Abstract
One of the major contributors to sickle cell disease (SCD) pathobiology is the hemolysis of sickle red blood cells (RBCs), which release free hemoglobin and platelet agonists including adenosine 5’-diphosphate (ADP) into the plasma. While platelet activation/aggregation may promote tissue ischemia and pulmonary hypertension in SCD, modulation of sickle platelet dysfunction remains poorly understood. Calpain-1, a ubiquitous calcium-activated cysteine protease expressed in the hematopoietic cells, mediates aggregation of platelets in healthy mice. We generated calpain-1 knockout Townes sickle (SSCKO) mice to investigate the role of calpain-1 in the steady state and hypoxia/reoxygenation (H/R)-induced sickle platelet activation and aggregation, clot retraction, and pulmonary arterial hypertension. Using multi-electrode aggregometry, which measures platelet adhesion and aggregation in whole blood, we determined that steady state SSCKO mice exhibit significantly impaired PAR4-TRAP-stimulated platelet aggregation as compared to Townes sickle (SS) and humanized control (AA) mice. Interestingly, the H/R injury induced platelet hyperactivity in SS and SSCKO, but not AA mice, partially rescued the aggregation defect in SSCKO mice. The PAR4-TRAP-stimulated GPIIb-IIIa/αIIbβ3 integrin activation was normal in SSCKO platelets suggesting that an alternate mechanism mediates the impaired platelet aggregation in steady state SSCKO mice. Taken together, we provide the first evidence that calpain-1 regulates platelet hyperactivity in sickle mice, and may offer a viable pharmacological target to reduce platelet hyperactivity in SCD.
Keywords: Calpain-1, platelets, sickle cell disease, aggregation, hypoxia, hypertension
Introduction
Sickle cell disease (SCD) was the first genetic disorder for which a molecular mechanism was identified [1]. SCD, caused by a glutamic acid to valine mutation in the sixth codon of the β-globin gene, results in sickle hemoglobin that polymerizes under low oxygen conditions forming sickle red blood cells (RBCs) [2, 3]. Reduced deformability of sickle RBCs in part contributes to blood vessel occlusion, leading to organ damage, vaso-occlusive crises (VOC), and pain, which are hallmark features of SCD [4]. Hemolysis of sickle RBCs releases adenosine 5’-diphosphate (ADP), a potent agonist of platelet activation and aggregation, in addition to free hemoglobin that acts as a scavenger of nitric oxide (NO), a known inhibitor of platelet activation [5]. Activation of the protease activated receptors PAR1 and PAR4 in humans (PAR3 and PAR4 in mice) by thrombin or a PAR-specific agonist triggers phospholipase-C β (PLC-β) activation resulting in calcium mobilization. Increased calcium flux leads to the expression of activated GPIIb-IIIa, GP1b, and P-selectin involved in platelet aggregation, adhesion, and rolling pathways, respectively [6]. Activated platelets, in addition to sickle RBCs and leukocytes, ultimately contribute to VOC [7, 8]. Moreover, increased platelet and thrombotic activity in SCD correlates with clinical complications such as pulmonary arterial hypertension (PAH), deep vein thrombosis, and stroke [9–11]. Patients with SCD and mouse models show platelet activation [7, 12, 13], yet mechanisms underlying platelet dysfunction in SCD remain poorly understood.
Calpains are neutral calcium-activated cysteine proteases involved in a variety of physiological processes, including cytoskeletal reorganization, platelet activation, and hemostasis [14–16]. Fourteen genes are known to encode calpains; however, calpain-1 (μ-calpain) and calpain-2 (m-calpain) are the two conventional calpains with 61% amino acid identity that are most intensely investigated in mammals [14]. While the micromolar calcium is sufficient to activate calpain-1 in vitro, calpain-2 requires millimolar concentrations of calcium for activation. Under resting in vivo conditions, both calpains are kept inactive presumably by their common endogenous inhibitor, calpastatin [14]. Although both calpains are widely expressed in most mammalian tissues, calpain-1 dominates in hematopoietic cells such as RBCs and platelets whereas calpain-2 is more prevalent in the nervous system [16, 17].
Sickle RBCs are known to exhibit elevated intracellular calcium [18], suggesting a role for calcium in the pathophysiology of SCD. Our previous studies tested this hypothesis using whole blood from SCD patients, and found that calpain-1 activity is elevated in dense/dehydrated RBCs [19], the RBC fraction that is thought to be mainly responsible for VOC [20, 21]. Furthermore, pharmacological inhibition of calpain-1 in the SAD mouse model of mild SCD reduced sickle RBC density/dehydration under both steady state and hypoxia conditions, which mimic some features of VOC [19]. Although the SAD sickle mouse model [22] has been used to elucidate several biological mechanisms of SCD [23, 24], its clinical relevance is limited for a number of reasons. Some of the features of SAD mice are: (i) it is a non-humanized model that expresses both mouse and human beta globin genes, (ii) it is a mild model of SCD without anemia in adult mice, (iii) it requires hypoxia/reoxygenation (H/R) treatment to induce RBC sickling, and (iv) it displays minimal organ damage [22]. Thus, to describe the role of calpain-1 more definitively in SCD, we acquired the Townes mouse model of severe SCD [25]. Townes Sickle mice offer several advantages such as: (i) it is a humanized mouse model of SCD that expresses exclusively human alpha, beta, and gamma globins, (ii) expresses 99% human sickle beta globin resulting in a severe disease phenotype with hemolytic anemia and multi-organ damage, and (iii) displays extensive RBC sickling at steady state without requiring hypoxia/reoxygenation treatment. Using established breeding and gene mapping techniques, we generated the first calpain-1 knockout Townes sickle (SSCKO) mice, which demonstrated reductions in all aspects of sickle chronic pain phenotype including mechanical hyperalgesia, sensitivity to heat and cold, and deep tissue hyperalgesia [26].
In the present study, we tested our hypothesis that platelets from the novel SSCKO mouse model [26] would display reduced platelet activation and aggregation, consistent with our previous studies on washed platelets from calpain-1 knockout C57BL/6 (CKO) mice, which display impaired platelet aggregation and clot retraction, but normal bleeding times [17, 27]. We further hypothesized that compared to control Townes AA mice, Townes sickle (SS) mice would display thrombosis in major organs such as the liver and lungs, which might contribute to pulmonary arterial hypertension (PAH) consistent with elevated markers of platelet activation that correlate with incidence of PAH in SCD patients [28]. Given the expected reduction in platelet function in SSCKO mice compared to SS and AA mice, we proposed that SSCKO mice would also display a reduction in multi-organ thrombosis and PAH. Our results demonstrate that the steady state platelet activation/aggregation is elevated in Townes sickle (SS) mice but reduced in their SSCKO counterparts. Interestingly, the hypoxia/reoxygenation induced platelet hyperactivity partially rescued the impaired platelet aggregation in SSCKO mice. Finally, despite exhibiting in vivo platelet activation and extensive ventricular hypertrophy, Townes SS and SSCKO mice neither display thrombosis in the liver and lungs or evidence of PAH. Together, our findings in the SSCKO mouse model reveal a functional role for calpain-1 in sickle platelet pathophysiology, and demonstrate that calpain-1 may present a viable pharmacological target in SCD.
Methods
Detailed methods are described in supplemental data
Animal studies
Townes sickle (SS) mice were obtained from the Jackson Laboratory and backcrossed with our calpain-1 knockout C57BL/6 (CKO) mice [17] to generate humanized calpain-1 knockout sickle (SSCKO) mice [26]. Humanized (AA) or wild type C57BL/6 mice were used as controls. Both male and female mice were used except otherwise indicated. Tufts University Institutional Animal Care and Use Committee approved all animal procedures.
Platelet counts
Due to RBC fragmentation in the Townes SS mice, platelet counts were determined by flow cytometry using the double staining of platelet markers CD41/GP1b as described [29]. Bulk hematological analyzers such as Hemavet 950FS and ADVIA 2120 were not suitable for such measurements.
Hypoxia/reoxygenation treatment
Mice were exposed to 3 hours of hypoxia (10.5% O2, 89.5% N2), followed by 4 hours of reoxygenation at room air (22% O2) prior to indicated experiments.
Platelet aggregation in whole blood (multiple electrode aggregometry)
Whole blood multi-electrode aggregometry (MEA) was performed using the Multiplate® analyzer (Roche Diagnostics) as previously described [30]. For steady state measurements, groups of AA, SS, SSCKO, and CKO mice were anesthetized with isoflurane and blood was collected from the vena cava into heparin-coated tubes (Becton Dickinson). MEA, in 300 µL of whole blood from each mouse, was performed over a 6-minute window following PAR4-TRAP (650 µM) agonist stimulation. Results reported as aggregation units (AU) are shown as area under the curve. A different group of AA, SS, and SSCKO mice was exposed to hypoxia/reoxygenation as described above, prior to platelet aggregation testing. Hypoxia/reoxygenation and aggregation testing were performed on the same group of CKO mice after one week of steady state testing.
Flow cytometry analyses of platelet surface activated GPIIb-IIIa/αIIbβ3
Basal and PAR4-TRAP-stimulated expression of GPIIb-IIIa was quantified by flow cytometry as previously described with slight modifications [13]. The mean fluorescence intensity (MFI) of all platelets is included in the data reported [30].
Clot retraction
Nine months old male AA, SS, and SSCKO mice were anesthetized with isoflurane (3.5%). Blood was collected from vena cava in ACD (15%) mixed with 0.5 µM PGE1, and HEPES-stabilized modified Tyrode’s solution was added to collect Platelet Rich Plasma (PRP) by centrifugation at 200×g for 15 minutes (brakes off at room temperature). PRP was used to isolate washed platelets by centrifugation at 600×g for 10 minutes as before. Washed platelets were resuspended in the Tyrode’s buffer for 1–2 hours at 37°C. Resting platelets were normalized by the addition of Tyrode’s buffer to 108 platelets/mL for clot retraction assay in glass borosilicate tubes. Platelet Poor Plasma (PPP) (50 µL) from the corresponding genotypes was added to each of the samples along with 1.0 mM CaCl2 and 0.5 unit/mL Thrombin to a final volume of 350 µl. Packed RBCs (5µL) from each genotype were added to visualize the clot formation in presence of Pasteur glass pipet. Experiments for clot retraction were performed three times in age and sex matched mice. Photographs were taken at 30-minute intervals until the clot retraction was complete at room temperature.
Thrombosis and experimental pulmonary arterial hypertension (PAH) in sickle mice
Standard H&E staining was performed on liver and lung tissues harvested from H/R-treated mice, and slides were examined for fibrin thrombi. For PAH, steady state and H/R-treated mice were examined for right ventricular systolic pressure (RVSP) as previously described [31, 32].
Statistical analyses
Differences between groups of mice in platelet aggregation studies were analyzed using Mann-Whitney U-test (GraphPad Prism version 5.0a). All other analyses were conducted using unpaired t-tests (Microsoft Excel 2010). Data are presented as mean ± SEM except as otherwise stated. The P value of < 0.05 was considered significant.
Results
Townes sickle platelets are activated in vivo
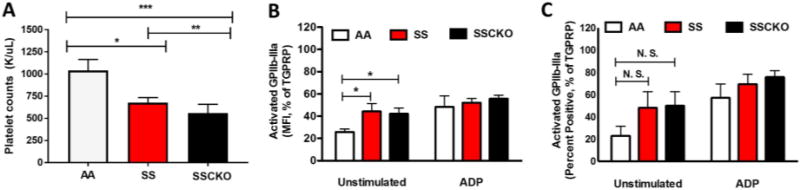
Flow cytometric analyses following CD41/GP1b staining of whole blood platelets indicated thrombocytopenia in the Townes SS (677 K/µL vs. 1040 K/µL in control AA mice, P < 0.05) and SSCKO (560 K/µL vs. 1040 K/µL in control AA mice, P < 0.001) mice (Figure 1A). Previous studies in the Berkeley sickle mice have demonstrated activated circulating platelets in vivo [13]. Analyses of platelet mean fluorescence intensity revealed that at baseline (unstimulated), the SS and SSCKO mice exhibit increased expression of activated GPIIb-IIIa compared to control AA mice (Figure 1B) (n = 6 mice per group; P < 0.05). At baseline, the percentage of platelets positive for activated GPIIb-IIIa was also higher in both SS and SSCKO mice, although the difference was not statistically significant (Figure 1C). However, there was no difference in the ADP-stimulated expression of activated GPIIb-IIIa in SS and SSCKO mice as compared to AA, suggesting that plasma ADP mediated activation might have rendered sickle platelets refractory to further ADP stimulation (Figure 1A – B). Both the mean fluorescent intensity and percentage of platelets positive for P-selectin were similar for AA, SS, and SSCKO mice (supplemental Figure 1A – B), consistent with studies in young Berkeley sickle mice showing no difference in the expression of P-selectin on circulating platelets compared to control mice [13].
Figure 1. Townes sickle mice display thrombocytopenia and platelet activation.
Whole blood collected from AA, SS, and SSCKO mice (n = 6 – 9 per genotype) was evaluated for platelet counts using flow cytometry (A). Expression of platelet surface activated GPIIb-IIIa reported as mean fluorescent intensity (per cell) of activated GPIIb-IIIa (B), and percentage of platelets positive for activated GPIIb-IIIa (C) is shown. Data in (B) and (C) were normalized to percent of maximal activation induced by TGPRP. All data are presented as mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001. Abbreviations: MFI = mean fluorescence intensity; ADP = Adenosine 5’-diphosphate (20 µM), TGPRP = Thrombin Gly-Pro-Arg-Pro (2U/mL).
Calpain-1 is required for whole blood platelet aggregation in steady state sickle mice
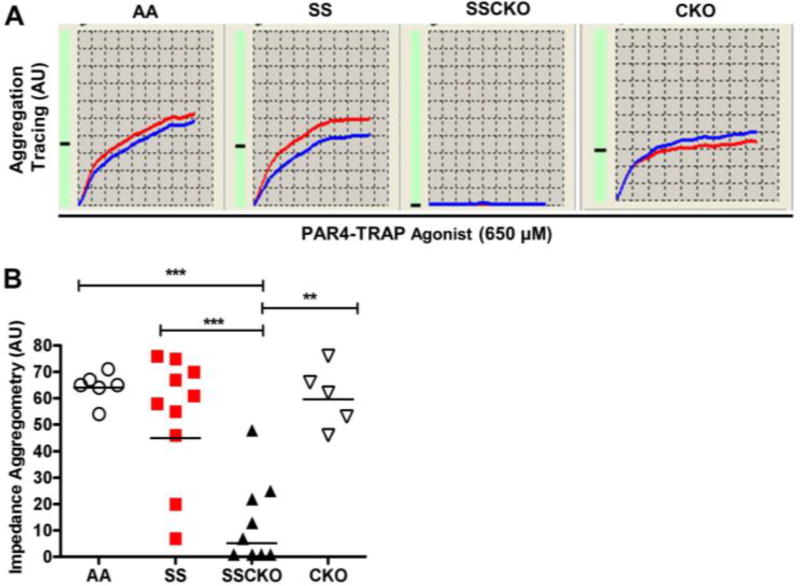
Platelets from SCD patients [30] and Berkeley sickle mice [13] display increased reactivity. Our previous studies on calpain-1 knockout C57BL/6 (CKO) mice demonstrated attenuated aggregation of isolated washed platelets by light transmission aggregometry [17, 27]. Due to the crucial role of sickle RBC hemolysis in platelet activation/aggregation, we employed impedance/multiple electrode aggregometry of platelets in whole blood to investigate the role of calpain-1 in sickle platelet aggregation. Results from each aggregation assay represent two simultaneous recordings (red and blue tracings) of the same blood sample (Figure 2A). At steady state (Normoxia), the PAR4-TRAP induced platelet aggregation was similar in AA and SS mice (64 AU vs. 54 AU, n = 6 – 10 mice per genotype, P = 0.6) (Figure 2A – B). However, SSCKO mice displayed significantly reduced platelet aggregation as compared to SS (13 AU vs. 54 AU, P < 0.005, n = 6 – 10 mice per genotype) and AA mice (13 AU vs. 64 AU P < 0.005, n = 6 – 10 mice per genotype) (Figure 2A – B). To determine whether the whole blood platelet aggregation defect in SSCKO mice was due to the lack of calpain-1, severe SCD or both, the CKO mice were also tested for whole blood platelet aggregation. In contrast to previous studies using washed platelets and PRP [17, 27], CKO platelets did not display significantly impaired platelet aggregation in whole blood compared to AA mice (46 AU vs. 58 AU, n = 5 – 6 mice per genotype, P = 0.5) (Figure 2A – B). One possible explanation is differential aggregation responses of CKO platelets to the maximal agonist concentration of 650 µM PAR4-TRAP (with respect to normal platelets) used in the current whole blood aggregation study as compared to the submaximal concentrations of thrombin (10 nM) used in our previous washed platelet aggregation studies [17]. Indeed, washed platelets from CKO mice were found to display a dose-dependent increase in aggregation response to thrombin concentrations ranging from 0.018 U/mL to 0.3 U/mL. Furthermore, when stimulated with thrombin concentrations greater than 0.3 U/mL, the defective aggregation phenotype in the washed CKO platelets was no longer observed [27]. Thus, our whole blood platelet aggregation data (Figure 2A – B) revealed that both CKO and SSCKO platelets have diminished platelet aggregation in response to stimulation of PAR4 receptor but that the PAR4-TRAP dose-dependence differs – SSCKO platelets that are less responsive to 650 µM PAR4-TRAP than CKO platelets. Taken together, our findings demonstrate that calpain-1 is required for whole blood platelet aggregation in steady state Townes sickle mice.
Figure 2. Calpain-1 is required for whole blood aggregation of sickle platelets at steady state.
Whole blood samples from AA, SS, SSCKO, and CKO mice at steady state were evaluated for PAR4-TRAP-stimulated MEA. (A) Representative aggregation profile of each mouse genotype represents two simultaneous recordings (red and blue tracings) of the same blood sample over a 6-minute window. (B) Cumulative results and mean aggregation unit (AU) of each group of mice (n = 6 – 10 mice per genotype). Each symbol shows data from a single mouse. Bars represent geometric mean. Mann-Whitney U-test. *P < 0.05; **P < 0.01; ***P < 0.005. PAR4-TRAP = 650 µM.
Hypoxia/reoxygenation induces platelet hyperactivity in sickle mice, and partly rescues impaired platelet aggregation in calpain-1 knockout sickle mice
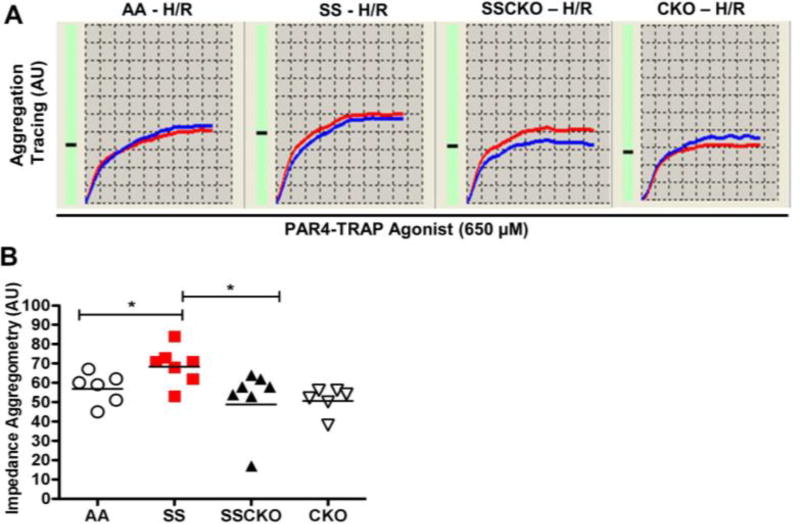
To test the hypothesis that H/R) injury, which mimics many features of sickle cell vaso-occlusive crises (VOC) [33–35], induces platelet hyperactivity in sickle mice, we exposed AA, SS, SSCKO, and CKO mice to H/R treatment prior to assessment of whole blood platelet aggregation. As expected, H/R induced platelet hyperactivity in SS mice but not AA mice (69 AU vs. 57 AU, P < 0.05, n = 6 – 7 mice per genotype) or CKO mice (69 AU vs. 51 AU, P < 0.05, n = 6 – 7 mice per genotype) (Figure 3A – B). Interestingly, H/R of SSCKO mice induced significant platelet hyperactivity that partly restored platelet aggregation to the levels observed in AA (52 AU vs. 57 AU, P = 0.7, n = 6 – 7 mice per genotype) and CKO (52 AU vs. 51 AU, P = 0.2, n = 6 – 7 mice per genotype), but not the high levels detected in SS mice (52 AU vs. 69 AU P < 0.05, n = 7 mice per genotype) (Figure 3A – B). These findings represent the first assessment of platelet aggregation in the whole blood of sickle mice, with several implications for sickle pathobiology. We conclude that calpain-1 is required for platelet aggregation in both steady state SCD and H/R injury simulating some features of sickle crises. Precise mechanisms including compensation by calpain-2, post-translational modifications of calpain-1, inflammatory pathways, and cell-cell adhesion events are some of the possibilities that might contribute to the partial rescue of impaired platelet aggregation in SSCKO mice exposed to H/R injury under these conditions.
Figure 3. Hypoxia/reoxygenation induces platelet hyperactivity in sickle mice, and partly rescues impaired platelet aggregation in calpain-1 knockout sickle mice.
AA, SS, SSCKO, and CKO mice were exposed to hypoxia/reoxygenation (H/R) (3 hours of hypoxia with 10.5% O2 followed by 4 hours of reoxygenation at room air) prior to whole blood collection for PAR4-TRAP stimulated MEA. (A) Representative aggregation profile of two simultaneous recordings (red and blue tracings) of the same blood sample over a 6-minute window. (B) Cumulative results and mean aggregation unit (AU) of each group of mice (n = 6 – 7 mice per genotype). Each symbol shows data from a single mouse. Bars represent geometric mean. Mann-Whitney U-test. *P < 0.05. PAR4-TRAP = 650 µM.
Activation of platelet surface GPIIb-IIIa by PAR4-TRAP is unaltered in steady state and hypoxia/reoxygenation-treated calpain-1 knockout sickle mice
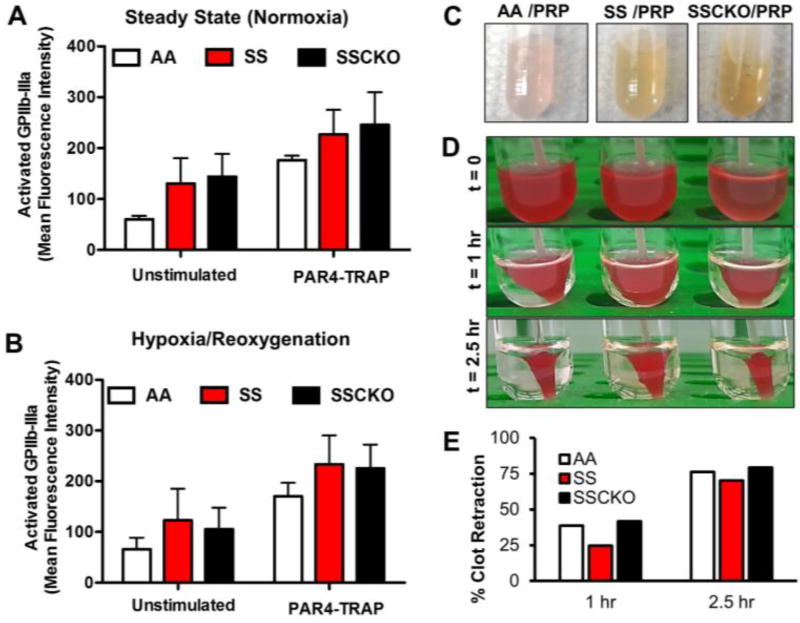
Since the multi-electrode aggregometry (MEA) provides a measure of both platelet adhesion and aggregation in whole blood, we investigated two platelet functions that might explain the impaired aggregation in SSCKO mice at steady state, and the partial rescue of the defect following hypoxia/reoxygenation. Surface expression of activated platelet GPIIb-IIIa, the critical mediator of fibrinogen binding and platelet aggregation [36], was quantified following the same maximal PAR4-TRAP (650 µM) stimulation that was used in the aggregation studies (Figures 2 and 3). Interestingly, platelets from the steady state and H/R-treated SSCKO mice exhibited similar expression levels of activated GPIIb-IIIa compared to their AA and SS counterparts (Figure 4A – B). These results suggest that the impaired platelet aggregation in steady state SSCKO mice (Figure 2) is not due to a lack of activation-dependent conformational change in GPIIb-IIIa.
Figure 4. Activation of platelet surface GPIIb-IIIa by PAR4-TRAP is unaltered in steady state and hypoxia/reoxygenation–treated calpain-1 knockout sickle mice. Rescue of the clot retraction defect.
Whole blood samples were collected from (A) steady state and (B) hypoxia/reoxygenation-treated AA, SS, and SSCKO mice, and tested for unstimulated (basal) and PAR4-TRAP stimulated GPIIb-IIIa expression. Graphs represent average mean fluorescent intensity (MFI) of each group of mice (n = 3 per genotype) ± SEM, PAR4-TRAP = 650 µM. (C) Platelet rich plasma (PRP) of AA, SS, and SSCKO mice shows evidence of in vivo hemolysis. (D) Washed resting platelets (108) were isolated from age and sex matched AA, SS, and SSCKO mice, and clot retraction was initiated in the presence of 1.0 mM CaCl2 and 0.5 U/mL Thrombin. (E) Clot retraction was quantified in AA, SS, and SSCKO mice. Experiments were repeated three times under different conditions, resulting in similar rescue of the clot retraction phenotype in SSCKO as compared to SS mice.
Calpain-1 gene inactivation corrects clot retraction defect in sickle mice
Since platelet outside-in signaling via GPIIb-IIIa plays a critical role in clot retraction, we evaluated the clot retraction phenotype in AA, SS, and SSCKO mice (Figure 4C – E). Because of the hemolysis in both SS and SSCKO mice (Figure 4C), washed platelets were isolated, normalized to equal number, and activated with 0.5 U/mL Thrombin. We found that the clot retraction is slower in Townes SS mice as compared to AA controls (Figure 4D, E). Interestingly, clot retraction phenotype in SSCKO mice was similar to that of AA mice indicating that calpain-1 deletion in SSCKO mice corrected the slower clot retraction in SS mice (Figure 4D, E). The rescue of the clot retraction defect in SSCKO mice was further confirmed at different doses of thrombin (data not shown). In previous studies, we have shown that lack of calpain-1 causes abnormal clot retraction in mice without the complications of hemolysis and SCD [27]. However, in the sickle background of SSCKO mice that are prone to hemolysis (Figure 4C) and endowed with RBC abnormalities, deletion of calpain-1 corrects the defective clot retraction phenotype. The RBCs display improved deformability in calpain-1 null mice [16], and show improved morphology in SAD mice pharmacologically treated with a calpain-1 inhibitor [19]. Recently, it was demonstrated that RBCs undergo characteristic polyhedral shape upon clot formation, and excessive membrane rigidity can interfere with this polyhedral shape under low thrombin condition [37].
Hypoxia/reoxygenation-induced platelet hyperactivity does not cause in vivo thrombosis or pulmonary arterial hypertension in Townes sickle mice
In SCD patients, platelet activation is correlated with the severity of pulmonary arterial hypertension (PAH), a clinical complication that increases mortality risk [28, 38, 39]. PAH is believed to be a complication of chronic hemolysis in SCD patients [40]. Thus, a phenomenon known as hemolysis-associated PAH posits that release of free hemoglobin upon RBC hemolysis potentiates: (i) activation of platelets, and (ii) scavenging nitric oxide (NO), which results in constricted blood vessels leading to pulmonary vessel thrombosis and PAH [39].
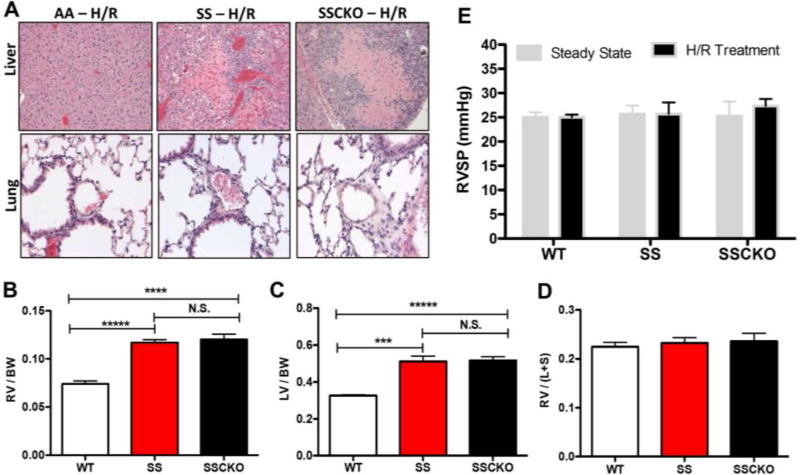
Since SS and SSCKO mice demonstrated H/R-induced platelet hyperactivity (Figure 3), we tested whether the increased propensity for platelet aggregation would result in in vivo thrombosis under these conditions. Histological analyses of liver and lung tissues revealed no evidence of fibrin thrombi in H/R-treated SS and SSCKO mice (Figure 5A). The absence of visible thrombi is consistent with previous studies in Berkeley sickle mice, which found evidence of hepatic ischemia but no thrombosis [41]. A number of studies have indicated the presence of PAH in Berkeley sickle mice using parameters such as elevated pulmonary artery pressure [42] and left and right ventricular end-diastole volumes [11]. In the present study, we observed severe hypertrophy of the right and left ventricles of SS and SSCKO mice (Figures 5B – C), resulting in a normal Fulton ratio (Figure 5D). Despite the ventricular hypertrophy, SS and SSCKO mice displayed normal right ventricular systolic pressure (RVSP) (Figure 5E), a common measure of PAH [31, 32]. Thus, our results suggest that the Townes sickle mice might be a model of ventricular dysfunction but not PAH. Future studies would delineate the mechanism of the ventricular hypertrophy observed in the Townes sickle mice.
Figure 5. Townes sickle mice display right ventricular dysfunction but no in vivo thrombosis or pulmonary arterial hypertension.
(A) Representative histological sections of liver and lung from hypoxia/reoxygenation-treated AA, SS, and SSCKO mice. (Top panel) Extensive focal hepatic necrosis with chronic inflammation in the livers of SS and SSCKO mice without visible fibrin thrombi. (Bottom panel) Normal lung histology in SS and SSCKO compared to WT mice exposed to hypoxia/reoxygenation treatment. Images were taken on an Olympus BX45 microscope with 20×/0.50 numerical aperture UPlanFl objective or 40×/0.75 NA UPlanFl objective, and using Infinity II camera and Infinity Capture software. H&E stained sections were captured at 20× (lung) and 10× (liver) magnification. (B) Right ventricular hypertrophy is present in SS (***P < 0.00001 vs. WT mice, n = 6 – 8 mice per genotype, unpaired t test) and SSCKO mice (** P < 0.01 vs. WT mice, n = 6 – 7 mice per genotype, unpaired t test). (C) Similarly, left ventricular hypertrophy is present in SS (***P < 0.001 vs. WT mice, n = 6 – 8 mice per genotype, unpaired t test) and SSCKO mice (** P < 0.01) vs. WT mice, n = 6 – 7 mice per genotype, unpaired t test). (D) The Fulton ratio of ventricular weights (right ventricle-left ventricle including septum) [63] is normal in SS and SSCKO compared to WT mice. By exhibiting hypertrophy in both left and right ventricles, the SS and SSCKO have a similar Fulton ratio compared to WT mice. (E) Right ventricular systolic pressure (RVSP), the key indicator of pulmonary arterial hypertension (PAH) in lungs was measured in steady state and H/R-treated C57BL/6 wild type (WT), SS, and SSCKO mice. RSVP values are not elevated in Townes SS and SSCKO mice as compared to their wild type counterparts. Data are shown as mean ± SEM of 3 – 6 mice per genotype. * P < 0.05.
Discussion
In this study, we report the first characterization of platelet function in the novel calpain-1 knockout Townes sickle (SSCKO) mouse model [26]. We provide evidence that calpain-1 is required for whole blood platelet aggregation in both steady state and hypoxia/reoxygenation (H/R)-treated SSCKO mice. We also show that H/R injury partially relieves the dependence on calpain-1 for platelet aggregation by an unidentified GPIIb-IIIa/αIIbβ3 integrin activation-independent mechanism. This study establishes calpain-1, a known modulator of platelet function [17, 27, 43], as the first platelet target to be differentially modulated by steady state SCD and H/R injury, which mimics features of sickle VOC [33–35].
Calpains are considered potential therapeutic targets in Alzheimer’s disease [44], aging [45], and diabetes [46]. Calpains regulate the functions of many proteins, thereby modulating multiple physiological processes including apoptosis, cytoskeletal reorganization, and hemostasis [14, 47]. Calpain-1, one of two conventional calpains [48], has been extensively investigated in hematopoietic cells. Global knockout of calpain-1 improved RBC deformability and fragility in CKO mice [16], while pharmacological inhibition of calpain-1 improved sickle RBC dehydration in the SAD mouse model of mild SCD [19]. Calpain-1 is known to regulate platelet functions [49, 50], including our previous studies demonstrating its role in the aggregation and spreading of washed platelets, clot retraction, and FeCl3-induced in vivo thrombosis [17, 27]. Hence, we hypothesized that genetic ablation of calpain-1 in a severe model of SCD such as the Townes mice [25] may unveil its functional role in the pathobiology of SCD.
During both steady state and painful crises, SCD is characterized by a hypercoagulable state [51] including platelet activation [30], elevated thrombin generation, decreased levels of anticoagulant proteins [52], procoagulant activity [53], and aberrant activation of the fibrinolytic system [54]. Fibrin D-dimer has also been described as a marker of SCD vaso-occlusive pain crises and other complications [55], pointing to a role of platelets and coagulation in SCD vascular occlusion [56]. While increased platelet activation and reactivity have been reported in the Berkeley sickle mice [13], a similar assessment has not been conducted in the Townes sickle mice.
Recent studies revealed a pivotal role for calpains in hypoxia-induced acute thrombosis [57, 58]. Platelets from hypoxia-treated rats demonstrated increased platelet reactivity, and significant upregulation of calpain small subunit 1 (CAPNS1), a regulatory component required for the activity of both calpain-1 and calpain-2. The hypoxia-induced platelet hyperactivity was abolished upon treatment with the pan-calpain inhibitor, PD150606 [57]. Consistent with these findings, we found that while whole blood platelet aggregation in steady state SSCKO mice was significantly impaired, the H/R injury induced platelet hyperactivity in sickle mice partially relieved the aggregation defect in SSCKO mice. Since platelet aggregation is known to be mediated by the binding of fibrinogen to platelet GPIIb-IIIa [59], we measured the levels of PAR4-TRAP-stimulated expression of activated GPIIb-IIIa on the surface of platelets from steady state and H/R-treated SSCKO mice. Interestingly, the expression of activated GPIIb-IIIa remains unaltered in SSCKO mice under both conditions, indicating that at least in sickle platelets, calpain-1 does not mediate translocation of GPIIb-IIIa to the platelet surface following agonist stimulation. Future studies would investigate whether calpain-1 affects mechanisms downstream of GPIIb-IIIa-fibrinogen binding that might participate in stable clot formation [36, 60]. The rescue of the clot retraction defect in SSCKO mice (Figure 4) in the presence of washed platelets and RBCs further underscores the complexities of clot formation in SCD influenced by hypoxia, hemolysis, and adhesive interactions involving platelets, WBCs, and RBCs [61, 62]. Therefore, it remains a possibility that the rescue of the clot retraction defect in SSCKO as compared to SS mice is influenced by RBC membrane stiffness, dehydration, and hemolytic episodes.
Consistent with previous findings in the Berkeley sickle mice [13, 41], we found little evidence for hepatic thrombi formation in Townes SS and SSCKO mice despite H/R-induced platelet hyperactivity. In contrast to previous studies in the Berkeley mice [42], we did not find evidence of pulmonary arterial hypertension (PAH), as measured by elevated right ventricular systolic pressure (RVSP) in the Townes sickle mice. However, we observed severe hypertrophy of the left and right ventricles of Townes sickle mice, which suggest ventricular dysfunction. Future studies would examine the underlying cause of the ventricular dysfunction in the absence of elevated RVSP. Further characterization of calpain-1 null Townes sickle mice as reported in this study will allow experimental evaluation of in vivo thrombosis in the blood vessels under conditions of systemic calpain-1 inactivation.
Supplementary Material
Highlights.
Gene knockout in sickle mice reveals a role for calpain-1 in platelet function
At steady state, calpain-1 is required for sickle cell platelet aggregation
Hypoxia/reoxygenation partly reduces reliance on calpain-1 for platelet aggregation
Acknowledgments
The authors thank members of the Center for Platelet Research Studies at Boston Children’s Hospital including Dr. Alan D. Michelson, Sabrina Carmichael, and Emma Forde, for their support of J.O.N. while she was conducting the platelet studies; Dr. Nancy Wandersee for helpful discussions about in vivo thrombosis in sickle mice; Drs. John Castellot, David J. Greenblatt, Henry H. Wortis, Alicia Rivera, Kalpna Gupta, William F. Dietrich, Carlo Brugnara, and Richard J. Labotka for guiding J.O.N in the completion of her doctoral dissertation research; Donna-Marie Mironchuk for helpful graphics and manuscript preparation support, and all members of the Chishti lab for supporting J.O.N. throughout her tenure as a graduate student.
Funding: This work was supported by the Howard Hughes Medical Institute International Student Research Fellowship (J.O.N), National Institutes of Health grants HL089517 and HL095050, American Society of Hematology Bridge Grant, and American Heart Association Grant-in-Aid (A.H.C). F.J.M was partly funded by R25GM066567 under the Post-Baccalaureate Research Education Program (PREP).
J.A.J is an employee (retired) and minor shareholder of Eli Lilly and Company; A.L.F. has received research funding from GE Global Research, GL Synthesis, Bristol-Myers Squibb, Pfizer, Baxalta, Celerion, Eisai, Megakaryon, and Sysmex.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest statement
All other authors report no declarations of interest.
References
- 1.Pauling L, Itano HA, et al. Sickle cell anemia, a molecular disease. Science. 1949;109(2835):443. [PubMed] [Google Scholar]
- 2.Eaton WA, Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987;70(5):1245–66. [PubMed] [Google Scholar]
- 3.Eaton WA, Hofrichter J. Sickle cell hemoglobin polymerization. Adv. Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 4.Ballas SK, Lieff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, Johnson CS, Rogers ZR, Smith-Whitley K, Wang WC, Telen MJ, C.S.C.C. Investigators Definitions of the phenotypic manifestations of sickle cell disease. Am. J. Hematol. 2010;85(1):6–13. doi: 10.1002/ajh.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaarder A, Jonsen J, Laland S, Hellem A, Owren PA. Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature. 1961;192:531–2. doi: 10.1038/192531a0. [DOI] [PubMed] [Google Scholar]
- 6.Jackson SP, Schoenwaelder SM. Antiplatelet therapy: in search of the 'magic bullet'. Nat Rev Drug Discov. 2003;2(10):775–89. doi: 10.1038/nrd1198. [DOI] [PubMed] [Google Scholar]
- 7.Lee SP, Ataga KI, Orringer EP, Phillips DR, Parise LV. Biologically active CD40 ligand is elevated in sickle cell anemia: potential role for platelet-mediated inflammation. Arterioscler. Thromb. Vac. Biol. 2006;26(7):1626–31. doi: 10.1161/01.ATV.0000220374.00602.a2. [DOI] [PubMed] [Google Scholar]
- 8.Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr. Opin. Hematol. 2002;9(2):101–6. doi: 10.1097/00062752-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Ataga KI, Moore CG, Hillery CA, Jones S, Whinna HC, Strayhorn D, Sohier C, Hinderliter A, Parise LV, Orringer EP. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica. 2008;93(1):20–6. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 10.Verduzco LA, Nathan DG. Sickle cell disease and stroke. Blood. 2009;114(25):5117–25. doi: 10.1182/blood-2009-05-220921. [DOI] [PubMed] [Google Scholar]
- 11.Arumugam PI, Mullins ES, Shanmukhappa SK, Monia BP, Loberg A, Shaw MA, Rizvi T, Wansapura J, Degen JL, Malik P. Genetic diminution of circulating prothrombin ameliorates multiorgan pathologies in sickle cell disease mice. Blood. 2015;126(15):1844–55. doi: 10.1182/blood-2015-01-625707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beurling-Harbury C, Schade SG. Platelet activation during pain crisis in sickle cell anemia patients. Am. J. Hematol. 1989;31(4):237–41. doi: 10.1002/ajh.2830310404. [DOI] [PubMed] [Google Scholar]
- 13.Ohno K, Tanaka H, Samata N, Jakubowski JA, Tomizawa A, Mizuno M, Sugidachi A. Platelet activation biomarkers in Berkeley sickle cell mice and the response to prasugrel. Thromb. Res. 2014;134(4):889–94. doi: 10.1016/j.thromres.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol. Rev. 2003;83(3):731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 15.Yamada KH, Kozlowski DA, Seidl SE, Lance S, Wieschhaus AJ, Sundivakkam P, Tiruppathi C, Chishti I, Herman IM, Kuchay SM, Chishti AH. Targeted gene inactivation of calpain-1 suppresses cortical degeneration due to traumatic brain injury and neuronal apoptosis induced by oxidative stress. J. Biol. Chem. 2012;287(16):13182–93. doi: 10.1074/jbc.M111.302612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieschhaus A, Khan A, Zaidi A, Rogalin H, Hanada T, Liu F, De Franceschi L, Brugnara C, Rivera A, Chishti AH. Calpain-1 knockout reveals broad effects on erythrocyte deformability and physiology. Biochem. J. 2012 doi: 10.1042/BJ20121008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azam M, Andrabi SS, Sahr KE, Kamath L, Kuliopulos A, Chishti AH. Disruption of the mouse mu-calpain gene reveals an essential role in platelet function. Mol. Cell. Biol. 2001;21(6):2213–20. doi: 10.1128/MCB.21.6.2213-2220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eaton JW, Skelton TD, Swofford HS, Kolpin CE, Jacob HS. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973;246(5428):105–6. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- 19.De Franceschi L, Franco RS, Bertoldi M, Brugnara C, Matte A, Siciliano A, Wieschhaus AJ, Chishti AH, Joiner CH. Pharmacological inhibition of calpain-1 prevents red cell dehydration and reduces Gardos channel activity in a mouse model of sickle cell disease. FASEB J. 2013;27(2):750–759. doi: 10.1096/fj.12-217836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serjeant GR, Serjeant BE, Milner PF. The irreversibly sickled cell; a determinant of haemolysis in sickle cell anaemia. Br. J. Haematol. 1969;17(6):527–33. doi: 10.1111/j.1365-2141.1969.tb01403.x. [DOI] [PubMed] [Google Scholar]
- 21.Clark MR, Mohandas N, Shohet SB. Deformability of oxygenated irreversibly sickled cells. J. Clin. Invest. 1980;65(1):189–96. doi: 10.1172/JCI109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trudel M, Saadane N, Garel MC, Bardakdjian-Michau J, Blouquit Y, Guerquin-Kern JL, Rouyer-Fessard P, Vidaud D, Pachnis A, Romeo PH, et al. Towards a transgenic mouse model of sickle cell disease: hemoglobin SAD. EMBO J. 1991;10(11):3157–65. doi: 10.1002/j.1460-2075.1991.tb04877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Paepe ME, Trudel M. The transgenic SAD mouse: a model of human sickle cell glomerulopathy. Kidney Int. 1994;46(5):1337–45. doi: 10.1038/ki.1994.403. [DOI] [PubMed] [Google Scholar]
- 24.De Franceschi L, Beuzard Y, Jouault H, Brugnara C. Modulation of erythrocyte potassium chloride cotransport, potassium content, and density by dietary magnesium intake in transgenic SAD mouse. Blood. 1996;88(7):2738–44. [PubMed] [Google Scholar]
- 25.Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278(5339):873–6. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 26.Nwankwo JO, Lei J, Xu J, Rivera A, Gupta K, Chishti AH. Genetic inactivation of calpain-1 attenuates pain sensitivity in a humanized mouse model of sickle cell disease. Haematologica. 2016;101(10):e397–e400. doi: 10.3324/haematol.2016.148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuchay SM, Kim N, Grunz EA, Fay WP, Chishti AH. Double knockouts reveal that protein tyrosine phosphatase 1B is a physiological target of calpain-1 in platelets. Mol. Cell. Biol. 2007;27(17):6038–52. doi: 10.1128/MCB.00522-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, Orringer EP. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br. J. Haematol. 2006;134(1):109–15. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 29.Alugupalli KR, Michelson AD, Barnard MR, Leong JM. Serial determinations of platelet counts in mice by flow cytometry. Thromb. Haemost. 2001;86(2):668–71. [PubMed] [Google Scholar]
- 30.Frelinger AL, 3rd, Jakubowski JA, Brooks JK, Carmichael SL, Berny-Lang MA, Barnard MR, Heeney MM, Michelson AD. Platelet activation and inhibition in sickle cell disease (pains) study. Platelets. 2014;25(1):27–35. doi: 10.3109/09537104.2013.770136. [DOI] [PubMed] [Google Scholar]
- 31.DiRaimondo TR, Klock C, Warburton R, Herrera Z, Penumatsa K, Toksoz D, Hill N, Khosla C, Fanburg B. Elevated transglutaminase 2 activity is associated with hypoxia-induced experimental pulmonary hypertension in mice. ACS Chem Biol. 2014;9(1):266–75. doi: 10.1021/cb4006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei L, Warburton RR, Preston IR, Roberts KE, Comhair SA, Erzurum SC, Hill NS, Fanburg BL. Serotonylated fibronectin is elevated in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302(12):L1273–9. doi: 10.1152/ajplung.00082.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96(1):314–20. [PubMed] [Google Scholar]
- 34.Belcher JD, Mahaseth H, Welch TE, Vilback AE, Sonbol KM, Kalambur VS, Bowlin PR, Bischof JC, Hebbel RP, Vercellotti GM. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2005;288(6):H2715–25. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- 35.Pritchard KA, Jr, Ou J, Ou Z, Shi Y, Franciosi JP, Signorino P, Kaul S, Ackland-Berglund C, Witte K, Holzhauer S, Mohandas N, Guice KS, Oldham KT, Hillery CA. Hypoxia-induced acute lung injury in murine models of sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L705–14. doi: 10.1152/ajplung.00288.2002. [DOI] [PubMed] [Google Scholar]
- 36.Ma YQ, Qin J, Plow EF. Platelet integrin alpha(IIb)beta(3): activation mechanisms. Journal of thrombosis and haemostasis : JTH. 2007;5(7):1345–52. doi: 10.1111/j.1538-7836.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- 37.Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, Rauova L, Lowery TJ, Weisel JW. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123(10):1596–603. doi: 10.1182/blood-2013-08-523860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307(12):1254–6. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110(6):2166–72. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N. Engl. J. Med. 2004;350(9):886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Wandersee NJ, Guo Y, Jones DW, Holzhauer SL, Hanson MS, Machogu E, Brousseau DC, Hogg N, Densmore JC, Kaul S, Hillery CA, Pritchard KA., Jr Sickle cell disease increases high mobility group box 1: a novel mechanism of inflammation. Blood. 2014;124(26):3978–81. doi: 10.1182/blood-2014-04-560813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109(7):3088–98. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuchay SM, Wieschhaus AJ, Marinkovic M, Herman IM, Chishti AH. Targeted gene inactivation reveals a functional role of calpain-1 in platelet spreading. Journal of thrombosis and haemostasis : JTH. 2012;10(6):1120–32. doi: 10.1111/j.1538-7836.2012.04715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Battaglia F, Trinchese F, Liu S, Walter S, Nixon RA, Arancio O. Calpain inhibitors, a treatment for Alzheimer's disease: position paper. J. Mol. Neurosci. 2003;20(3):357–62. doi: 10.1385/JMN:20:3:357. [DOI] [PubMed] [Google Scholar]
- 45.Nabeshima Y, Washida M, Tamura M, Maeno A, Ohnishi M, Shiroishi T, Imura A, Razzaque MS, Nabeshima Y. Calpain 1 inhibitor BDA-410 ameliorates alpha-klotho-deficiency phenotypes resembling human aging-related syndromes. Sci Rep. 2014;4:5847. doi: 10.1038/srep05847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE, del Bosque-Plata L, Horikawa Y, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat. Genet. 2000;26(2):163–75. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 47.Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23(1):20–6. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- 48.Sorimachi H, Ishiura S, Suzuki K. Structure and physiological function of calpains. Biochem. J. 1997;328(Pt 3):721–32. doi: 10.1042/bj3280721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraemer BF, Weyrich AS, Lindemann S. Protein degradation systems in platelets. Thromb. Haemost. 2013;110(5):920–4. doi: 10.1160/TH13-03-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuchay SM, Chishti AH. Calpain-mediated regulation of platelet signaling pathways. Curr. Opin. Hematol. 2007;14(3):249–54. doi: 10.1097/MOH.0b013e3280ef68f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ataga KI, Orringer EP. Hypercoagulability in sickle cell disease: a curious paradox. Am. J. Med. 2003;115(9):721–8. doi: 10.1016/j.amjmed.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Peters M, Plaat BE, ten Cate H, Wolters HJ, Weening RS, Brandjes DP. Enhanced thrombin generation in children with sickle cell disease. Thromb. Haemost. 1994;71(2):169–72. [PubMed] [Google Scholar]
- 53.Key NS, Slungaard A, Dandelet L, Nelson SC, Moertel C, Styles LA, Kuypers FA, Bach RR. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood. 1998;91(11):4216–4223. [PubMed] [Google Scholar]
- 54.Nsiri B, Gritli N, Bayoudh F, Messaoud T, Fattoum S, Machghoul S. Abnormalities of coagulation and fibrinolysis in homozygous sickle cell disease. Hematol. Cell Ther. 1996;38(3):279–84. doi: 10.1007/s00282-996-0279-2. [DOI] [PubMed] [Google Scholar]
- 55.Devine DV, Kinney TR, Thomas PF, Rosse WF, Greenberg CS. Fragment D-dimer levels: an objective marker of vaso-occlusive crisis and other complications of sickle cell disease. Blood. 1986;68(1):317–9. [PubMed] [Google Scholar]
- 56.Francis RB., Jr Platelets, coagulation, and fibrinolysis in sickle cell disease: their possible role in vascular occlusion. Blood Coagul. Fibrinolysis. 1991;2(2):341–53. doi: 10.1097/00001721-199104000-00018. [DOI] [PubMed] [Google Scholar]
- 57.Tyagi T, Ahmad S, Gupta N, Sahu A, Ahmad Y, Nair V, Chatterjee T, Bajaj N, Sengupta S, Ganju L, Singh SB, Ashraf MZ. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced prothrombotic phenotype. Blood. 2014;123(8):1250–60. doi: 10.1182/blood-2013-05-501924. [DOI] [PubMed] [Google Scholar]
- 58.Pawlinski R. Inhibit the calpain to climb the mountain. Blood. 2014;123(8):1123–4. doi: 10.1182/blood-2013-12-543397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ginsberg MH, Frelinger AL, Lam SC, Forsyth J, McMillan R, Plow EF, Shattil SJ. Analysis of platelet aggregation disorders based on flow cytometric analysis of membrane glycoprotein IIb-IIIa with conformation-specific monoclonal antibodies. Blood. 1990;76(10):2017–23. [PubMed] [Google Scholar]
- 60.Ginsberg MH, Xiaoping D, O'Toole TE, Loftus JC, Plow EF. Platelet integrins. Thromb. Haemost. 1993;70(1):87–93. [PubMed] [Google Scholar]
- 61.Litvinov RI, Weisel JW. Role of red blood cells in haemostasis and thrombosis. ISBT Sci Ser. 2017;12(1):176–183. doi: 10.1111/voxs.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801–9. doi: 10.1182/blood-2015-09-618538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fulton RM, Hutchinson EC, Jones AM. Ventricular weight in cardiac hypertrophy. Br. Heart J. 1952;14(3):413–20. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.