Introduction
Both the Clinical Pharmacogenetics Implementation Consortium (CPIC®) and Dutch Pharmacogenetics Working Group provide therapeutic recommendations for well-known gene-drug pairs. Published recommendations show a high rate of concordance. However, as a result of different guideline development methods used by these two consortia, differences between the published guidelines exist. The aim of this paper is to compare both initiatives and explore these differences, with the objective to achieve harmonization.
Background
An important barrier for the implementation of pharmacogenetics in clinical practice is the translation of the results of a genetic test into clinical action (1–3). Kirchheiner et al. were among the first to extract dosing recommendations based on pharmacokinetic (PK) data of patients with known CYP2D6 and CYP2C19 genotypes (4). Anticipating a proximate future in which both pharmacists and physicians would be confronted with patients with a known genotype, two consortia, the Dutch Pharmacogenetics Working Group (DPWG) and the Clinical Pharmacogenetics Implementation Consortium (CPIC®), provide widely recognized therapeutic recommendations for specific gene-drug pairs (5–32).
The DPWG was founded by the Royal Dutch Pharmacists Association (KNMP) in 2005 and in the last decade has reviewed 86 potential gene-drug pairs of which 47 guidelines provide therapeutic recommendations for one or more aberrant phenotypes (see Table 1 & Box 1 for additional information) (7).
Table 1.
Characteristics of the two consortia
| CPIC | DPWG | |
|---|---|---|
| Founded | 2009 | 2005 |
| Type of membership | Open for application of new members with a clinical interest in pharmacogenetics, N=206 as of March 2017 | By invitation, N=14 |
| Composition | Multidisciplinary | Multidisciplinary |
| Objectives | 1) To address the barriers to implementation of pharmacogenetic tests into clinical practice 2) To provide guidelines that enable the translation of genetic laboratory test results into actionable prescribing decisions for specific drugs |
1) To develop pharmacogenetics-based therapeutic (dose) recommendations 2) To assist drug prescribers and pharmacists by integrating the recommendations into computerized systems for drug prescription and automated medication surveillance |
| Number of gene-drug pairs covered | 40 | 86 |
| Number of gene-drug pairs with therapeutic recommendation | 40 | 47 |
| Frequency of scheduled updates | As needed, reviewed at least every 2 years | If needed, max 4 years |
| Funding | National Institutes of Health | Royal Dutch Pharmacist’s Association and H2020 contract number 668353-I |
Box 1. Integration of therapeutic recommendations of the DPWG into clinical care.
The DPWG guidelines are available at point of care in the Netherlands through all electronic prescribing and medication surveillance systems and continuously updated and distributed through the G-standard. The G-standard is the Dutch national drug database which contains information used in medication surveillance. The information of the G-standard supports the prescribing, dispensing, ordering and reimbursement of drugs and is used by physicians, pharmacists, health insurers, government and drug wholesalers in the Netherlands (https://www.knmp.nl/producten-en-diensten/gebruiksrecht-g-standaard/informatie-over-de-g-standaard/the-g-standaard-the-medicines-standard-in-healthcare). English versions of the DPWG guidelines have been published in 2008 and 2011 in the international literature, and a subset is currently available at the PharmGKB website: https://www.pharmgkb.org/ (5;6;33).
The CPIC, established in 2009 as a joint project between the Pharmacogenomics Research Network (PGRN) and the Pharmacogenomics Knowledgebase (PharmGKB), has a similar goal to provide actionable, genotype-based prescribing recommendations for known gene-drug pairs (see Table 1) (3). To date, CPIC has published 19 guidelines (eight that have been updated since the original publication) covering 40 gene-drug pairs which are publicly available through both the PharmGKB (https://www.pharmgkb.org/) and CPIC websites (https://cpicpgx.org/) (8–17;19–32).
The aim of this paper is to compare both initiatives and explore differences in the methodology and therapeutic recommendations of both consortia.
Methodology of CPIC
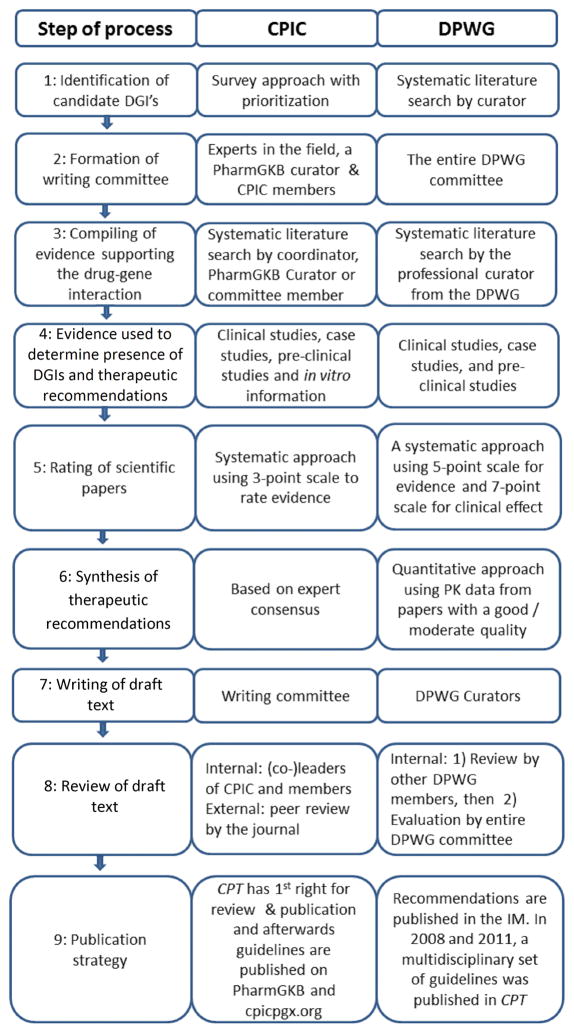
To select relevant gene-drug pairs, the CPIC uses a survey-based approach supplemented with nominations from members and external experts and is informed by actions such as the U.S. Food and Drug Administration labeling (3). The CPIC also takes into account the actionability of a gene-drug pair (i.e., genetic information should/could be used to change prescribing of affected drug) and the degree of testing for variations in the gene. For each new gene-drug pair, the CPIC coordinator forms a multidisciplinary writing committee consisting of experts with a relevant track record of related publications and/or other expertise. To assist in the literature search, compiling and evaluation of identified evidence, a scientific curator from PharmGKB is added to the team. The curator and coordinator are responsible for drafting the gene background information, phenotype assignments, and compilation of the tabular materials necessary for clinical implementation of the guideline (34;35). Clinical studies, case studies, pre-clinical studies and in vitro information of the drug(s) of interest with a genetic variant are evaluated and are systematically rated as weak, moderate or high. Based on this body of evidence, as well as the evidence for the alternative therapy being recommended, the writing committee derives clinical recommendations stratified by phenotype. Each recommendation is scored using a system based on that by Valdes et al (34–36) as Strong, Moderate, Optional, or No Recommendation. A draft of the guideline is written by the committee and reviewed by other CPIC members. Feedback from this process is incorporated into the guideline before it is subjected to external peer review (34;35). The guideline is considered for update whenever new evidence impacts prescribing recommendations (see Figure 1).
Figure 1.
A comparison of the methodology for guideline synthesis of the two consortia. DGI = Drug-Gene Interaction
Methodology of DPWG
To select relevant gene-drug pairs, curators from the DPWG perform systematic searches in PubMed on known gene variants that affect drug pharmacokinetics and pharmacodynamics. For each gene-drug pair identified papers are rated by two independent DPWG members based on a scoring system (37). Based on the scores the DPWG assesses whether a gene-drug pair is indeed present and whether a therapeutic (dose) recommendation is required. Recommendations can include a dose adjustment or a therapeutic strategy (i.e., therapeutic drug monitoring or stricter clinical monitoring of patients). Dose-adjustments are calculated using PK-data from available papers with evidence rated 3 or 4 on a 0–4 point scale. All evidence is condensed into a final report containing the DPWG conclusion whether a gene-drug pair is indeed present, whether action is required and if so, the therapeutic recommendation. These reports are then integrated into a database for electronic medication surveillance, the G-standard, which feeds all available electronic drug prescribing and dispensing systems in the Netherlands (5;6). Gene-drug pairs are updated if needed but at least every four years (see Figure 1).
Differences in methodology
An overview of the characteristics and objectives of both consortia is presented in Table 1, and the methodology of both consortia in selection of relevant gene-drug interactions, literature review and guideline synthesis are in Figure 1. Although the initial selection of the relevant gene-drug pairs was different, the general process of guideline synthesis by the DPWG and the CPIC is highly similar. Both consortia use professional curators to systematically search and evaluate scientific evidence (36;37). Yet, there are some minor differences. DPWG reviews the level of evidence and the level of clinical relevance on separate scales using a five-point (0–4) and seven-point (AA-F) scale, respectively, while the CPIC rates the level of evidence on a three-point (weak-moderate-high) scale (36;37).
A second difference is the sources of information considered for guideline development. The DPWG only provides a recommendation if data from at least one clinical study of good or moderate quality are available, whereas the CPIC also considers data from preclinical studies and case-reports. A third difference is the process used to synthesize a dose recommendation. The DPWG applies a quantitative method, whereas CPIC applies an approach based on expert consensus.
Differences in terminology for allele function and phenotype assignment
Differences in the terminology used to describe allele function also exist. An example is the difference in the word used to describe an allele that has a “greater than normal function”. The CPIC uses the term “increased function”, whereas the DPWG uses “gain-of-function”. A similar difference in terminology is seen for alleles with decreased function (see Table 2).
Table 2.
Discordances in terminology for allele function and phenotypes
| Category | Functional definition | Consortium | Term |
|---|---|---|---|
| Allele | Greater than normal function | CPIC | Increased function |
| DPWG | Gain-of-function* | ||
| Allele | Less than normal function | CPIC | Decreased function |
| DPWG | Decreased activity* | ||
| Phenotype | Individuals who carry two alleles which encode for a fully functional enzyme or individuals with a combination of an allele which encodes for a fully functional enzyme and an allele which encodes for an enzyme with decreased function | CPIC | Normal metabolizer |
| DPWG | Extensive metabolizer* | ||
| Phenotype | An individual carrying one normal function allele and one increased function allele | CPIC | Rapid metabolizer |
| DPWG | - |
In this article the terminology of the CPIC will be used.
With the publication of the first therapeutic guideline of CYP2D6, CPIC opted for the historical term “extensive metabolizer” to describe individuals who carry one or two alleles which encode for a fully functional enzyme (9;16). However, based on the results from the recent CPIC term standardization project, normal metabolizer will replace extensive metabolizer in all new and updated CPIC guidelines (38). The term extensive metabolizer is also used by the DPWG in the published guidelines and is currently still used in clinical practice (5–7). In this comparison the term normal metabolizer will be used to describe individuals who were previously (CPIC) or are currently (DWPG) categorised as extensive metabolizers (see Table 2).
The CPIC term standardization project also resulted in the addition of phenotypes such as CYP2C19 rapid metabolizer with a functional definition of “increased enzyme activity compared to normal metabolizers, but less than ultra-rapid metabolizers” and the SLCO1B1 increased function with a functional definition of “increased transporter function compared to normal function” (38).
Differences in allele classification and genotype to phenotype conversion
Both CPIC and DPWG provide therapeutic recommendations at the phenotype level. As a result, a genotype-predicted phenotype (gPhenotype) needs to be inferred from the results of the genetic test. This process requires “translation tables” provided by both consortia. For the genes CYP2C9, CYP2C19, CYP2D6 and DPYD, differences in both the classification of alleles (category I) and the translation of genotype to phenotype (category II) can be observed.
CYP2C9
Based on a publication from 2004, the DPWG guideline categorizes the CYP2C9*8 allele as a “gain-of-function” allele (39). CPIC categorizes the same allele as a “possible decreased function” allele based on 2 more recent reports by Liu et al and Allabi et al (40;41). The allele frequency of CYP2C9*8 is ~ 0% in Caucasians and 4.70% in African-Americans (42;43). As a result of the low allele frequency, this difference in allele classification does not appear to have clinical consequences for Caucasians. However, in African-American patients this difference could result in different therapeutic recommendations.
CYP2C19
Both consortia recognize the CYP2C19*17 allele as an allele with a function greater than normal and categorize the genotypes *17/*17 as “ultra-rapid metabolizer” and *2/*17 and *3/*17 as intermediate metabolizers, respectively. However, a difference exists between the genotype to phenotype translation of the *1/*17 genotype. In guidelines of CPIC published before July 2016 the diplotype *1/*17 is categorized as the phenotype ultra-rapid metabolizer (24;26;44–50), while the DPWG classifies this diplotype as normal metabolizer based on the same literature (5;6;44–50) (see Table 2). As of July 2016 CPIC introduced the additional phenotype “rapid metabolizer” to fill the need to distinguish between individuals with a *1/*17 and *17/*17 on a phenotype level. This new phenotype was introduced in the CPIC guideline providing information and therapeutic recommendations on the gene-drug interaction of CYP2C19 and voriconazole (32;38).
Based on the *17 allele frequency of 18% among African populations and 18–24% among Caucasian populations, this difference in genotype to phenotype translation can result in a difference in treatment recommendations for many individuals (6;24). For example, a prescription with amitriptyline for a patient with a CYP2C19*1/*17 genotype results in a recommendation to switch to an alternate therapy based on the CPIC guideline, while the DPWG guidelines advise the normal starting dose for the same genotype (6;24).
DPYD
CPIC provides fluoropyrimidine dosing recommendations for normal/high, intermediate, and deficient dihydropyrimidine dehydrogenase (DPD) activity phenotypes based on DPYD genotypes (14). In contrast the DPWG uses an activity-score (AS) to accommodate the increasing number of DPYD allelic variants and their difference in function (7;51). Further differences can be seen in the amount of variants that are discussed in the guidelines. For example, the 496A>G, 1156G>T, 1651G>A, and 1845G>T variants are not mentioned in the CPIC guideline, while the IVS10-15TC variant is mentioned without a classification of the status. In contrast, the DPWG indicates that the variant alleles 496A>G and IVS10-15TC are only associated with toxicity in a single study and the 1156G>T, 1651G>A and 1845G>T variant alleles are mentioned as cause of toxicity in case reports. Inversely, the 1129-5923C>G is mentioned by the CPIC, but not by the DPWG.
Finally, a difference between the two guidelines exists in the evidence supporting the allele classification of the *13 and the 1236G>A/haplotype B3 variant. CPIC reports that there is a clear association of the *13 allele with reduced clearance of capecitabine and 5-FU in addition to evidence from case-reports (14). In contrast, DPWG concludes that the evidence supporting a decreased activity of the *13 allele is limited and only described in case-reports (7). Inversely, the DPWG categorizes the 1236G>A/haplotype B3 as a reduced function variant with a body of evidence similar to the 2846A>T variant (7). In contrast, CPIC mentions this variant without assigned status, similar to the IVS10-15TC variant (14).
CYP2D6
Both consortia classify the CYP2D6*36 allele as a variant allele; however, there is a difference in the interpretation of the functionality of this allele between the two consortia. The DPWG classifies the activity of the *36 allele as reduced (52–57), while the CPIC classifies the allele as non-functional based on four articles published after 2002 (58–61). The difference in allele classification of functionality can potentially be explained by the distinction between the single variant which has no residual CYP2D6 activity and the *36 +*10 tandem allele with residual activity (of the *10 allele). In contrast, the DPWG still uses the old classification of the *36 and has not yet made a difference between the single and tandem variants. Due to the low allele frequency of the *36 and the *36+*10 tandem in African Americans and Europeans (0.00 – 0.98%) this difference in allele status will not have much clinical consequences for these populations. However, in patients from Asian descent the frequency of these two variant alleles of CYP2D6 is much higher, 1.52% (0.00 – 16.40) and 26.41% (22.45 – 32.65) for the single and tandem variant of the *36, respectively, and this could have implications for the therapy of Asian individuals carrying the single variant of CYP2D6 (6;26).
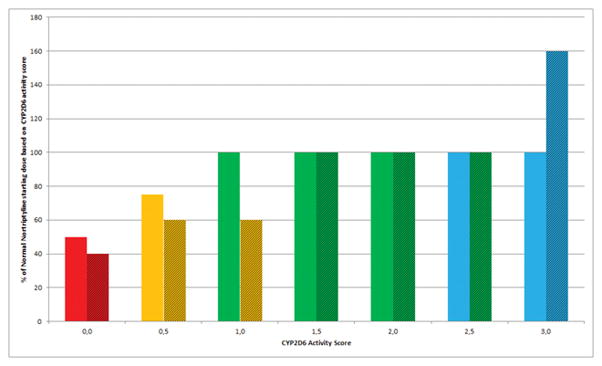
A second more important difference between the DPWG and the CPIC guidelines concerns the translation of the genotype to the CYP2D6 phenotype. Both consortia use the AS of CYP2D6 proposed by Gaedigk et al to attribute the scores of 0, 0.5, 1.0, and N to alleles with no function, decreased function, normal function, and alleles with normal function and a duplication of N times, respectively, to calculate a gene-activity score for CYP2D6. Although DPWG and CPIC agree on the diplotype score of 0 for poor metabolizers, they use a different conversion of the AS to the intermediate metabolizer gPhenotype (62;63). The DPWG has assigned the scores of 1.0 (combination of a functional and a non-functional allele or a combination of two alleles with reduced function) and 0.5 to intermediate metabolizer phenotype and the scores of 1.5 and 2.0 to the normal metabolizer phenotype (57), whereas CPIC assigned the scores of 1.0–2.0 to the normal metabolizer phenotype and the score of 0.5 to the intermediate metabolizer phenotype, respectively (see Figure 2) (15;16).
Figure 2. Phenotype translation and nortriptyline dose recommendations of the CPIC and DPWG based on CYP2D6 activity scores.
Solid bars: CPIC interpretation of phenotype and dosing recommendation. Hatched bars: DPWG interpretation of phenotype and dosing recommendation. Red: poor metabolizer, Orange: intermediate metabolizer, Green: normal metabolizer, Blue: ultra-rapid metabolizer. Note: CPIC provides no specific dose adjustment for amitriptyline but recommends to consider increasing the dose and using therapeutic drug monitoring to guide dose adjustments.
Due to the relatively high frequency of null alleles as the *3, *4 and *5 among African, American and European populations, and the high occurrence of the *10 allele among Asian individuals, a large group of patients will have an AS of 1.0. These patients will be classified as either normal metabolizers or intermediate metabolizers and may receive different treatment recommendations. The different translation of CYP2D6 genotype to phenotype between the DPWG and CPIC guidelines has a potentially significant impact on the treatment with drugs of individuals with an AS of 1.0 (see Figure 2).
Therapeutic recommendations
A total of 40 and 86 gene-drug pairs were reviewed by CPIC and the DPWG, respectively. For 27 gene-drug pairs both CPIC and DPWG provide guidelines which were included in the comparison. For 5 gene-drug pairs the rating of the evidence and the therapeutic recommendations were equal. For 8 gene-drug pairs, differences in the rating of the body of evidence supporting the same therapeutic recommendation were observed, but no clinical relevant differences in the therapeutic recommendations were identified (see Table 4). An example of this difference is the therapeutic recommendation for the CYP2C19 intermediate metabolizer and clopidogrel. Both consortia recommend to switch to a different platelet inhibitor for the CYP2C19 intermediate metabolizer phenotype but the rating of the available evidence by CPIC and the DPWG are moderate and strong, respectively (6;21). For 16 of the 27 gene-drug pairs with a total of 31 individual gene-drug-phenotype combinations, relevant differences (see definition) were observed in the therapeutic recommendations. In the case of 6 gene-drug pairs relevant differences in therapeutic recommendations were seen for only one aberrant phenotype, whereas for 8 gene-drug pairs differences were seen for two aberrant phenotypes and for 2 gene-drug pairs differences were seen in >2 aberrant phenotypes. All discordant therapeutic recommendations can be found in Table 4. Some of the discordances will be highlighted below.
Table 4.
Gene-drug pairs and dose recommendations by CPIC and the DPWG (differences in dosing recommendations of ≥20% are marked bold)
| Gene | Drug | Gene-drug Interaction | Phenotype | Action Required? | Therapeutic Recommendations + Classification of Evidence | Cat. | Ref. | |
|---|---|---|---|---|---|---|---|---|
| CYP2C9 | Phenytoin | CPIC: Yes | IM | Yes | Consider 25% reduction of recommended starting maintenance dose. Subsequent doses should be adjusted according to therapeutic drug monitoring and response. | M | (19) | |
| PM | Yes | Consider 50% reduction of recommended starting maintenance dose. Subsequent doses should be adjusted according to therapeutic drug monitoring and response. | S | |||||
| DPWG: Yes | IM | Yes | Standard loading dose. Reduce maintenance dose by 25%. Evaluate response and serum concentration after 7–10 days. Be alert to ADEs (e.g., ataxia, nystagmus, dysarthria, sedation). | 4D | (5–7) | |||
| PM | Yes | Standard loading dose. Reduce maintenance dose by 50–60%. Evaluate response and serum concentration after 7–10 days. Be alert to ADEs (e.g., ataxia, nystagmus, dysarthria, sedation). | 4D | |||||
| CYP2C9 | Warfarin | CPIC: Yes | *1/*2 | Yes | Calculate dose based on validated published pharmacogenetic algorithm | S | V | (17;18) |
| *1/*3 | Yes | Calculate dose based on validated published pharmacogenetic algorithm | S | V | ||||
| *2/*2 | Yes | Calculate dose based on validated published pharmacogenetic algorithm | S | V | ||||
| *2/*3 | Yes | Calculate dose based on validated published pharmacogenetic algorithm | S | V | ||||
| *3/*3 | Yes | Calculate dose based on validated published pharmacogenetic algorithm | S | V | ||||
| DPWG: Yes | *1/*2 | Yes | Initiate therapy with recommended starting dose | 4A | V | (7) | ||
| *1/*3 | Yes | Consider a reduction to 65% of the normal starting dose | 4D | V | ||||
| *2/*2 | Yes | Consider a reduction to 65% of the normal starting dose | 4A | V | ||||
| *2/*3 | Yes | Consider a reduction to 45% of the normal starting dose | 4A | V | ||||
| *3/*3 | Yes | Consider a reduction to 45% of the normal starting dose | 4C | V | ||||
| CYP2C19 | Amitriptyline | CPIC: Yes | IM | No | Initiate therapy with recommended starting dose | S | (24;25) | |
| PM | Yes |
1) Avoid amitriptyline use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include nortriptyline and desipramine. 2) Consider 50% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. |
M | III | ||||
| UM or RM | Yes |
1) Avoid amitriptyline use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include nortriptyline and desipramine. 2) If amitriptyline is warranted, utilize therapeutic drug monitoring to guide dose adjustments. |
O | III | ||||
| DPWG: Yes | IM | No | Initiate therapy with recommended starting dose. | IN | (7) | |||
| PM | No | Initiate therapy with recommended starting dose. | IN | III | ||||
| UM | No | Initiate therapy with recommended starting dose. | IN | III | ||||
| CYP2C19 | Citalopram / Escitalopram | CPIC: Yes | IM | No | Initiate therapy with recommended starting dose. | S | III | (26) |
| PM | Yes | 1) Consider a 50% reduction of recommended starting dose and titrate to response. 2) Select alternative drug not predominantly metabolized by CYP2C19. |
M | |||||
| UM | Yes | Consider an alternative drug not predominantly metabolized by CYP2C19. | M | III | ||||
| DPWG: Yes | IM | Yes |
1) Consider a maximum daily dose of 20 mg for age < 65 or 10 mg for ≥ 65 years. 2) Consider a 50% reduction in starting dose and raise to normal dose under monitoring of ECG to 40 mg for age < 65 or 20 mg for ≥ 65 years. |
4A | III | (6;7) | ||
| PM | Yes | Consider a maximum daily dose of 20 mg for age < 65 or 10 mg for ≥ 65 years. | 4A | |||||
| UM | No | Initiate therapy with recommended starting dose. | 3AA | III | ||||
| CYP2C19 | Clopidogrel | CPIC: Yes | IM | Yes | Consider alternative drug not metabolized by CYP2C19. | M | (20;21) | |
| PM | Yes | Consider alternative drug not metabolized by CYP2C19. | S | |||||
| UM | No | Initiate therapy with recommended starting dose. | S | |||||
| DPWG: Yes | IM | Yes | Consider alternative drug not metabolized by CYP2C19. | 4F | (6;7) | |||
| PM | Yes | Consider alternative drug not metabolized by CYP2C19. | 4F | |||||
| UM | No | Initiate therapy with recommended starting dose. | 4A | |||||
| CYP2C19 | Clomipramine | CPIC: Yes | IM | No | Initiate therapy with recommended starting dose. | O | (24;25) | |
| PM | Yes |
1) Avoid clomipramine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include nortriptyline and desipramine. 2) Consider 50% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. |
O | III | ||||
| UM or RM | Yes |
1) Avoid clomipramine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include nortriptyline and desipramine. 2) If clomipramine is warranted, utilize therapeutic drug monitoring to guide dose adjustments. |
O | III | ||||
| DPWG: Yes | IM | No | Initiate therapy with recommended starting dose. | IN | (7) | |||
| PM | No | Initiate therapy with recommended starting dose. | IN | III | ||||
| UM | No | Initiate therapy with recommended starting dose. | IN | III | ||||
| CYP2C19 | Doxepin | CPIC: Yes | IM | No | Initiate therapy with recommended starting dose | O | (24;25) | |
| PM | Yes |
1) Avoid doxepin use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include nortriptyline and desipramine. 2) Consider 50% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. |
O | III | ||||
| UM or RM | Yes |
1) Avoid doxepin use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include nortriptyline and desipramine. 2) If doxepin is warranted, utilize therapeutic drug monitoring to guide dose adjustments. |
O | III | ||||
| DPWG: No | IM | No | Initiate therapy with recommended starting dose. | IN | (7) | |||
| PM | No | Initiate therapy with recommended starting dose. | IN | III | ||||
| UM | No | Initiate therapy with recommended starting dose. | IN | III | ||||
| CYP2C19 | Imipramine | CPIC: Yes | IM | No | Initiate therapy with recommended starting dose. | O | (24;25) | |
| PM | Yes |
1) Avoid imipramine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include nortriptyline and desipramine. 2) Consider 50% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. |
O | III | ||||
| UM or RM | Yes |
1) Avoid imipramine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include nortriptyline and desipramine. 2) If imipramine is warranted, utilize therapeutic drug monitoring to guide dose adjustments. |
O | III | ||||
| DPWG: Yes | IM | No | Initiate therapy with recommended starting dose. | 4A | (6;7) | |||
| PM | Yes |
1) Consider a 30% reduction of recommended starting dose and utilize therapeutic drug monitoring of imipramine and desipramine. 2) Consider alternative drug not metabolized by CYP2C19. |
4A | III | ||||
| UM | No | Initiate therapy with recommended starting dose | 4A | III | ||||
| CYP2C19 | Sertraline | CPIC: Yes | IM | No | Initiate therapy with recommended starting dose. | S | III | (26) |
| PM | Yes |
1) Consider a 50% reduction of recommended starting dose and titrate to response. 2) Select alternative drug not predominantly metabolized by CYP2C19. |
O | III | ||||
| UM | Yes | Initiate therapy with recommended starting dose. If patient does not respond to recommended maintenance dosing, consider alternative drug not predominantly metabolized by CYP2C19. | O | |||||
| DPWG: Yes | IM | Yes | Consider a maximum daily dose of 100 mg and utilize clinical monitoring on response/side effects or therapeutic drug monitoring of sertraline + desmethylsertraline to guide dose adjustments. | 4A | III | (6;7) | ||
| PM | Yes | Consider a maximum daily dose of 50 mg and utilize clinical monitoring on response/side effects or therapeutic drug monitoring of sertraline + desmethylsertraline to guide dose adjustments. | 4C | III | ||||
| UM | No | Initiate therapy with recommended starting dose. | 4AA | |||||
| CYP2C19 | Voriconazole | CPIC: Yes | IM | No | Initiate therapy with recommended starting dose. | M | V | (32) |
| PM | Yes | Choose an alternative agent that is not dependent on CYP2C19 metabolism as primary therapy in lieu of voriconazole | M | V | ||||
| RM | Yes | Choose an alternative agent that is not dependent on CYP2C19 metabolism as primary therapy in lieu of voriconazole | M | II | ||||
| UM | Yes | Choose an alternative agent that is not dependent on CYP2C19 metabolism as primary therapy in lieu of voriconazole | M | V | ||||
| DPWG: Yes | IM | Yes | Monitor serum concentration | 4A | V | (5–7) | ||
| PM | Yes | Monitor serum concentration | 4A | V | ||||
| UM | Yes | Monitor serum concentration | 4A | V | ||||
| CYP2D6 | Amitriptyline | CPIC: Yes | IM | Yes | Consider 25% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. | M | III | (24;25) |
| PM | Yes | 1) Avoid amitriptyline use due to potential for side effects. Consider alternative drug not metabolized by CYP2D6. 2) If amitriptyline is warranted, consider 50% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. |
S | |||||
| UM | Yes | 1) Avoid amitriptyline use due to potential lack of efficacy. Consider alternative drug not metabolized by CYP2D6. 2) If amitriptyline is warranted, consider titrating to a higher target dose (compared to normal metabolizers). . Utilize therapeutic drug monitoring to guide dose adjustments |
S | |||||
| DPWG: Yes | IM | Yes |
1) Consider alternative drug not metabolized by CYP2D6. 2) If an alternative is not possible consider a decrease of up to 60% of the recommended dose under therapeutic drug monitoring of amitriptyline and nortriptyline. |
3C | III | (6;7) | ||
| PM | Yes | 1) Consider alternative drug not metabolized by CYP2D6. 2) If an alternative is not possible consider a decrease of up to 50% of the recommended dose under therapeutic drug monitoring of amitriptyline and nortriptyline. |
3A | |||||
| UM | Yes | 1) Consider alternative drug not metabolized by CYP2D6. 2) If an alternative is not possible consider an increase of up to 125% of the recommended dose under therapeutic drug monitoring of amitriptyline and nortriptyline. Be alert of a possible decrease in therapeutic levels and an increase of active cardiotoxic hydroxymetabolites. |
3C | |||||
| CYP2D6 | Clomipramine | CPIC: Yes | IM | Yes | Consider 25% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. | O | (24;25) | |
| PM | Yes | 1) Avoid clomipramine use due to potential for side effects. Consider alternative drug not metabolized by CYP2D6. 2) If clomipramine is warranted, consider 50% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. |
O | III | ||||
| UM | Yes | 1) Avoid clomipramine use due to potential lack of efficacy. Consider alternative drug not metabolized by CYP2D6. 2) If clomipramine is warranted, consider titrating to a higher target dose (compared to normal metabolizers). Utilize therapeutic drug monitoring to guide dose adjustments. |
O | |||||
| DPWG: Yes | IM | Yes | 1) Consider 30% reduction of recommended starting dose. 2) Utilize therapeutic drug monitoring of clomipramine and desmethylclomipramine. |
4C | (5–7) | |||
| PM | Yes | Depression: 1) Consider 60% reduction of recommended starting dose. 2) Utilize therapeutic drug monitoring of clomipramine and desmethylclomipramine. Anxiety: 1) Consider alternative drug not metabolized by CYP2D6. 2) If an alternative is not possible consider a decrease of up to 50% of the recommended dose under therapeutic drug monitoring of clomipramine and desmethylclomipramine. |
4C | III | ||||
| UM | Yes | 1) Consider alternative drug not metabolized by CYP2D6. 2) If an alternative is not possible consider an increase of up to 150% of the recommended dose under therapeutic drug monitoring of amitriptyline and nortriptyline. Be alert of a possible decrease in therapeutic levels and an increase of cardiotoxic active hydroxymetabolites. |
3C | |||||
| CYP2D6 | Codeine | CPIC: Yes | IM | Yes | Use label-recommended age- or weight-specific dosing. If no response, consider alternative analgesics such as morphine or a non-opioid. | M | (15;16) | |
| PM | Yes | Avoid codeine use due to lack of efficacy. | S | |||||
| UM | Yes | Avoid codeine use due to potential for toxicity. | S | |||||
| DPWG: Yes | IM | Yes | Cough: no action required Pain: Be on alert for a lack of clinical effect. In case of a lack of clinical effect consider a raise in daily dose or consider an alternative drug |
3A | (5–7) | |||
| PM | Yes | Cough: no action required / Pain: Consider an alternative drug | 4B | |||||
| UM | Yes | Contraindicated | 3F | |||||
| CYP2D6 | Doxepin | CPIC: Yes | IM | Yes | Consider 25% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. | O | (24;25) | |
| PM | Yes | 1) Avoid doxepin use due to potential for side effects. Consider alternative drug not metabolized by CYP2D6. 2) If doxepin is warranted, consider 50% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. |
O | |||||
| UM | Yes | 1) Avoid doxepin use due to potential lack of efficacy. Consider alternative drug not metabolized by CYP2D6. 2) If doxepin is warranted, consider titrating to a higher target dose (compared to normal metabolizers). Utilize therapeutic drug monitoring to guide dose adjustments. |
O | |||||
| DPWG: Yes | IM | Yes | Consider a 20% reduction of recommended starting dose. Utilize therapeutic drug monitoring to monitor doxepin and nordoxepin to guide dose adjustments. | 3A | (6;7) | |||
| PM | Yes | Consider a 60% reduction of recommended starting dose. Utilize therapeutic drug monitoring to monitor doxepin and nordoxepin to guide dose adjustments. | 3F | |||||
| UM | Yes | 1) Consider alternative drug not metabolized by CYP2D6. 2) If an alternative is not possible consider an increase of up to 200% of the recommended dose under therapeutic drug monitoring of doxepin and nordoxepin. |
3A | |||||
| CYP2D6 | Fluvoxamine | CPIC: Yes | IM | No | Initiate therapy with recommended starting dose. | M | (26) | |
| PM | No |
1) Consider a 25–50% reduction of recommended starting dose and titrate to response. 2) Use an alternative drug not metabolized by CYP2D6. |
O | IV | ||||
| UM | No | No recommendation due to lack of evidence. | O | |||||
| DPWG: No | IM | No | Initiate therapy with recommended starting dose | IN | (7) | |||
| PM | No | Initiate therapy with recommended starting dose | 3AA | IV | ||||
| UM | No | Initiate therapy with recommended starting dose | IN | |||||
| CYP2D6 | Imipramine | CPIC: Yes | IM | Yes | Consider 25% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. | O | (24;25) | |
| PM | Yes |
1) Avoid imipramine use due to potential for side effects. Consider alternative drug not metabolized by CYP2D6. 2) If imipramine is warranted, consider 50% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. |
O | III | ||||
| UM | Yes | 1) Avoid imipramine use due to potential lack of efficacy. Consider alternative drug not metabolized by CYP2D6. 2) If imipramine is warranted, consider titrating to a higher target dose (compared to normal metabolizers). Utilize therapeutic drug monitoring to guide dose adjustments. |
O | |||||
| DPWG: Yes | IM | Yes | Consider 30% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. | 4A | (5–7) | |||
| PM | Yes | Consider 70% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. | 4C | III | ||||
| UM | Yes | 1) Consider alternative drug not metabolized by CYP2D6. 2) If an alternative is not possible consider an increase of up to 170% of the recommended dose under therapeutic drug monitoring of imipramine and desipramine. |
4A | |||||
| CYP2D6 | Nortriptyline | CPIC: Yes | IM | Yes | Consider 25% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. | M | (24;25) | |
| PM | Yes | 1) Avoid nortriptyline use due to potential for side effects. Consider alternative drug not metabolized by CYP2D6. 2) If nortriptyline is warranted, consider 50% reduction of recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. |
S | |||||
| UM | Yes | 1) Avoid nortriptyline use due to potential lack of efficacy. Consider alternative drug not metabolized by CYP2D6. 2) If nortriptyline is warranted, consider titrating to a higher target dose (compared to normal metabolizers). Utilize therapeutic drug monitoring to guide dose adjustments. |
S | |||||
| DPWG: Yes | IM | Yes | Consider a 40% reduction of recommended starting dose. Utilize therapeutic drug monitoring of nortriptyline and 10-hydroxytriptyline to guide dose adjustments | 4C | (5–7) | |||
| PM | Yes | Consider a 60% reduction of recommended starting dose. Utilize therapeutic drug monitoring of nortriptyline and 10-hydroxytriptyline to guide dose adjustments | 3C | |||||
| UM | Yes | 1) Consider alternative drug not metabolized by CYP2D6 2) If an alternative is not possible consider an increase of up to 60% of the recommended dose. Utilize therapeutic drug monitoring of nortriptyline and 10-hydroxytriptyline to guide dose adjustments. |
3C | |||||
| CYP2D6 | Paroxetine | CPIC: Yes | IM | No | Initiate therapy with recommended starting dose. | M | (26) | |
| PM | Yes |
1) Consider alternative drug not predominantly metabolized by CYP2D6. 2) If paroxetine use warranted, consider a 50% reduction of recommended starting dose and titrate to response. |
O | III | ||||
| UM | Yes | Consider alternative drug not predominantly metabolized by CYP2D6. | S | |||||
| DPWG: Yes | IM | No | Initiate therapy with recommended starting dose | 4A | (5–7) | |||
| PM | No | Initiate therapy with recommended starting dose | 4A | III | ||||
| UM | Yes | Consider alternative drug not metabolized by CYP2D6 | 4C | |||||
| CYP3A5 | Tacrolimus | CPIC: Yes | IM (heterozygous expressor) | Yes | Increase starting dose 1.5 to 2 times recommended starting dose. Total starting dose should not exceed 0.3 mg/kg/day. Use therapeutic drug monitoring to guide dose adjustments. | S | (30) | |
| NM (homozygous expressor) | Yes | Increase starting dose 1.5 to 2 times recommended starting dose. Total starting dose should not exceed 0.3 mg/kg/day. Use therapeutic drug monitoring to guide dose adjustments. | S | III | ||||
| DPWG: Yes | Heterozygous expressor | Yes | Increase starting dose 1.75 times recommended starting dose. Total starting dose should not exceed 0.3 mg/kg/day. Use therapeutic drug monitoring to guide dose adjustments. | 4E | (6;7) | |||
| Homozygous expressor | Yes | Increase starting dose 2.5 times recommended starting dose. Total starting dose should not exceed 0.3 mg/kg/day. Use therapeutic drug monitoring to guide dose adjustments. | 4E | III | ||||
| DPYD | Capecitabine/5-Fluorouracil | CPIC: Yes | IM | Yes | Start with at least a 50% reduction in starting dose followed by titration of dose based on toxicity or pharmacokinetic test (if available). | M | (14) | |
| PM | Yes | Select alternate drug. | S | |||||
| DPWG: Yes | 1.5 | Yes | Start with at least a 75% reduction in star ting dose followed by titration of dose based on toxicity and efficacy. | 4F | II | (6;7;51) | ||
| 1.0 | Yes | Start with at least a 50% reduction in starting dose followed by titration of dose based on toxicity and efficacy. | 4F | |||||
| 0.5 | Yes | Start with at least a 25% reduction in starting dose followed by titration of dose based on toxicity and efficacy. | 4F | II | ||||
| 0.0 | Yes | Select alternate drug. | 4F | |||||
| DPYD | Tegafur | CPIC: Yes | IM | Yes | Start with at least a 50% reduction in starting dose followed by titration of dose based on toxicity or pharmacokinetic test (if available). | M | III | (14) |
| PM | Yes | Select alternate drug. | S | |||||
| DPWG: Yes | 1.5 | Yes | Select alternate drug. | 2E | (6;7;51) | |||
| 1.0 | Yes | Select alternate drug. | 2D | III | ||||
| 0.5 | Yes | Select alternate drug. | 0E | |||||
| 0.0 | Yes | Select alternate drug. | 0E | |||||
| HLA-B | Abacavir | CPIC: Yes | *57:01/* X; *57:01/*57:01 | Yes | Abacavir is not recommended. | S | (12;13) | |
| DPWG: Yes | *57:01/* X; *57:01/*57:01 | Yes | Abacavir is contraindicated. | 4E | (6;7) | |||
| HLA-B | Carbamazepine | CPIC: Yes | *15:02/* X; *15:02/*15:02 | Yes | A. If patient is carbamazepine-naive, do not use carbamazepine B. If patient has previously used carbamazepine for longer than 3 months without incidence of cutaneous adverse reactions, cautiously consider use of carbamazepine |
S(A) O(B) |
(11) | |
| DPWG: Yes | *15:02/* X; *15:02/*15:02 *15:11/* X; *15:11/*15:11 *31:01/* X; *31:01/*31:01 |
Yes | For HLA-B*1502, the recommendation is to choose an alternative. If an alternative is possible, choosing an alternative is also recommended for HLA-A*3101 and HLA-B*151 | 4E (ALL | (7) | |||
| SLCO1B1 | Simvastatin | CPIC: Yes | Decreased Function (521TC) | Yes | 1) Prescribe a lower dose. 2) Consider an alternative statin (e.g., pravastatin or rosuvastatin). 3) Consider routine CK surveillance. |
S | (22;23) | |
| Poor Function (521CC) | Yes | 1) Prescribe a lower dose. 2) Consider an alternative statin (e.g., pravastatin or rosuvastatin). 3) Consider routine CK surveillance. |
S | |||||
| DPWG: Yes | 521TC | Yes | 1) Consider alternative drug. 2) If simvastatin is warranted, prescribe a maximum dose of 40 mg/day. |
4D | (7) | |||
| 521CC | Yes | Select alternative drug. | 4D | |||||
| TPMT | Azathioprine/mercaptopurine | CPIC: Yes | IM | Yes | If disease treatment normally starts at the “full dose”, consider starting at 30–70% of target dose (e.g., 1–1.5 mg/kg/d), and titrate based on tolerance. Allow 2–4 weeks to reach steady state after each dose adjustment. | S | (8;9) | |
| PM | Yes | 1) Consider alternative agents. 2) If using azathioprine start with drastically reduced doses (reduce daily dose by 10-fold and dose thrice weekly instead of daily) and adjust doses of azathioprine based on degree of myelosuppression and disease-specific guidelines. Allow 4–6 weeks to reach steady state after each dose adjustment. Azathioprine is the likely cause of myelosuppression. |
S | |||||
| DPWG: Yes | IM | Yes | 1) Select alternative drug. 2) Reduce dose by 50%. Increase dose in response of hematologic monitoring and efficacy. |
4E | (6;7) | |||
| PM | Yes | 1) Select alternative drug. 2) Reduce dose by 90%. Increase dose in response of hematologic monitoring and efficacy. |
4F | |||||
| TPMT | Thioguanine | CPIC: Yes | IM | Yes | Start with reduced doses (reduce by 30–50%) and adjust doses of thioguanine based on degree of myelosuppression and disease-specific guidelines. Allow 2–4 weeks to reach steady state after each dose adjustment. In setting of myelosuppression, and depending on other therapy, emphasis should be on reducing thioguanine over other agents. | M | V | (8;9) |
| PM | Yes | Start with drastically reduced doses (reduce daily dose by 10-fold and dose thrice weekly instead of daily) and adjust doses of thioguanine based on degree of myelosuppression and disease-specific guidelines. Allow 4–6 weeks to reach steady state after each dose adjustment. In setting of myelosuppression, emphasis should be on reducing thioguanine over other agents. For nonmalignant conditions, consider alternative nonthiopurine immunosuppressant therapy. | S | |||||
| DPWG: Yes | IM | Yes | 1) Select alternative drug, 2) Reduce dose by 25%. Increase dose in response of hematologic monitoring and efficacy. |
3E | V | (6;7) | ||
| PM | Yes | 1) Select alternative drug. 2) Reduce dose to 6–7% of starting dose. Increase dose in response of hematologic monitoring and efficacy. |
2F | |||||
| VKORC1 | Warfarin | CPIC: Yes | -1639GA | Yes | Calculate dose based on validated published pharmacogenetic algorithm | S | V | (17;18) |
| -1639AA | Yes | Calculate dose based on validated published pharmacogenetic algorithm | S | V | ||||
| DPWG: Yes | -1639GA | Yes | Initiate therapy with recommended starting dose | 4A | V | (7) | ||
| -1639AA | Yes | Consider a reduction to 60% of the normal starting dose | 4A | V | ||||
IM = intermediate metabolizer; NM = normal metabolizer; PM = poor metabolizer; RM = rapid metabolizer; UM = ultra-rapid metabolizer; ADE = adverse drug event; M = moderate; S = strong; O = Optional; IN = Insufficient evidence; 0 = data on file; 1 = published incomplete case reports; 2 = well documented case reports / case series; 3 = published controlled studies of moderate quality; 4 = published controlled studies of good quality; A = minor clinical effect; B = clinical effect : short-lived discomfort (<48 h) without permanent injury; C = clinical effect: long-standing discomfort (48–168 h) without permanent injury; D = clinical effect: long-standing effect (>168) and permanent symptom or invalidating injury; E = Increased risk of failure of lifesaving therapy / expected bone marrow depression; F = death, arrhythmia, unexpected bone marrow depression
Category of discordance: I = discordance in allele classification, II = discordance in genotype to phenotype translation, III = discordance in therapeutic recommendation attributed to a difference in methodology of the two consortia, IV = discordance in therapeutic recommendation attributed to a time-effect, V = discordance in therapeutic recommendation attributed to a difference in clinical practice.
CYP2C19 & CYP2D6 + Tricyclic antidepressants
The DPWG has provided individual therapeutic recommendations for CYP2D6 and the tricyclic antidepressants (TCAs) amitriptyline, clomipramine, doxepin, nortriptyline, and imipramine, as well as CYP2C19 and imipramine based on AUC and steady-state concentrations (7). CPIC additionally provides recommendations for CYP2C19 and amitriptyline, clomipramine, doxepin, and trimipramine as well as CYP2D6 and desipramine, while the DPWG has not (yet) provided therapeutic recommendations because the consortium has categorized these gene-drug pairs as low clinical impact based on the scientific literature (7). As a result of the used methodology, the CPIC has provided equal therapeutic recommendations for the TCAs amitriptyline, clomipramine, doxepin, imipramine and trimipramine to avoid these drugs in CYP2C19 ultra-rapid, rapid and poor metabolizers. In addition, the same therapeutic recommendation of a 25% dose reduction is provided for CYP2D6 intermediate metabolizers and a recommendation to avoid TCAs in CYP2D6 ultra-rapid and poor metabolizers (Table 4).
Other differences in the therapeutic recommendations for TCAs can be seen in the dosing recommendations for CYP2D6 ultra-rapid metabolizers. In this case, CPIC recommends to avoid using a TCA due to the potential lack of efficacy and to consider an alternative drug not metabolized by CYP2D6. If a TCA is warranted, CPIC recommends to consider titrating to a higher target dose and using therapeutic drug monitoring to guide dose adjustments. In contrast, the DPWG provides specific, PK-based dosing advice. If a TCA is warranted, the DPWG recommends starting dosages for amitriptyline, clomipramine, doxepin, imipramine, and nortriptyline (see Figure 2) of 125%, 150%, 200%, 170% and 160% of the normal starting doses, respectively, followed by a recommendation to utilize therapeutic drug monitoring (6;7;24) (Table 4).
DPYD + fluoropyrimidines
As mentioned previously, the DPWG uses an AS for DPYD while the CPIC uses phenotypes of normal/high, intermediate and deficient activity (soon to be changed to normal, intermediate, and poor metabolizers, respectively, in the next DPYD guideline update based on the results of the CPIC term standardization project) (14;51). The gene activity model scores alleles with a reduced function as 0.5, while fully dysfunctional alleles are classified as 0. The AS model allows scores of 1.5 and 0.5 in addition to the scores of 2.0 (EM), 1.0 (IM) and 0.0 (PM). Both guidelines include an initial 50% dose-reduction for intermediate metabolizers (AS = 1) and a recommendation to switch to an alternative drug for poor metabolizers (AS = 0). The DPWG also contains a recommendation for 25% and 75% dose reduction of the starting dose for the AS of 1.5 and 0.5, respectively (7;14) (Table 4).
TPMT + thiopurines
In the guidelines of the DPWG the dosing advice for azathioprine and 6-mercaptopurine are a 50% dose-reduction and 90% dose-reduction for TPMT intermediate metabolizers and poor metabolizers, respectively. The CPIC recommend a mean starting dose of 50% (range 30–70%) and 10% of the conventional dose for TPMT intermediate metabolizers and poor metabolizers, respectively. Thrice weekly dosing instead of the normal daily dosing is also recommended for poor metabolizers. In the case of thioguanine the DPWG recommends a slightly (15%) smaller dose reduction for intermediate metabolizers compared to the 30–50% dose reduction and thrice weekly dosing advised in the CPIC guideline (Table 4).
Analysis of differences
From the 19 guidelines published by CPIC (covering 40 gene-drug pairs) and 86 guidelines by the DPWG, 27 guidelines cover the same gene-drug pairs. Based upon the comparison of the guidelines, there is substantial agreement between the recommendations given by the two consortia. However, for 13 gene-drug pairs there are differences (≥20%) in therapeutic recommendations for one or more aberrant phenotypes. Most of the observed differences in therapeutic recommendations probably result from differences in applied methodologies. In some cases this results in a situation where CPIC provides a recommendation for a gene-drug combination based on an expert consensus formed for a group of drugs (e.g. TCAs), whereas the DPWG finds the evidence for certain individual gene-drug combinations insufficient and is unable to calculate a dosing recommendation (e.g. CYP2C19 and amitriptyline). In other cases both consortia recognize a gene-drug interaction, but come to a different dosing recommendation for a certain phenotype as a result of the used methodology (e.g. the CYP2C19 poor metabolizer and imipramine). A second explanation of the differences between the CPIC and DPWG are the result of a “time effect,” as literature searches are performed at different time points by the two consortia and new articles are published continuously (category IV, see Table 4). For example, a difference exists in the therapeutic recommendation for fluvoxamine between the guideline for CYP2D6 and CYP2C19 genotypes and dosing of SSRIs of CPIC from 2015 and the DPWG guideline for fluvoxamine. This difference can partially be explained by the article of Suzuki et al. which was not included in the DPWG guidelines because it was published after the literature search of the DPWG (64). This article showed a significant effect of CYP2D6 genotype on fluvoxamine steady-state concentration. This example underscores the need to update existing recommendations.
Additionally, differences in therapeutic recommendations are sometimes the result of differences in clinical practices between countries (category V, see Table 4). An example of this difference can be seen in the recommendations for the gene-drug combination of CYP2C19 and voriconazole (6;7;32). The CPIC recommends to choose an alternative agent that is not dependent on CYP2C19 metabolism in poor and ultra-rapid metabolizers and a standard regimen in intermediate metabolizers. Therapeutic drug monitoring is mentioned as a factor that can warrant a change in dosing regimen or choice of drug similar to other clinical factors (e.g. drug-drug interactions or impaired renal/hepatic function) that can lead to change in selection of therapy or dose adjustments. Therapeutic drug monitoring is only mentioned specifically in the therapeutic recommendation for a poor metabolizer in the event that voriconazole is considered more appropriate than alternative agents. In contrast, DPWG provides the recommendation to start with a standard of care dosing and always follow-up with therapeutic drug monitoring in case of poor, intermediate and ultra-rapid metabolizers. These differences between the guidelines clearly show a difference in the place of therapeutic monitoring within voriconazole therapy between the different practice settings.
Another example of differences as a result of clinical practice can be seen in the recommendations for coumarins. The DPWG and CPIC both provide recommendations for the gene-drug pairs of CYP2C9 and VKORC1 and warfarin. In addition the DPWG also provides therapeutic recommendations for acenocoumarol & phenprocoumon which are mainly used in the Netherlands (6;7;17;18). The CPIC provides recommended daily maintenance dosing regimen for warfarin in mg/day based on specific algorithms, while the DPWG guidelines only provides a decrease in the loading dose. In the Netherlands, patients using coumarins are strictly monitored by anticoagulation clinics using the international normalized ratio (INR). With this strict control of the INR, the need for a predicted daily coumarin dose based on pharmacogenetic information is limited. Due to the large difference in the monitoring of patients the added clinical value of pharmacogenetics is different in both settings, resulting in other therapeutic recommendations of the DPWG and CPIC, respectively.
Specifically for CYP2D6, differences in the translation of genotypes to phenotypes that exist between the guidelines of the two consortia can be explained by the different interpretations of certain genotypes throughout literature. For the gene CYP2D6, some consider an AS of 1.0 an intermediate metabolizer, while the package insert of the Amplichip categorizes this score as a normal metabolizer (56). In part this is due to variability in how AS is translated into phenotype for different probe drugs. Studies using tramadol, dextromethorphan and sparteïne as the probe drug show no difference in the kinetic profile between individuals with and AS of 1.0 and individuals with an AS of 2.0 (54;55;65–69). In contrast, using trimipramine, doxepine, haloperidol and debrisoquine as the probe drug shows a significant difference between individuals with an AS 1.0 and 2.0 (54;70–75). Specifically for CYP2D6, it can be concluded that the use of different model substrates have led to mixed interpretations of genotypes which in turn have led to different interpretations of the AS of 1.0 by the two consortia. In fact, an international team of CPIC and DPWG members has recently agreed to try to impose standards on how AS are interpreted into phenotypes for major CYP2D6 substrates.
As previously mentioned, the differences between guidelines can potentially lead to differences in dosages or choice of drugs for patients with the same genotype. In case of some discordances a minor update (e.g., an update of the status of the CYP2C9*8 and CYP2D6*36 alleles in the DPWG guidelines) (category I, IV) can solve discrepancies, while in some cases harmonization is warranted to create uniform interpretations of genotypes into phenotypes (category II).
Finally, the difference in publication strategy should also be addressed. A current disadvantage of the DPWG guidelines is the limited availability in English. In addition, the available English versions date back to 2011 and do not contain the most recent information. Currently, as a part of the EU granted Ubiquitous Pharmacogenomics (U-PGx) project, the DPWG is working on the English translation of their therapeutic recommendations and it is anticipated that in the near future these documents will be made available to clinicians of other nations in the form of European guidelines which further strengthens the need for harmonization with CPIC (http://upgx.eu/) (76).
In conclusion, this comparison shows that the CPIC and the DPWG guidelines are generally similar in terms of allele classification, genotype to phenotype translations and therapeutic recommendations for most gene-drug pairs. However, some differences between the guidelines of the two consortia exist and should be harmonized where possible, especially in the case of different allele classifications and genotype to phenotype translations.
Methodology of comparison
The process of guideline synthesis was compared based on the information provided in papers describing the two initiatives (5;34). A list of gene-drug pairs evaluated by both DPWG and CPIC was created. CPIC guidelines published up to March 2017 were extracted from https://cpicpgx.org/guidelines/. Information on DPWG guidelines was extracted from the G-standard on the 1st of March 2017. For each gene-drug pair, guidelines were systematically compared for used terminology (used for the determination of allele function and phenotypes), allele classification / genotype, dose recommendations, evidence, and clinical relevance scores. Differences in allele classification were labeled as a category I difference and differences in genotype to phenotype translation were labeled as a category II difference. Relevant differences in therapeutic recommendations were defined as different therapeutic strategies (e.g., no adjustment vs. changes in dose vs. alternate therapy) or a ≥ 20% difference in the recommended dose between the two guidelines for a specific genotype. The found relevant differences were then subdivided based on the attributed explanation for the differences. Discordances in therapeutic recommendations presumably explained by differences in the methodologies of the two consortia were allocated to category III, discordances presumably explained by a time-effect were classified as category IV, and discordances in recommendations which were presumably explained by differences in clinical practice between nations were allocated to category V.
Table 3.
Discordances in genotype to phenotype translation
| Gene | Genotype / activity score | Classification* | |
|---|---|---|---|
| CYP2C19 | *1/*17 | CPIC: Rapid metabolizer | (24;26;44–50) |
| DPWG: Normal metabolizer | (5;6;44–50) | ||
| CYP2D6 | AS 1.0 | CPIC: Normal metabolizer | (15;16) |
| DPWG: Intermediate metabolizer | (57) | ||
| CYP2D6 | AS 2.5 | CPIC: Ultra-rapid metabolizer | (15;16) |
| DPWG: Normal metabolizer | (7) | ||
| DPYD | 2846AT / 1236GA | CPIC: Normal metabolizer | (14) |
| DPWG: AS 1.5 / Intermediate metabolizer | (7;14;51) | ||
| DPYD | e.g. (*2A + (2846AT or 1236GA)) | CPIC: Intermediate metabolizer | (14) |
| DPWG: AS 0.5 / Intermediate metabolizer | (7;14;51) |
As a result of a consensus in a CPIC project to standardize terms for PGx test results the CPIC has adopted the term normal metabolizer to replace the historical term extensive metabolizer as experts participating in the CPIC project found it less confusing for clinicians. In this comparison the term normal metabolizer is used (See Table 2) (38).
Acknowledgments
We would like to thank A. Pastor-Clériques for his assistance in collecting parts of data for this study.
Footnotes
Author contributions
PB, JS and HJG designed the research; PB performed the research; PB, JS, HJG, KC, RG, MW, TK and MR analysed the data; PB, JS, HJG, KC, RG, MW, TK and MR wrote the manuscript.
Disclosures
This work was funded by the National Institutes of Health (NIH) CPIC (R24GM115264), PharmGKB (R24 GM61374) and European Community H2020 Programme (U-PGx - 668353).
Reference List
- 1.Swen JJ, Huizinga TW, Gelderblom H, de Vries EG, Assendelft WJ, Kirchheiner J, et al. Translating pharmacogenomics: challenges on the road to the clinic. PLoS Med. 2007;4(8):e209. doi: 10.1371/journal.pmed.0040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Relling MV, Altman RB, Goetz MP, Evans WE. Clinical implementation of pharmacogenomics: overcoming genetic exceptionalism. Lancet Oncol. 2010;11(6):507–9. doi: 10.1016/S1470-2045(10)70097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89(3):464–7. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirchheiner J, Brosen K, Dahl ML, Gram LF, Kasper S, Roots I, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173–92. doi: 10.1034/j.1600-0447.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 5.Swen JJ, Wilting I, de Goede AL, Grandia L, Mulder H, Touw DJ, et al. Pharmacogenetics: from bench to byte. Clin Pharmacol Ther. 2008;83(5):781–7. doi: 10.1038/sj.clpt.6100507. [DOI] [PubMed] [Google Scholar]
- 6.Swen JJ, Nijenhuis M, de BA, Grandia L, Maitland-van der Zee AH, Mulder H, et al. Pharmacogenetics: from bench to byte--an update of guidelines. Clin Pharmacol Ther. 2011;89(5):662–73. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 7.Geneesmiddel Informatie Centrum. Informatorium Medicamentorum. The Hague: KNMP; 2016. [Google Scholar]
- 8.Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93(4):324–5. doi: 10.1038/clpt.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89(3):387–91. doi: 10.1038/clpt.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hershfield MS, Callaghan JT, Tassaneeyakul W, Mushiroda T, Thorn CF, Klein TE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther. 2013;93(2):153–8. doi: 10.1038/clpt.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leckband SG, Kelsoe JR, Dunnenberger HM, George AL, Jr, Tran E, Berger R, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for HLA-B genotype and carbamazepine dosing. Clin Pharmacol Ther. 2013;94(3):324–8. doi: 10.1038/clpt.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin MA, Hoffman JM, Freimuth RR, Klein TE, Dong BJ, Pirmohamed M, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for HLA-B Genotype and Abacavir Dosing: 2014 update. Clin Pharmacol Ther. 2014;95(5):499–500. doi: 10.1038/clpt.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin MA, Klein TE, Dong BJ, Pirmohamed M, Haas DW, Kroetz DL. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing. Clin Pharmacol Ther. 2012;91(4):734–8. doi: 10.1038/clpt.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caudle KE, Thorn CF, Klein TE, Swen JJ, McLeod HL, Diasio RB, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther. 2013;94(6):640–5. doi: 10.1038/clpt.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95(4):376–82. doi: 10.1038/clpt.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crews KR, Gaedigk A, Dunnenberger HM, Klein TE, Shen DD, Callaghan JT, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther. 2011;91(2):321–6. doi: 10.1038/clpt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90(4):625–9. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SA, et al. Clinical pharmacogenetics implementation consortium (cpic) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther. 2017 doi: 10.1002/cpt.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caudle KE, Rettie AE, Whirl-Carrillo M, Smith LH, Mintzer S, Lee MT, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther. 2014;96(5):542–8. doi: 10.1038/clpt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317–23. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90(2):328–32. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsey LB, Johnson SG, Caudle KE, Haidar CE, Voora D, Wilke RA, et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther. 2014;96(4):423–8. doi: 10.1038/clpt.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilke RA, Ramsey LB, Johnson SG, Maxwell WD, McLeod HL, Voora D, et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther. 2012;92(1):112–7. doi: 10.1038/clpt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicks JK, Swen JJ, Thorn CF, Sangkuhl K, Kharasch ED, Ellingrod VL, et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther. 2013;93(5):402–8. doi: 10.1038/clpt.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hicks JK, Sangkuhl K, Swen JJ, Ellingrod VL, Muller DJ, Shimoda K, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2016 doi: 10.1002/cpt.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicks JK, Bishop JR, Sangkuhl K, Muller DJ, Ji Y, Leckband SG, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin Pharmacol Ther. 2015;98(2):127–34. doi: 10.1002/cpt.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Relling MV, McDonagh EM, Chang T, Caudle KE, McLeod HL, Haidar CE, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clin Pharmacol Ther. 2014;96(2):169–74. doi: 10.1038/clpt.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clancy JP, Johnson SG, Yee SW, McDonagh EM, Caudle KE, Klein TE, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for ivacaftor therapy in the context of CFTR genotype. Clin Pharmacol Ther. 2014;95(6):592–7. doi: 10.1038/clpt.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muir AJ, Gong L, Johnson SG, Lee MT, Williams MS, Klein TE, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for IFNL3 (IL28B) genotype and PEG interferon-alpha-based regimens. Clin Pharmacol Ther. 2014;95(2):141–6. doi: 10.1038/clpt.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther. 2015;98(1):19–24. doi: 10.1002/cpt.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gammal RS, Court MH, Haidar CE, Iwuchukwu OF, Gaur AH, Alvarellos M, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing. Clin Pharmacol Ther. 2016;99(4):363–9. doi: 10.1002/cpt.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moriyama B, Obeng AO, Barbarino J, Penzak SR, Henning SA, Scott SA, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC(R)) Guideline for CYP2C19 and Voriconazole Therapy. Clin Pharmacol Ther. 2016 doi: 10.1002/cpt.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414–7. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caudle KE, Klein TE, Hoffman JM, Muller DJ, Whirl-Carrillo M, Gong L, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209–17. doi: 10.2174/1389200215666140130124910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caudle KE, Gammal RS, Whirl-Carrillo M, Hoffman JM, Relling MV, Klein TE. Evidence and resources to implement pharmacogenetic knowledge for precision medicine. Am J Health Syst Pharm. 2016;73(23):1977–85. doi: 10.2146/ajhp150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valdes R. General Introduction and Scope. In: Valdes R, Payne D, Linder MW, editors. LABORATORY MEDICINE PRACTICE GUIDELINES - Laboratory Analysis and Application of Pharmacogenetics to Clinical Practice. The National Academy of Clinical Biochemistry (NACB); 2010. pp. 1–2. [Google Scholar]
- 37.van Roon EN, Flikweert S, le CM, Langendijk PN, Kwee-Zuiderwijk WJ, Smits P, et al. Clinical relevance of drug-drug interactions : a structured assessment procedure. Drug Saf. 2005;28(12):1131–9. doi: 10.2165/00002018-200528120-00007. [DOI] [PubMed] [Google Scholar]
- 38.Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet Med. 2016 doi: 10.1038/gim.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaisdell J, Jorge-Nebert LF, Coulter S, Ferguson SS, Lee SJ, Chanas B, et al. Discovery of new potentially defective alleles of human CYP2C9. Pharmacogenetics. 2004;14(8):527–37. doi: 10.1097/01.fpc.0000114759.08559.51. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Jeong H, Takahashi H, Drozda K, Patel SR, Shapiro NL, et al. Decreased warfarin clearance associated with the CYP2C9 R150H (*8) polymorphism. Clin Pharmacol Ther. 2012;91(4):660–5. doi: 10.1038/clpt.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allabi AC, Gala JL, Horsmans Y. CYP2C9, CYP2C19, ABCB1 (MDR1) genetic polymorphisms and phenytoin metabolism in a Black Beninese population. Pharmacogenet Genomics. 2005;15(11):779–86. doi: 10.1097/01.fpc.0000174787.92861.91. [DOI] [PubMed] [Google Scholar]
- 42.Scott SA, Jaremko M, Lubitz SA, Kornreich R, Halperin JL, Desnick RJ. CYP2C9*8 is prevalent among African-Americans: implications for pharmacogenetic dosing. Pharmacogenomics. 2009;10(8):1243–55. doi: 10.2217/pgs.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cespedes-Garro C, Fricke-Galindo I, Naranjo ME, Rodrigues-Soares F, Farinas H, de AF, et al. Worldwide interethnic variability and geographical distribution of CYP2C9 genotypes and phenotypes. Expert Opin Drug Metab Toxicol. 2015;11(12):1893–905. doi: 10.1517/17425255.2015.1111871. [DOI] [PubMed] [Google Scholar]
- 44.Rudberg I, Mohebi B, Hermann M, Refsum H, Molden E. Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther. 2008;83(2):322–7. doi: 10.1038/sj.clpt.6100291. [DOI] [PubMed] [Google Scholar]
- 45.Ohlsson RS, Mwinyi J, Andersson M, Baldwin RM, Pedersen RS, Sim SC, et al. Kinetics of omeprazole and escitalopram in relation to the CYP2C19*17 allele in healthy subjects. Eur J Clin Pharmacol. 2008;64(12):1175–9. doi: 10.1007/s00228-008-0529-z. [DOI] [PubMed] [Google Scholar]
- 46.Baldwin RM, Ohlsson S, Pedersen RS, Mwinyi J, Ingelman-Sundberg M, Eliasson E, et al. Increased omeprazole metabolism in carriers of the CYP2C19*17 allele; a pharmacokinetic study in healthy volunteers. Br J Clin Pharmacol. 2008;65(5):767–74. doi: 10.1111/j.1365-2125.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li-Wan-Po A, Girard T, Farndon P, Cooley C, Lithgow J. Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol. 2010;69(3):222–30. doi: 10.1111/j.1365-2125.2009.03578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schenk PW, van VM, Mathot RA, van GT, Vulto AG, van Fessem MA, et al. The CYP2C19*17 genotype is associated with lower imipramine plasma concentrations in a large group of depressed patients. Pharmacogenomics J. 2010;10(3):219–25. doi: 10.1038/tpj.2009.50. [DOI] [PubMed] [Google Scholar]
- 49.de VA, van der Weide J, Loovers HM. Association between CYP2C19*17 and metabolism of amitriptyline, citalopram and clomipramine in Dutch hospitalized patients. Pharmacogenomics J. 2011;11(5):359–67. doi: 10.1038/tpj.2010.39. [DOI] [PubMed] [Google Scholar]
- 50.Harmsze AM, van Werkum JW, Hackeng CM, Ruven HJ, Kelder JC, Bouman HJ, et al. The influence of CYP2C19*2 and *17 on on-treatment platelet reactivity and bleeding events in patients undergoing elective coronary stenting. Pharmacogenet Genomics. 2012;22(3):169–75. doi: 10.1097/FPC.0b013e32834ff6e3. [DOI] [PubMed] [Google Scholar]
- 51.Henricks LM, Lunenburg CA, Meulendijks D, Gelderblom H, Cats A, Swen JJ, et al. Translating DPYD genotype into DPD phenotype: using the DPYD gene activity score. Pharmacogenomics. 2015;16(11):1277–86. doi: 10.2217/pgs.15.70. [DOI] [PubMed] [Google Scholar]
- 52.Raimundo S, Fischer J, Eichelbaum M, Griese EU, Schwab M, Zanger UM. Elucidation of the genetic basis of the common ‘intermediate metabolizer’ phenotype for drug oxidation by CYP2D6. Pharmacogenetics. 2000;10(7):577–81. doi: 10.1097/00008571-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Zanger UM, Fischer J, Raimundo S, Stuven T, Evert BO, Schwab M, et al. Comprehensive analysis of the genetic factors determining expression and function of hepatic CYP2D6. Pharmacogenetics. 2001;11(7):573–85. doi: 10.1097/00008571-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(1):23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- 55.Chou WH, Yan FX, Robbins-Weilert DK, Ryder TB, Liu WW, Perbost C, et al. Comparison of two CYP2D6 genotyping methods and assessment of genotype-phenotype relationships. Clin Chem. 2003;49(4):542–51. doi: 10.1373/49.4.542. [DOI] [PubMed] [Google Scholar]
- 56.Kirchheiner J, Nickchen K, Bauer M, Wong ML, Licinio J, Roots I, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442–73. doi: 10.1038/sj.mp.4001494. [DOI] [PubMed] [Google Scholar]
- 57.Schaik RHN, Grandia L, de Goede A, Bet PM, Bekers O, Guchelaar HJ, et al. Nederlandse consensus: CYP2D6 genotype ‘1-0’ ingedeeld als intermediaire metaboliseerder. Ned Tijdschr Klin Chem Labgeneesk. 2008;33:52–3. [Google Scholar]
- 58.Chida M, Ariyoshi N, Yokoi T, Nemoto N, Inaba M, Kinoshita M, et al. New allelic arrangement CYP2D6*36 × 2 found in a Japanese poor metabolizer of debrisoquine. Pharmacogenetics. 2002;12(8):659–62. doi: 10.1097/00008571-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Soyama A, Saito Y, Ohno Y, Komamura K, Kamakura S, Kitakaze M, et al. Diverse structures of chimeric CYP-REP7/6-containing CYP2D6 and a novel defective CYP2D6 haplotype harboring single-type *36 and CYP-REP7/6 in Japanese. Drug Metab Pharmacokinet. 2006;21(5):395–405. doi: 10.2133/dmpk.21.395. [DOI] [PubMed] [Google Scholar]
- 60.Soyama A, Saito Y, Kubo T, Miyajima A, Ohno Y, Komamura K, et al. Sequence-based analysis of the CYP2D6*36-CYP2D6*10 tandem-type arrangement, a major CYP2D6*10 haplotype in the Japanese population. Drug Metab Pharmacokinet. 2006;21(3):208–16. doi: 10.2133/dmpk.21.208. [DOI] [PubMed] [Google Scholar]
- 61.Gaedigk A, Bradford LD, Alander SW, Leeder JS. CYP2D6*36 gene arrangements within the cyp2d6 locus: association of CYP2D6*36 with poor metabolizer status. Drug Metab Dispos. 2006;34(4):563–9. doi: 10.1124/dmd.105.008292. [DOI] [PubMed] [Google Scholar]
- 62.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83(2):234–42. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 63.Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: a critical assessment and call for standardization. Curr Drug Metab. 2014;15(2):218–32. doi: 10.2174/1389200215666140202215316. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki Y, Sugai T, Fukui N, Watanabe J, Ono S, Inoue Y, et al. CYP2D6 genotype and smoking influence fluvoxamine steady-state concentration in Japanese psychiatric patients: lessons for genotype-phenotype association study design in translational pharmacogenetics. J Psychopharmacol. 2011;25(7):908–14. doi: 10.1177/0269881110370504. [DOI] [PubMed] [Google Scholar]
- 65.Eichelbaum M, Spannbrucker N, Steincke B, Dengler HJ. Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. Eur J Clin Pharmacol. 1979;16(3):183–7. doi: 10.1007/BF00562059. [DOI] [PubMed] [Google Scholar]
- 66.Gaedigk A, Bradford LD, Marcucci KA, Leeder JS. Unique CYP2D6 activity distribution and genotype-phenotype discordance in black Americans. Clin Pharmacol Ther. 2002;72(1):76–89. doi: 10.1067/mcp.2002.125783. [DOI] [PubMed] [Google Scholar]
- 67.Schmid B, Bircher J, Preisig R, Kupfer A. Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther. 1985;38(6):618–24. doi: 10.1038/clpt.1985.235. [DOI] [PubMed] [Google Scholar]
- 68.Wojtczak A, Rychlik-Sych M, Krochmalska-Ulacha E, Skretkowicz J. CYP2D6 phenotyping with dextromethorphan. Pharmacol Rep. 2007;59(6):734–8. [PubMed] [Google Scholar]
- 69.Frank D, Jaehde U, Fuhr U. Evaluation of probe drugs and pharmacokinetic metrics for CYP2D6 phenotyping. Eur J Clin Pharmacol. 2007;63(4):321–33. doi: 10.1007/s00228-006-0250-8. [DOI] [PubMed] [Google Scholar]
- 70.Mahgoub A, Idle JR, Dring LG, Lancaster R, Smith RL. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977;2(8038):584–6. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- 71.Sachse C, Brockmoller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60(2):284–95. [PMC free article] [PubMed] [Google Scholar]
- 72.Dalen P, Dahl ML, Eichelbaum M, Bertilsson L, Wilkinson GR. Disposition of debrisoquine in Caucasians with different CYP2D6-genotypes including those with multiple genes. Pharmacogenetics. 1999;9(6):697–706. doi: 10.1097/01213011-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Brockmoller J, Kirchheiner J, Schmider J, Walter S, Sachse C, Muller-Oerlinghausen B, et al. The impact of the CYP2D6 polymorphism on haloperidol pharmacokinetics and on the outcome of haloperidol treatment. Clin Pharmacol Ther. 2002;72(4):438–52. doi: 10.1067/mcp.2002.127494. [DOI] [PubMed] [Google Scholar]
- 74.Kirchheiner J, Meineke I, Muller G, Roots I, Brockmoller J. Contributions of CYP2D6, CYP2C9 and CYP2C19 to the biotransformation of E- and Z-doxepin in healthy volunteers. Pharmacogenetics. 2002;12(7):571–80. doi: 10.1097/00008571-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Kirchheiner J, Muller G, Meineke I, Wernecke KD, Roots I, Brockmoller J. Effects of polymorphisms in CYP2D6, CYP2C9, and CYP2C19 on trimipramine pharmacokinetics. J Clin Psychopharmacol. 2003;23(5):459–66. doi: 10.1097/01.jcp.0000088909.24613.92. [DOI] [PubMed] [Google Scholar]
- 76.van der Wouden CH, Cambon-Thomsen A, Cecchin E, Cheung KC, Lucia Davila-Fajardo C, Deneer VH, et al. Implementing Pharmacogenomics in Europe: Design and Implementation Strategy of the Ubiquitous Pharmacogenomics Consortium. Clin Pharmacol Ther. 2016 doi: 10.1002/cpt.602. [DOI] [PubMed] [Google Scholar]