Abstract
Background
Airway obstruction is a physiologic feature of asthma and IL-15 may have an important role in the pathogenesis of asthma.
Objective
We tested the hypothesis that regulation of IL-15 is critical for the preservation of allergen-induced airway hyperresponsiveness (AHR), airway resistance and compliance in response to methacholine.
Methods
Airway inflammation, AHR, resistance and compliance were assessed in IL-15- gene deficient mice and IL-15 overexpressing mice in an allergen-induced murine model of asthma. We assessed eosinophils by anti-MBP immunostaining, goblet cells hyperplasia by PAS staining, and cytokines and chemokine levels by performing qPCR and ELISA analyses.
Results
Herein, we made a novel observation that IL-15 deficiency promotes baseline airway resistance in naïve mice. Moreover, rIL-15 delivery to the lung downregulates expression of proinflammatory cytokines, and improves allergen-induced AHR, resistance and compliance. These observations were further validated in DOX-inducible CC-10-IL-15 transgenic mice. DOX exposed Aspergillus extract challenged CC-10-IL-15 bitransgenic mice exhibited significantly reduced levels of proinflammatory cytokines (IL-4, IL-5, IL-13) and decreased goblet cell hyperplasia. Airway obstruction including AHR and resistance was diminished in allergen challenged DOX exposed mice compared to non-DOX exposed CC-10-IL-15 bitransgenic mice. Mechanistically, we observed that IL-15-mediated protection of airway obstruction is associated with induced IFN-γ and IL-10-producing regulatory CD4+CD25+Foxp3+ T cells. Additionally, we found that a human IL-15 agonist (ALT-803) improved airway resistance and compliance in an experimental asthma model.
Conclusion
We report our novel finding that IL-15 has a potent inhibitory effect on the airway obstruction that occurs in response to environmental allergens.
Keywords: Allergen, Asthma, Airway Hyperresponsiveness (AHR), Methacholine, IL-10, IL-15
Introduction
The occurrence of asthma is increasing throughout the world, including the United States. Nearly 25 million people, 8.6 % of adults and 14 % of children are suffering from this chronic inflammatory pulmonary disorder, which is also responsible for over 5000 deaths/year (http://www.aafa.org). 1, 2 Hence, there is a great need to continue with innovative fundamental studies to uncover new possibilities for therapeutic interventions that will prevent or treat airway obstruction. Currently, anti-inflammatory corticosteroid inhalers and other medications are available to treat airway inflammation and obstruction. 3, 4 These treatments bring about a significant reduction in inflammation, but fail to restrict or reverse the progression of the bronchial airway obstruction and fibrosis. Airway obstruction is not a disease per se, but rather represents a physiologic manifestation of airway narrowing as a consequence of inflammation and scarring. Airway reactivity is an important component of the diagnostic criteria for examining bronchial asthma using a variety of stimuli including methacholine, histamine, hypertonic saline, distilled water, exercise, or eucapnic hyperventilation. 5–7 Asthma is a chronic, inflammatory airway disease characterized by activation of inflammatory cells and mediators, variable obstruction, hyperresponsiveness and airway remodelling. 8–10 Substantial research in the field of bronchial asthma within the last two decades was aimed at identifying inflammatory cell products responsible for the pathogenesis of bronchial asthma, including airway hyperresponsiveness (AHR), and airway resistance (RI). 11, 12 Measurements of AHR have traditionally been used to identify individuals with intermittent obstruction. 12 Presently, it is believed that AHR can result from the coordination of several mechanisms, some or all of which might be operative in individual asthmatics. In asthma, there is a relationship between the inflammatory state of the airways and the severity of hyperresponsiveness. Clinical and experimental investigations have demonstrated a strong correlation between the presence of CD4+ T helper (Th) 2 and Th3 lymphocytes and disease severity, suggesting an integral role for these cells in the pathophysiology of asthma. 13, 14 Studies have identified elevated production of IgE, mucus hypersecretion, and eosinophilic inflammation, which lead to obstructive lung function abnormalities and airway fibrosis. 15–20 Th2 cells are thought to induce asthma through secretion of an array of cytokines [interleukin (IL)-4, IL-5, IL-6, IL-9, IL-10, and IL-13] that activate inflammatory and resident effector pathways in the lung both directly and indirectly. 21, 22 In particular, investigations have identified IL-13 as a potential key regulator of the pathogenic mechanism(s) underlying allergic disease. IL-13 is expressed at exaggerated levels in atopic and non-atopic asthma. 23, 24 IL-15 is a cytokine implicated in innate and acquired antiviral immunity, and its association with pediatric, asthma has been reported. 25 Another manuscript indicated that IL-15 deficiency exacerbates asthma in children. 26 What is more, higher levels of IL-15 were found in steroid treated asthmatic patients. 25 However, to date the role of IL-15 in the pathogenesis of asthma has not yet been explored. Importantly, IL-15 is a requisite for the generation or maintenance of specific hematopoietic lineages, and IL-15 receptors are expressed on dendritic cells, monocytes, NK cells and number of T cells subtypes including natural killer T (NKT), naïve and memory CD8+ T cells and γδ T cells 27, 28. Therefore, we focused our studies on understanding the modulatory effects of IL-15 on allergen-induced airway obstruction, including airway AHR, RI and Cydn. Accordingly, we first examined whether endogenous IL-15 deficiency exacerbates baseline airway resistance, and next whether rescue use of IL-15 in IL-15 gene-deficient (IL-15−/−) mice would improve airway resistance and compliance. In addition, we provide evidence that both pharmacological delivery of recombinant (r) IL-15 to the lung and lung targeted transgenic IL-15 overexpression protects mice against allergen-induced initiation of AHR, RI and Cydn. Taken together, our findings show that IL-15 is critical for the development of asthma and has the potential to serve as a therapeutic agent for the treatment of airway obstruction in asthma.
Materials and Methods
Mice
rtTA-CC-10 IL-15 bitransgenic mice were generated as per the construct shown in (Figure 3, A), and back crossed into the Balb/c background in our laboratory. IL-15 gene-deficient (IL-15−/−) Balb/c mice were kindly donated by Dr. Fred D. Finkelman (Cincinnati Children’s Hospital Medical Centre, Cincinnati, OH, USA). Specific pathogen-free wild type Balb/c mice (8–10 weeks old) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). The Tulane Institutional Animal Care and Use Committee (IACUC) approved the animal protocols that were employed in accordance with National Institute of Health (NIH) guidelines.
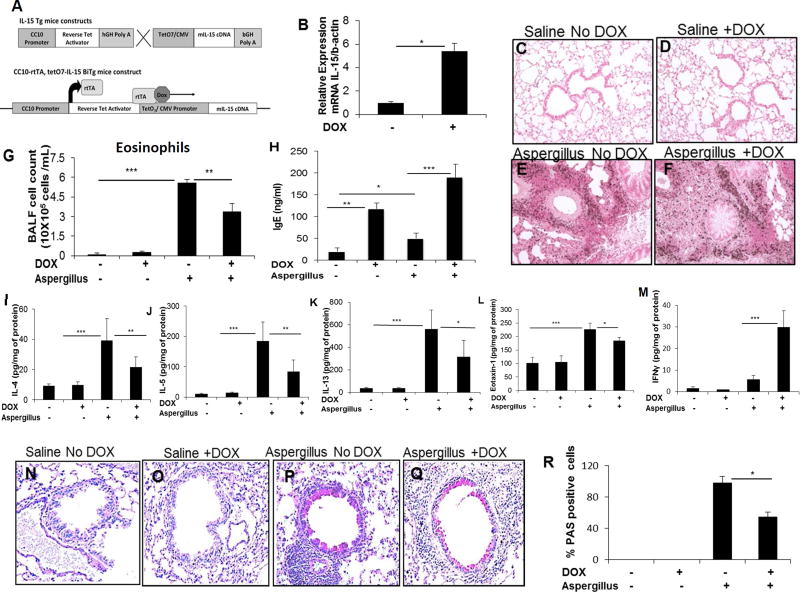
Figure 3. Generation of DOX regulated IL-15 transgenic mice and the analysis of eosinophils, proinflammatory cytokines, and goblet cell hyperplasia in Aspergillus exposed IL-15 transgenic mice.
The construct of (tetO)7 CMV-IL15 transgenic mice and rtTA-CC10 transgenic mice to generate rtTA-CC10-IL-15 bi-transgenic mice is shown (A). Induction of IL-15 mRNA levels following 3 weeks of DOX exposure to rtTA-CC10-IL-15 bi-transgenic mice is shown (B). A representative photomicrograph (original magnification x400) of anti-MBP-stained saline and Aspergillus challenged CC-10 IL-15 bitransgenic mice (C-F), BALF eosinophils (G), blood IgE (H), cytokine and chemokine levels of IL-4, IL-5, IL-13, IFN-γ, eotaxin-1 (I-M) in saline or Aspergillus challenged DOX non-DOX exposed mice are shown. A representative photomicrograph of goblet cell hyperplasia (N-Q) and respective cell numbers in saline and Aspergillus challenge DOX and non-DOX exposed IL-15 bitransgenic mice are shown (R). Data is expressed as mean ± SD, n=12. *p<0.04 **p<0.001, *** p<0.0001, NS, not significant. A high-resolution version of this slide for use with the Virtual Microscope is available as eSlide: VM03992 (3C, saline-anti-MBP); VM03970 (3E, Aspergillus, anti-MBP); VM03993 (3N, saline challenged PAS stained); VM03971 (3P, Aspergillus, PAS stained showing goblet cell hyperplasia).
Induction of airway allergic inflammation
A murine model of allergic lung disease was employed using three different allergenshouse dust mite /cockroach/ aspergillus extracts as per the methods described previously. 29–31 In brief, mice were lightly anesthetized with isoflurane inhalation (Methoxy-fluorane; Pittman-Moore, Mundelein, IL) and 100 µg (50 µg (50 µl) of Aspergillus fumigatus, or cockroach, or house l) of Aspergillus fumigatus, or cockroach, or house dust mites extracts (Greer Laboratories, Lenoir, NC) or 50 µl of normal saline alone was applied to the nares using a micropipette 3 times per week for 3 weeks to induce lung inflammation.
rlL-15 or ALT-803 pre-treatment
rlL-15 (PeproTech, Rocky Hill, NJ) 10µg/50 µl or ALT-803 5µg/50 µl (Alter biosciences, Miramar, FL) or 50 µl saline alone were used to pretreat the mice via intranasal route, 2 times a week for 3 weeks in allergen challenged mice during the experimental regimen as described above. Mice were sacrificed between 18 to 20 hours after the last intranasal challenge.
Anti-IL-15 Neutralization
Anti-mouse rabbit polyclonal IL-15 neutralization antibody (1mg/mL) (PeproTech, Inc., Rocky Hill, NJ) or rabbit-IgG (control) was administered intraperitoneally to mice 30 min before challenging them with Aspergillus extract following the protocol describe above.
Measurement of Airway hyperreactivity (AHR)
AHR to methacholine (MCh) was assessed in conscious, unrestrained mice by barometric plethysmography, using the apparatus and software supplied by Buxco Electronics Ltd., (Troy, NY). This system yields a dimensionless parameter known as enhanced pause (Penh), reflecting changes in waveform of the pressure signal from the plethysmography chamber combined with a timing comparison of early and late expiration. Measurement was performed as previously described. 32, 33 Briefly, mice were placed in the plethysmography chamber and exposed to an aerosol of water (baseline readings) and then cumulative concentrations of methacholine (MCh) (Sigma-Aldrich, St. Louis, MO) ranging from 3.125 to 25 mg/ml. The aerosol was generated by an ultrasonic nebulizer and drawn through the chamber for 2 min. The inlet was then closed and Penh readings taken over 3 min were averaged. Values were reported as the increase over baseline.
Measurement of airway resistance and compliance
The trachea was surgically exposed in anesthetized mice and a cannula was inserted and tied with a suture to prevent air leakage. 34 Mice were placed in the Finepoint RC system chamber (Data Sciences International [DSI] St. Paul, MN) and connected to the ventilator. Mechanical ventilation was started with the appropriate respiratory rate and tidal/stroke volume. After two baseline measurements lasting 3 min, mice were exposed to PBS or MCh (3.125 to 50 mg/ml) and their RI and Cydn were measured as per the manufacturer’s defined protocol.
Analysis of eosinophils
Paraffin embedded lung tissue sections (5µm) were immunostained with antiserum against mouse eosinophil major basic protein (anti-MBP) as described previously. 33, 35–37 In brief, endogenous peroxidase in the tissues was quenched with 0.3% hydrogen peroxide in methanol followed by non-specific protein blocking using 3 % normal goat serum. Tissue sections were then incubated with rat anti-MBP antibody (1:6000 dilution) overnight at 4°C (kindly provided by Drs. James and Nancy Lee, Mayo Clinic, Scottsdale, AZ, USA), followed by a 1:250 dilution of biotinylated goat anti-rat IgG secondary antibody and avidin-peroxidase complex (Vector Laboratories, Burlingame, CA) for 30 min each. Slides were further developed with nickel diaminobenzidine-cobalt chloride solution to form a black precipitate, and counterstained with nuclear fast red. Negative controls included replacing the primary antibody with normal rabbit serum to control for endogenous biotin and peroxidase activity.
Analysis of Goblet cell hyperplasia
Lung tissue samples were fixed with 10 % paraformaldehyde in phosphate buffer (PBS) pH 7.4, embedded in paraffin, cut into 5 µm sections, and fixed to positively charge slides. Periodic Acid Schiff (PAS) reaction staining (Poly Scientific R&D Corp, Bay Shore, NY) was performed on the tissue sections according to the manufacturer’s recommendations. Quantification of mucus stained cells was assessed with morphometric analysis using the Metamorph Imaging System (Universal Imaging Corporation, West Chester, PA) as described. 34 Lung sections were taken from the same position in each set of mice and at least 4–5 random sections/mouse were analysed. Using digital image capture, tissue regions associated with the entire perivascular/peribroncheal region were viewed and goblet cells were quantified as the percentage of total mucus producing cells relative to the total number of airway epithelial cells.
Bronchoalveolar lavage fluid (BALF) and lung tissue collection for analysis
The trachea was surgically exposed and cannulated using 27-gauge silicon tubing attached to a 23-gauge needle. Bronchoalveolar lavage (BAL) was performed three times with 0.5 ml of PBS per mouse. The BALF return from individual animals were pooled in plastic tubes on ice and centrifuged (250 g) at 4 °C for 5 min. Total cell counts in the BALF were analysed using a haemocytometer. Thin-layer preparations of BALF were generated using a Cytospin and stained using a Diff-Quik stain kit (IMEB inc, San Marcos, CA, USA), and differential cells counts were obtained based on the morphology and staining characteristics of at least 200 cells. BALF supernatants were stored at −80°C for cytokine quantification. Murine lung tissue was collected in M-PER protein buffer (Invitrogen, Indianapolis, IN) and homogenized, then stored at −80°C for future cytokine measurements.
ELISA analysis
Cytokine levels in BALF supernatants and lung homogenates were measured using ELISA kits (IL-4, IL-5, IL-10, IL-13, IL-15 and IFN-γ from eBiosciences, San Diego, CA). The detection limits were 4 pg/ml for IL-4, IL-5, IL-13, IL-15 and 15 pg/ml for IFN-γ, IL-10. Eotaxin-1, −2 protein concentrations in the BALF and lung homogenates were quantified using the DuoSet ELISA kit (R & D Systems, Minneapolis, MN).
Real-time PCR analysis
Total RNA was isolated from the lung samples using TRIZOL (Invitrogen, Indianapolis, IN). RNA samples (500 ng) were subjected to reverse transcription analysis using iScript reverse transcriptase (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Transcripts of IL-5, IL-4, IL-13, IL-15, eotaxin-1, eotaxin-2 and IL-10 were quantified by real-time PCR using the IQ5 (Bio-Rad, Hercules, CA) instrument. Results were then normalized to GAPDH amplified from the same cDNA mix and expressed as relative expression induction compared with the GAPDH. Specific transcripts from cDNA were amplified using the primers listed in table 1.
Table 1.
Primers used for real time PCR analysis.
| Gene name |
Forward | Reverse |
|---|---|---|
| mGAPDH | 5′-ACCCAGAAGACTGTGGATGG-3′ | 5′-CACATTGGGGGTAGGAACAC-3′ |
| mIL-4 | 5′-CCTCACAGCAACGAAGAACA-3′ | 5′-ATCGAAAAGCCCGAAAGAGT-3′ |
| mIL-5 | 5′-TCCCATGAGCACAGTGGTGAAAG-3′ | 5′-CACAGTACCCCCACGGACAGTTT-3′ |
| mIL-13 | 5′-CATGGCGCTCTGGGTGACTG-3′ | 5′-CGGCCAGGTCCACACTCCATAC-3′ |
| mIL-15 | 5′-TTGCAGTGCATCTCCTTACG-3′ | 5′-CCTTCCAAACACAGCAGGAT-3′ |
| mIL-10 | 5′-CAGAGAAGCATGGCCCAGAAATC-3′ | 5′-TCTTCACCTGCTCCACTGCCTTG-3′ |
| mEotaxin-1 | 5′-GGCTCACCCAGGCTCCATCC-3′ | 5′-TTTGGTCCAGGTGCTTTGTGG-3′ |
| mEotaxin-2 | 5′-GTGATGAAGATGACCCCTGCCTT-3′ | 5′-CTCCTTCTCCTGGTAGCCTGC-3′ |
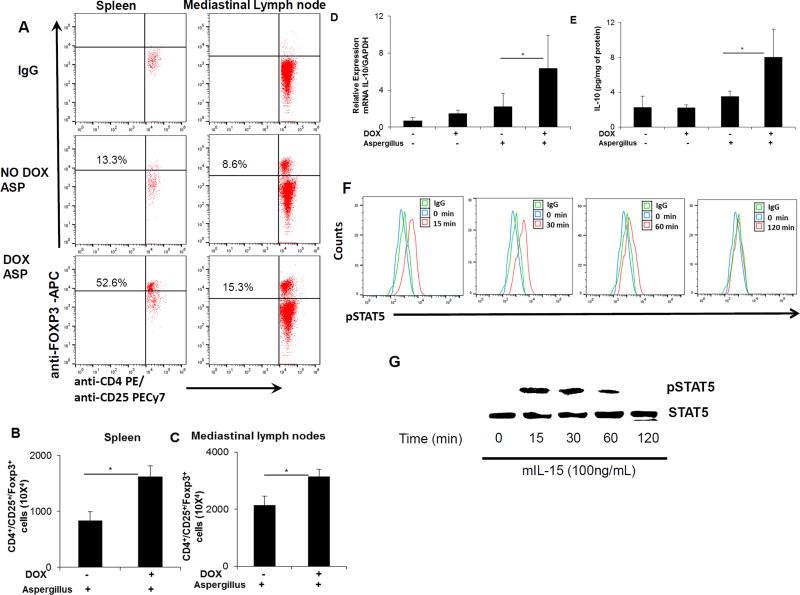
Flow cytometric analysis of CD4+CD25+Foxp3+ T regulatory cells
Our hypothesis was tested using IL-15 inducible lung-specific (rtTA-CC-10-IL-15) bi-transgenic experimental asthmatic mice. Cells were isolated from the spleen and mediastinal lymph nodes of DOX and non-DOX exposed CC-10-IL-15 bi-transgenic mice in our experimental asthma model. Isolated cells were stained with anti-CD45-FITC, anti-CD4-PE, anti-CD25-PE-Cy7, and anti-Foxp3-APC and examined by flow cytometry. Cells were first stained with anti-CD45- FITC, anti-CD4-PE and anti-CD25-PE-Cy7 for cell surface markers followed by intracellular Foxp3 Staining Set (eBioscience, San Diego, CA) using an anti-Foxp3-APC antibody. Intracellular IL-10 in regulatory T cells was analyzed using an IL-10 Staining Set (eBioscience, San Diego, CA). The samples were analyzed using FACSCalibur flow cytometer (BD Biosciences, San Diego, CA). 38 STAT5 activation was examined in purified regulatory T cells. Regulatory T cells were isolated using the regulatory T cell Isolation Kit (Miltenyi Biotec, San Diego, CA) from the mouse splenocytes. The isolated regulatory T cells were exposed to 100ng/ml rIL-15 for 0, 15, 30, 60, or 120 min and STAT5 phosphorylation (pSTAT5) was examined using a pSTAT5 antibody (BD Biosciences, San Diego, CA) by flow cytometry and western blot analysis as per the protocol described earlier.39
Statistical analysis
Data are expressed as mean ± Standard Deviation (S.D.). Statistical significance comparing different groups of mice was determined using unpaired InStat GraphPad Prism5 Version 5.03 (San Diego, CA) software and the nonparametric one-way ANOVA Kruskal-Wallis test followed by Dunn’s corrections for multiple comparisons. p-value < 0.05 was considered statistically significant.
RESULTS
Endogenous IL-15 deficiency exacerbates airway obstruction and is rescued bypharmacological delivery of rIL-15 in mice
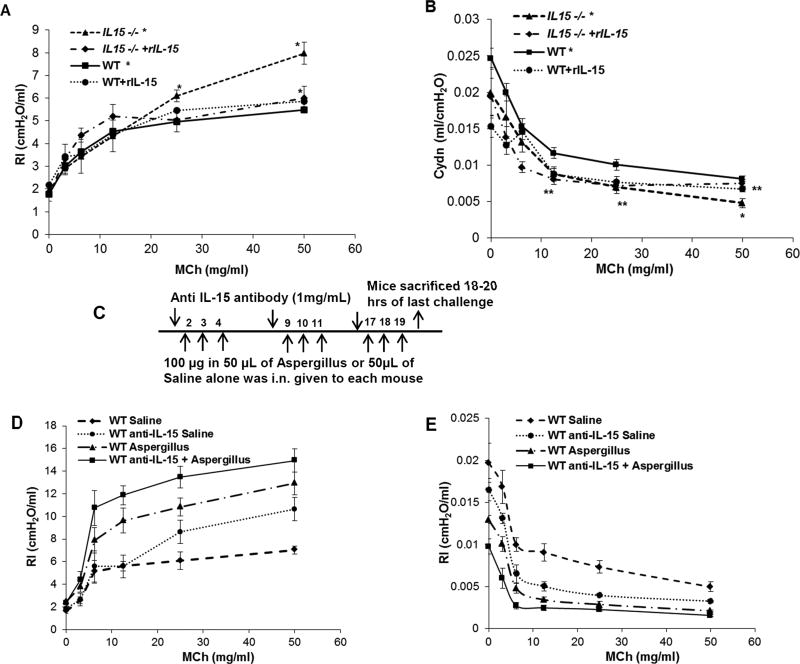
IL-15 polymorphism and IL-15 deficiency has been reported in asthmatic children. 26 Notably, higher levels of IL-15 were observed in steroid treated asthmatic patients. 25 Therefore, we tested the hypothesis that endogenous deficiency of IL-15 induces baseline airway resistance and this effect can be rescued by administration of rIL-15 in IL-15 endogenous deficient mice. Baseline airway resistance and compliance in wild type (WT) and IL-15 gene-deficient (IL-15−/−) mice were measured using the Finepoint RC system (Data Sciences International, DSI St. Paul, MN). Our experiments showed that baseline resistance was increased (Figure 1, A) and compliance (Figure 1, B) was decreased in IL-15−/− mice compared to WT mice. Further, we observed that rescue of IL-15−/− mice using rIL-15, 10µg/two times a week over a period of 3 weeks, decreased resistance (Figure 1, A) and increased compliance (Figure 1, B) back to the values measured in WT mice. Saline-treated IL-15−/− mice demonstrated high resistance that was comparable to naïve IL-15−/− mice, whereas WT rIL-15 treated mice did not show any significant change in resistance or compliance. Further, to establish that IL-15 deficiency indeed exuberates asthma, we followed a different approach by neutralizing the IL-15 in mice and inducing experimental asthma in mice. The anti-IL-15 neutralization and Aspergillus challenged protocol we employed is shown in Figure 1, C. We observed that IL-15 neutralize mice (anti-IL-15, 1mg/week over a period of 3 weeks) following allergen challenge exhibit increased resistance (Figure 1, D) and decreased compliance (Figure 1, E) compared to non-neutralized Aspergillus challenged mice. Saline-treated IL-15 neutralized mice also showed enhanced baseline resistance and decreased compliance compare to naïve non-neutralized mice (Figure 1, D and E). These data confirm that IL-15 deficiency promotes the pathogenesis of asthma.
Figure 1. Analysis of baseline airway obstruction in endogenous IL-15gene deficient (IL-15−/−) mice and rIL-15 treated mice.
Baseline airway resistance (RI) and compliance (Cydn) in anesthetized WT, IL-15 gene deficient (IL-15−/−) mice rIL-15 rescued IL-15−/− mice in response to various concentrations (0, 3.125, 6.25, 12.5, 25, 50 mg/ml) of methacholine were measured and comparison data is presented (A, B). The protocol followed for IL-15 level modulation and induction of asthma in mice is shown (C). Anti-IL-15 neutralized mice show exuberated resistance and compliance at baseline and with allergen-induced asthma compared to non-neutralized saline and Aspergillus challenged mice (D, E). Data is expressed as mean ± SD n=6 mice/group in each group, *p<0.04 **p<0.001.
rIL-15 treatment downregulates inflammatory cytokine levels and tissue eosinophilia in experimental asthma
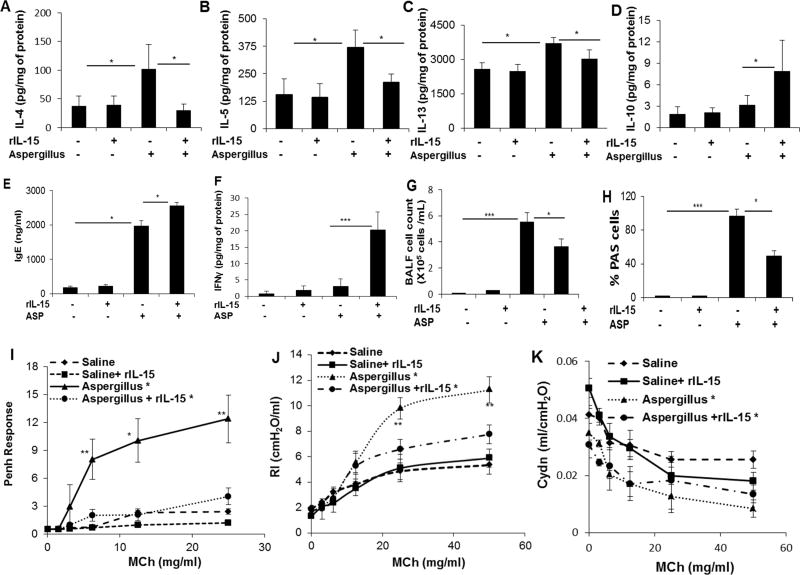
IL-15−/− mice showed enhanced airway resistance; therefore, we assessed whether IL-15 pharmacological delivery protects mice from the induction of airway obstruction in allergen challenged asthmatic mice. Accordingly, we pre-treated WT mice with saline or rIL-15 and challenging the mice with Aspergillus extract as per the protocol described (Supplementary Figure 1, A). Anti-MBP immunostaining detected eosinophils in the lungs of both Aspergillus challenged rIL-15 treated and non-treated mice compared to their respective controls (Supplementary Figure 1, B-E). Real-time PCR analysis revealed reduced IL-4, IL-5, and IL-13, but not IL-10, mRNA levels in rIL-15 treated mice compared to non-treated Aspergillus challenged mice (Supplementary Figure 1, F-I) and protein levels of the respective cytokines were also diminished except for the levels of IL-10, IgE and IFN-γ (Figure 2, A-F). However, enumeration of BALF eosinophils indicated reduced numbers in rIL-15 treated mice compared to Aspergillus challenged mice (Figure 2, G). Induced PAS stained goblet cell hyperplasia was observed in Aspergillus challenged mice compared to saline control, and there was a notable reduction in goblet cells in rIL-15 treated Aspergillus challenged mice (Supplementary Figure 1, J-M). Mucus cell producing goblet cells were counted along the airway epithelium and are shown as the percentage of PAS positive cells (Figure 2, H).
Figure 2. Consequences of rIL-15 pretreatment on proinflammatory cytokine levels, goblet cell hyperplasia and airway obstruction in Aspergillus-induced experimental asthma.
ELISA analysis of IL-4, IL-5, IL-13, IL-10 IFNγ and IgE levels in control and rIL-15 treated saline or Aspergillus-challenge mice are shown (A-F). BALF eosinophil and PAS+ goblet cell numbers in control and rIL-15 treated saline or Aspergillus-challenge mice are shown (G, H). Airway hyperreactivity (AHR), airway resistance (RI) and compliance (Cydn) in response to methacholine were measured in saline challenged, rIL-15 pretreated saline, Aspergillus challenged alone and rIL-15 pretreated Aspergillus challenged groups of mice are shown (I, J, K). Data is expressed as mean ± SD, n=12 mice/group. *p<0.05, ** p<0.001
rIL-15 pre-treatment protects mice from airway hyperreactivity (AHR) and bronchial resistance (RI) in Aspergillus-induced experimental asthma
We examined AHR and RI in response to methacholine in Aspergillus challenged mice, with or without administration of rIL-15. Allergen challenged mice demonstrated AHR when compared with saline challenged mice. The reactivity to methacholine is presented as the percentage increase in enhanced pause (Penh) over baseline reactivity in the absence of cholinergic stimuli. Heightened reactivity was seen in response to methacholine in Aspergillus challenged mice when compared with the corresponding saline challenged mice. Mice treated with rIL-15 and challenged with Aspergillus showed significantly diminished AHR (Figure 2, I), RI (Figure 2, J) and increased compliance, Cydn (Figure 2, K) in response to methacholine compared to sham rIL-15-treated Aspergillus-challenged mice. Baseline AHR, airway resistance and compliance in response to methacholine have been shown in rIL-15 treated and saline challenged mice (Figure 2, I-K). Further, to establish that the rIL-15 mediated protection of RI in response to methacholine is not specific to Aspergillus, we also examined the response to methacholine in dust mite and cockroach allergen challenged mice. Herein, we show that both dust mites and cockroach allergen challenged mice demonstrated induced methacholine responses to RI and reduced Cydn compared to their respective saline challenged controls. However, both the RI and Cydn responses to methacholine in rIL-15 treated dust mites and cockroach allergen challenged mice were significantly improved compared to saline treated dust mite or cockroach allergen challenged mice (Supplementary Figure 2, B-E). These findings indicate that rIL-15 treatment responses are not allergen specific.
Generation of rtTA-CC10-IL-15 Bi-transgenic mice
Doxycycline (DOX) regulated rtTA-CC-10-IL-15 bitransgenic mice were generated in our laboratory to further test our hypothesis. Responder transgenic mice [tetO-IL-15 containing germline IL-15 cDNA under the regulation of the tetracycline operator (tetO7)] were generated and breed to a tetracycline reverse-transactivator transgenic mouse line CC10-rtTA (expressing rtTA cDNA under the regulation of the lung-specific rat 2.3-kb CC10 promoter) to establish tetracycline-induced rtTA-CC10-IL-15 bitransgenic mice as shown (Figure 3, A). rtTA-CC10-IL-15 bitransgenic mice were phenotyped following DOX or sham-DOX food for 3 weeks. The expression of IL-15 mRNA was analysed using real time PCR. The analysis showed an ∼6-fold increase in IL-15 mRNA (Figure 3, B) and protein, similar to earlier reports of lung transgene augmentation in other CC10 promoter driven bitransgenic mice.40, 41 In vivo overexpression of IL-15 in these mice will be free from any external endotoxin contamination. Lung sections and BALF were analysed for eosinophil accumulation in DOX and non-DOX exposed saline or Aspergillus challenged IL-15 overexpressing mice. The anti-MBP immunostaining detected no eosinophils in saline challenged non-DOX or DOX exposed IL-15 overexpressing mice (Figure 3, C and F). Whereas, increased and comparable tissue eosinophils were detected in both DOX and non-DOX Aspergillus exposed IL-15 overexpressing mice (Figure 3, E and F). Of note, induced blood IgE levels and reduced BALF airway eosinophilia in the BALF of DOX exposed Aspergillus challenged IL-15 transgenic mice were observed compared to the non-DOX Aspergillus challenged IL-15 transgenic mice (Figure 3, G, H). DOX exposed saline challenged IL-15 transgenic mice showed IgE induction compared to non-DOX saline exposed mice; but the level of IgE in IL-15 transgenic mice was low compared to allergen challenged DOX and non-DOX mice (Figure 3H) or within the normal range for IgE compared to wild type mice (Figure 2E). Pro-inflammatory cytokines and chemokines, specifically IL-4, IL-5, IL-13, eotaxin-1 and eotaxin-2 mRNA (Supplementary Figure 3, A-E) and protein levels of all of these cytokines were significantly reduced, excluding anti-inflammatory cytokines like IFN-γ was found to be elevated, in DOX exposed Aspergillus challenged mice compared to the non-DOX Aspergillus challenged mice (Figure 3, I-M). Saline challenged DOX and non-DOX exposed mice did not exhibit any mucus producing goblet cells (Figure 3, N and Q); however, increased numbers of goblet cells were detected in non-DOX Aspergillus challenged mice compared to the DOX exposed Aspergillus challenged mice (Figure 3, P and Q). The percentage of PAS-positive cells in the airway epithelium of the Aspergillus exposed group in DOX exposed mice was significantly reduced compared to the Aspergillus challenged non-DOX exposed mice (Figure 3, R).
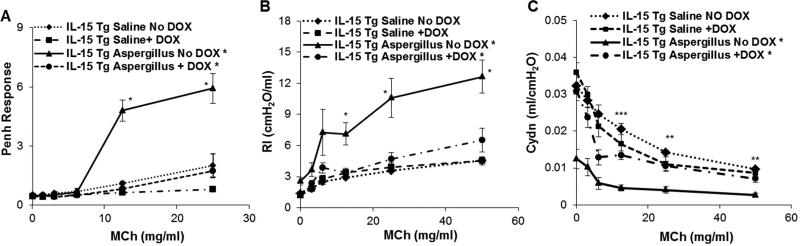
DOX regulated IL-15 overexpressing mice show reduced airway hyperreactivity (AHR) and airway resistance (RI) in response to methacholine in the mouse model of Aspergillus-induced experimental asthma
DOX and non-DOX exposed saline or Aspergillus challenged mice were examined for AHR, RI and Cydn in response to methacholine. DOX exposed Aspergillus challenged mice compared to the non-DOX exposed Aspergillus challenged mice showed significantly reduced AHR in response to methacholine at the doses of 12.5 and 25 mg/ml of methacholine (Figure 4, A). Similarly, DOX exposed Aspergillus challenged IL-15 transgenic mice also showed a significant decrease in RI (Figure 4, B) and improved Cydn (Figure 4, C) compared to non-DOX exposed Aspergillus challenged mice in response methacholine. No significant change was observed in AHR, RI or Cydn in saline challenged DOX exposed mice and non-DOX-exposed mice (Figure 4, A-C).
Figure 4. Analysis of airway hyperreactivity, resistance and compliance in DOX regulated CC10-IL-15 overexpressed mice.
Airway hyperreactivity (PENH), resistance (RI) and compliance (Cydn) in response to methacholine were measured in DOX and non-DOX diet saline or Aspergillus challenged CC10-IL-15 mice are shown (A, B, C). Data are expressed as mean ± SD, n = 10/group. *p<0.001,
Regulatory T cells and IL-10 are increased in DOX exposed IL-15 overexpressing mice following the induction of experimental asthma
Regulatory T cells are proposed to play a central role in the maintenance of immunological tolerance by effectively suppressing induction of inflammatory IgE and Th2 responses in allergic inflammation. 43 Therefore, we next tested the hypothesis that overexpression of IL-15 may induce regulatory T cells. Accordingly, we enumerated regulatory T cells in the spleen and mediastinal lymph nodes of Aspergillus challenged DOX and non-DOX exposed IL-15 transgenic mice. Spleen and mediastinal lymph nodes were harvested and single cell suspensions were analyzed for quantification of regulatory T cells using a flow cytometer in Aspergillus challenged mice that were treated +/− DOX for 3 weeks prior to challenge. The cells were stained with anti-CD4-PE, anti-CD25-PECy7 and anti-Foxp3-APC. The CD4 and CD25 expressed cells were first examined by flow cytometer and then double positive CD4+CD25+ expressed cells were gated and analyzed for Foxp3 expression. Foxp3 matched anti-IgG was used as isotype match control to correctly identify regulatory T cells. The regulatory T cells are identified as CD4+CD25+Foxp3+. As shown in Figure 5 A, the CD4+CD25+Foxp3+ regulatory T cell population in the spleen (52.6%) and mediastinal lymph nodes (15.3%) was significantly increased in DOX exposed Aspergillus challenged mice compared to non-DOX exposed Aspergillus challenged mice [spleen (13.3%) and mediastinal lymph nodes (8.6%)]. DOX exposed Aspergillus challenged mice displayed significantly higher absolute numbers of CD4+CD25+Foxp3+ in both spleen and mediastinal lymph nodes (Figure 5, B and C). Further to establish that induced T regulatory cells are the source of IL-10, we analyzed IL-10 producing regulatory T cells in the mediastinal lymph nodes of Aspergillus challenged non-DOX and 3 weeks DOX exposed IL-15 bitransgenic mice using intracellular IL-10 cytokine detection kit (eBiosciences). The flow cytometer analysis showed that indeed DOX exposed mice induce regulatory T cell and are the source of IL-10 (Supplementary Figure 4, B-E). However, 5 µg of rlL-10 delivery to the lung (similar to rlL-15) did not improve Aspergillus induced airway obstruction in mice and showed comparable resistance and compliance to non-treated Aspergillus challenged mice (supplementary Figure 5, A, B). Real-time PCR and ELISA analysis demonstrated significantly higher mRNA and protein levels of IL-10 in the lungs of DOX exposed Aspergillus challenged mice compared to non-DOX exposed Aspergillus challenged mice (Figure 5, D and E). Further, our interest was to understand that signal transducer and activator of transcription 5 (STAT5) regulate rlL-15 induced regulatory T cells induction and activation. Therefore, STAT5 activation analysis was examined by both flow cytometer and western blot analysis. The regulatory T cells were isolated from the spleen and exposed to 100ng/ml rIL-15 for 0, 15, 30, 60, 120 min and STAT5 phosphorylation was examined by flow cytometer and western blot analyses. Both flow cytometer and western blot analyses showed that rIL-15 activates STAT5 in regulatory T cells within 15 and 30 min post exposure (Figure 5, F and G). These analyses indicate that IL-15 induced regulatory T cells responses are regulated by STAT5.
Figure 5. Regulatory T cells and IL-10 analysis in IL-15 bitransgenic mice following the induction of experimental asthma.
Regulatory T cells in the spleen and mediastinal lymph nodes of Aspergillus challenged DOX and non-DOX exposed CC10-IL-15 bi-transgenic mice were examined by flow cytometry using fluorochrome conjugated anti-CD4-PE, anti-CD25-PECy7 and anti-Foxp3-APC along with matched IgG antibodies (A). The average percent positive (CD4+CD25+Foxp3+) regulatory T cells from 3 independent experiments are shown for the spleen (B) and mediastinal lymph nodes (C). Real time PCR and ELISA analysis from the lungs of 3 independent experiments of saline or Aspergillus challenged DOX and non-DOX exposed mice for IL-10 mRNA (D) and protein (E) are shown. In vitro IL-15 induced STAT5 activation in regulatory T cells was analyzed by performing flow cytometry and western blot analysis are shown (F, G). Data are expressed as mean ± SD, *p<0.05
Further, to establish that IL-15 deficiency induced airway obstruction is associated with reduced regulatory T cells, we examined regulatory T cells in saline and Aspergillus challenged anti-IL-15 neutralized and non-neutralized mice. We observed a baseline regulatory T cells reduction in anti-IL-15 treated mice compare to the wild type mice (35% and 56%) and reduced regulatory T cells in Aspergillus challenged anti-IL-15 treated mice compare to non-treated Aspergillus challenged mice (63% to 78%). The data is shown in Supplementary Figure 6, A-E. Furthermore, to establish that rescued IL-15 in IL-15−/− mice increases regulatory T cells in IL-15−/− mice, we examined the status of regulatory T cells in rIL-15 treated WT and IL-15−/− mice. The flow cytometer analysis using anti-CD4-PE, anti-CD25- PECy7 and anti-Foxp3-APC indicated that rIL-15 given WT and IL-15−/− mice show increase levels of regulatory T cells in the spleen compare non treated WT and IL-15−/− mice (Supplementary Figure 6, F-J). These data indicates that IL-15 regulation is critical asthma.
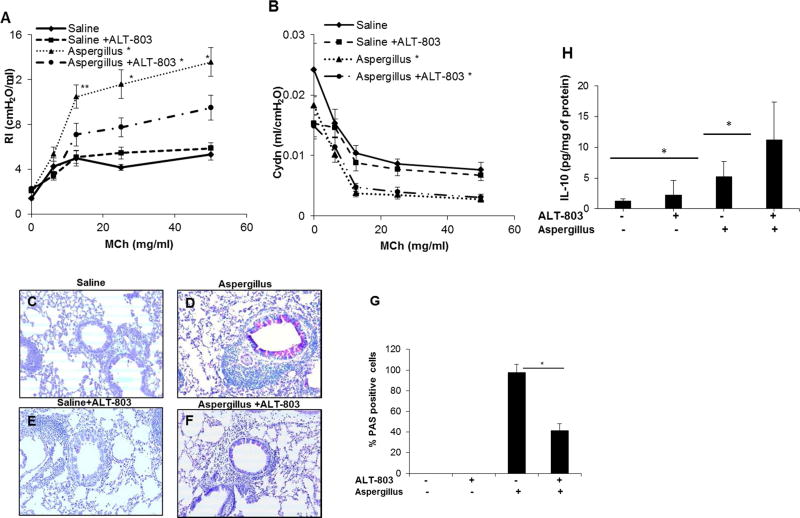
IL-15 agonist complex (ALT-803) may be a novel therapeutic agent to reduce airway resistance
Lastly, we examined whether an IL-15 agonist (ALT-803), obtained from Altor Biosciences (Altor Bioscience Corporation, Miramar, FL), could serve to reduce allergen-induced airway obstruction. Aspergillus challenged mice displayed a significant increase in airway resistance in response to (12.5, 25 and 50 mg/mL) methacholine compared to saline challenged mice. ALT-803 pre-treatment resulted in a decrease in AHR in Aspergillus challenged mice (Figure 6, A and B). ALT-803 pre-treatment also gave rise to a decrease in the number of mucus producing goblet cells observed in lung sections from Aspergillus challenged mice (Figure 6, C-F). The quantitation of PAS positive mucus-producing lung cells demonstrated a highly significant reduction in ALT-803 treated Aspergillus challenged mice compared to the Aspergillus challenged mice without ALT-803 treatment (Figure 6, G). Further, we show that ALT-803 treated allergen challenged mice exhibit significantly higher IL-10 levels compared to allergen challenged mice that did not receive the compound (Figure 6, H). However, we observed that ALT-803 treatment did not improve allergen-induced compliance in this experimental model of asthma. This phenomenon may occur on account of the mouse developing venous congestion or incomplete filling of alveoli. To help understand this finding further investigations are needed using different concentrations of ALT-803 in experimental model of asthma.
Figure 6. An improvement in allergen-induced airway obstruction is observed in response to pretreatment with the hIL-15 agonist (ALT-803).
The potential of a human IL-15 agonist as a therapeutic agent was examined on allergen induced airway resistance and compliance in an experimental model of asthma. Improvements in resistance and compliance are shown in response to ALT-803 treatment (5ug, 2 times per day for three weeks) in Aspergillus challenged mice compared to saline control mice in response to various concentrations of methacholine (A, B). A representative light photomicrograph of lungs sections for goblet cell hyperplasia in saline and Aspergillus challenged mice with and without ALT-803 (C-F, original magnification x200) and respective murine PAS positive cells are shown (G). ALT-803-induced IL-10 in BALF of Aspergillus challenged mice (H). Data is expressed as mean ± SD, n=8 mice/group, *p<0.001.
Discussion
Airway inflammation manifests as increased numbers of inflammatory cells and higher expression of associated cytokines and chemokines. 44, 45 Allergen induced airway inflammation (mainly eosinophilic) is associated with an increase in AHR and mucus production. 46–48 Both the influx of eosinophils and increase in AHR precede the development of the late phase response. 49, 50 In the current study, we demonstrated that reduced levels of IL-15 fosters airway obstruction in mice and that rescue using rIL-15 in IL-15 gene deficient (IL-15−/−) mice mitigates the airway obstruction. Additionally, using a chronic Aspergillus induced mouse model of asthma, we provide evidence that overexpression of IL-15 can suppress fundamental features of allergic airway inflammation as well as airway obstruction. IL-15 is a cytokine implicated in innate and acquired antiviral immunity and clinical reports implicate an association with pediatric asthma. 25 IL-15 has a critical role in the growth and survival of natural killer (NK) and memory phenotype CD8+ T cells that sustain innate immunity. 51, 52 IL-15 functions through its specific receptor IL-15Ra and also binds to the common cytokine γ-chain (CD132) IL-2/IL-15 receptor that shares receptor β (IL-15Rβ) (CD122). The rationale to investigate IL-15 in asthma is based on the fact that paediatric asthma patients exhibit a relative IL-15 deficiency, 26 and because levels of IL-15 are increased following steroid therapy in adult asthmatic patients. 25 Accordingly, we analyzed multiple strategies to test the hypothesis that IL-15 regulation is critical for the induction and protection of airway obstruction in asthmatic mice. Herein, we used both non-invasive and invasive methods to examine airway function in allergen induced experimental models. Whole body plethysmography (Penh), a non-invasive method for measurement of airway responsiveness, has been frequently used in the past, but its validity is now under debate. Therefore, to establish that IL-15 overexpression indeed diminishes allergen induced airway obstruction, we also used an invasive lung resistance (RI) measurement. Measurements of airway resistance are considered to be a more accurate and sensitive procedure for analysis of pulmonary mechanics. 53, 54 Our finding that endogenous IL-15 deficiency enhances airway resistance and reduces lung compliance in the absence of perturbation are consistent with the previously reported clinical findings. 55 Accordingly, we focused our current studies on the protective role of IL-15 on lung inflammation and mechanics in murine asthma models. Our experimental results showed that both pharmacological lung delivery of rIL-15 and lung targeted transgenic IL-15 overexpression down regulated the levels of Th2 cytokines and decreased mucus producing goblet cell hyperplasia in asthmatic mice. Induction of IL-4, IL-5 and IL-13 are implicated in the pathogenesis of asthma, including the features of inflammation, airway resistance and diminished compliance. 56–58
Our studies strongly support the concept that IL-15 protects mice from allergen induced increases in airway resistance and AHR. Moreover, we demonstrate that this effect is not specific to particular allergen, as the Aspergillus, dust mite and cockroach allergen induced increase in airway resistance and decrease in compliance was improved by rIL-15 treatment for all of these allergens. Mechanistically, we show that the IL-15 induced improvement in airway obstruction may be associated with higher numbers of IL-10 producing CD4+CD25+ T cells. We discovered that transgene-induced IL-15 expression results in increased numbers of CD4+CD25+Foxp3+ regulatory T cells in mediastinal lymph nodes that circulate and induce anti-inflammatory cytokine IL-10 in the lungs of asthma mouse model. Notably, it has been previously documented that IL-10 59, 60 or IL-10 producing CD4+CD25+ cells can inhibit Th2-associated airway inflammation. 61, 62 Therefore, the induction of both regulatory T cells and IL-10 by IL-15 overexpression may be a key mechanism for the observed IL-15 mediated improvement of lung inflammation and airway obstruction. However, our experimentation indicates that direct delivery of IL-10 to the lung, is not able to significantly improve airway obstruction induced by allergen in mice. This may be because either the tested dose of IL-10 is not sufficient or IL-10 requires induction of NK cell derived IFN-γ or other mediators for the protection of airway obstruction. Notably, IL-15 promotes NK cells proliferation and survival. 27, 28 In addition, IL-15 overexpression-induced IgE may also be beneficial and mechanistically contribute to the IL-15 induced protection for airway obstruction. This concept is based on an earlier report that indicates that a small amount of IgE has a significant role in the immune system’s recognition that is required for strong cytotoxic responses.42
Furthermore, we provided clinically relevant supportive data illustrating the effectiveness of an IL-15 agonist (ALT-803) in reducing mucus-producing cells and improving airway resistance in asthmatic mice. These investigations provide a strong preclinical rationale to consider regulation of IL-15 signalling for therapeutic trials in asthmatic patients. IL-15 agonists are currently in phase I clinical trials to evaluate the safety, dosing, and anti-tumor effects of IL-15 in humans with metastatic melanoma and renal cell carcinoma. 63, 64 ALT-803 is a complex of an interleukin (IL)-15 superagonist mutant and dimeric IL-15 receptor aSu/Fc fusion protein, and was found to exhibit significantly stronger in vivo biologic activity than rIL-15. Additionally, dose-response studies indicated that a single dose of ALT-803, as low as 0.05 mg/kg, was capable of reducing 90% of the BM 5T33P myeloma cells in mice.65 These reports support the usability of ALT-803 in mouse models of asthma. Based on our findings herein, we propose IL-15 immunotherapy may be useful to reduce airway obstruction in people suffering with asthma.
Conclusions
Herein, we firstly showed that IL-15-deficiency results in increased baseline airway resistance and decreased airway compliance. Secondly, we report that rIL-15 treatment diminishes allergen-induced airway obstruction in an experimental murine model of asthma. Thirdly, we provide evidence that transgenic IL-15 overexpression in murine lung is protective against allergen induced AHR, significantly enhanced the levels of IFNγ and IL-10, and reduced pro-inflammatory cytokines, specifically IL-4, IL-5, and IL-13, as well as goblet cell hyperplasia. Furthermore, we provide mechanistic evidence that the IL-15-regulated reduction in Th2 cytokine induced airway inflammation and airway obstruction is associated with higher numbers of IL-10 producing regulatory T cells and induced IFNγ. Taken together, these studies indicate that IL-15 agonists may be a novel approach for non-steroidal therapy to improve airway obstruction, resistance and compliance in chronic asthma.
Supplementary Material
Clinical Implications.
Our studies indicate that IL-15 agonists may be a novel non-steroidal approach to treat asthma, including airway obstruction.
Acknowledgments
This work was supported in part by an NIH grant R01 AI080581 (AM). We thank Dr. Hing C. Wang, Ph.D. of Altor Biosciences Corporation for providing human ALT803 (a p53 TCR/IL-15 fusion molecule) to test our hypothesis and to validate our rIL-15 data for airway diseases. Altor Biosciences has no claim on our findings and we do not promote the use of ALT-803 for any clinical trials on asthma.
Abbreviations used
- AHR
Airway hyperresponsiveness
- BALF
Bronchoalveolar lavage fluid
- IL-15
Interleukin (IL)-15
- DOX
Doxycycline
- RI
Resistance
- Cydn
Compliance
- PAS
Periodic Acid Schiff
- MCh
Methacholine
- PBS
Phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any financial interests to be disclosed.
References
- 1.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 (vol 380, pg 2197, 2012) Lancet. 2013;381:628–628. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. Bmc Public Health. 2012:12. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morosco G, Kiley J. National asthma education and prevention program - Expert panel report 3: Guidelines for the diagnosis and management of asthma summary report 2007 - Preface. Journal of Allergy and Clinical Immunology. 2007;120:S93–S93. [Google Scholar]
- 4.Barnes PJ, Adcock IM. How do corticosteroids work in asthma? Annals of Internal Medicine. 2003;139:359–370. doi: 10.7326/0003-4819-139-5_part_1-200309020-00012. [DOI] [PubMed] [Google Scholar]
- 5.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. New England Journal of Medicine. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC. Citation Classic-Site and Nature of Airway-Obstruction in Chronic Obstructive Lung-Disease. Current Contents/Clinical Practice. 1982:22–22. [Google Scholar]
- 7.Yanai M, Sekizawa K, Ohrui T, et al. Site of Airway-Obstruction in Pulmonary-Disease - Direct Measurement of Intrabronchial Pressure. Journal of Applied Physiology. 1992;72:1016–1023. doi: 10.1152/jappl.1992.72.3.1016. [DOI] [PubMed] [Google Scholar]
- 8.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postma DS, Kerstjens HA. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:S187–92. doi: 10.1164/ajrccm.158.supplement_2.13tac170. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet J, Jeffery PK, Busse WW, et al. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–45. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 11.Holgate S. Mediator and cytokine mechanisms in asthma. Thorax. 1993;48:103–9. doi: 10.1136/thx.48.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129:S65–87. doi: 10.1016/j.jaci.2011.12.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan SP, Mould AW, Young JM, et al. Cellular and molecular regulation of eosinophil trafficking to the lung. Immunol Cell Biol. 1998;76:454–60. doi: 10.1046/j.1440-1711.1998.00766.x. [DOI] [PubMed] [Google Scholar]
- 14.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal DK, Shao Z. Pathogenesis of allergic airway inflammation. Curr Allergy Asthma Rep. 2010;10:39–48. doi: 10.1007/s11882-009-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad T, Mabalirajan U, Hasija K, et al. Mepacrine treatment attenuates allergic airway remodeling segregated from airway inflammation in mice. Int Immunopharmacol. 2011;11:74–8. doi: 10.1016/j.intimp.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Al Heialy S, McGovern TK, Martin JG. Insights into asthmatic airway remodelling through murine models. Respirology. 2011;16:589–97. doi: 10.1111/j.1440-1843.2011.01974.x. [DOI] [PubMed] [Google Scholar]
- 18.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–62. doi: 10.1016/j.jaci.2011.04.047. quiz 463-4. [DOI] [PubMed] [Google Scholar]
- 19.Akuthota P, Xenakis JJ, Weller PF. Eosinophils: offenders or general bystanders in allergic airway disease and pulmonary immunity? J Innate Immun. 2011;3:113–9. doi: 10.1159/000323433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araujo LM, Lefort J, Nahori MA, et al. Exacerbated Th2-mediated airway inflammation and hyperresponsiveness in autoimmune diabetes-prone NOD mice: a critical role for CD1d–dependent NKT cells. Eur J Immunol. 2004;34:327–35. doi: 10.1002/eji.200324151. [DOI] [PubMed] [Google Scholar]
- 21.Cohn L, Tepper JS, Bottomly K. Cutting edge: IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. Journal of Immunology. 1998;161:3813–3816. [PubMed] [Google Scholar]
- 22.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annual Review of Immunology. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 23.Humbert M, Durham SR, Kimmitt P, et al. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucose of atopic and nonatopic subjects with asthma. Journal of Allergy and Clinical Immunology. 1997;99:657–665. doi: 10.1016/s0091-6749(97)70028-9. [DOI] [PubMed] [Google Scholar]
- 24.Huang SK, Xiao HQ, Kleinetebbe J, et al. Il-13 Expression at the Sites of Allergen Challenge in Patients with Asthma. Journal of Immunology. 1995;155:2688–2694. [PubMed] [Google Scholar]
- 25.Komai-Koma M, McKay A, Thomson L, et al. Immuno-regulatory cytokines in asthma: IL-15 and IL-13 in induced sputum. Clin Exp Allergy. 2001;31:1441–8. doi: 10.1046/j.1365-2222.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 26.Bierbaum S, Nickel R, Zitnik S, et al. Confirmation of association of IL-15 with pediatric asthma and comparison of different controls. Allergy. 2006;61:576–80. doi: 10.1111/j.1398-9995.2006.01059.x. [DOI] [PubMed] [Google Scholar]
- 27.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy MK, Park LS. Characterization of interleukin-15 (IL-15) and the IL-15 receptor complex. J Clin Immunol. 1996;16:134–43. doi: 10.1007/BF01540911. [DOI] [PubMed] [Google Scholar]
- 29.Mishra A, Hogan SP, Brandt EB, et al. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogan SP, Mishra A, Brandt EB, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–60. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 31.Rayapudi M, Mavi P, Zhu X, et al. Indoor insect allergens are potent inducers of experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2010;88:337–46. doi: 10.1189/jlb.0110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pope SM, Fulkerson PC, Blanchard C, et al. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005;280:13952–61. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Hogan SP, Mahalingam S, et al. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J Allergy Clin Immunol. 2003;112:935–43. doi: 10.1016/j.jaci.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Rothenberg ME, Wen T, Shik D, et al. IL-13 receptor alpha1 differentially regulates aeroallergen-induced lung responses. J Immunol. 2011;187:4873–80. doi: 10.4049/jimmunol.1004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niranjan R, Rayapudi M, Mishra A, et al. Pathogenesis of allergen-induced eosinophilic esophagitis is independent of interleukin (IL)-13. Immunol Cell Biol. 2013;91:408–15. doi: 10.1038/icb.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulkerson PC, Fischetti CA, McBride ML, et al. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci U S A. 2006;103:16418–23. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fulkerson PC, Fischetti CA, Hassman LM, et al. Persistent effects induced by IL-13 in the lung. Am J Respir Cell Mol Biol. 2006;35:337–46. doi: 10.1165/rcmb.2005-0474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia J, Liu W, Hu B, et al. IL-15 promotes regulatory T cell function and protects against diabetes development in NK-depleted NOD mice. Clin Immunol. 2010;134:130–9. doi: 10.1016/j.clim.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Wang MQ, Mavi P, et al. Interleukin-15 Expression Is Increased in Human Eosinophilic Esophagitis and Mediates Pathogenesis in Mice. Gastroenterology. 2010;139:182–U286. doi: 10.1053/j.gastro.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra A, Wang M, Schlotman J, et al. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L305–13. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 41.Zuo L, Fulkerson PC, Finkelman FD, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R alpha 2-inhibited pathway. J Immunol. 2010;185:660–9. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karagiannis SN, Wang Q, East N, et al. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol. 2003;33:1030–40. doi: 10.1002/eji.200323185. [DOI] [PubMed] [Google Scholar]
- 43.Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr Opin Immunol. 2006;18:727–32. doi: 10.1016/j.coi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 44.O’Byrne PM, Inman MD. Airway hyperresponsiveness. Chest. 2003;123:411S–6S. doi: 10.1378/chest.123.3_suppl.411s. [DOI] [PubMed] [Google Scholar]
- 45.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–92. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 46.Lam S, LeRiche J, Phillips D, et al. Cellular and protein changes in bronchial lavage fluid after late asthmatic reaction in patients with red cedar asthma. J Allergy Clin Immunol. 1987;80:44–50. doi: 10.1016/s0091-6749(87)80189-6. [DOI] [PubMed] [Google Scholar]
- 47.De Monchy JG, Kauffman HF, Venge P, et al. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985;131:373–6. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- 48.Brusasco V, Crimi E, Gianiorio P, et al. Allergen-induced increase in airway responsiveness and inflammation in mild asthma. J Appl Physiol (1985) 1990;69:2209–14. doi: 10.1152/jappl.1990.69.6.2209. [DOI] [PubMed] [Google Scholar]
- 49.Rossi GA, Crimi E, Lantero S, et al. Late-phase asthmatic reaction to inhaled allergen is associated with early recruitment of eosinophils in the airways. Am Rev Respir Dis. 1991;144:379–83. doi: 10.1164/ajrccm/144.2.379. [DOI] [PubMed] [Google Scholar]
- 50.Durham SR, Craddock CF, Cookson WO, et al. Increases in airway responsiveness to histamine precede allergen-induced late asthmatic responses. J Allergy Clin Immunol. 1988;82:764–70. doi: 10.1016/0091-6749(88)90077-2. [DOI] [PubMed] [Google Scholar]
- 51.Dubois S, Waldmann TA, Muller JR. ITK and IL-15 support two distinct subsets of CD8+ T cells. Proc Natl Acad Sci U S A. 2006;103:12075–80. doi: 10.1073/pnas.0605212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 53.Glaab T, Taube C, Braun A, et al. Invasive and noninvasive methods for studying pulmonary function in mice. Respir Res. 2007;8:63. doi: 10.1186/1465-9921-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoymann HG. Lung function measurements in rodents in safety pharmacology studies. Front Pharmacol. 2012;3:156. doi: 10.3389/fphar.2012.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laza-Stanca Vasile, Message Simon D, Edwards Michael R, et al. The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations. PLoS Pathog. 2011;8(4):1–10. doi: 10.1371/journal.ppat.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattes J, Yang M, Siqueira A, et al. IL-13 induces airways hyperreactivity independently of the IL-4R alpha chain in the allergic lung. J Immunol. 2001;167:1683–92. doi: 10.4049/jimmunol.167.3.1683. [DOI] [PubMed] [Google Scholar]
- 57.Shim JJ, Dabbagh K, Ueki IF, et al. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol Lung Cell Mol Physiol. 2001;280:L134–40. doi: 10.1152/ajplung.2001.280.1.L134. [DOI] [PubMed] [Google Scholar]
- 58.Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 59.van Scott MR, Justice JP, Bradfield JF, et al. IL-10 reduces Th2 cytokine production and eosinophilia but augments airway reactivity in allergic mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L667–74. doi: 10.1152/ajplung.2000.278.4.L667. [DOI] [PubMed] [Google Scholar]
- 60.Tournoy KG, Kips JC, Pauwels RA. Endogenous interleukin-10 suppresses allergen-induced airway inflammation and nonspecific airway responsiveness. Clin Exp Allergy. 2000;30:775–83. doi: 10.1046/j.1365-2222.2000.00838.x. [DOI] [PubMed] [Google Scholar]
- 61.Hadeiba H, Locksley RM. Lung CD25 CD4 regulatory T cells suppress type 2 immune responses but not bronchial hyperreactivity. Journal of Immunology. 2003;170:5502–5510. doi: 10.4049/jimmunol.170.11.5502. [DOI] [PubMed] [Google Scholar]
- 62.Kearley J, Barker JE, Robinson DS, et al. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4(+) CD25(+) regulatory T cells is interleukin 10 dependent. Journal of Experimental Medicine. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.A Phase I Study of Intravenous Recombinant Human IL-15 in Adults With Refractory Metastatic Malignant Melanoma and Metastatic Renal Cell Cancer”. ClinicalTrials.gov. ClinicalTrials.gov [Google Scholar]
- 64.Morris JC, Janik JE, White JD, et al. Preclinical and phase I clinical trial of blockade of IL-15 using Mikbeta1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proc Natl Acad Sci U S A. 2006;103:401–6. doi: 10.1073/pnas.0509575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu W, Jones M, Liu B, et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013;73:3075–86. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.