Abstract
Papillary adenocarcinoma of the lungs is the most common primary lung adenocarcinoma, with the feature of papillary-like structure formation by cells. A dog was presented with the primary complaint of vomiting, hyporexia and increased respiratory effort. Thoracic radiography revealed increased soft tissue radiopacity of the right cranial lung lobe suggestive of possible consolidation or collapsed lung lobe, with generalised miliary nodular pattern throughout the other lung fields. The dog was euthanized humanely and necropsy was performed. Histopathology confirmed the diagnosis of primary pulmonary lung neoplasm (papillary adenocarcinoma) with Aspergillus versicolor infection identified through fungal culture and PCR. There have been several reports on humans and dogs with fungal infections that often mimic or coexist with pulmonary neoplasm. This is the first documented report of A. versicolor isolated from a lung neoplasm in a dog in Malaysia.
Keywords: Canine, Aspergillus versicolor, Lung neoplasm, Radiography, Histopathology, Post-mortem
1. Introduction
Pulmonary tumours are not as common in domestic animals compared to humans [15]. Dogs with lung neoplasm commonly present with dyspnoea, coughing and lethargy with other comorbidities such as loss of body weight, anorexia, occasional vomiting and paraneoplastic syndrome specific to the type of neoplasm. Lung neoplasms in dogs are diagnosed through diagnostic imaging, mainly through radiography of the thorax and computed tomography [19]. Lung neoplasms are most often described as solitary masses that involve a single or multiple lung lobes, and can be diffuse in appearance.
Papillary adenocarcinoma of the lung is the most common type of lung neoplasm involving the adenocarcinoma histopathology type among domestic carnivores and ruminants [8]. Based on data from 1975 to 1985, a total 74.8% of all canine primary lung neoplasms are adenocarcinoma [12]. In humans, primary lung cancer is the most common type of cancer in several countries [13]. There are four common patterns of pulmonary adenocarcinoma in humans, namely papillary, acinar, bronchoalveolar and solid tumour with mucin production [8]; in veterinary medicine, adenocarcinoma is only divided into two patterns: papillary and bronchoalveolar [15]. The incidence of pulmonary neoplasm is relatively high among old-age dogs. The pathogenesis of the development of lung neoplasm in dogs is still not well established.
Nasal aspergillosis is a common form of aspergillosis in dogs, but pulmonary aspergillosis can occur without involvement of the upper respiratory tract. Aspergillus spores can disseminate to other visceral organs [2]. Aspergillosis caused by Aspergillus terreus has been reported in German shepherd dogs, where it has been suggested that the genetic factors of this particular breed play a significant role in the acquisition of this disease, as disseminated A. terreus infections occur more often in this breed than in others [17]. The common clinical signs in dogs with disseminated aspergillosis are weight loss, pyrexia, inflammatory ocular disease, neurological deficits, muscular pain and/or weakness, spinal column pain and lameness [14]. The definitive diagnosis of disseminated or systemic aspergillosis is microbiological culture of the associated tissues and fluid samples. Early presentation of animals for diagnostic investigation can help with early treatment, and if treatment is delayed, the advanced stages of systemic aspergillosis could lead to the animal's death [2].
2. Case
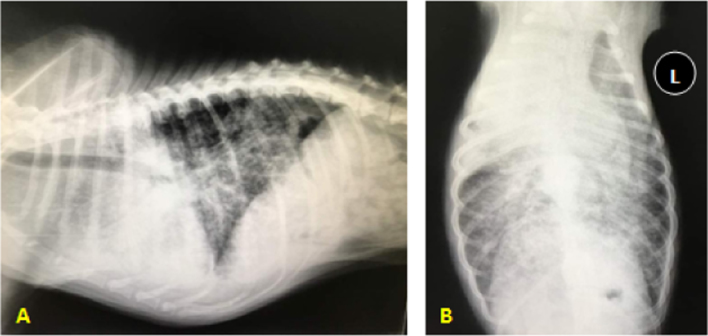
An 8-year-old, male local dog was presented to a small animal practice in Kuala Lumpur, Malaysia, with the primary complaint of vomiting, hyporexia, increased respiratory efforts and epistaxis. The dog had tremendous weight lost from its last visit 1 year ago for vaccination, where body weight decreased from 20.2 to 15.4 kg (25%). The clinical symptoms related to respiratory system began about 3 months prior to the clinic visit from early coughing and signs of becoming easily fatigue due to dyspnoea with open-mouth breathing during presentation. Thoracic radiography revealed, on lateral view, increased radiopacity at the cranioventral lung field with generalised miliary lung pattern (Fig. 1A), while on the ventrodorsal view there was increased radiopacity on the right cranial lung lobe with mild involvement of the medial lobe (Fig. 1B). An intact diaphragm was observed and there was a mildly obscured cardiac silhouette, most likely due to the generalised intense miliary pattern and increased radiopacity superimposing a silhouette.
Fig. 1.
(A) Lateral radiograph of the thorax. There is increased radiopacity at the cranioventral region of the lung field suggestive of pneumonia, atelectasis or primary lung mass, which may either be abscess or neoplasm. (B) Ventrodorsal radiograph of the thorax. There is increased radiopacity at the right cranial lung lobe with generalised intense miliary pattern throughout the lung suggestive of fungal infection or tumour metastasis.
Due to the severity of the lung lesions and poor quality of life, the dog was euthanized and post-mortem was conducted. Upon general inspection, the dog had poor body condition, which was below the ideal body condition for dogs (Fig. 2A). Bilateral epistaxis was also noted. During the post-mortem examination of the thoracic cavity, the lung was diffusely tan, dark red and heavy. There were numerous multifocal to confluent, circumscribed, firm, greyish, variable-sized pyogranulomatous nodules varying from 1 to 10 mm in diameter disseminated throughout the lung lobes (Fig. 2B), including the lung parenchyma (Fig. 2C). Abscess affecting the whole right cranial lung lobe was observed and was consolidated and firm upon palpation (Fig. 2D). New formation of blood vessels was observed on the right lung lobe (Fig. 2D). As the lung lobes were cross-sectioned, pus and purulent material oozed out (Fig. 2E). The liver had a few whitish pyogranulomatous nodules on its parenchyma surface (Fig. 2F), which were suggestive of abscess or metastasis of neoplasm. In addition, the liver was mottled, which was highly suggestive of congestion due to euthanasia. The other organs appeared normal during the examination. The differential diagnosis based on the post-mortem inspection was possibly neoplasm, lung abscess or pneumonia, which could be bacterial or fungal pneumonia.
Fig. 2.
(A) General inspection. The body condition score of the dog was 1/9 (under ideal) according to the World Small Animal Veterinary Association (WSAVA) chart. (B) Lung. The lung was dark red with numerous multifocal to confluent, circumscribed, firm, grey, variable sized pyogranulomatous nodules ranging 1–10 mm in diameter disseminated throughout. (C) Dissected section of the lung parenchyma. Multiple whitish pyogranulomatous nodules were observed. (D) Abscess affecting the whole right cranial lung lobe. Arrow indicates new blood vessel formation. (E) Dissected section of right cranial lung lobe. Arrow shows pus and purulent material oozing out from the lung lobe parenchyma. (F) Liver. Several whitish multiple pyogranulomatous nodules (arrows) were observed on the liver parenchyma surface. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
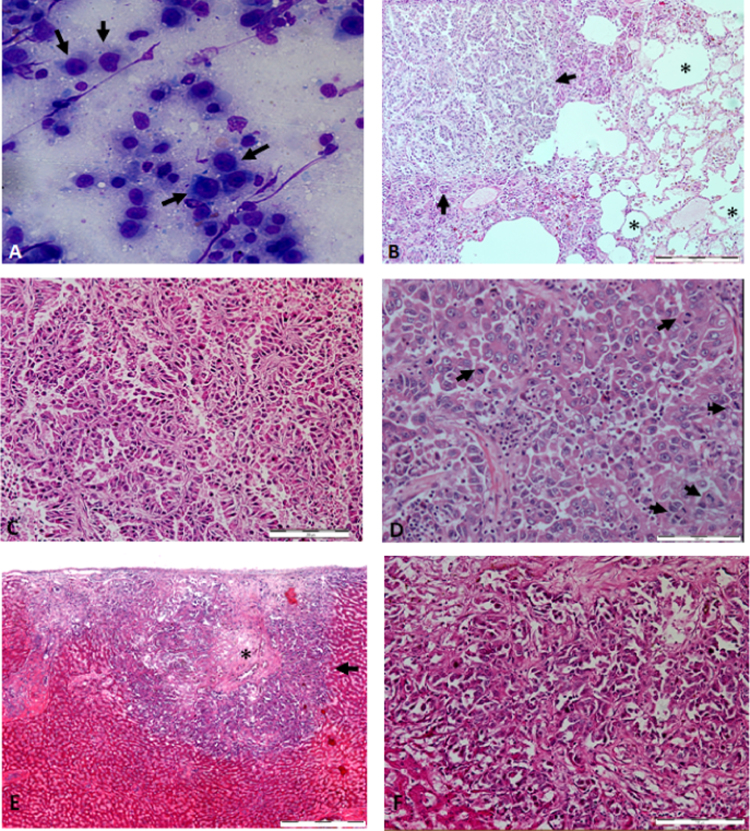
For quick identification, a lung smear was obtained for cytological assessment to determine whether infection or neoplasm was involved. The cytology results showed cellular components, with cuboidal to polygonal cells of various sizes containing multiple nucleoli and large nuclei with fine vacuolated cytoplasm and high nucleus-to-cytoplasm ratio (N/C), which were highly suggestive of adenocarcinoma (Fig. 3A). Further diagnostic workup was performed; the lung and liver samples were evaluated by histopathology, bacterial culture and identification as well as fungal culture and identification. Histopathology revealed abnormal papillary structures infiltrating the lung tissues, which led to the loss of normal lung architecture (Fig. 3B). On higher magnification (×200), a few mitotic figures were observed per high power field (hpf) at the papillary structures infiltrating the lung tissue, indicating rapid division of neoplastic cells (Fig. 3D). This suggested that the cells exhibited malignant characteristic of neoplasia. The histopathology of the liver revealed that the liver nodule had a necrotic centre with a similar papillary structure histomorphology as in the lung (Fig. 3E). This suggested that the neoplasia from the lung had metastasised to the liver. As the tumour tissue was predominantly papillary-shaped, we concluded that the neoplasm was in the category of primary papillary adenocarcinoma.
Fig. 3.
(A) Cytology of the lung smear. Arrows shows the variously sized cuboidal to polygonal cells with multiple nucleoli, large nuclei and fine vacuolated cytoplasm. (B) Histopathology section of lung (×40 magnification). There was infiltration of papillary structures to the lung tissue, leading to loss of lung structure (arrows) and identifiable alveolar structure (*). Bar, 500 µm. (C) Histopathology section of the lung (×100 magnification) shows papillary structure of the neoplasm infiltrating the lung. Bar, 200 µm. (D) Histopathology section of the lung (×200 magnification). The presence of several mitotic figures (arrows) under one hpf indicates malignancy. Bar, 100 µm. Histopathology section of the liver (×40 magnification). A liver nodule (arrow) contains a necrotic centre (*) with similar papillary structure histomorphology as found in the lung, surrounded by identifiable liver structure. Bar, 500 µm. (F) Histopathology section of the liver (×200 magnification). Similar papillary structure histomorphology was found in the lung. Bar, 100 µm.
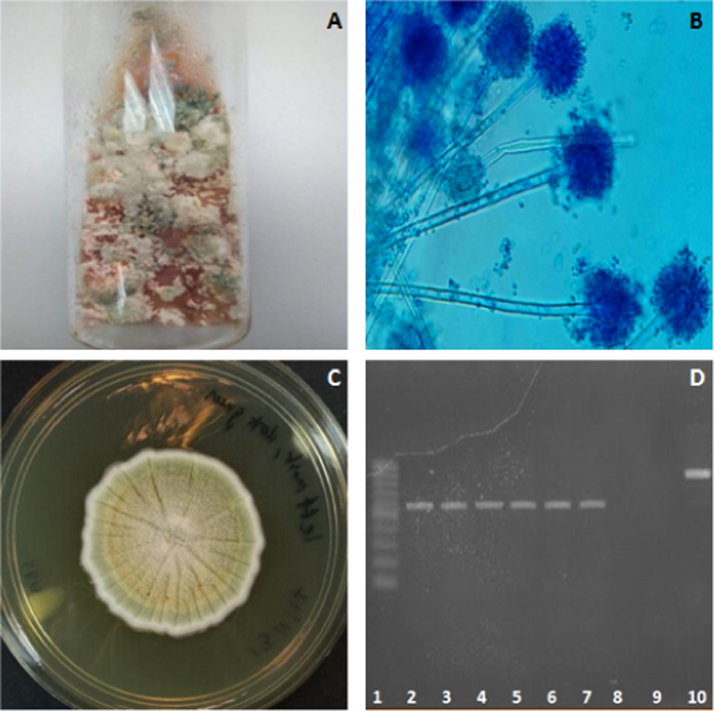
Swabs from the purulent materials were cultured on blood and McConkey agar at 37◦C; and Sabouraud dextrose agar (SDA) at room temperature for 2 weeks. Bacterial culture revealed the isolation and identification of Acinetobacter baumannii (1+), Enterobacter cloacae (1+) and Chromobacterium sp. (1+). The preliminary result of fungal culture was Aspergillus spp. (Fig. 4A). Subsequent lactophenol blue staining revealed brush-like and radiate conidial heads with round vesicles and biseriate phialides, which were similar to Aspergillus spp. morphology (Fig. 4B). The primary fungal culture was then sub-cultured on SDA and incubated for another 21 days at room temperature to obtain pure single fungal colonies (Fig. 4C). DNA was extracted from the pure fungal colonies by collecting a small piece of mycelia tissue from the colonies grown on the SDA subculture, which then underwent conventional phenol extraction.
Fig. 4.
(A) Primary fungal culture. Colonies of different colours were observed in the primary culture. (B) Lactophenol blue staining of lung sample. The morphological criteria matches that of Aspergillus spp., with brush-like and radiate conidial heads with round vesicles and biseriate phialides. (C) Pure fungal colonies from subculture at day 21. (D) PCR product. A single band was obtained from the two mycelia tissue samples collected from different subcultured pure colonies. Lanes 1: 100-bp ladder marker; lane 2–4, DNA from first mycelia tissue; lane 5–7, DNA from second mycelia tissue; lane 8, empty well; lane 9, no-template control PCR water; lane 10, positive control (Candida glabrata ATCC 2001 DNA). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
PCR was performed using the extracted DNA and universal internal transcribed spacer (ITS) primers: ITS1, 5′-TCCGTAGGTGAACCTGCGG-3′ and ITS4, 5′-TCCTCCGCTTATTGATATGC-3′. A single ~550-bp band was obtained on agarose gel electrophoresis (Fig. 4D) and the PCR products were sent for DNA sequencing. The nucleotide sequences were subjected to NCBI (National Center for Biotechnological Information) Nucleotide BLAST (Basic Local Alignment Search Tool) analysis for species identification. The organism that had the closest homology to the sequences was A. versicolor (530/530, 100%). The BLAST result suggested that A. versicolor is consistent with the macroscopic and microscopic morphology information of the fungus, where it displayed slow growth (25-mm colony diameter after 14 days’ incubation at room temperature) and formed white colonies in the first week of incubation that turned yellow and green as they matured. In addition, it showed a lower growth rate when incubated at 37 °C. Microscopic observation showed smooth and colourless stipe with spathulate vesicles as well as smooth and globose conidia [9]. Thus, the final diagnosis, according to the diagnostics workup, was pulmonary papillary adenocarcinoma concurrent with A. versicolor and secondary bacterial infection.
3. Discussion
This is the first case reported in Malaysia of primary pulmonary papillary adenocarcinoma concurrent with A. versicolor and secondary bacterial infection in a dog. Identification of A. versicolor can be confirmed based on culture and microscopic morphology together with PCR using the universal ITS primers through DNA sequencing. In humans, pulmonary fungal, mycobacterial, parasitic and indolent bacterial infection has always coexisted with pulmonary neoplasia [10]. Thus far, there has been no report on the co-occurrence of pulmonary neoplasia with aspergillosis in dogs.
The genus Aspergillus consists of hyphomycetes, which are ubiquitous in the air and the environment and are commonly found in soil, water and organic matter. They are opportunistic saprophytic pathogens that cause infections in dogs, horses, mammals, birds as well as humans [17]. A. versicolor is a resilient organism and can grow at temperatures of 4–40 °C and on any surface, including those with nutrient deficiency [3]. A. fumigates, A. flavus, A. terreus, A. niger and A. deflectus cause disease in dogs [14], such as nasal aspergillosis or disseminated aspergillosis. A case of disseminated A. versicolor infection in a 2.5-year-old male castrated German shepherd has been reported [18]. Similarly, A. versicolor was isolated in a case of invasive pulmonary aspergillosis in a patient on mechanical ventilation [3]. In the present case, it is unlikely that the A. versicolor was from a source of contamination during tissue sampling at post-mortem examination, mainly because the culture and PCR results revealed pure isolates of A. versicolor and without mixing with other Aspergillus spp., which are more ubiquitous in the environment, nor with other types of fungi from the environment.
The neoplasm in this case is of papillary adenocarcinoma type, which affected all of the lung lobes and metastasised to the liver. Pulmonary adenocarcinoma is categorised as papillary based on the features of the neoplasm, which infiltrates more than 75% of the lungs, causing loss of normal lung architecture with more exaggerated papillary appearance with columnar cells and large ovoid nuclei ([11], [15]). The risk factors for the development of lung neoplasm due to aspergillosis in dogs are not well studied. However, it is believed that dogs with lung infections, experiencing stress or are immunosuppressed have a higher risk of acquiring tumours [18]. As in this case, the neoplasm was concurrent with A. versicolor infection, it may have been the risk factor that triggered the pulmonary neoplasm in this dog. Several supporting studies conducted in multiple institutions in Europe, Japan and Canada revealed that fungal infection occurred twice as frequently in human patients with leukaemia, lymphoma and solid tumours [1]. As Aspergillus spp. are respiratory tract opportunistic pathogens, infection of the lung by these fungi are involved in nearly 80% of neoplasm cases [1]. Aspergillus spp. can cause irritation to the bronchial cell lining and enhance metaplasia of the ciliated cuboidal epithelium and non-ciliated cuboidal cells of the lungs [18]. Furthermore, A. versicolor releases a carcinogenic mycotoxin called sterigmatocystin [5] which has been reported to cause colon cancer and liver cancer in humans [16].
In this case, the bacterial infection was considered secondary to the primary lung neoplasm and fungal infection, as the dog was already inappetant and immunosuppressed, which led to the development of both diseases. This can be explained by the generalised body weight loss (more than 20% over a 6-month period) and prolonged inappetence observed in this dog. Chromobacterium spp. is a soil and water inhabitant and can be found in food but is not considered pathogenic [4], while E. cloacae causes infection due to poor hygiene and is a common pathogen in the gastrointestinal tract [6]. The 1+ growth of Acinetobacter baumannii is notable. This organism is not commonly found in the environment and is mostly acquired from nosocomial infections [7]. In this case, it may have been acquired from repeated visits to the veterinary clinic or from the environment, where the immunosuppression could have facilitated the infection. Further investigations on the isolation of A. versicolor from the veterinary clinical setting are warranted.
Acknowledgements
The authors extend great appreciation to Krisnammah Kuppusamy, Nur Rabiatuladawiyah Rosli, Jamilah Jahari, Latifah Hanan, Zainatulaisyah Abdul Manap for their technical assistance. We also thank Shu Yih Chew for his assistance in the PCR assays and Lee Veterinary Clinic, Kuala Lumpur, Malaysia, for referring this case to our diagnostic laboratory.
Acknowledgments
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Bodey G., Bueltmann B., Duguid W., Gibbs D., Hanak H., Hotchi M., Mall G., Martino P., Meunier F., Milliken S., Naoe S. Fungal infections in cancer patients: an international autopsy survey. Eur. J. Clin. Microbiol. Infect. Dis. 1992;11(2):99–109. doi: 10.1007/BF01967060. [DOI] [PubMed] [Google Scholar]
- 2.Bruchim Y., Elad D., Klainbart S. Disseminated aspergillosis in two dogs in Israel. Mycoses. 2006;49(2):130–133. doi: 10.1111/j.1439-0507.2006.01168.x. [DOI] [PubMed] [Google Scholar]
- 3.Charles M.P., Noyal M.J., Easow J.M. Invasive pulmonary aspergillosis caused by Aspergillus versicolor in a patient on mechanical ventilation. Australas. Med. J. 2011;4(11):632. doi: 10.4066/AMJ.2011.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa P.S., Nascimento A., Lima-Bittencourt C.I., Chartone-Souza E., Santos F.R., Vilas-Boas A. Chromobacterium sp. from the tropics: detection and diversity of phytase activity. Braz. J. Microbiol. 2011;42(1):84–88. doi: 10.1590/S1517-83822011000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Ruyck K., De Boevre M., Huybrechts I., De Saeger S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: short review. Mutat. Res./Rev. Mutat. Res. 2015;766:32–41. doi: 10.1016/j.mrrev.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Feglo P., Sakyi K. Bacterial contamination of street vending food in Kumasi, Ghana. J. Med. Biomed. Sci. 2012;1(1):1–8. [Google Scholar]
- 7.Hsueh P.R., Teng L.J., Chen C.Y., Chen W.H., Ho S.W., Luh K.T. Pandrug-resistant Acinetobacter baumannii causing nosocomial infections in a university hospital, Taiwan. Emerg. Infect. Dis. 2002;8(8):827. doi: 10.3201/eid0808.020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerr K.M. Current issues in pulmonary adenocarcinoma. Diagn. Histopathol. 2008;14(10):509–518. [Google Scholar]
- 9.Klich M.A. Morphological studies of Aspergillus section Versicolores and related species. Mycologia. 1993:100–107. [Google Scholar]
- 10.Moran C.A. Pulmonary adenocarcinoma: the expanding spectrum of histologic variants. Arch. Pathol. Lab. Med. 2006;130(7):958–962. doi: 10.5858/2006-130-958-PATESO. [DOI] [PubMed] [Google Scholar]
- 11.Moulton J.E., Tscharner C.V., Schneider R. Classification of lung carcinomas in the dog and cat. Vet. Pathol. 1981;18(4):513–528. doi: 10.1177/030098588101800409. [DOI] [PubMed] [Google Scholar]
- 12.Ogilvie G.K., Haschek W.M., Withrow S.J., Richardson R.C., Harvey H.J., Henderson R.A., Fowler J.D., Norris A.M., Tomlinson J., McCaw D. Classification of primary lung tumors in dogs: 210 cases (1975–1985) J. Am. Vet. Med. Assoc. 1989;195(1):106–108. [PubMed] [Google Scholar]
- 13.Park W.Y., Kim M.H., Shin D.H., Lee J.H., Choi K.U., Kim J.Y., Lee C.H., Sol M.Y. Ciliated adenocarcinomas of the lung: a tumor of non-terminal respiratory unit origin. Mod. Pathol. 2012;25(9):1265. doi: 10.1038/modpathol.2012.76. [DOI] [PubMed] [Google Scholar]
- 14.Schultz R.M., Johnson E.G., Wisner E.R., Brown N.A., Byrne B.A., Sykes J.E. Clinicopathologic and diagnostic imaging characteristics of systemic aspergillosis in 30 dogs. J. Vet. Intern. Med. 2008;22(4):851–859. doi: 10.1111/j.1939-1676.2008.0125.x. [DOI] [PubMed] [Google Scholar]
- 15.Stünzi H., Head K.W., Nielsen S.W. Tumours of the lung. Bull. World Health Organ. 1974;50(1–2):9. [PMC free article] [PubMed] [Google Scholar]
- 16.Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis. 2000;21(3):387–395. doi: 10.1093/carcin/21.3.387. [DOI] [PubMed] [Google Scholar]
- 17.Tell L.A. Aspergillosis in mammals and birds: impact on veterinary medicine. Med. Mycol. 2005;43(Suppl_1):S71–S73. doi: 10.1080/13693780400020089. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S., Corapi W., Quist E., Griffin S., Zhang M. Aspergillus versicolor, a new causative agent of canine disseminated aspergillosis. J. Clin. Microbiol. 2012;50(1):187–191. doi: 10.1128/JCM.05388-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y.Z., Li W.P., Wang Z.Y., Yang H.F., He Q.L., Zhu H.G., Zheng G.J. Primary pulmonary adenocarcinoma mimicking papillary thyroid carcinoma. J. Cardiothorac. Surg. 2013;8(1):131. doi: 10.1186/1749-8090-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]