Abstract
Objectives
This study assessed whether coronary artery calcium (CAC) can be used to optimize statin allocation among individuals for whom trial-based evidence supports efficacy of statin therapy.
Background
Recently, it was proposed to allocate statins for primary prevention of atherosclerotic cardiovascular disease (ASCVD) based on proven efficacy from randomized controlled trials (RCT) of statin therapy – a so-called “trial-based” approach.
Methods
The study used MESA (Multi-ethnic study of Atherosclerosis) with 5600 men and women aged 45–84 years, all free of clinical ASCVD, lipid-lowering therapy or missing information on risk factors at baseline examination.
Results
During 10 years of follow-up, 354 ASCVD and 219 hard coronary heart disease (CHD) events occurred. Based on enrollment criteria for 7 RCTs of statin therapy in primary prevention, 73% of MESA participants (91% of those aged >55 years) were eligible for statins according to a trial-based approach. Among these individuals, CAC=0 was common (44%) and associated with low rates of ASCVD and CHD (3.9 and 1.7 per 1000 person-years). There was a graded increase in event rates with increasing CAC score, and in individuals with CAC>100 (27% of participants) the rates of ASCVD and CHD were 18.9 and 12.7. Consequently, the estimated number needed to treat (NNT) in 10 years to prevent 1 event varied greatly according to CAC score. For ASCVD events, the NNT was 87 for CAC=0 and 19 for CAC>100. For CHD events, the NNT was 197 for CAC=0 and 28 for CAC>100.
Conclusions
The vast majority of MESA participants qualified for trial-based primary prevention with statins. Among these individuals for whom trial-based evidence supports efficacy of statin therapy, CAC=0 and CAC>100 were common and associated with low and high cardiovascular risk, respectively. This information may guide shared decision-making aimed at targeting evidence-based statins to those who are likely to benefit the most.
Keywords: Primary prevention, cardiovascular disease, guideline, statin, lipoproteins
Introduction
Low-density lipoprotein cholesterol (LDL-C) lowering with HMG-CoA reductase inhibitors, also known as statins, constitutes the cornerstone of pharmacological prevention of atherosclerotic cardiovascular disease (ASCVD). While it is widely accepted that statins should be offered to patients with clinical ASCVD (secondary prevention), controversies exist in whom to treat for primary prevention. Leading international guidelines on ASCVD prevention agree on the principle of allocating statin therapy based on absolute 10-year risk estimates of future ASCVD(1–3). This long-held principle, however, was recently questioned by leading cardiovascular investigators who proposed a paradigm shift in ASCVD prevention in which statin eligibility is based on randomized controlled trials (RCT) of statin therapy(4–7). In this alternative proposal, allocation of statins is based strictly on proven trial evidence (“trial-based approach”), that is, on the principle of “what works” and “in whom”. The rationale behind such a trial-based approach is clear: no RCTs of statin therapy have ever enrolled participants based on 10-year ASCVD risk assessment – the approach recommended for statin allocation by current guidelines - and abundant data from large scale RCTs have now proven the efficacy and safety of statin therapy in a wide range of different patient populations. Unfortunately, as recently highlighted(8), most individuals eligible for statin therapy with a trial-based approach are at low absolute risk of ASCVD in whom the net benefit of treatment may be questioned.
Nevertheless, accepting the rationale behind a trial-based approach to statin therapy, we sought to investigate if assessment of subclinical atherosclerosis – the root cause of ASCVD - could be used to improve trial-based statin allocation. Specifically, we hypothesized that assessment of coronary artery calcium (CAC) among individuals for whom trial-based evidence supports efficacy of statin therapy, could be used to identify subgroups with high and low ASCVD event rates, and thereby individuals expected to benefit the most, and least, from trial-based evidence supporting primary prevention with statin therapy.
Methods
Study participants
Multi-ethnic study of atherosclerosis (MESA) is a National Institutes of Health/National Heart, Lung and Blood Institute-funded study of the characteristics of subclinical atherosclerosis and designed to identify risk factors involved in progression of atherosclerosis to clinical ASCVD. A total of 6814 men and women aged 45 to 84, free of clinical ASCVD at baseline examination, were recruited between July 2000 and September 2002. Enrollment was at 6 sites in the United States (Baltimore (Maryland), Chicago (Illinois), Forsyth County (North Carolina), Los Angeles (California), New York (New York) and St. Paul (Minnesota)). Details on the design and organization have been published previously(9).
Risk factor assessment
The baseline examination in MESA included an interview/questionnaire, physical examination and blood sampling for biochemical measurements. In the interview, MESA staff collected information on traditional as well as non-traditional risk factors. Systolic and diastolic blood pressure was measured at rest using Dinamap Pro 1000 automated oscillometric spyghmomanometer (Critikan), using the mean of the last 2 measurements for analysis. Blood samples were drawn after 12 hours of fast and used for measurement of total cholesterol, LDL cholesterol and triglycerides at the collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis, Minnesota). Smoking was defined as current smoking by self-report. Diabetes was defined as self-reported diabetes, a fasting glucose ≥7.0 mmol/L or use of anti-diabetic drugs.
CAC score measurements
All MESA participants underwent noncontrast cardiac-gated computed tomography (CT) at baseline examination to determine the Agatston coronary artery calcium (CAC) score. Participants were scanned twice, with mean CAC score used for analysis. The estimated average radiation dose was 0.89 mSv.
Trial-based recommendations for statin therapy
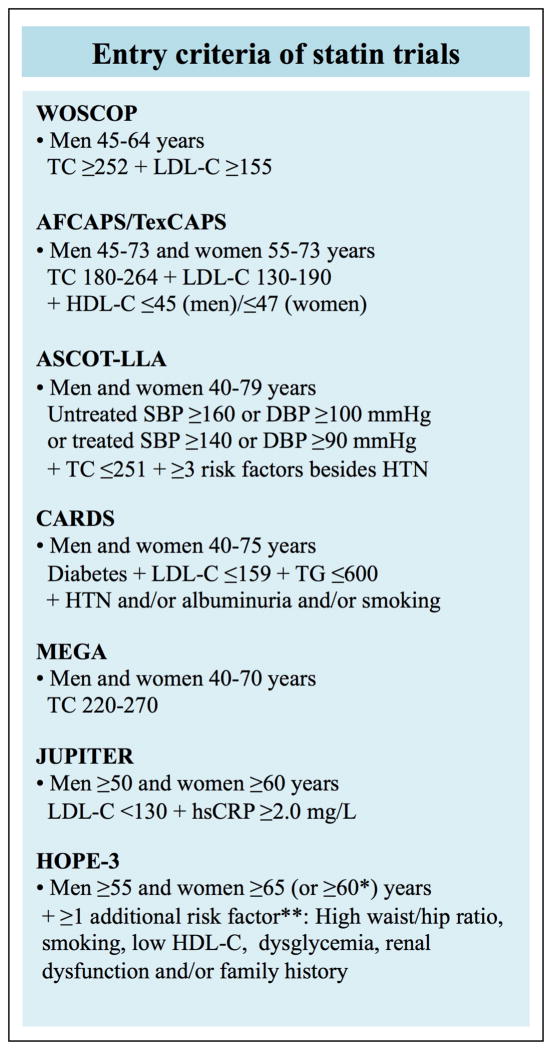
A trial-based approach to statin therapy for primary prevention based on currently available evidence is guided by enrollment criteria in the following 7 large RCTs (named in chronological order by publication year): WOSCOPS (West of Scotland Coronary Prevention Study)(10), AFCAPS/TexCAPS (Air Force/Texas Coronary Atherosclerosis Prevention Study)(11), ASCOT-LLA (Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm)(12), CARDS (Collaborative Atorvastatin Diabetes Study)(13), MEGA (Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese)(14), JUPITER (Justification for the Use of Statins in prevention: An Intervention Trial Evaluating Rosuvastatin)(15) and HOPE-3 (Heart Outcomes Prevention Evaluation-3)(16). Characteristics of these 7 RCTs to guide trial-based allocation of statins in primary prevention of ASCVD are shown in Figure 1.
Figure 1. Enrollment criteria for primary prevention with statins under the trial-based approach.
The figure summarizes the criteria for initiation of statin therapy in people free of ASCVD as defined by a trial-based approach to statin therapy.
ASCVD=atherosclerotic cardiovascular disease;WOSCOPS=West of Scotland Coronary Prevention Study; AFCAPS/TexCAPS=Air Force/Texas Coronary Atherosclerosis Prevention Study;ASCOT-LLA=Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm;CARDS=Collaborative Atorvastatin Diabetes Study;MEGA=Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese; JUPITER=Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; HOPE-3=Heart Outcomes Prevention Evaluation-3.
TC=total cholesterol; LDL-C=low-density lipoprotein cholesterol; HDL-C=high-density lipoprotein cholesterol; SBP=systolic blood pressure; DBP=diastolic blood pressure; HTN=hypertension; hsCRP=high-sensitivity C-reactive protein; Cholesterol concentrations are shown in mg/dL(to convert to mmol/L, divide by 38.6).
*Women 60–65 years of age were eligible for statins with HOPE-3 trial if they had at least two additional risk factors.
**High waist/hip ratio, ≥0.90 in men and ≥0.85 in women; Low HDL-cholesterol, < 1.0 mmol/L in men and <1.3 mmol/L in women; Dysglycemia, impaired fasting glucose, impaired glucose tolerance or uncomplicated diabetes treated with diet only; Renal dysfunction, microalbuminuria, eGFR<60 ml/min/1.73m2 or creatinine >124 μmol/L.
Cardiovascular disease endpoints
Ascertainment of events has been described previously, and is available at the MESA website(17). Briefly, at intervals of 9 to 12 months, trained MESA personal contacted participants or family members to inquire about ASCVD diagnosis, including hospital admissions, outpatient diagnoses and deaths. Follow-up was completed in 92% of living participants. Medical records were obtained for approximately 98% of hospital admissions and 95% of outpatient diagnoses. A MESA study committee, including cardiologists, neurologists and epidemiologists, adjudicated every event.
For this study, we defined CHD events as myocardial infarction, resuscitated cardiac arrest and CHD death. ASCVD was defined as CHD plus fatal and nonfatal strokes. Myocardial infarction was diagnosed based on the combination of symptoms, electrocardiographic findings and levels of cardiac biomarkers. Hospital records as well as family interviews were used to determine if a death was related to CHD. Stroke was diagnosed based on a documented focal neurological deficit lasting 24 hours or until death, or if <24 hours, with imaging evidence of relevant brain lesions. In this study, participants were followed for 10 years (i.e. data truncated at 10 years).
Statistical analysis
Baseline characteristics are presented as proportions for categorical variables and as medians (interquartile range) for continuous variables.
We calculated the number and percentage of participants eligible for statin therapy under the described trial-based approach. Among these trial-based eligible individuals, for whom RCT evidence supports efficacy of statin therapy, we assessed the distribution of CAC using three well-defined CAC groups: 0, 1–100 and >100(18–21).
To determine if CAC could be used to risk stratify trial-based eligible individuals, we calculated the 10-year ASCVD and CHD event rates across the three CAC groups as well as used Cox regression modeling (analyzing time to event) to obtain multivariable-adjusted hazard ratios (HR). Analyses were adjusted for race and MESA site. Further, we used Kaplan-Meier estimates to describe the occurrence of ASCVD and CHD events over time stratified by the CAC groups. Finally, we calculated a 10-year number needed to treat (NNT10) to prevent 1 ASCVD or CHD event by assuming a 30% relative risk reduction with statin therapy in primary prevention(22, 23). The NNT10 was calculated for each CAC group as the reciprocal of the absolute risk difference in 10-year event rates. In a sensitivity analysis, we also estimated 5-year NNT (NNT5) using 5-year Kaplan-Meier estimates(24) to better comply with follow-up length in the RCTs. Further, in a second sensitivity analysis we recalculated NNT10 after assuming a more optimistic benefit of long-term statin therapy for primary prevention (40% relative risk reduction). Analyses were performed using Stata version 13.1 SE.
Results
A total of 6814 men and women were included in MESA. After exclusion of individuals on lipid-lowering medication (n=1100) or with missing information (n=114), 5600 individuals were available for this study. Baseline characteristic of the study population are shown in Table 1. Median age was 61 years, and 53% were women.
Table 1.
Baseline characteristics and observed events in MESA study population
| Multi-Ethic Study of Atherosclerosis
|
|||
|---|---|---|---|
| Characteristics | All | Men | Women |
| Participants, n | 5600 | 2635 | 2965 |
| Age, median (IQR), year | 61 (53–70) | 61 (53–70) | 61 (53–69) |
| Systolic blood pressure, median (IQR), mmHg | 123 (111– 139) | 122 (112– 138) | 123 (109– 140) |
| Diastolic blood pressure, median (IQR), mmHg | 72 (65–79) | 75 (69–81) | 69 (63–76) |
| Plasma cholesterol, median (IQR) | |||
| Total cholesterol, mmol/L | 5.0 (4.5–5.6) | 4.9 (4.3–5.4) | 5.1 (4.6–5.7) |
| HDL cholesterol, mmol/L | 1.2 (1.0–1.5) | 1.1 (1.0–1.3) | 1.4 (1.2–1.7) |
| LDL cholesterol, mmol/L | 3.1 (2.6–3.6) | 3.1 (2.6–3.5) | 3.1 (2.6–3.6) |
| Current smokers, % | 13 | 15 | 12 |
| C-reactive protein, median (IQR), mg/L | 1.9 (0.8–4.3) | 1.5 (0.7–3.2) | 2.6 (1.0–5.9) |
| Diabetes, % | 11 | 12 | 9 |
| Hypertension, % | 44 | 43 | 46 |
| 10-year ASCVD risk, median (IQR), % | 8.4 (3.6–18.4) | 12.0 (6.0– 22.0) | 5.4 (1.9–13.7) |
| 10-year ASCVD events, n | 354 | 205 | 149 |
| 10-year CHD events, n | 219 | 142 | 77 |
HDL = High-density lipoprotein; LDL = Low-density lipoprotein; 10-year ASCVD risk calculated with the pooled cohort equations;
ASCVD = Atherosclerotic cardiovascular disease; CHD = Coronary heart disease; IQR = interquartile range.
Statin eligibility based on randomized statin trials
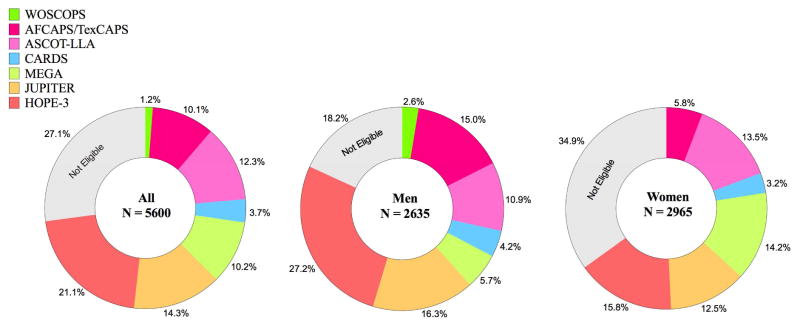
Based on enrollment criteria used in 7 high quality RCTs of statin therapy (“trial-based approach”), 4085 individuals (73%) were eligible for primary prevention with statins (Figure 2). More men than women met enrollment criteria (82 vs 65%). Notably, among those >55 years of age, 91% qualified for trial-based statin therapy (Supplementary Figure 1). Baseline characteristics of participants stratified by statin eligibility are presented in Table 2. Individuals eligible for statins were older and had a higher burden of cardiovascular risk factors, including higher blood pressure and more atherogenic lipid profile compared to individuals who did not fulfill enrollment criteria in any RCT of statin therapy. Assessing statin eligibility by each trial individually, 55% of MESA participants qualified for statin therapy based on HOPE-3 trial alone. For comparison, statin eligibility varied from 1% (WOSCOPS) to 18% (JUPITER) in the other 6 trials (Supplementary Figure 2 and Supplementary Table 1). More than half of statin eligible individuals met enrollment criteria in 2 or more of the 7 RCTs.
Figure 2. Statin eligibility in MESA using a trial-based approach.
Diagram illustrating the fraction of individuals from MESA meeting enrollment criteria in RCT’s of statin therapy. Individuals were selected consecutively in chronological order clockwise starting 12 o’clock, that is, first we selected individuals according to WOSCOPS criteria(1995), then we selected additional individuals according to AFCAPS/TexCAPS criteria(1998), and so on. Abbreviations as in Figure 1.
Table 2.
Baseline characteristics of MESA participants stratified by eligibility for trial-based statin therapy.
| All
|
Men
|
Women
|
||||
|---|---|---|---|---|---|---|
| Characteristics | Trial- based eligible | Not trial eligible | Trial- based eligible | Not trial eligible | Trial- based eligible | Not trial eligible |
| Participants, n | 4085 | 1515 | 2156 | 479 | 1929 | 1036 |
| Age, median (IQR), year | 65 (58–72) | 51 (48–55) | 64 (56–71) | 50 (47–53) | 66 (60–73) | 52 (48–56) |
| Systolic blood pressure, median (IQR), mmHg | 127 (114–144) | 114 (103–125) | 125 (114–140) | 115 (107–125) | 131 (115–148) | 113 (102–125) |
| Diastolic blood pressure, median (IQR), mmHg | 73 (66–80) | 70 (64–76) | 75 (70–82) | 73 (68–79) | 69 (63–76) | 68 (62–75) |
| Plasma cholesterol, median (IQR) | ||||||
| Total cholesterol, mmol/L | 5.1 (4.6– 5.8) | 4.8 (4.3– 5.2) | 4.9 (4.4– 5.6) | 4.6 (4.2– 5.0) | 5.4 (4.8– 6.0) | 4.8 (4.4–5.2) |
| HDL cholesterol,mmol/L | 1.2 (1.0– 1.5) | 1.3 (1.1– 1.6) | 1.1 (1.0– 1.3) | 1.2 (1.0– 1.4) | 1.4 (1.2– 1.7) | 1.4 (1.2–1.7) |
| LDL cholesterol,mmol/lL | 3.2 (2.6– 3.7) | 2.8 (2.4– 3.2) | 3.1 (2.6– 3.7) | 2.8 (2.4– 3.2) | 3.3 (2.7– 3.8) | 2.8 (2.4–3.2) |
| Current smokers, % | 13 | 15 | 15 | 16 | 10 | 15 |
| C-reactive protein, median (IQR), mg/L | 2.2 (1.0– 4.5) | 1.4 (0.6– 3.8) | 1.7 (0.8– 3.5) | 0.8 (0.4– 1.7) | 3.0 (1.3– 6.2) | 1.9 (0.8–4.9) |
| Diabetes, % | 14 | 3 | 14 | 3 | 13 | 2 |
| Hypertension, % | 53 | 21 | 49 | 16 | 58 | 23 |
| 10-year ASCVD risk, median (IQR), % | 12.5 (6.4– 22.3) | 2.2 (1.1– 4.1) | 14.7 (8.3– 24.1) | 3.9 (2.5– 6.3) | 10.0 (4.8– 19.3) | 1.5 (0.8–2.9) |
HDL=High-density lipoprotein; LDL=Low-density lipoprotein; ASCVD=Atherosclerotic cardiovascular disease. IQR=interquartile range; 10-year ASCVD risk calculated with the pooled cohort equations.
CAC distribution among statin eligible individuals
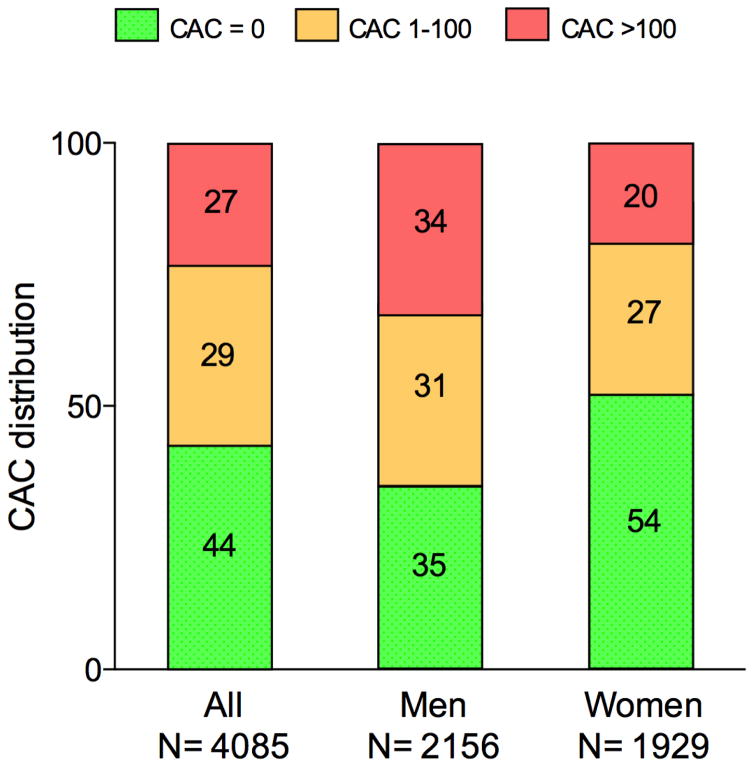
Among the 4085 individuals meeting enrollment criteria in RCTs of statin therapy, nearly half had no detectable CAC (CAC=0) and more than one-fourth had CAC>100 (Figure 3). The burden of CAC differed in a sex-specific manner. CAC=0 was more common among women than men, the opposite was the case for CAC>100. Overall, the number needed to screen (NNS) to identify one person with CAC=0 was 2.3, and the NNS to find one with CAC>100 was 3.7 (Table 3). The NNS to identify one person with either CAC=0 or CAC>100 was less than 2. The distribution of CAC among individuals meeting enrollment criteria in each of the 7 statin trials varied considerably. Using MEGA criteria would include the most individuals (59%) with no CAC, while using WOSCOPS criteria would include the fewest (38%) with no CAC (Supplementary Figure 3).
Figure 3. Distribution of CAC among individuals eligible for statin therapy based on a trial-based approach.
In individuals for whom trial-based evidence supports efficacy of statin therapy, 44% had no sign of CAC.
CAC=coronary artery calcium score.
Table 3.
Number needed to screen for subclinical atherosclerosis among individuals eligible for trial-based statin therapy.
| All (n=4085) | Men (n=2156) | Women (n=1929) | |
|---|---|---|---|
| CAC=0, NNS | 2.3 | 2.8 | 1.9 |
| CAC >100 NNS | 3.7 | 3.0 | 5.1 |
| CAC=0 or CAC>100 NNS | 1.4 | 1.4 | 1.4 |
NNS = Number needed to screen to identify 1 individual with the value(s) in question; CAC=coronary artery calcium score.
Clinical events in statin eligible individuals
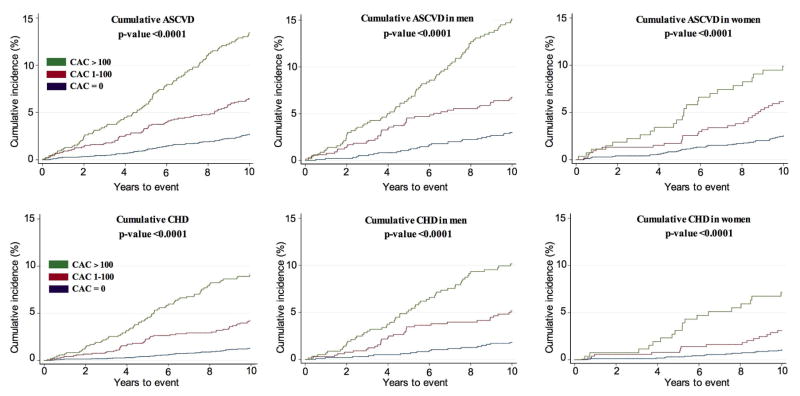
Among those who qualified for trial-based statin therapy, we observed 332 ASCVD events during 10-year follow-up, out of which 208 were CHD events (Table 4). Kaplan Meier cumulative-event curves for ASCVD and CHD by CAC score (0, 1–100 and >100) are shown in Figure 4. There was a strong relationship between the burden of CAC and both ASCVD and CHD events (Table 4 and Supplementary Table 2). Among individuals with no CAC, the event rates were low (3.9 for ASCVD and 1.7 for CHD per 1000 person-years) whereas the event rates were considerably higher in individuals with CAC>100 (18.9 for ASCVD and 12.7 for CHD per 1000 person-years). Compared with a CAC score of 0, CAC scores above 100 were associated with an adjusted hazard ratio of 5.2 (95% CI: 3.9–6.4) for ASCVD and 8.0 (95% CI: 5.3–12.1) for CHD. Interestingly, event rates were similar in men and women separately when stratified by CAC score (Table 4).
Table 4.
Relationship between coronary artery calcium and clinical events in individuals eligible for trial-based statin therapy.
| ASCVD events and hazard ratio (95% CI)
|
CHD events and hazard ratio (95% CI)
|
||||||
|---|---|---|---|---|---|---|---|
| Subclinical atherosclerosis | N (%) | N (%) | Event rate per 1000 person-years | Hazard ratio1 | N (%) | Event rate per 1000 person-years | Hazard ratio1 |
| All | |||||||
| CAC = 0 | 1798 (44) | 64 (3.6%) | 3.85 (3.02– 4.93) | 1 (reference) | 28 (1.6%) | 1.67 (1.16– 2.43) | 1 (reference) |
| CAC 1- 100 | 1185 (29) | 98 (8.3%) | 9.44 (7.74– 11.50) | 2.53 (1.84– 3.46) | 63 (5.3%) | 5.99 (4.68– 7.70) | 3.69 (2.36–5.76) |
| CAC >100 | 1102 (27) | 170 (15.4%) | 18.86 (16.22– 21.91) | 5.19 (3.88– 6.94) | 117 (10.6%) | 12.68 (10.58– 15.20) | 8.01 (5.29– 12.13) |
| Men | |||||||
| CAC = 0 | 765 (35) | 28 (3.7%) | 3.97 (2.74– 5.75) | 1 (reference) | 17 (2.2%) | 2.40 (1.49– 3.86) | 1 (reference) |
| CAC 1-100 | 667 (31) | 55 (8.2%) | 9.49 (7.29–12.37) | 2.49 (1.58–3.93) | 40 (6.0%) | 6.83 (5.01–9.31) | 2.98 (1.69–5.26) |
| CAC >100 | 724 (34) | 115 (15.9%) | 19.35 (16.12– 23.23) | 5.23 (3.44– 7.94) | 81 (11.2%) | 13.36 (10.75– 16.61) | 6.01 (3.54–10.20) |
| Women | |||||||
| CAC = 0 | 1033 (54) | 36 (3.5%) | 3.77 (2.72– 5.23) | 1 (reference) | 11 (1.1%) | 1.14 (0.63– 2.06) | 1 (reference) |
| CAC 1-100 | 518 (27) | 43 (8.3%) | 9.37 (6.95– 12.63) | 2.54 (1.63– 3.96) | 23 (4.4%) | 4.94 (2.28– 7.43) | 4.39 (2.14–9.00) |
| CAC >100 | 378 (20) | 55 (14.6%) | 17.89 (13.74– 23.31) | 4.99 (3.27– 7.62) | 36 (9.5%) | 11.37 (8.20– 15.77) | 10.31 (5.23– 20.33) |
Adjusted for race and MESA site; CHD = Coronary heart disease; ASCVD = Atherosclerotic cardiovascular disease; CAC= coronary artery calcium score.
Figure 4. Cumulative incidence of ASCVD and CHD stratified by CAC burden, among individuals eligible for statin therapy under a trial-based approach.
ASCVD=atherosclerotic cardiovascular disease; CHD=coronary heart disease; CAC=coronary artery calcium score.
NNT stratified by CAC group
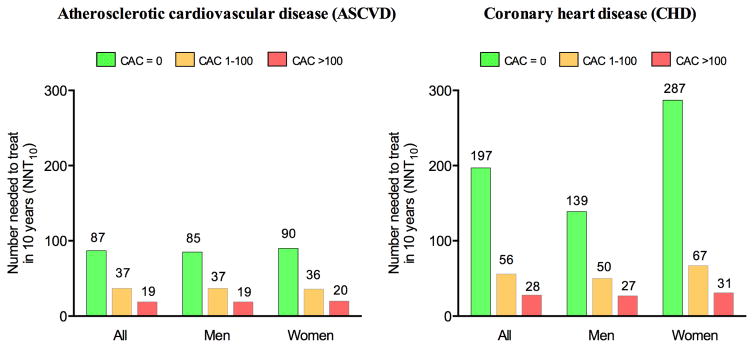
Assuming a 30% relative risk reduction with statin therapy, the NNT for 10 years (NNT10) to prevent one ASCVD event among individuals meeting enrollment criteria in RCTs of statin therapy was 87 for those with CAC=0 compared to 19 for those with CAC>100 (Figure 5). For CHD events the NNT10 to prevent 1 event was 197 for those without CAC compared to 28 for those with CAC>100. For ASCVD events there were no sex difference, but women with CAC=0 had a higher NNT to prevent 1 CHD event than men. As most statin trials have mean follow-up time of 5 years or less, we also calculated NNT for 5 years (NNT5) (Supplementary Figure 4). NNT5 ranged from 194 (CAC=0) to 40 (CAC>100) for ASCVD and from 725 (CAC=0) to 54 (CAC>100) for CHD events. Notably, among women with CAC=0 (54% of all women), the estimated NNT5 to prevent 1 CHD event was >1000. In a secondary sensitivity analysis, we recalculated the NNT10 assuming 40% relative risk reduction by long-term statin therapy. In this analysis, the NNT10 was as low as 14 for ASCVD events and 21 for CHD events in those with CAC>100 (Supplementary Figure 5).
Figure 5. Estimated number needed to treat in 10 years to prevent 1 ASCVD or CHD event stratified by CAC burden, among individuals eligible for statin therapy under a trial-based approach.
ASCVD=atherosclerotic cardiovascular disease; CHD=coronary heart disease; CAC=coronary artery calcium score.
Discussion
Among MESA participants, as many as 73% met enrollment criteria in one or more of 7 high quality RCTs of statin therapy for primary prevention and would therefore be eligible for trial-based statin therapy. In these trial-based eligible individuals, the burden of CAC differed substantially with nearly half having CAC=0 and, thus, very low event rates. In those with CAC=0, the NNT to prevent 1 event (ASCVD or CHD) was unfavorably high, with a NNT10 of 87 for ASCVD and 197 for CHD. In contrast, in the large subpopulation with CAC>100, event rates were much higher and associated with considerably more favorable NNT10 (19 for ASCVD and 28 for CHD). Hence, for health care providers who prefer a trial-based approach to primary prevention with statins, knowing the CAC score may help targeting of treatment to those at highest risk for ASCVD and, thus, to those who are likely to benefit the most from statin therapy.
Trial-based statin therapy for primary prevention – reasonable to treat all?
In 1995 the first larger RCT of statin use in primary prevention was published (WOSCOPS) in which the efficacy of statin therapy was documented in a selected subgroup of high-risk men with hypercholesterolemia(10). Only 1% of MESA participants met enrollment criteria for WOSCOPS. Since then, primary prevention with statins has proven effective in other carefully selected individuals with specific risk-factor profiles, progressively expanding the indication for primary prevention with statins. Based on enrollment criteria used in the first 6 (10–15) of the 7 RCTs, 52% of MESA participants were eligible for primary prevention with statin therapy. Similar results were recently reported from 2 European population-based cohort studies, the Copenhagen General Population Study (56%)(8) and the Rotterdam Study (53%)(25). In 2016, RCT evidence for primary prevention with statins became stronger and more inclusive with publication of the HOPE-3 trial(16). This pragmatic trial had enrolled “intermediate-risk” persons without known ASCVD in whom clear trial-based evidence for efficacy of statins was still lacking. The HOPE-3 trial provided important missing evidence, and 55% of MESA participants were statin eligible based solely on enrollment criteria for this trial. Considering not only HOPE-3 but the totality of evidence from all the 7 randomized statin trials, 73% of MESA participants aged 40–84 qualified for trial-based primary prevention with statins, increasing to 91% when considering only those >55 years of age. This proportion would most likely be even higher in a real-world population(26).
Hence, after publication of the statin arm of HOPE-3, near-complete RCT evidence has now been provided for a universal, pragmatic approach to primary prevention of ASCVD based on a fixed low-to-moderate statin dose from age 55 – a provocative concept introduced by Wald and Law in 2003(27). However, a critical question is whether such a pragmatic approach is reasonable in countries where a simple test is available that could distinguish between those who need or don’t need to take a statin pill every day for the rest of their lives.
Precision medicine: CAC to guide trial-based statin allocation
Over the short term, people without atherosclerosis are at low risk for ASCVD and the higher the burden of atherosclerosis, the higher the risk for ASCVD. Although CAC is not a marker of the earliest coronary atherosclerotic lesions, those with CAC=0 are at very low risk for ASCVD and mortality for up to 15 years(18, 21, 28–33). At the other end of the risk spectrum, those with CAC >100 have a risk for a first ASCVD event that approach that seen for a recurrent event in patients with established ASCVD (secondary prevention)(19). Thus, in primary prevention, it makes sense to identify those with CAC=0 to avoid overtreatment and those with CAC>100 to avoid undertreatment and ensure long-term adherence to a cost-effective treatment(34). In the present study we confirmed that CAC=0 at baseline examination was associated with very low CHD and ASCVD event rates for at least 10 years, known as the power of zero(35). Of course, to prevent events there need to be events to prevent(19). Nearly half of MESA participants who were eligible for trial-based statin therapy had CAC=0. In this low-risk population (CAC=0), the NNT5 to prevent 1 CHD event was high (>500 in men and >1000 in women, assuming 30% event reduction with statin therapy). Screening just 3 persons who were eligible for trial-based statin therapy would identify one who did not need this treatment. At the other end of the risk spectrum, more than one quarter of MESA participants had CAC>100 and a high 10-year event rate. Most events occurred in this high-risk subpopulation. Overall, just 2 persons need to be screened to find just 1 with either CAC=0 (don’t treat) or CAC >100(treat and ensure long-term adherence).
The size of study population and length of follow-up allowed us to assess the benefit of CAC assessment in men and women separately. The NNS to find one person with CAC=0 or CAC>100 was similar in men and women (lower than 2), but more women than men had CAC=0 while the opposite was the case for CAC>100. Notably, the 10-year cumulative ASCVD risk was >15% in both men and women with CAC>100, that is, far above the current 7.5% 10-year ASCVD risk threshold for statin therapy identified by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines. Thus, as the ACC/AHA guidelines currently recommend that statin therapy might be considered in selected individuals with CAC ≥300 (class IIb recommendation), our results suggest that this cutpoint may reasonable be lowered to 100 in future guidelines. Given that the price for CAC testing is now low (≈100$), the additional information on ASCVD risk that CAC provides (especially when treatment decision is uncertain) may be worth the expense and, in some circumstances, even be cost-effective(36). In patients with detectable CAC, knowing this may increase adherence to preventive medication (37), which may further improve ASCVD outcome(38). These considerations are obvious topics for evidence-based and meaningful patient-physician discussions on initiation of statin therapy for primary prevention(39).
Limitations
Our study has potential limitations. First, our ability to consider all exclusion criteria used in the randomized statin trials was limited. However, potential exclusion criteria were not mentioned in the trial-based proposal, and are often ignored in routine clinical practices(40). Second, although we excluded individuals taking lipid-lowering medication at baseline examination, MESA participants were informed about their CAC scores, which may have led to selective uptake of preventive measures (including statin) among individuals with high CAC scores that could influence eventrates. However, this would be expected to weaken the association of CAC with ASCVD and CHD events and, thus, can not explain our results. Third, we assumed a 30% relative risk reduction with statin therapy based on Cochrane analyses(22), although this may vary according to both treatment time and dose/type of statin. Fourth, most statin trials have follow-up of 5 years or less. However, using 5 years of follow-up instead of 10 years did not affect the main results or conclusion.
Strengths of our study include the high-quality assessment of risk factors at baseline (enabling assessment of enrollment criteria in the 7 RCTs), adjudicated events over 10years of follow-up and the size of the study population that allowed sex-specific assessment of the trial-based approach to statin therapy in a modern, multiethnic population.
Conclusions
After the HOPE-3 trial, the great majority of middle-aged and elderly MESA participants free of ASCVD would meet enrollment criteria used in at least one randomized statin trial. Evidence from RTCs now supports primary prevention with statins in nearly all men and women >55 years of age. However, nearly half of those considered statin-eligible based on RCTs had CAC=0 and a very low event rate, and one quarter had CAC>100 and a high event rate. For health care providers and patients who are reluctant to treat all with statins from age 55, this information may help in shared decision making aimed at targeting prevention to those at highest risk.
Supplementary Material
Clinical Perspectives.
Competency in medical knowledge
Among individuals with trial evidence supporting statin efficacy, quantification of subclinical atherosclerosis using the Agatston CAC score can be used to identify individuals with questionable (if CAC=0) and substantial (if CAC>100) benefit of statin therapy.
Translational outlook
Future research is needed to evaluate how best to incorporate CAC-guided allocation of statin therapy in routine clinical practice.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR.
Abbreviations and acronyms
- ACC/AHA
American College of Cardiology/American Heart Association
- AFCAPS/TexCAPS
Air Force/Texas Coronary Atherosclerosis Prevention Study
- ASCOT-LLA
Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- HOPE-3
Heart Outcomes Prevention Evaluation-3
- JUPITER
Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin
- MEGA
Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese
- MESA
Multi-Ethnic Study of Atherosclerosis
- PCEs
pooled cohort equations
- RCT
randomized controlled trial
- WOSCOPS
West of Scotland Coronary Prevention Study
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016 doi: 10.1080/13814788.2017.1398320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.JBS3 Board. Joint British Societies' consensus recommendations for the prevention of cardiovascular disease (JBS3) Heart. 2014;100(Suppl 2):ii1–ii67. doi: 10.1136/heartjnl-2014-305693. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Wilson PWF. A trial-based approach to statin guidelines. JAMA. 2013;310:1123–1124. doi: 10.1001/jama.2013.276529. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Rose L, Cook NR. A Proposal to Incorporate Trial Data Into a Hybrid ACC/AHA Algorithm for the Allocation of Statin Therapy in Primary Prevention. Journal of the American College of Cardiology. 2015;65:942–948. doi: 10.1016/j.jacc.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM. What works and in whom? A simple, easily applied, evidence-based approach to guidelines for statin therapy. Circ Cardiovasc Qual Outcomes. 2012;5:592–593. doi: 10.1161/CIRCOUTCOMES.112.966556. [DOI] [PubMed] [Google Scholar]
- 8.Mortensen MB, Afzal S, Nordestgaard BG, Falk E. Primary Prevention With Statins: ACC/AHA Risk-Based Approach Versus Trial-Based Approaches to Guide Statin Therapy. Journal of the American College of Cardiology. 2015;66:2699–2709. doi: 10.1016/j.jacc.2015.09.089. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 11.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 12.Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 13.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 17.MESA Coordinating Center. MESA Website. 2015 Available at: http://www.mesa-nhlbi.org.
- 18.Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin SS, Blaha MJ, Blankstein R, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bittencourt MS, Blaha MJ, Blankstein R, et al. Polypill therapy, subclinical atherosclerosis, and cardiovascular events-implications for the use of preventive pharmacotherapy: MESA (Multi-Ethnic Study of Atherosclerosis) Journal of the American College of Cardiology. 2014;63:434–443. doi: 10.1016/j.jacc.2013.08.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis) Journal of the American College of Cardiology. 2015;66:1657–1668. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 22.Taylor FC, Huffman M, Ebrahim S. Statin therapy for primary prevention of cardiovascular disease. JAMA. 2013;310:2451–2452. doi: 10.1001/jama.2013.281348. [DOI] [PubMed] [Google Scholar]
- 23.Ebrahim S, Taylor FC, Brindle P. Statins for the primary prevention of cardiovascular disease. BMJ. 2014;348:g280. doi: 10.1136/bmj.g280. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, MacFadyen JG, Fonseca FAH, et al. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER) Circ Cardiovasc Qual Outcomes. 2009;2:616–623. doi: 10.1161/CIRCOUTCOMES.109.848473. [DOI] [PubMed] [Google Scholar]
- 25.Pavlovic J, Greenland P, Deckers JW, et al. Comparison of ACC/AHA and ESC Guideline Recommendations Following Trial Evidence for Statin Use in Primary Prevention of Cardiovascular Disease: Results From the Population-Based Rotterdam Study. JAMA Cardiol. 2016 doi: 10.1001/jamacardio.2016.1577. [DOI] [PubMed] [Google Scholar]
- 26.Leening MJG, Heeringa J, Deckers JW, et al. Healthy volunteer effect and cardiovascular risk. Epidemiology. 2014;25:470–471. doi: 10.1097/EDE.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 27.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2009;158:554–561. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht HS. A zero coronary artery calcium score: priceless. Journal of the American College of Cardiology. 2010;55:1118–1120. doi: 10.1016/j.jacc.2009.09.064. [DOI] [PubMed] [Google Scholar]
- 30.Greenland P. When Nothing Is Really Something: New Evidence of the Importance of Zero Coronary Calcium. JACC Cardiovasc Imaging. 2015;8:910–912. doi: 10.1016/j.jcmg.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 31.Valenti V, Hartaigh ÓB, Heo R, et al. A 15-Year Warranty Period for Asymptomatic Individuals Without Coronary Artery Calcium: A Prospective Follow-Up of 9,715 Individuals. JACC Cardiovasc Imaging. 2015;8:900–909. doi: 10.1016/j.jcmg.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen MB, Fuster V, Muntendam P, et al. A Simple Disease-Guided Approach to Personalize ACC/AHA-Recommended Statin Allocation in Elderly People: The BioImage Study. Journal of the American College of Cardiology. 2016;68:881–891. doi: 10.1016/j.jacc.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 33.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Rossello X, Pocock SJ, Julian DG. Long-Term Use of Cardiovascular Drugs: Challenges for Research and for Patient Care. Journal of the American College of Cardiology. 2015;66:1273– 1285. doi: 10.1016/j.jacc.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Blaha MJ, Silverman MG, Budoff MJ. Is there a role for coronary artery calcium scoring for management of asymptomatic patients at risk for coronary artery disease?: Clinical risk scores are not sufficient to define primary prevention treatment strategies among asymptomatic patients. Circ Cardiovasc Imaging. 2014;7:398–408. doi: 10.1161/CIRCIMAGING.113.000341. – discussion 408. [DOI] [PubMed] [Google Scholar]
- 36.Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7:276–284. doi: 10.1161/CIRCOUTCOMES.113.000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamudu HM, Paul TK, Veeranki SP, Budoff M. The effects of coronary artery calcium screening on behavioral modification, risk perception, and medication adherence among asymptomatic adults: a systematic review. Atherosclerosis. 2014;236:338–350. doi: 10.1016/j.atherosclerosis.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Bansilal S, Castellano JM, Garrido E, et al. Assessing the Impact of Medication Adherence on Long-Term Cardiovascular Outcomes. Journal of the American College of Cardiology. 2016;68:789–801. doi: 10.1016/j.jacc.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. Journal of the American College of Cardiology. 2015;65:1361–1368. doi: 10.1016/j.jacc.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pocock SJ, Gersh BJ. Do current clinical trials meet society's needs?: a critical review of recent evidence. Journal of the American College of Cardiology. 2014;64:1615–1628. doi: 10.1016/j.jacc.2014.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.