 2016 doi: 10.1097/IAE.0000000000001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournaras CJ, Riva CE. Retinal Blood Flow Evaluation. Ophthalmologica. 2012;229(2):61–74. doi: 10.1159/000338186. [DOI] [PubMed] [Google Scholar]
- Reif R, Qin J, An L, Zhi Z, Dziennis S, Wang R. Quantifying Optical Microangiography Images Obtained from a Spectral Domain Optical Coherence Tomography System. Journal of Biomedical Imaging. 2012;2012:9. doi: 10.1155/2012/509783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva CE, Logean E, Falsini B. Visually Evoked Hemodynamical Response and Assessment of Neurovascular Coupling in the Optic Nerve and Retina. Progress in Retinal and Eye Research. 2005;24(2):183–215. doi: 10.1016/j.preteyeres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS. Update on Animal Models of Diabetic Retinopathy: From Molecular Approaches to Mice and Higher Mammals. Disease Models and Mechanisms. 2012;5(4):444–456. doi: 10.1242/dmm.009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz DA, Talisa E, Adhi M, Moult E, Choi W, Baumal CR, Witkin AJ, Duker JS, Fujimoto JG, Waheed NK. Select Features of Diabetic Retinopathy on Swept-Source Optical Coherence Tomographic Angiography Compared with Fluorescein Angiography and Normal Eyes. JAMA Ophthalmology. 2016 doi: 10.1001/jamaophthalmol.2016.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samara WA, Shahlaee A, Adam MK, Khan MA, Chiang A, Maguire JI, Hsu J, Ho AC. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology. 2016 doi: 10.1016/j.ophtha.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Savastano MC, Federici M, Falsini B, Caporossi A, Minnella AM. Detecting Papillary Neovascularization in Proliferative Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Acta Ophthalmologica. 2016 doi: 10.1111/aos.13166. [DOI] [PubMed] [Google Scholar]
- Scarinci F, Nesper PL, Fawzi AA. Deep Retinal Capillary Non-Perfusion Is Associated with Photoreceptor Disruption in Diabetic Macular Ischemia. American Journal of Ophthalmology. 2016 doi: 10.1016/j.ajo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenhamml J, Moult EM, Ploner S, Lee B, Novais EA, Cole E, Dang S, Lu CD, Husvogt L, Waheed NK. An Automatic, Intercapillary Area-Based Algorithm for Quantifying Diabetes-Related Capillary Dropout Using Optical Coherence Tomography Angiography. Retina. 2016 doi: 10.1097/IAE.0000000000001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RS, Soetikno BT, Yi J, Liu W, Skondra D, Zhang HF, Fawzi AA. Visible-Light Optical Coherence Tomography Angiography for Monitoring Laser-Induced Choroidal Neovascularization in Micedetecting Cnv Via Vis-Octa. Investigative Ophthalmology & Visual Science. 2016;57(9):OCT86–OCT95. doi: 10.1167/iovs.15-18891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahlaee A, Samara WA, Hsu J, Say EAT, Khan MA, Sridhar J, Hong BK, Shields CL, Ho AC. In Vivo Assessment of Macular Vascular Density in Healthy Human Eyes Using Optical Coherence Tomography Angiography. American Journal of Ophthalmology. 2016;165:39–46. doi: 10.1016/j.ajo.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Weinhaus RS, Choi J. Neural-Vascular Relationships in Central Retina of Macaque Monkeys (Macaca Fascicularis) Journal of Neuroscience. 1992;12(4):1169–1193. doi: 10.1523/JNEUROSCI.12-04-01169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetikno BT, Yi J, Shah R, Liu W, Purta P, Zhang HF, Fawzi AA. Inner Retinal Oxygen Metabolism in the 50/10 Oxygen-Induced Retinopathy Model. Scientific Reports. 2015;5 doi: 10.1038/srep16752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan VJ, Chan AC, Lam EY. Doppler Oct and Oct Angiography for in Vivo Imaging of Vascular Physiology. INTECH Open Access Publisher; 2012. [Google Scholar]
- Su JP, Chandwani R, Gao SS, Pechauer AD, Zhang M, Wang J, Jia Y, Huang D, Liu G. Calibration of Optical Coherence Tomography Angiography with a Microfluidic Chip. Journal of Biomedical Optics. 2016;21(8):086015–086015. doi: 10.1117/1.JBO.21.8.086015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of Foveal Avascular Zone in Diabetic Eyes Evaluated by En Face Optical Coherence Tomography Angiography. Retina. 2015;35(11):2377–2383. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- Tokayer J, Jia Y, Dhalla AH, Huang D. Blood Flow Velocity Quantification Using Split-Spectrum Amplitude-Decorrelation Angiography with Optical Coherence Tomography. Biomedical Optics Express. 2013;4(10):1909–1924. doi: 10.1364/BOE.4.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RK, An L, Francis P, Wilson DJ. Depth-Resolved Imaging of Capillary Networks in Retina and Choroid Using Ultrahigh Sensitive Optical Microangiography. Optics letters. 2010;35(9):1467–1469. doi: 10.1364/OL.35.001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bower BA, Izatt JA, Tan O, Huang D. In Vivo Total Retinal Blood Flow Measurement by Fourier Domain Doppler Optical Coherence Tomography. Journal of Biomedical Optics. 2007;12(4) doi: 10.1117/1.2772871. 041215. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bower BA, Izatt JA, Tan O, Huang D. Retinal Blood Flow Measurement by Circumpapillary Fourier Domain Doppler Optical Coherence Tomography. Journal of Biomedical Optics. 2008;13(6) doi: 10.1117/1.2998480. 064003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA. Retinal Oxygen: Fundamental and Clinical Aspects. Archives of Ophthalmology. 2003;121(4):547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- Werkmeister RM, Schmidl D, Aschinger G, Doblhoff-Dier V, Palkovits S, Wirth M, Garhöfer G, Linsenmeier RA, Leitgeb RA, Schmetterer L. Retinal Oxygen Extraction in Humans. Scientific Reports. 2015;5 doi: 10.1038/srep15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B, Pierce M, Nassif N, Cense B, Park B, Tearney G, Bouma B, Chen T, de Boer J. In Vivo Dynamic Human Retinal Blood Flow Imaging Using Ultra-High-Speed Spectral Domain Optical Coherence Tomography. Optics Express. 2003;11(25):3490–3497. doi: 10.1364/oe.11.003490. [DOI] [PubMed] [Google Scholar]
- Wolf S, Jung F, Kiesewetter H, Körber N, Reim M. Video Fluorescein Angiography: Method and Clinical Application. Graefe’s Archive for Clinical and Experimental Ophthalmology. 1989;227(2):145–151. doi: 10.1007/BF02169788. [DOI] [PubMed] [Google Scholar]
- Yi J, Chen S, Backman V, Zhang HF. In Vivo Functional Microangiography by Visible-Light Optical Coherence Tomography. Biomedical Optics Express. 2014;5(10):3603–3612. doi: 10.1364/BOE.5.003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Chen S, Shu X, Fawzi AA, Zhang HF. Human Retinal Imaging Using Visible-Light Optical Coherence Tomography Guided by Scanning Laser Ophthalmoscopy. Biomedical Optics Express. 2015a;6(10):3701–3713. doi: 10.1364/BOE.6.003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Liu W, Chen S, Backman V, Sheibani N, Sorenson CM, Fawzi AA, Linsenmeier RA, Zhang HF. Visible Light Optical Coherence Tomography Measures Retinal Oxygen Metabolic Response to Systemic Oxygenation. Light: Science & Applications. 2015b;4(9):e334. doi: 10.1038/lsa.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Wei Q, Liu W, Backman V, Zhang HF. Visible-Light Optical Coherence Tomography for Retinal Oximetry. Optics letters. 2013;38(11):1796–1798. doi: 10.1364/OL.38.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Lu J, Cao D, Liu R, Liu B, Li T, Luo Y, Lu L. The Role of Optical Coherence Tomography Angiography in Fundus Vascular Abnormalities. BMC ophthalmology. 2016;16(1):107. doi: 10.1186/s12886-016-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid S, Dolz-Marco R, Freund KB, Balaratnasingam C, Dansingani K, Gilani F, Mehta N, Young E, Klifto MR, Chae B. Fractal Dimensional Analysis of Optical Coherence Tomography Angiography in Eyes with Diabetic Retinopathyfractal Analysis of Octa Imaging. Investigative Ophthalmology & Visual Science. 2016;57(11):4940–4947. doi: 10.1167/iovs.16-19656. [DOI] [PubMed] [Google Scholar]
- Zhang A, Zhang Q, Chen CL, Wang RK. Methods and Algorithms for Optical Coherence Tomography-Based Angiography: A Review and Comparison. Journal of Biomedical Optics. 2015a;20(10):100901–100901. doi: 10.1117/1.JBO.20.10.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Zhang Q, Wang RK. Minimizing Projection Artifacts for Accurate Presentation of Choroidal Neovascularization in Oct Micro-Angiography. Biomedical Optics Express. 2015;6(10):4130–4143. doi: 10.1364/BOE.6.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Hwang TS, Campbell JP, Bailey ST, Wilson DJ, Huang D, Jia Y. Projection-Resolved Optical Coherence Tomographic Angiography. Biomedical Optics Express. 2016a;7(3):816–828. doi: 10.1364/BOE.7.000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Hwang TS, Dongye C, Wilson DJ, Huang D, Jia Y. Automated Quantification of Nonperfusion in Three Retinal Plexuses Using Projection-Resolved Optical Coherence Tomography Angiography in Diabetic Retinopathyquantification of Retinal Ischemia in Three Plexuses. Investigative Ophthalmology & Visual Science. 2016b;57(13):5101–5106. doi: 10.1167/iovs.16-19776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Huang Y, Zhang T, Kubach S, An L, Laron M, Sharma U, Wang RK. Wide-Field Imaging of Retinal Vasculature Using Optical Coherence Tomography-Based Microangiography Provided by Motion Tracking. Journal of Biomedical Optics. 2015b;20(6):066008–066008. doi: 10.1117/1.JBO.20.6.066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Petrig BL, Qi X, Burns SA. In Vivo Measurement of Erythrocyte Velocity and Retinal Blood Flow Using Adaptive Optics Scanning Laser Ophthalmoscopy. Optics Express. 2008;16(17):12746–12756. doi: 10.1364/oe.16.012746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
En face OCTA allows visualization of the superficial (A), middle (B), and deep (C) capillary plexuses. The extent of non-perfusion and degree of vascular abnormalities differ between the capillary plexuses on en face OCTA. The red and green lines on cross-sectional OCTA are the segmentation boundaries for each layer.
En face OCTA of the (A) superficial (SCP) and (B) deep capillary plexus (DCP) with segmentation boundaries on the cross-sectional OCT below. The yellow line in B shows the location of the cross-sectional OCT. The green line shows an area of rarefied DCP capillaries on OCTA corresponding to reduced reflectivity of the inner / outer segment junction on OCT.
(A) OCTA of the superficial capillary plexus (SCP). (B) OCT of the SCP. (C and D) Enlarged insets from the yellow box in A and B, respectively. The arrows point to blood vessels that appear on structural OCT, but not on OCTA. These may represent vessels with no flow or with flow rates below the OCTA threshold.
(A) OCTA of the optic nerve head reveals abnormal NV vasculature in the upper right quadrant surrounded by normal appearing vasculature. The red segmentation boundaries are seen on the cross-sectional OCT below. FA of the corresponding location shows dye leakage and less distinct NV morphology in the early phase (B) and more leakage and indistinct boundaries in the late phase (C).
(A) Media opacity. (B) Projection artifacts, the top arrow on cross-sectional OCT points to actual location of flow inside the vessel, while the bottom arrow points to the flow projected onto the retinal pigment epithelium. Red lines on cross-sectional OCT are segmentation boundaries for the en face image. (C) Segmentation error caused by epiretinal membrane (arrow). Red and green lines on cross-sectional OCT show the segmentation boundaries following the membrane. (D) Stretch artifact (arrow) from software correction of eye motion. (E, F) Eye motion artifacts before (E) and after (F) motion correction technology (MCT). (G) ‘Quilting’ artifact from motion and MCT correction. (H) ‘Vessel doubling’ artifact from motion and MCT correction.
(A) An example en face OCTA image of the superficial capillary plexus. (B) An en face OCTA image of the middle capillary plexus with many projection artifacts resembling vessels from (A). (C) A thresholded image of A is multiplied with A and a weighting factor. (D) After subtraction of the major superficial retinal vessels, the middle capillary plexus is now free of projection artifact.
(A) An en face vis-OCT image from a healthy rat eye. The white solid circles indicate the scanning paths for the dual-circle Doppler vis-OCT method. The arrow indicates a clockwise scanning direction. (B) The vis-OCT circular B-scan corresponding to the inner circular scan path in (A). (C) A zoomed view of an artery-vein pair corresponding to the solid yellow box in (B). Vessel diameter was measured using the high axial resolution of OCT, as indicated by the yellow arrows. (D) Doppler shifts on the OCT signal were used to recover the blood flow speed within the artery and vein respectively. The artery had a positive velocity, while the vein had a negative velocity, consistent with the direction of flow in these respective vessels.


