Abstract
Hormones with anabolic properties such as growth hormone (GH), insulin-like growth factor-1 (IGF-I), and insulin are commonly abused among professional and recreational athletes to enhance physical ability. Performance enhancing drugs (PEDs) such as these are also commonly used by recreational athletes to improve body aesthetics. The perception of increased muscle mass due to supraphysiologic hormone supplementation, or doping, is widespread among PED users despite a paucity of evidence-based data in humans. Even still, athletes will continue to abuse PEDs in hopes of replicating anecdotal results. It is important to educate the general public and potential treating physicians of the risks of PED use, including the dangers of polypharmacy and substance dependence. It will also be important for the research community to address the common challenges associated with studying PED use such as the ethical considerations of PED administration, the general reticence of the PED-using community to volunteer information, and the constant need to improve or create new detection methods as athletes continually attempt to circumvent current methods. This review highlights the anabolic mechanisms and suggestive data implicating GH, IGF-I, and insulin for use as PEDs, the specific detection methods with cutoff ranges that may be utilized to diagnose abuse of each substance, and their respective side effects.
Keywords: drug abuse, performance enhancing drugs, muscle, side effects, doping, sports
Introduction
Skeletal muscle is critical for execution of movement, thermogenesis, and nutrient metabolism. Proficiency of these processes is dependent on skeletal muscle mass which is largely regulated by exercise, nutrition, hormones, and to a lesser extent, genetics and ethnicity. As skeletal muscle is a plastic tissue, it responds to progressive overload, such as resistance training, or amino acid ingestion by altering protein synthesis and degradation in favor of tissue growth, or anabolism. Hormones with anabolic properties induce similar responses in skeletal muscle, increasing protein synthesis and/or decreasing protein degradation through a variety of downstream pathways after binding their respective receptors.
Reported enhancements of muscle mass and/or performance from supplementation with exogenous anabolic hormones have encouraged athletes to seek out performance enhancing drugs (PED) for a competitive edge. In an effort to protect elite and professional athletes from the unknown health consequences of PED abuse, the 2004 Anabolic Steroid Control Act expanded the list of controlled substances regulated by the federal government to include naturally occurring precursors of testosterone, growth hormone (GH), and insulin-like growth factor 1 (IGF-I). Although efforts have been made to protect athletes from doping, PED use has also spread to non-professional athletes and to the general population due to ease of access via the web and black market. These individuals have turned to PEDs primarily to improve body aesthetics (Pope, Khalsa and Bhasin, 2017) but also to improve energy levels, sex drive, and athletic performance (Creado and Reardon, 2016).
The use of anabolic-androgenic steroids (AAS) such as testosterone and its derivatives has been described extensively (Creado and Reardon, 2016, Pope, Wood, Rogol et al., 2014); however, less attention has been placed on the use of other common PEDs such as GH, IGF-I, and insulin. In 1992, 5% of male high school students admitted to taking GH at some point in their high school sport careers, and about one-third of the participants knew a classmate who had taken GH (Rickert, Pawlakmorello, Sheppard et al., 1992). Middle-aged and elderly people often seek GH with hopes of improving muscle mass and obtaining more youthful physical qualities. PED abuse has also grown in the weightlifting community with 27 of 231 (12%) weightlifters polled reporting past and/or present GH or IGF-I use with over 80% of those polled also exhibiting signs of past or present AAS dependency (Brennan, Kanayama, Hudson et al., 2011). Insulin is also a commonly used PED by body builders for its purported anabolic properties such as stimulation of glycogen formation, which is important for muscle recovery after exercise, and its accessibility from local pharmacies (Dawson and Harrison, 1997, Evans and Lynch, 2003).
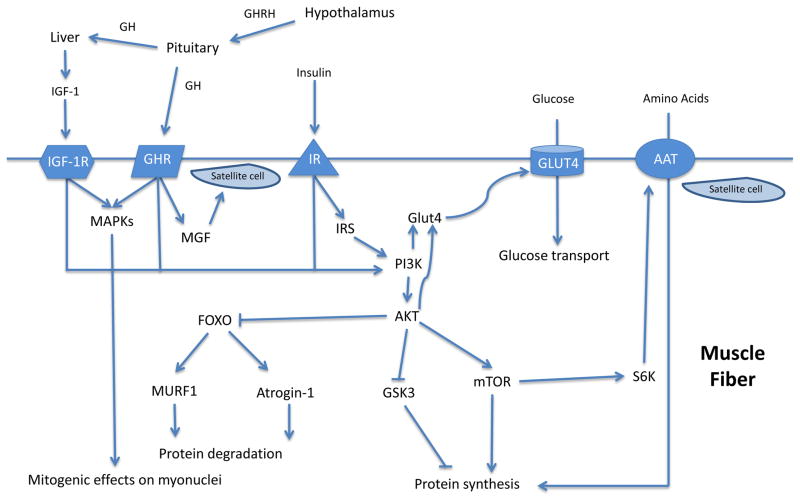
At the molecular level, the balance of anabolic and catabolic (protein degrading) processes is coordinated primarily by the phosphoinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR)/Forkhead boxO3a (FOXO3a) pathway among others (Glass, 2005). mTOR is a key mediator contributing to muscle protein synthesis and can be activated by PI3K/Akt (Gulati, Gaspers, Dann et al., 2008), which are, in turn, activated by growth factor signaling, such as insulin and IGF-I (Sandri, 2008). Akt phosphorylates, thereby inhibiting, FOXO3a which is a transcription factor that induces the proteosomal pathway by up-regulating the ubiquitin ligases atrogin-1 and muscle RING finger 1 (MuRF-1) (Salih and Brunet, 2008, Stitt, Drujan, Clarke et al., 2004). Akt also inhibits glycogen synthase kinase 3 (GSK3), removing the GSK3-induced inhibition of mRNA translation initiation (Leger, Cartoni, Praz et al., 2006). Importantly, when the size of a muscle fiber increases, activation of muscle precursor cells, or satellite cells, is required for provision of additional nuclear machinery to support the growing tissue (Hawke and Garry, 2001). Activation of Akt also affects glucose metabolism inducing an increase in glucose and amino acid uptake (Edinger and Thompson, 2002, Kohn, Summers, Birnbaum et al., 1996). Growth hormone, IGF-I, and insulin are known to influence these pathways, which have provided the basis for their use as PEDs (see figure 1).
Figure 1. Mechanisms of action of GH, IGF-1, and insulin in skeletal muscle.
AAT, amino acid transporter. Intramuscular anabolic mechanisms mediated by GH, IGF-1, and insulin providing the theoretical basis for use of these agents as PEDs.
This review will highlight the pharmacologic basis for and misuse, detection, and side effects of GH, IGF-I, and insulin as PED in sports and the larger recreational community. The impact of polypharmacy will also be addressed as this is an important factor in doping regimens.
Anabolic potential of GH, IGF-I, and Insulin use
Growth Hormone
The extent to which GH itself induces significant anabolism is still a matter of debate. Growth hormone’s anabolic effects in muscle are mediated in part via IGF-I (Kim, Barton, Muja et al., 2005), although IGF-I-independent pathways have also been proposed (Daughaday, 1989, Isaksson, Lindahl, Nilsson et al., 1988, Le Roith, Bondy, Yakar et al., 2001, Sotiropoulos, Ohanna, Kedzia et al., 2006). IGF-I is made in the liver but also in other tissues including skeletal muscle which express the GH receptor (Jorgensen, Jessen, Pedersen et al., 2006, Mathews, Norstedt and Palmiter, 1986). The interplay between circulating IGF-I and IGF-I locally produced in muscle is not fully understood but it is likely that both forms play a role in muscle growth. After administration of recombinant human (rh)GH in combination with resistance exercise, GH release after exercise was associated with increased expression of muscle-specific IGF-I (MGF) (Hameed, Lange, Andersen et al., 2004) which activates satellite cells for fusion with muscle fibers (Hill and Goldspink, 2003).
Adult GH deficiency (AGHD) is characterized by a decrease in muscle mass and strength along with increased adiposity (Jorgensen et al., 2006, Cuneo, Salomon, Wiles et al., 1990, Hoffman, Osullivan, Freund et al., 1995). Exercise capacity is also decreased and these patients often complain of fatigue being one of the main contributors to reduced quality of life. GH replacement using physiologic doses of 0.2–1.0 mg/day (estimated range for an 80 kg person based on various units used to report dosing regimens among studies) in this setting is associated with improvements in body composition (increases in lean mass and decreases in fat mass) in some studies (Bengtsson, Eden, Lonn et al., 1993, Salomon, Cuneo, Hesp et al., 1989, Snel, Brummer, Doerga et al., 1995, Lonn, Johansson, Sjostrom et al., 1996, Jorgensen, Thuesen, Muller et al., 1994, Woodhouse, Asa, Thomas et al., 1999, Blackman, Sorkin, Munzer et al., 2002), although other reports looking directly at muscle cross sectional area showed no changes over a 6-month period (Cuneo, Salomon, Mcgauley et al., 1992). GH [0.5–2.2 mg/day] has been shown to increase exercise capacity and improve fatigue scores and overall quality of life in this population (Gilchrist, Murray and Shalet, 2002, Widdowson and Gibney, 2008, Rubeck, Bertelsen, Vestergaard et al., 2009). Also, GH has been shown to increase muscle mass and strength after 3 years (Jorgensen et al., 1994) and after 10–15 years (Elbornsson, Gotherstrom, Bosaeus et al., 2013, Gotherstrom, Elbornsson, Stibrant-Sunnerhagen et al., 2009) although no effect was detected on fiber type with 6 months of treatment on a different report (Cuneo, Salomon, Wiles et al., 1992). In addition, two meta-analyses reported no effect on muscle strength after less than 12 months of treatment (Rubeck et al., 2009, Widdowson and Gibney, 2010). Regarding its effects at the molecular level, GH does not affect protein degradation directly but it appears to enhance protein synthesis in skeletal muscle via IGF-I-dependent mechanisms (Le Roith et al., 2001). Nevertheless, other studies have failed to see an effect of GH at the muscle level (Copeland and Nair, 1994, Yarasheski, Zachwieja, Angelopoulos et al., 1993). In summary, the extent to which GH can have an anabolic effect and the potential mechanisms mediating such effects at physiologic doses remain controversial.
Insulin-like growth factor-1
IGF-I receptor activation promotes muscle anabolism by activating PI3K/Akt (Velloso, 2008). In addition to these anabolic effects, IGF-I-induced activation of PI3K/Akt is also the reported mechanism by which IGF-I inhibits mitochondrial apoptosis (Pang, Zheng, Fan et al., 2007). Other effects on transcription have also been described via activation of mitogen activated protein kinases (MAPKs) (Leinninger, Backus, Sastry et al., 2005, Palacios, Sanchez-Franco, Fernandez et al., 2005, Song, Li, Du et al., 2005) which relay hypertrophic signals to myonuclei after contraction; however, explicit mechanisms are not well characterized (Martineau and Gardiner, 2001). IGF-I also promotes glycogen synthesis (Park, Kido and Accili, 1999), tendon collagen synthesis (Abrahamsson and Lohmander, 1996) and may indirectly promote GH-induced lipolysis (Vikman, Isgaard and Eden, 1991). Given the similarities between the IGF-I receptor and the insulin receptor, there is some cross-reactivity that accounts for similar side effects such as hypoglycemia (Ullrich, Gray, Tam et al., 1986). IGF-I has also been shown to induce proliferation and differentiation of satellite cells into myocytes (Musaro, McCullagh, Naya et al., 1999). Administration of IGF-I down-regulates proteolysis but does not appear to alter protein synthesis (Hussain, Schmitz, Mengel et al., 1994). This data is in contrast with the effects of GH on protein metabolism described above and suggest that these actions of GH are IGF-I independent and complimentary.
Insulin
The anabolic potential of insulin has been recognized since it was first used for the treatment of diabetes (Cefalu, 2004, Peterson, 1982). Insulin exerts these anabolic effects by increasing the transport of glucose and amino acids into skeletal muscle fibers, thereby increasing protein synthesis and decreasing protein degradation (Biolo, Fleming and Wolfe, 1995). Insulin binds to its receptor, causing phosphorylation of insulin receptor substrate (IRS) proteins, which in turn, activates the PI3K/Akt pathway. This process may be enhanced by administering insulin after a bout of exercise since this is known to increase insulin sensitivity in muscle for up to 24–48 h (Dawson and Harrison, 1997). Insulin may also promote muscle anabolism indirectly by increasing appetite via its hypoglycemic effects (Sprague, 2011), and by inhibiting fatty acid oxidation in muscle (Sidossis, Stuart, Shulman et al., 1996). In contrast, insulin may act centrally to reduce appetite and increase energy expenditure long term (Dallman, Akana, Strack et al., 1995, Ikeda, West, Pustek et al., 1986). As most reports of insulin-induced anabolism are anecdotal, there are no in-vivo reports in humans studying its anabolic potential. Insulin also induces lipogenesis leading to an increase in fat mass and body weight. This may be undesirable for certain activities where leanness is sought.
GH, IGF-I and insulin used as PED
Growth Hormone
The emergence of GH as a PED began sometime before 1985 when the first description of its purported effects and recommendations for performance enhancement was published (Duchaine, 1982). Shortly thereafter, Ben Johnson, who won the 100m gold medal in the Seoul Olympics in 1988, was stripped of his medal after admitting under oath to using GH and anabolic steroids. GH was banned by the International Olympic Committee in 1989 and “off-label” distribution/prescription was declared felonious by Congress with the Crime Control Act of 1990. Various scandals and reports have surfaced over the ensuing years which continue to highlight the prevalence of GH usage by professional athletes (Holt and Sonksen, 2008). Despite being one of the most widely abused agents both professionally and recreationally (Holt and Sonksen, 2008, Barroso, Mazzoni and Rabin, 2008, Chikani and Ho, 2014), there is little clinical evidence that GH in isolation has any significant effect on performance enhancement.
Aerobic exercise capacity is not affected by GH at physiologic doses in healthy individuals (reviewed here (Chikani and Ho, 2014)) or in AGHD patients (Chikani, Cuneo, Hickman et al., 2016). However, physiologic doses may improve anaerobic capacity in AGHD patients (Chikani and Ho, 2014, Chikani et al., 2016). A study using supraphysiologic doses of GH [2mg/day] in recreational athletes showed no effect on muscle strength or VO2 max although it did improve anaerobic capacity (Meinhardt, Nelson, Hansen et al., 2010). A different study using the same dose showed increases in lipid oxidation and lipolysis which contribute to a decrease in adiposity and may make more nutrients available to muscle (Krag, Gormsen, Guo et al., 2007). It is also important to note that states of GH-excess, such as acromegaly, are associated with muscle weakness and fatigue (Flitsch, Spitzner and Ludecke, 2000), suggesting that chronically elevated levels of GH or IGF-I have a deleterious effect on muscle. GH [0.2–2 mg/day] has also been tested in conjunction with exercise for up to 6 months and was found to be similar to exercise alone (Hennessey, Chromiak, DellaVentura et al., 2001, Lange, Andersen, Beyer et al., 2002, Yarasheski, Campbell, Smith et al., 1992, Yarasheski, Zachwieja, Campbell et al., 1995). Despite these data, it should be noted that hypopituitarism remains the only indication for GH administration in adults (Clemmons, Molitch, Hoffman et al., 2014).
GH’s popularity remains evident through its increased affordability and online availability to consumers (Brennan et al., 2011). From a poll of 231 male weightlifters from the United States, 100 admitted past and/or present PED use of any kind, and 26 admitted past and/or present GH use (Brennan et al., 2011). Its prevalence was reported to be just below 10% among recreational and professional bodybuilders in Iran and was 34% (GH or GH-releasing peptide) among recreational weightlifters in the UK (Haerinejad, 2016, Chandler, 2014). GH is administered subcutaneously at doses of 0.2–1.0 mg/day for AGHD based on an 80 kg person (Carroll, Christ and Comm, 1998). The dosage for improving muscle mass, typically for bodybuilders, is reported in the range of 3–8 mg/day, three to four times a week in cycles of four to six weeks (Saugy, Robinson, Saudan et al., 2006). Relatively little is known about the typical GH regimen used by endurance athletes.
IGF-I
IGF-I is typically used to enhance the anabolic effects of concurrent GH and/or anabolic steroid use. It has been on the World Anti-Doping Association’s prohibited list since the World Anti-Doping Code was established in 2003. Prevalence of its use as a PED was reported to be 16% of patients admitted to a Swedish addiction clinic, over 6% (including IGF-I and MGF) in a UK survey disseminated to online weightlifting forums to participants of needle exchange programs, and 7% in a poll of weightlifters from the United States (Brennan et al., 2011, Chandler, 2014, Skarberg, Nyberg and Engstrom, 2009). The lower incidence of IGF-I use, in comparison to GH use, is partly attributed to the difficulty in drug preparation and accessibility. However, there have been many accounts of its availability on the black market (Holt and Sonksen, 2008, Baumann, 2012, Guha, Cowan, Sonksen et al., 2013). IGF-I is only approved for treatment of patients with primary severe IGF-I deficiency or with GH gene deletion that have developed neutralizing antibodies to GH at a dose of 40–120 μg/kg twice daily subcutaneously. When administered to patients with Type 2 Diabetes at doses of 200–240 μg/kg/day, it significantly reduced blood glucose levels (Moses, Young, Morrow et al., 1996, Zenobi, Jaeggigroisman, Riesen et al., 1992). Consequently, IGF-I is administered with a meal to avoid hypoglycemia.
The typical regimen of IGF-I use among the American weightlifters was 50–75 μg/day with median lifetime duration of 9 weeks (Brennan et al., 2011). This dose appears particularly low in comparison to those used in Type 2 Diabetes; however, the authors were fairly skeptical of the accuracy of these doses reported. Marked anabolism occurred in mice over-expressing IGF-I (Coleman, Demayo, Yin et al., 1995) and IGF-I administration (30–60 μg/kg twice a day, ~3600–8600 μg/day based on reported mean body weight) improved whole body and muscle protein synthesis in elderly women (Butterfield, Thompson, Rennie et al., 1997). However, there is currently no direct in vivo human evidence to suggest IGF-I significantly increases muscle mass. One year of IGF-I treatment (15 μg/kg bid, ~2200 μg/day based on reported mean body weight) failed to improve muscle mass in postmenopausal women (Friedlander, Butterfield, Moynihan et al., 2001).
Insulin
Although there is a paucity of data on the use of insulin as a PED, it appears to be commonly abused because it is inexpensive and readily available in most settings. Its prevalence was reported to be just below 10% among recreational and professional bodybuilders in Iran (Haerinejad, 2016) and among recreational weightlifters in the UK (Chandler, 2014) and 25% among a small group of recreational bodybuilders in the US (Rich, 1998). The International Olympic Committee banned its use in 1998 for those without diabetes (“International Olympic Committee and Medical Commission,”) but its use also appears to be increasing among recreational weight lifters. Insulin is usually obtained from local sources (e.g., friends, training partners, gym member/dealer) or from community pharmacies where it is available without a prescription (Evans and Lynch, 2003, Elkin, Brady and Williams, 1997). It is usually administered right before a post-workout meal or along with glucose or with amino acids with the purpose of preventing hypoglycemia while shutting off proteolysis and increasing protein synthesis (Evans and Lynch, 2003). Short acting insulin or insulin analogs (regular, lispro, aspart, etc.) appear to be the most common forms of insulin used once a day subcutaneously or intramuscularly in the range of 2–15 IU/dose (Dawson and Harrison, 1997). Among the group of weightlifters polled in the US, a mean of 10 IU per injection was reported with users obtaining dosing information by word of mouth (Rich, 1998). Insulin administration (1.5 μU/kg/min, mean 45 days) after severe burn significantly increased total, trunk, and peripheral lean mass (Thomas, Morimoto, Herndon et al., 2002); however, there is no evidence of improved muscle mass after insulin administration in healthy adults at this time.
Methods of Detection
Growth Hormone
Unlike anabolic steroids which can be detected by mass spectroscopy from urine samples, GH is measured by immunoassay from blood samples because of low and variable GH excretion levels. However, due to the pulsatile release of GH, detection of elevated GH levels may simply reflect peak circulating levels and not necessarily indicate exogenous GH use. In addition, recombinant human GH is virtually indistinguishable from the endogenous 22-KDa form, including having a very short half-life, with levels returning to normal 8–20 hours after administration (Barroso et al., 2008). Consequently, an isoform differential immunoassay method, also considered the “direct” method, was derived to detect exogenous GH use (Bidlingmaier, Wu and Strasburger, 2000, Wu, Bidlingmaier, Dall et al., 1999). Under normal conditions, circulating GH levels occur as a mixture of different isoforms in specific proportions. Between 75–80% of circulating GH is the 22-KDa isoform, 5–10% is the 20-KDa isoform, and the remaining levels occur as dimers, oligomers, and various other isoforms (Baumann, 2012). In contrast, rhGH levels only occur in the 22-KDa form. Endogenous GH is suppressed after administration of rhGH, thereby increasing total 22-KDa levels and levels relative to the other isoforms for up to 4 days (Wallace, Cuneo, Bidlingmaier et al., 2001). One differential immunoassay recognizes only the 22-KDa isoform while the other recognizes a combination of isoforms. As a result, the ratio of the two detection levels can be used to determine if rhGH was administered within 36 hours but it is recommended that sampling time should be less than 24 hours for increased accuracy (Pope et al., 2014, Baumann, 2012). The ratio of 22-KDa-only levels to level of multiple isoforms has a median value of 0.8. The ratio limits for determination of rhGH abuse by the World Anti-Doping Agency range from 1.68–1.81 for men and 1.46–1.55 for women (“2010 World Anti-Doping Agency Guidelines: hGH isoform differential immunoassays for anti-doping analyses, version 1.0, June 2010.,”).
Alternatively, there is an “indirect” or “biomarker” method of detecting rhGH abuse which examines the downstream biomarkers of GH activity. The predominantly tested biomarkers are IGF-I and procollagen type III amino-terminal propeptide (P-III-NP) due to their particular responsiveness to GH. Circulating IGF-I, measured my immunoassay or liquid mass spectroscopy, rises rapidly within 2 weeks after rhGH use and then falls to baseline levels within 1 week after rhGH cessation. Levels of P-III-P, measured by immunoassay, increase gradually within 4–6 weeks of rhGH initiation then return to normal after 2–8 weeks (Pope et al., 2014, Baumann, 2012, Dall, Longobardi, Ehrnborg et al., 2000). The results of these two assays are incorporated into a formula taking into account age and gender (Powrie, Bassett, Rosen et al., 2007) to differentiate between those using or not using rhGH when carried out within 7 days of rhGH administration, which is a much longer window of opportunity than the direct method (Pope et al., 2014). Other biomarkers explored, but ultimately not determined suitable, for this method were the liver factors IGF binding protein (IGFBP)-2, IGFBP-3, and acid-labile subunit (ALS), and bone turnover markers procollagen type I carboxyl-terminal propeptide and type 1 collagen cross-linked carboxy-terminal telopeptide (Barroso et al., 2008). Detection by using the biomarker method may highlight disruption of the GH/IGF-I axis for various exogenous GH-related agents (i.e. rhGH, GH-releasing hormone analogs, GH secretagogues, etc.).
Insulin-like growth factor-1
While there is no current standard method for determination of IGF-I abuse, detection of IGF-I is typically accomplished by immunometric, noncompetitive assays because of increased specificity and speed of completion. However, these assays do require acid-ethanol precipitation or addition of excess IGF-2 to minimize binding protein interference (Guha et al., 2013). Current investigations of detection methods for IGF-I abuse in athletes are formulated to also detect IGFBP-3 abuse and are based on the same principle as the biomarker method for determination of GH abuse. Markers of IGF-I/IGFBP-3 administration include increased IGF-I and IGFBP-2, decreased P-III-NP and IGF-2, and decreased ALS in women (Guha et al., 2013, Guha, Erotokritou-Mulligan, Bartlett et al., 2014, Holt, 2017).
Insulin
Assays for measurements of insulin are readily available given their use in the evaluation of hypoglycemia. When insulin abuse is suspected, evaluation should be performed when the patient is hypoglycemic, which may be challenging in this population unless they seek medical care during such an episode. A plasma insulin concentration of 3 IU/mL (20.8 pmol/L) or more by immunochemiluminometric assay (ICMA) when the plasma glucose concentration is below 55 mg/dL (3.0 mmol/L) suggests inappropriate hyperinsulinemia. Measurements of plasma C-peptide and proinsulin levels will help distinguish endogenous from exogenous hyperinsulinemia. C-peptide <200 pmol/L (0.6 ng/mL) and proinsulin <5pmol/L when glucose is <45 mg/dL and elevated insulin levels suggest exogenous insulin use. With the development of insulin analogs, one important caveat is that insulin concentrations may be falsely low depending on the cross-reactivity with the particular assay used. Specific assays now detect a variety of insulins including human insulin, animal insulin, and insulin analogs (Andersen, Jorgensen, Jensen et al., 2000, Bowsher, Lynch, Brown-Augsburger et al., 1999, Moriyama, Hayashi, Ohyabu et al., 2006, Neal, 2008, Walfish, Feig and Bauman, 1987). Urinary liquid chromatography/tandem mass spectroscopy has also been proposed to detect insulin analogs (Holt and Sonksen, 2008).
Adverse Effects
Growth Hormone
A recent workshop comprised of the European Society of Paediatric Endocrinology, Growth Hormone Research Society, and Pediatric Endocrine Society determined that the safety profile of GH administration for indicated purposes (i.e. GH deficiency) was satisfactory (Allen, Backeljauw, Bidlingmaier et al., 2016). In contrast, GH administration is not advised for anti-aging in healthy elderly due to increased adverse events (Liu, Bravata, Olkin et al., 2007). However, there is little systemic evidence of adverse effects related to GH abuse in humans. Most accounts of side effects are anecdotal and/or are related to abuse of multiple substances. These effects are believed to be similar to those observed in acromegaly which may result in hypertension, carpal tunnel syndrome, diabetes, and neuropathy among many others (Table 1) (Pope et al., 2014, Ezzat, Forster, Berchtold et al., 1994, Wass JAH, 2002, Bengtsson, Eden, Ernest et al., 1988, Colao, Marzullo, Di Somma et al., 2001, Colao, Pivonello, Di Somma et al., 2007, Jenkins, Mukherjee and Shalet, 2006, Kreze, Kreze-Spirova and Mikulecky, 2001). Nevertheless, overuse/abuse may be associated with unknown side effects given that it is often used in combination with other agents and at higher doses. In animals, administration of GH in supraphysiologic doses leads to an increase in many organs, most noticeably, cardiomegaly, which also mimics that seen in acromegaly (Kopchick, Bellush and Coschigano, 1999, Penney, Dunbar and Baylerian, 1985). Edema, orthostatic hypotension, myositis, carpal tunnel, and gynecomastia have also been reported during GH administration to frail elderly (Cohn, Feller, Draper et al., 1993, Sullivan, Carter, Warr et al., 1998) while carpal tunnel and hyperglycemia were reported during GH administration to healthy adults (Blackman, 2004). Edema, decreased glucose tolerance, paresthesias, and in rare cases, macular degeneration were reported after GH administration during AGHD (Reed, 2013). The first documented report of rhGH abuse-induced diabetes was reported in 2007 when a 36-year old man with a presented to the emergency department with acute renal failure (Young and Anwar, 2007). The man admitted to a 15 year history of steroid abuse and 3 years of rhGH use. In addition, GH was originally extracted from the pituitary glands of human cadavers for treatment of short stature until its association with Creutzfeldt–Jakob Disease was discovered in 1985. Cadaveric GH is still available overseas and its use can potentially expose individuals to this deadly disease (Brown, Gajdusek, Gibbs et al., 1985).
Table 1.
Side effects of excess GH due to disease, supplement, or use for anabolic purposes.
| Organ System/Effect | Excess due to acromegaly | Physiologic Supplementation | Overuse/abuse |
|---|---|---|---|
| Cardiovascular/Lymphatic | Cardiomyopathy Hypertension |
Excessive sweating Edema |
Congestive heart failure Hypertension |
| Metabolic | Diabetes Insulin resistance |
Decreased glucose tolerance | Diabetes |
| Neurologic | Peripheral neuropathy Sleep apnea |
Paresthesia | |
| Musculoskeletal | Osteoarthritis Acral enlargement |
Arthropathy Carpal tunnel Myositis |
|
| Reproductive | Menstrual disturbance Erectile dysfunction |
Gynecomastia | |
| Dermatologic | Coarsening of skin | ||
| Increased Cancer Risk | Thyroid Colorectal |
Insulin-like growth factor-1
Most features of IGF-I misuse will not be distinguishable from those that develop from GH abuse, since IGF-I production is also promoted by increased GH levels. However, hypoglycemia, seizures, jaw pain, myalgia, edema, headaches, increased liver and kidney mass, and altered liver function among others (Table 2) have been reported after rhIGF-I administration (Sullivan et al., 1998, Williams, McDonald, O’Savage et al., 2008, Laron, 1999, Major, Laughlin, Kritz-Silverstein et al., 2010). The most common adverse side effects are erythema and lipohypertrophy at the injection-site (Williams et al., 2008).
Table 2.
Side effects of excess IGF-I and insulin due to supplement or use for anabolic purposes.
| Organ System/Effect | Physiologic Supplementation | Overuse/abuse |
|---|---|---|
| IGF-I | ||
| Cardiovascular/Lymphatic | Edema | Edema |
| Metabolic | Insulin resistance Orthostatic hypotension |
Hyperandrogenism Hypoglycemia |
| Musculoskeletal | Myositis Arthralgia Jaw pain |
|
| Insulin | ||
| Metabolic | Hypoglycemia | |
| Neurologic | Loss of consciousness Coma Seizures Potentially death |
|
Insulin
Hypoglycemia is the most common complication of insulin use. It is a dose-dependent effect of insulin and it occurs commonly in PED users since they tend to be individuals without diabetes or insulin resistance and do not use glucometers for capillary glucose measurements. It is also more likely to happen with the use of short-acting insulin or insulin analogs (i.e. lispro, aspart, regular, etc). Hypoglycemia can lead to loss of consciousness, coma, seizures, and potentially death. Insulin-induced hypoglycemia, seizures and severe chronic brain damage have been reported after prolonged neuroglycopenia in two cases after chronic use of insulin for doping (Elkin et al., 1997). In a different report of 41 insulin users, hypoglycemia was reported by most of the subjects (56.8%), and one individual reported unconsciousness (Ip, Barnett, Tenerowicz et al., 2012). Acutely, signs and symptoms of hypoglycemia are due to increased adrenergic tone (tachycardia, palpitations, anxiety, sweatiness, tremors) and neuroglycopenia (confusion, sleepiness, hunger, coma, seizures). Chronic hypoglycemia has been associated with hypoglycemia unawareness due to a decrease in adrenergic response and cognitive problems.
As with other injectable agents, improper aseptic techniques and unsafe use of needles can lead to skin infections, abscesses, and transmission of serious infections including hepatitis B and C and HIV (Larance, Degenhardt, Copeland et al., 2008). Insulin can also induce hypokalemia as it induces a shift of potassium into the cells leading to muscle cramping, respiratory paralysis, ventricular arrhythmias, and death. This may be particularly troublesome for individuals performing aerobic exercise which may have hypokalemia due to dehydration. As insulin also increases lipogenesis, it can lead to an increase in adiposity and overall weight gain. Other minor side effects include peripheral edema and the potential for bruising or localized lipodystrophy at the site of injection.
The impact of polypharmacy
The vast majority of individuals using anabolic agents for recreational purposes use more than one substance. Substances frequently used as supplements to AAS include alcohol, amphetamine, caffeine, cannabinoids, clenbuterol, cocaine, codeine, creatine, ephedrine, erythropoietin, gamma hydroxybutyrate, GH, heroin, human chorionic gonadotropin, insulin, IGF-I, tamoxifen, tobacco, and many others (Ip et al., 2012, Sagoe, McVeigh, Bjornebekk et al., 2015, Borjesson, Garevik, Dahl et al., 2016). In one US report of 41 insulin users, 95% also used AAS concomitantly and practiced polypharmacy by incorporating 16.2 ± 5.6 PEDs in their yearly routine (Ip et al., 2012). In the UK, polypharmacy of GH and AAS is increasing as production costs decrease with one third of AAS users reportedly also using GH (Hope, McVeigh, Marongiu et al., 2013, McVeigh and Begley, 2017). In addition, 1 in 8 female AAS users in Sweden, also reported GH use (Borjesson et al., 2016).
Unfortunately, AAS use can lead to dependence and usage of opioids and other illicit drugs unrelated to physical performance or fitness (Skarberg et al., 2009). In addition, use of multiple off-label or black market substances can increase unrelated health risks. For example, a young multi-PED user recently died from severe arsenic poisoning from a contaminated pill bottle (Perera, Steinbeck and Shackel, 2013). While the arsenic exposure was the root cause of his symptoms, it is unclear how his multiple PED use exacerbated or hastened his ultimately fatal condition. Liver disease was reported in two other young multi-PED users; both regimens included GH and one also included insulin (Solimini, Rotolo, Mastrobattista et al., 2017). Polypharmacy is also reported in individuals who cycle (dose-stop-dose intervals) or pyramid (intervals of slow dosing and tapering) PED use as non-performance related substances such as are often required for transitioning off of PEDs (i.e. aromatase inhibitors) or to combat side effects of PEDs (i.e. medication to treat hair loss), namely from AAS use.
Conclusions
Although there is plenty of data on the anabolic potential of GH, IGF-I, and insulin and on their safety and efficacy at physiologic doses, most of this information comes from their therapeutic use in other settings (AGHD, diabetes), or from pathological disorders characterized by hormone hypersecretion (acromegaly, insulinoma). The majority of the data about the abuse of these and other PEDs comes from case reports or uncontrolled studies, underscoring the need for more research in this area. This would be challenging as randomized controlled studies would be unethical and certain subpopulations such as professional athletes may not be willing to volunteer information about PED use. Establishing a long-term prospective registry study has been suggested in a recent Endocrine Society Scientific Statement on this topic (Pope et al., 2014) and this could prove very valuable in determining the long-term safety of PEDs. Other challenges in interpreting the data available and designing future studies include the extremely high rate of polypharmacy and substance abuse linked to PED use.
In recent years, new formulations of GH (i.e. clinicaltrials.gov identifiers NCT01909479, NCT02693522, NCT01909479, NCT02229851, NCT02410356, etc.) and insulin (insulin degludec and insulin degludec/aspart) have completed phase III clinical trials or have been approved by the FDA. In addition, new compounds known to stimulate the release of GH, known as GH secretagogues or ghrelin mimetics, are also in clinical development (NCT02558829). As these drugs become commercially available more resources will be needed to develop commercially available tests to detect them. In addition, efforts to educate the medical community and the general public to recognize the deleterious effects of PEDs and to develop strategies to help individuals currently using PEDs are also desperately needed.
Highlights.
PEDs are used professionally & recreationally but can be appropriately detected
Evidence does not suggest that GH, IGF-1, or insulin doping enhances performance
Community education is needed regarding negative side effects of recreational doping
Many PED users engage in polypharmacy, increasing the risk of adverse health events
Acknowledgments
We thank the University of Washington DERC (P30 DK017047) and NORC (P30 DK035816) for their help. We also thank Dorota Migula for her help in generating the figure.
Funding: This work was funded by the U.S. Dept of Veterans Affairs (MERIT grants BX002807 and CX000174) and NIH Grant AG040583 to JMG. Dr Anderson is supported by a training grant from the Puget Sound VA RnD office.
Abbreviations
- PED
performance enhancing drug
- AAS
anabolic-androgenic steroids
- PI3K
phosphoinositol 3-kinase
- mTOR
mammalian target of rapamycin
- FOXO3a
Forkhead boxO3a
- MuRF-1
muscle RING finger 1
- GSK3
glycogen synthase kinase 3
- MGF
muscle-specific IGF-I
- MAPK
mitogen activated protein kinase
- IRS
insulin receptor substrate
- P-III-NP
procollagen type III amino-terminal propeptide
- ALS
acid-labile subunit
Footnotes
Conflicts of Interests: JMG receives research support from Pfizer, Inc. LJA receives research support from the Endocrine Society; Endocrine Scholars Award in Growth Hormone Research supported by Genentec.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pope HG, Khalsa JH, Bhasin S. Body Image Disorders and Abuse of Anabolic-Androgenic Steroids Among Men. Jama-Journal of the American Medical Association. 2017;317:23–24. doi: 10.1001/jama.2016.17441. [DOI] [PubMed] [Google Scholar]
- 2.Creado S, Reardon C. The sports psychiatrist and performance-enhancing drugs. International Review of Psychiatry. 2016;28:564–571. doi: 10.1080/09540261.2016.1190690. [DOI] [PubMed] [Google Scholar]
- 3.Pope HG, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse Health Consequences of Performance-Enhancing Drugs: An Endocrine Society Scientific Statement. Endocrine Reviews. 2014;35:341–375. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rickert VI, Pawlakmorello C, Sheppard V, Jay MS. Human Growth-Hormone - a New Substance of Abuse among Adolescents. Clinical Pediatrics. 1992;31:723–726. doi: 10.1177/000992289203101206. [DOI] [PubMed] [Google Scholar]
- 5.Brennan BP, Kanayama G, Hudson JI, Pope HG. Human Growth Hormone Abuse in Male Weightlifters. American Journal on Addictions. 2011;20:9–13. doi: 10.1111/j.1521-0391.2010.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson RT, Harrison MW. Use of insulin as an anabolic agent. British Journal of Sports Medicine. 1997;31:259–259. doi: 10.1136/bjsm.31.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans PJ, Lynch RM. Insulin as a drug of abuse in body building. British Journal of Sports Medicine. 2003;37:356–357. doi: 10.1136/bjsm.37.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. International Journal of Biochemistry & Cell Biology. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR Complex 1 via Ca2+/CaM signaling to hVps34. Cell Metabolism. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology. 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 11.Salih DAM, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Current Opinion in Cell Biology. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents short article expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Molecular Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 13.Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Akt signalling through GSK-3 beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. Journal of Physiology-London. 2006;576:923–933. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. Journal of Applied Physiology. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 15.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Molecular Biology of the Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. Journal of Biological Chemistry. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Barton E, Muja N, Yakar S, Pennisi P, LeRoith D. Intact insulin and insulin-like growth factor-I receptor signaling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology. 2005;146:1772–1779. doi: 10.1210/en.2004-0906. [DOI] [PubMed] [Google Scholar]
- 18.Daughaday WH. A Personal History of the Origin of the Somatomedin Hypothesis and Recent Challenges to Its Validity. Perspectives in Biology and Medicine. 1989;32:194–211. doi: 10.1353/pbm.1989.0006. [DOI] [PubMed] [Google Scholar]
- 19.Isaksson OGP, Lindahl A, Nilsson A, Isgaard J. Action of Growth-Hormone - Current Views. Acta Paediatrica Scandinavica. 1988:12–18. doi: 10.1111/j.1651-2227.1988.tb10794.x. [DOI] [PubMed] [Google Scholar]
- 20.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocrine Reviews. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 21.Sotiropoulos A, Ohanna M, Kedzia C, Menon RK, Kopchick JJ, Kelly PA, Pende M. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7315–7320. doi: 10.1073/pnas.0510033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen JOL, Jessen N, Pedersen SB, Vestergaard E, Gormsen L, Lund SA, Billestrup N. GH receptor signaling in skeletal muscle and adipose tissue in human subjects following exposure to an intravenous GH bolus. American Journal of Physiology-Endocrinology and Metabolism. 2006;291:E899–E905. doi: 10.1152/ajpendo.00024.2006. [DOI] [PubMed] [Google Scholar]
- 23.Mathews LS, Norstedt G, Palmiter RD. Regulation of Insulin-Like Growth Factor-I Gene-Expression by Growth-Hormone. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:9343–9347. doi: 10.1073/pnas.83.24.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hameed M, Lange KHW, Andersen JL, Schjerling P, Kjaer M, Harridge SDR, Goldspink G. The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. Journal of Physiology-London. 2004;555:231–240. doi: 10.1113/jphysiol.2003.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. Journal of Physiology-London. 2003;549:409–418. doi: 10.1113/jphysiol.2002.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuneo RC, Salomon F, Wiles CM, Sonksen PH. Skeletal-Muscle Performance in Adults with Growth-Hormone Deficiency. Hormone Research. 1990;33:55–60. doi: 10.1159/000181585. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman DM, Osullivan AJ, Freund J, Ho KKY. Adults with Growth-Hormone Deficiency Have Abnormal Body-Composition but Normal Energy-Metabolism. Journal of Clinical Endocrinology & Metabolism. 1995;80:72–77. doi: 10.1210/jcem.80.1.7829643. [DOI] [PubMed] [Google Scholar]
- 28.Bengtsson BA, Eden S, Lonn L, Kvist H, Stokland A, Lindstedt G, Bosaeus I, Tolli J, Sjostrom L, Isaksson OGP. Treatment of Adults with Growth-Hormone (Gh) Deficiency with Recombinant Human Gh. Journal of Clinical Endocrinology & Metabolism. 1993;76:309–317. doi: 10.1210/jcem.76.2.8432773. [DOI] [PubMed] [Google Scholar]
- 29.Salomon F, Cuneo RC, Hesp R, Sonksen PH. The Effects of Treatment with Recombinant Human Growth-Hormone on Body-Composition and Metabolism in Adults with Growth-Hormone Deficiency. New England Journal of Medicine. 1989;321:1797–1803. doi: 10.1056/NEJM198912283212605. [DOI] [PubMed] [Google Scholar]
- 30.Snel YEM, Brummer RJM, Doerga ME, Zelissen PMJ, Bakker CJG, Hendriks MJ, Koppeschaar HPF. Adipose-Tissue Assessed by Magnetic-Resonance-Imaging in Growth Hormone-Deficient Adults - the Effect of Growth-Hormone Replacement and a Comparison with Control Subjects. American Journal of Clinical Nutrition. 1995;61:1290–1294. doi: 10.1093/ajcn/61.6.1290. [DOI] [PubMed] [Google Scholar]
- 31.Lonn L, Johansson G, Sjostrom L, Kvist H, Oden A, Bengtsson BA. Body composition and tissue distributions in growth hormone deficient adults before and after growth hormone treatment. Obesity Research. 1996;4:45–54. doi: 10.1002/j.1550-8528.1996.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 32.Jorgensen JOL, Thuesen L, Muller J, Ovesen P, Skakkebaek NE, Christiansen JS. 3 Years of Growth-Hormone Treatment in Growth Hormone-Deficient Adults - near Normalization of Body-Composition and Physical Performance. European Journal of Endocrinology. 1994;130:224–228. doi: 10.1530/eje.0.1300224. [DOI] [PubMed] [Google Scholar]
- 33.Woodhouse LJ, Asa SL, Thomas SG, Ezzat S. Measures of submaximal aerobic performance evaluate and predict functional response to growth hormone (GH) treatment in GH-deficient adults. Journal of Clinical Endocrinology & Metabolism. 1999;84:4570–4577. doi: 10.1210/jcem.84.12.6196. [DOI] [PubMed] [Google Scholar]
- 34.Blackman MB, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men - A randomized controlled trial. Jama-Journal of the American Medical Association. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 35.Cuneo RC, Salomon F, Mcgauley GA, Sonksen PH. The Growth-Hormone Deficiency Syndrome in Adults. Clinical Endocrinology. 1992;37:387–397. doi: 10.1111/j.1365-2265.1992.tb02347.x. [DOI] [PubMed] [Google Scholar]
- 36.Gilchrist FJ, Murray RD, Shalet SM. The effect of long-term untreated growth hormone deficiency (GHD) and 9 years of GH replacement on the quality of life (QoL) of GH-deficient adults. Clinical Endocrinology. 2002;57:363–370. doi: 10.1046/j.1365-2265.2002.01608.x. [DOI] [PubMed] [Google Scholar]
- 37.Widdowson WM, Gibney J. The Effect of Growth Hormone Replacement on Exercise Capacity in Patients with GH Deficiency: A Metaanalysis. Journal of Clinical Endocrinology & Metabolism. 2008;93:4413–4417. doi: 10.1210/jc.2008-1239. [DOI] [PubMed] [Google Scholar]
- 38.Rubeck KZ, Bertelsen S, Vestergaard P, Jorgensen JOL. Impact of GH substitution on exercise capacity and muscle strength in GH-deficient adults: a meta-analysis of blinded, placebo-controlled trials. Clinical Endocrinology. 2009;71:860–866. doi: 10.1111/j.1365-2265.2009.03592.x. [DOI] [PubMed] [Google Scholar]
- 39.Elbornsson M, Gotherstrom G, Bosaeus I, Bengtsson BA, Johannsson G, Svensson J. Fifteen years of GH replacement improves body composition and cardiovascular risk factors. European Journal of Endocrinology. 2013;168:745–753. doi: 10.1530/EJE-12-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gotherstrom G, Elbornsson M, Stibrant-Sunnerhagen K, Bengtsson BA, Johannsson G, Svensson J. Ten Years of Growth Hormone (GH) Replacement Normalizes Muscle Strength in GH-Deficient Adults. Journal of Clinical Endocrinology & Metabolism. 2009;94:809–816. doi: 10.1210/jc.2008-1538. [DOI] [PubMed] [Google Scholar]
- 41.Cuneo RC, Salomon F, Wiles CM, Round JM, Jones D, Hesp R, Sonksen PH. Histology of Skeletal-Muscle in Adults with Gh Deficiency - Comparison with Normal Muscle and Response to Gh Treatment. Hormone Research. 1992;37:23–28. doi: 10.1159/000182276. [DOI] [PubMed] [Google Scholar]
- 42.Widdowson WM, Gibney J. The effect of growth hormone (GH) replacement on muscle strength in patients with GH-deficiency: a meta-analysis. Clinical Endocrinology. 2010;72:787–792. doi: 10.1111/j.1365-2265.2009.03716.x. [DOI] [PubMed] [Google Scholar]
- 43.Copeland KC, Nair KS. Acute Growth-Hormone Effects on Amino-Acid and Lipid-Metabolism. Journal of Clinical Endocrinology & Metabolism. 1994;78:1040–1047. doi: 10.1210/jcem.78.5.8175957. [DOI] [PubMed] [Google Scholar]
- 44.Yarasheski KE, Zachwieja JJ, Angelopoulos TJ, Bier DM. Short-Term Growth-Hormone Treatment Does Not Increase Muscle Protein-Synthesis in Experienced Weight Lifters. Journal of Applied Physiology. 1993;74:3073–3076. doi: 10.1152/jappl.1993.74.6.3073. [DOI] [PubMed] [Google Scholar]
- 45.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. British Journal of Pharmacology. 2008;154:557–568. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang Y, Zheng BY, Fan LW, Rhodes PG, Cai Z. IGF-1 protects oligodendrocyte progenitors against TNF alpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55:1099–1107. doi: 10.1002/glia.20530. [DOI] [PubMed] [Google Scholar]
- 47.Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, Feldman EL. IGF-I inhibits high glucose-induced mitochondrial dysfunction in DRG neurons via Bim, Bax and the fission protein DRP1. Journal of the Peripheral Nervous System. 2005;10:52–52. [Google Scholar]
- 48.Palacios N, Sanchez-Franco F, Fernandez M, Sanchez I, Cacicedo L. Intracellular events mediating insulin-like growth factor I-induced oligodendrocyte development: modulation by cyclic AMP. Journal of Neurochemistry. 2005;95:1091–1107. doi: 10.1111/j.1471-4159.2005.03419.x. [DOI] [PubMed] [Google Scholar]
- 49.Song YH, Li YX, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. Journal of Clinical Investigation. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martineau LC, Gardiner PF. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. Journal of Applied Physiology. 2001;91:693–702. doi: 10.1152/jappl.2001.91.2.693. [DOI] [PubMed] [Google Scholar]
- 51.Park BC, Kido Y, Accili D. Differential signaling of insulin and IGF-1 receptors to glycogen synthesis in murine hepatocytes. Biochemistry. 1999;38:7517–7523. doi: 10.1021/bi9830718. [DOI] [PubMed] [Google Scholar]
- 52.Abrahamsson SO, Lohmander S. Differential effects of insulin-like growth factor-I on matrix and DNA synthesis in various regions and types of rabbit tendons. Journal of Orthopaedic Research. 1996;14:370–376. doi: 10.1002/jor.1100140305. [DOI] [PubMed] [Google Scholar]
- 53.Vikman K, Isgaard J, Eden S. Growth-Hormone Regulation of Insulin-Like Growth Factor-I Messenger-Rna in Rat Adipose-Tissue and Isolated Rat Adipocytes. Journal of Endocrinology. 1991;131:139–145. doi: 10.1677/joe.0.1310139. [DOI] [PubMed] [Google Scholar]
- 54.Ullrich A, Gray A, Tam AW, Yangfeng T, Tsubokawa M, Collins C, Henzel W, Lebon T, Kathuria S, Chen E, Jacobs S, Francke U, Ramachandran J, Fujitayamaguchi Y. Insulin-Like Growth Factor-I Receptor Primary Structure - Comparison with Insulin-Receptor Suggests Structural Determinants That Define Functional Specificity. Embo Journal. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Musaro A, McCullagh KJA, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 56.Hussain MA, Schmitz O, Mengel A, Glatz Y, Christiansen JS, Zapf J, Froesch ER. Comparison of the Effects of Growth-Hormone and Insulin-Like Growth-Factor-I on Substrate Oxidation and on Insulin Sensitivity in Growth Hormone-Deficient Humans. Journal of Clinical Investigation. 1994;94:1126–1133. doi: 10.1172/JCI117427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cefalu WT. Concept, strategies, and feasibility of noninvasive insulin delivery. Diabetes Care. 2004;27:239–246. doi: 10.2337/diacare.27.1.239. [DOI] [PubMed] [Google Scholar]
- 58.Peterson CM. Symposium on Optimal Insulin Delivery - Introduction - History and Goals of Insulin-Treatment. Diabetes Care. 1982;5:1–5. [PubMed] [Google Scholar]
- 59.Biolo G, Fleming RYD, Wolfe RR. Physiological Hyperinsulinemia Stimulates Protein-Synthesis and Enhances Transport of Selected Amino-Acids in Human Skeletal-Muscle. Journal of Clinical Investigation. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sprague J, Arbelaez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev. 2011;9:463–473. [PMC free article] [PubMed] [Google Scholar]
- 61.Sidossis LS, Stuart CA, Shulman GI, Lopaschuk GD, Wolfe RR. Glucose plus insulin regulate fat oxidation by controlling the rate of fatty acid entry into the mitochondria. Journal of Clinical Investigation. 1996;98:2244–2250. doi: 10.1172/JCI119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann N Y Acad Sci. 1995;771:730–42. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- 63.Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MR, Porte D, Jr, Woods SC. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7:381–6. doi: 10.1016/s0195-6663(86)80006-x. [DOI] [PubMed] [Google Scholar]
- 64.Duchaine D. Underground Steroid Handbook. 1. HLR Technical Books; Venice, CA, USA: 1982. [Google Scholar]
- 65.Holt RIG, Sonksen PH. Growth hormone, IGF-I and insulin and their abuse in sport. British Journal of Pharmacology. 2008;154:542–556. doi: 10.1038/bjp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barroso O, Mazzoni I, Rabin O. Hormone abuse, in sports: the antidoping perspective. Asian Journal of Andrology. 2008;10:391–402. doi: 10.1111/j.1745-7262.2008.00402.x. [DOI] [PubMed] [Google Scholar]
- 67.Chikani V, Ho KKY. Action of GH on skeletal muscle function: molecular and metabolic mechanisms. Journal of Molecular Endocrinology. 2014;52:R107–R123. doi: 10.1530/JME-13-0208. [DOI] [PubMed] [Google Scholar]
- 68.Chikani V, Cuneo RC, Hickman I, Ho KKY. Growth hormone (GH) enhances anaerobic capacity: impact on physical function and quality of life in adults with GH deficiency. Clinical Endocrinology. 2016;85:660–668. doi: 10.1111/cen.13147. [DOI] [PubMed] [Google Scholar]
- 69.Meinhardt U, Nelson AE, Hansen JL, Birzniece V, Clifford D, Leung KC, Graham K, Ho KKY. The Effects of Growth Hormone on Body Composition and Physical Performance in Recreational Athletes A Randomized Trial. Annals of Internal Medicine. 2010;152:568-+. doi: 10.7326/0003-4819-152-9-201005040-00007. [DOI] [PubMed] [Google Scholar]
- 70.Krag MB, Gormsen LC, Guo ZK, Christiansen JS, Jensen MD, Nielsen S, Jorgensen JOL. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. American Journal of Physiology-Endocrinology and Metabolism. 2007;292:E920–E927. doi: 10.1152/ajpendo.00374.2006. [DOI] [PubMed] [Google Scholar]
- 71.Flitsch J, Spitzner S, Ludecke DK. Emotional disorders in patients with different types of pituitary adenomas and factors affecting the diagnostic process. Experimental and Clinical Endocrinology & Diabetes. 2000;108:480–485. doi: 10.1055/s-2000-8144. [DOI] [PubMed] [Google Scholar]
- 72.Hennessey JV, Chromiak JA, DellaVentura S, Reinert SE, Puhl J, Kiel DP, Rosen CJ, Vandenburgh H, MacLean DB. Growth hormone administration and exercise effects on muscle fiber type and diameter in moderately frail older people. Journal of the American Geriatrics Society. 2001;49:852–858. doi: 10.1046/j.1532-5415.2001.49173.x. [DOI] [PubMed] [Google Scholar]
- 73.Lange KHW, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, Juul A, Bulow J, Kjaer M. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. Journal of Clinical Endocrinology & Metabolism. 2002;87:513–523. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- 74.Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy JO, Bier DM. Effect of Growth-Hormone and Resistance Exercise on Muscle Growth in Young Men. American Journal of Physiology. 1992;262:E261–E267. doi: 10.1152/ajpendo.1992.262.3.E261. [DOI] [PubMed] [Google Scholar]
- 75.Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of Growth-Hormone and Resistance Exercise on Muscle Growth and Strength in Older Men. American Journal of Physiology-Endocrinology and Metabolism. 1995;268:E268–E276. doi: 10.1152/ajpendo.1995.268.2.E268. [DOI] [PubMed] [Google Scholar]
- 76.Clemmons DR, Molitch M, Hoffman AR, Klibanski A, Strasburger CJ, Kleinberg DL, Ho K, Webb SM, Bronstein MD, Bouillon R, Ben-Shlomo A, Hamrahian AH, Chanson P, Barkan AL, Merriam GR, Blackman MR, Salvatori R. Growth Hormone Should Be Used Only for Approved Indications. Journal of Clinical Endocrinology & Metabolism. 2014;99:409–411. doi: 10.1210/jc.2013-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haerinejad MJ, Ostovar A, Farzaneh MR, Mojtaba Keshavarz M. The Prevalence and Characteristics of Performance-Enhancing Drug Use Among Bodybuilding Athletes in the South of Iran, Bushehr. Asian Journal of Sports Medicine. 2016;7:e35018. doi: 10.5812/asjsm.35018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chandler M, McVeigh J. Steroids and image enhancing drugs 2013 survey results. Liverpool: LJMU Centre for Public Health; 2014. [Google Scholar]
- 79.Carroll PV, Christ ER, Comm GHRSS. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: A review. Journal of Clinical Endocrinology & Metabolism. 1998;83:382–395. doi: 10.1210/jcem.83.2.4594. [DOI] [PubMed] [Google Scholar]
- 80.Saugy M, Robinson N, Saudan C, Baume N, Avois L, Mangin P. Human growth hormone doping in sport. British Journal of Sports Medicine. 2006;40:35–39. doi: 10.1136/bjsm.2006.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skarberg K, Nyberg F, Engstrom I. Multisubstance Use as a Feature of Addiction to Anabolic-Androgenic Steroids. European Addiction Research. 2009;15:99–106. doi: 10.1159/000199045. [DOI] [PubMed] [Google Scholar]
- 82.Baumann GP. Growth Hormone Doping in Sports: A Critical Review of Use and Detection Strategies. Endocrine Reviews. 2012;33:155–186. doi: 10.1210/er.2011-1035. [DOI] [PubMed] [Google Scholar]
- 83.Guha N, Cowan DA, Sonksen PH, Holt RIG. Insulin-like growth factor-I (IGF-I) misuse in athletes and potential methods for detection. Analytical and Bioanalytical Chemistry. 2013;405:9669–9683. doi: 10.1007/s00216-013-7229-y. [DOI] [PubMed] [Google Scholar]
- 84.Moses AC, Young SCJ, Morrow LA, OBrien M, Clemmons DR. Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes. 1996;45:91–100. doi: 10.2337/diab.45.1.91. [DOI] [PubMed] [Google Scholar]
- 85.Zenobi PD, Jaeggigroisman SE, Riesen WF, Roder ME, Rudolffroesch E. Insulin-Like Growth Factor-I Improves Glucose and Lipid-Metabolism in Type-2 Diabetes-Mellitus. Journal of Clinical Investigation. 1992;90:2234–2241. doi: 10.1172/JCI116109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coleman ME, Demayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic Vector Expression of Insulin-Like Growth-Factor-I Stimulates Muscle-Cell Differentiation and Myofiber Hypertrophy in Transgenic Mice. Journal of Biological Chemistry. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 87.Butterfield GE, Thompson J, Rennie MJ, Marcus R, Hintz RL, Hoffman AR. Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. American Journal of Physiology-Endocrinology and Metabolism. 1997;272:E94–E99. doi: 10.1152/ajpendo.1997.272.1.E94. [DOI] [PubMed] [Google Scholar]
- 88.Friedlander AL, Butterfield E, Moynihan S, Grillo J, Pollack M, Holloway L, Friedman L, Yesavage J, Matthias DF, Lee S, Marcus R, Hoffman AR. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. Journal of Clinical Endocrinology & Metabolism. 2001;86:1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- 89.Rich JD, Dickinson BP, Merriman NA, Thule PM. Insulin use by bodybuilders [letter] Jama-Journal of the American Medical Association. 1998;279:1613. doi: 10.1001/jama.279.20.1613. [DOI] [PubMed] [Google Scholar]
- 90.International Olympic Committee and Medical Commission. Olympic movement anti-doping code. Prohibited classes of substances and prohibited methods. 2001–2002 www.olympic.org/uk/organisation/commissions/medical/antidoping.
- 91.Elkin SL, Brady S, Williams IP. Bodybuilders find it easy to obtain insulin to help them in training. British Medical Journal. 1997;314:1280–1280. doi: 10.1136/bmj.314.7089.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomas SJ, Morimoto K, Herndon DN, Ferrando AA, Wolfe RR, Klein GL, Wolf SE. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery. 2002;132:341–347. doi: 10.1067/msy.2002.126871. [DOI] [PubMed] [Google Scholar]
- 93.Bidlingmaier M, Wu ZD, Strasburger CJ. Test method: GH. Best Practice & Research Clinical Endocrinology & Metabolism. 2000;14:99–109. doi: 10.1053/beem.2000.0057. [DOI] [PubMed] [Google Scholar]
- 94.Wu Z, Bidlingmaier M, Dall R, Strasburger CJ. Detection of doping with human growth hormone. Lancet. 1999;353:895–895. doi: 10.1016/S0140-6736(99)00775-8. [DOI] [PubMed] [Google Scholar]
- 95.Wallace JD, Cuneo RC, Bidlingmaier M, Lundberg PA, Carlsson L, Boguszewski CL, Hay J, Boroujerdi M, Cittadini A, Dall R, Rosen T, Strasburger CJ. Changes in non-22-kilodalton (kDa) isoforms of growth hormone (GH) after administration of 22-kDa recombinant human GH in trained adult males. Journal of Clinical Endocrinology & Metabolism. 2001;86:1731–1737. doi: 10.1210/jcem.86.4.7379. [DOI] [PubMed] [Google Scholar]
- 96.2010 World Anti-Doping Agency Guidelines: hGH isoform differential immunoassays for anti-doping analyses, version 1.0. 2010 Jun; http://www.wada-ama.org/Documents/Science_Medicine/Anti-Doping-Labs-Guidelines/WADA_SCGuidelines_hGH%20Differential%20Immunoassays_EN_June10.pdf.
- 97.Dall R, Longobardi S, Ehrnborg C, Keay N, Rosen T, Jorgensen JOL, Cuneo RC, Boroujerdi MA, Cittadini A, Napoli R, Christiansen JS, Bengtsson BA, Sacca L, Baxter RC, Basset EE, Sonksen PH, Grp G-S. The effect of four weeks of supraphysiological growth hormone administration on the insulin-like growth factor axis in women and men. Journal of Clinical Endocrinology & Metabolism. 2000;85:4193–4200. doi: 10.1210/jcem.85.11.6964. [DOI] [PubMed] [Google Scholar]
- 98.Powrie JK, Bassett EE, Rosen T, Jorgensen JO, Napoli R, Sacca L, Christiansen JS, Bengtsson BA, Sonksen PH. Detection of growth hormone abuse in sport. Growth Hormone & Igf Research. 2007;17:220–226. doi: 10.1016/j.ghir.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 99.Guha N, Erotokritou-Mulligan I, Bartlett C, Nevitt SP, Francis M, Bassett EE, Cowan DA, Sonksen PH, Holt RIG. Biochemical Markers of Insulin-Like Growth Factor-I Misuse in Athletes: The Response of Serum IGF-I, Procollagen Type III Amino-Terminal Propeptide, and the GH-2000 Score to the Administration of rhIGF-I/rhIGF Binding Protein-3 Complex. Journal of Clinical Endocrinology & Metabolism. 2014;99:2259–2268. doi: 10.1210/jc.2013-3897. [DOI] [PubMed] [Google Scholar]
- 100.Holt R, Guha N, Bohning W, Bartlett C, Cowan DA, Sonksen PH, Bohning D. Novel markers to detect recombinant human insulin-like growth factor-I (rhIGF-I)/rhIGF binding protein-3 (rhIGFBP-3) misuse in athletes. Drug Test Anal. 2017;9:30–37. doi: 10.1002/dta.1941. [DOI] [PubMed] [Google Scholar]
- 101.Andersen L, Jorgensen PN, Jensen LB, Walsh D. A new insulin immunoassay specific for the rapid-acting insulin analog, insulin aspart, suitable for bioavailability, bioequivalence, and pharmacokinetic studies. Clinical Biochemistry. 2000;33:627–633. doi: 10.1016/s0009-9120(00)00183-1. [DOI] [PubMed] [Google Scholar]
- 102.Bowsher RR, Lynch RA, Brown-Augsburger P, Santa PF, Legan WE, Woodworth JR, Chance RE. Sensitive RIA for the specific determination of insulin lispro. Clinical Chemistry. 1999;45:104–110. [PubMed] [Google Scholar]
- 103.Moriyama M, Hayashi N, Ohyabu C, Mukai M, Kawano S, Kumagai S. Performance evaluation and cross-reactivity from insulin analogs with the ARCHITECT insulin assay. Clinical Chemistry. 2006;52:1423–1426. doi: 10.1373/clinchem.2005.065995. [DOI] [PubMed] [Google Scholar]
- 104.Neal JaHW. Insulin immunoassays in the detection of insulin analogues in factitious hypoglycemia. Endocr Practice. 2008;14:1006. doi: 10.4158/EP.14.8.1006. [DOI] [PubMed] [Google Scholar]
- 105.Walfish PG, Feig DS, Bauman WA. Factitious Hyperinsulinemic Hypoglycemia - Confirmation of the Diagnosis by a Species-Specific Insulin Radioimmunoassay. Journal of Endocrinological Investigation. 1987;10:601–604. doi: 10.1007/BF03347007. [DOI] [PubMed] [Google Scholar]
- 106.Allen DB, Backeljauw P, Bidlingmaier M, Biller BMK, Boguszewski M, Burman P, Butler G, Chihara K, Christiansen J, Cianfarani S, Clayton P, Clemmons D, Cohen P, Darendeliler F, Deal C, Dunger D, Erfurth EM, Fuqua JS, Grimberg A, Haymond M, Higham C, Ho K, Hoffman AR, Hokken-Koelega A, Johannsson G, Juul A, Kopchick J, Lee P, Pollak M, Radovick S, Robison L, Rosenfeld R, Ross RJ, Savendahl L, Saenger P, Sorensen HT, Stochholm K, Strasburger C, Swerdlow A, Thorner M. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. European Journal of Endocrinology. 2016;174:P1–P9. doi: 10.1530/EJE-15-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: The safety and efficacy of growth hormone in the healthy elderly. Annals of Internal Medicine. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- 108.Ezzat S, Forster MJ, Berchtold P, Redelmeier DA, Boerlin V, Harris AG. Acromegaly - Clinical and Biochemical Features in 500 Patients. Medicine. 1994;73:233–240. [PubMed] [Google Scholar]
- 109.Wass JAH, SS, Gale E, Amiel SA. Oxford Textbook of Endocrinology and Diabetes. 1. Oxford: Oxford University Press; 2002. [Google Scholar]
- 110.Bengtsson BA, Eden S, Ernest I, Oden A, Sjogren B. Epidemiology and Long-Term Survival in Acromegaly - a Study of 166 Cases Diagnosed between 1955 and 1984. Acta Medica Scandinavica. 1988;223:327–335. doi: 10.1111/j.0954-6820.1988.tb15881.x. [DOI] [PubMed] [Google Scholar]
- 111.Colao A, Marzullo P, Di Somma C, Lombardi G. Growth hormone and the heart. Clinical Endocrinology. 2001;54:137–154. doi: 10.1046/j.1365-2265.2001.01218.x. [DOI] [PubMed] [Google Scholar]
- 112.Colao A, Pivonello R, Di Somma C, Tauchmanova L, Savastano S, Lombardi G. Growth hormone excess with onset in adolescence: clinical appearance and long-term treatment outcome. Clinical Endocrinology. 2007;66:714–722. doi: 10.1111/j.1365-2265.2007.02809.x. [DOI] [PubMed] [Google Scholar]
- 113.Jenkins PJ, Mukherjee A, Shalet SM. Does growth hormone cause cancer? Clinical Endocrinology. 2006;64:115–121. doi: 10.1111/j.1365-2265.2005.02404.x. [DOI] [PubMed] [Google Scholar]
- 114.Kreze A, Kreze-Spirova E, Mikulecky M. Risk factors for glucose intolerance in active acromegaly. Brazilian Journal of Medical and Biological Research. 2001;34:1429–1433. doi: 10.1590/s0100-879x2001001100009. [DOI] [PubMed] [Google Scholar]
- 115.Kopchick JJ, Bellush LL, Coschigano KT. Transgenic models of growth hormone action. Annual Review of Nutrition. 1999;19:437–461. doi: 10.1146/annurev.nutr.19.1.437. [DOI] [PubMed] [Google Scholar]
- 116.Penney DG, Dunbar JC, Baylerian MS. Cardiomegaly and Hemodynamics in Rats with a Transplantable Growth Hormone-Secreting Tumor. Cardiovascular Research. 1985;19:270–277. doi: 10.1093/cvr/19.5.270. [DOI] [PubMed] [Google Scholar]
- 117.Cohn L, Feller AG, Draper MW, Rudman IW, Rudman D. Carpal-Tunnel Syndrome and Gynecomastia during Growth-Hormone Treatment of Elderly Men with Low Circulating Igf-I Concentrations. Clinical Endocrinology. 1993;39:417–425. doi: 10.1111/j.1365-2265.1993.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 118.Sullivan DH, Carter WJ, Warr WR, Williams LH. Side effects resulting from the use of growth hormone and insulin-like growth factor-I as combined therapy to frail elderly patients. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 1998;53:M183–M187. doi: 10.1093/gerona/53a.3.m183. [DOI] [PubMed] [Google Scholar]
- 119.Blackman M, Sorkin JD, Munzer T, Bellantoni MF. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. BMJ. 2004;328:907–908. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 120.Reed M, Merriam GR, Kargi AY. Adult growth hormone deficiency - benefits, side effects, and risks of growth hormone replacement. Front Endocrinol (Lausanne) 2013;4:64. doi: 10.3389/fendo.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Young J, Anwar A. Strong diabetes. British Journal of Sports Medicine. 2007;41:335–336. doi: 10.1136/bjsm.2006.030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown P, Gajdusek DC, Gibbs CJ, Asher DM. Potential Epidemic of Creutzfeldt-Jakob Disease from Human Growth-Hormone Therapy. New England Journal of Medicine. 1985;313:728–731. doi: 10.1056/NEJM198509193131205. [DOI] [PubMed] [Google Scholar]
- 123.Williams RM, McDonald A, O’Savage M, Dunger DB. Mecasermin rinfabate: rhIGF-I/rhIGFBP-3 complex: iPLEX (TM) Expert Opinion on Drug Metabolism & Toxicology. 2008;4:311–324. doi: 10.1517/17425255.4.3.311. [DOI] [PubMed] [Google Scholar]
- 124.Laron Z. Commentary - The essential role of IGF-I: Lessons from the long-term study and treatment of children and adults with Laron syndrome. Journal of Clinical Endocrinology & Metabolism. 1999;84:4397–4404. doi: 10.1210/jcem.84.12.6255. [DOI] [PubMed] [Google Scholar]
- 125.Major JM, Laughlin GA, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Insulin-Like Growth Factor-I and Cancer Mortality in Older Men. Journal of Clinical Endocrinology & Metabolism. 2010;95:1054–1059. doi: 10.1210/jc.2009-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ip EJ, Barnett MJ, Tenerowicz MJ, Perry PJ. Weightlifting’s Risky New Trend: A Case Series of 41 Insulin Users. Current Sports Medicine Reports. 2012;11:176–179. doi: 10.1249/JSR.0b013e31825da97f. [DOI] [PubMed] [Google Scholar]
- 127.Larance B, Degenhardt L, Copeland J, Dillon P. Injecting risk behaviour and related harm among men who use performance- and image-enhancing drugs. Drug and Alcohol Review. 2008;27:679–686. doi: 10.1080/09595230802392568. [DOI] [PubMed] [Google Scholar]
- 128.Sagoe D, McVeigh J, Bjornebekk A, Essilfie MS, Andreassen CS, Pallesen S. Polypharmacy among anabolic-androgenic steroid users: a descriptive metasynthesis. Substance Abuse Treatment Prevention and Policy. 2015:10. doi: 10.1186/s13011-015-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Borjesson A, Garevik N, Dahl ML, Rane A, Ekstrom L. Recruitment to doping and help-seeking behavior of eight female AAS users. Subst Abuse Treat Prev Policy. 2016;11:11. doi: 10.1186/s13011-016-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hope VD, McVeigh J, Marongiu A, Evans-Brown M, Smith J, Kimergard A, Croxford S, Beynon CM, Parry JV, Bellis MA, Ncube F. Prevalence of, and risk factors for, HIV, hepatitis B and C infections among men who inject image and performance enhancing drugs: a cross-sectional study. Bmj Open. 2013;3:e003207. doi: 10.1136/bmjopen-2013-003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McVeigh J, Begley E. Anabolic steroids in the UK: an increasing issue for public health. Drugs-Education Prevention and Policy. 2017;24:278–285. [Google Scholar]
- 132.Perera NJ, Steinbeck KS, Shackel N. The Adverse Health Consequences of the Use of Multiple Performance-Enhancing Substances-A Deadly Cocktail. Journal of Clinical Endocrinology & Metabolism. 2013;98:4613–4618. doi: 10.1210/jc.2013-2310. [DOI] [PubMed] [Google Scholar]
- 133.Solimini R, Rotolo MC, Mastrobattista L, Mortali C, Minutillo A, Pichini S, Pacifici R, Palmi I. Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping. Eur Rev Med Pharmacol Sci. 2017;21:7–16. [PubMed] [Google Scholar]