Abstract
Primary and secondary hypertension is associated with kidney redox imbalance resulting in enhanced reactive oxygen species (ROS) and enzymes dependent phospholipid metabolism. The fatty acid amide hydrolase inhibitor, URB597, modulates the levels of endocannabinoids, particularly of anandamide, which is responsible for controlling blood pressure and regulating redox balance. Therefore, this study aimed to compare the effects of chronic URB597 administration to spontaneously hypertensive rats (SHR) and rats with secondary hypertension (DOCA-salt rats) on the kidney metabolism associated with the redox and endocannabinoid systems. It was shown fatty acid amide hydrolase (FAAH) inhibitor decreased the activity of ROS-generated enzymes what resulted in a reduction of ROS level. Moreover varied changes in antioxidant parameters were observed with tendency to improve antioxidant defense in SHR kidney. Moreover, URB597 administration to hypertensive rats decreased pro-inflammatory response, particularly in the kidneys of DOCA-salt hypertensive rats. URB597 had tendency to enhance ROS-dependent phospholipid oxidation, estimated by changes in neuroprostanes in the kidney of SHR and reactive aldehydes (4-hydroxynonenal and malondialdehyde) in DOCA-salt rats, in particular. The administration of FAAH inhibitor resulted in increased level of endocannabinoids in kidney of both groups of hypertensive rats led to enhanced expression of the cannabinoid receptors type 1 and 2 in SHR as well as vanilloid receptor 1 receptors in DOCA-salt rats. URB597 given to normotensive rats also affected kidney oxidative metabolism, resulting in enhanced level of neuroprostanes in Wistar Kyoto rats and reactive aldehydes in Wistar rats. Moreover, the level of endocannabinoids and cannabinoid receptors were significantly higher in both control groups of rats after URB597 administration.
In conclusion, because URB597 disturbed the kidney redox system and phospholipid ROS-dependent and enzymatic-dependent metabolism, the administration of this inhibitor may enhance kidney disorders depending on model of hypertension, but may also cause kidney disturbances in control rats. Therefore, further studies are warranted.
Abbreviations: 2-AG, 2-arachidonoylglycerol; 4-HNE, 4-hydroxynonenal; 8-isoPGF2α, F2 -isoprostanes; 8-OHdG, 8-hydroxy-2’-deoxyguanosine; AA, arachidonic acid; AEA, N-arachidonoylethanolamine, anandamide; AM3506, 5-(4-hydroxyphenyl) pentanesulfonyl fluoride; Bach1, basic leucine zipper transcription factor 1; CAT, catalase; CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; CO, carbonyl groups; COX1, cyclooxygenase 1; COX-2, cyclooxygenase 2; cPLA2, cytosolic phospholipase A2; Cu/Zn–SOD, superoxide dismutase; DHA, docosahexaenoic acid; DOCA, deoxycorticosterone acetate; FAAH, fatty acid amide hydrolase; GSH, reduced glutathione; GSH-Px, glutathione peroxidase; GSSG-R, glutathione reductase; HO-1, heme oxygenase 1; KAP1, KRAB-associated protein-1; Keap1, Kelch-like ECH-associated protein 1; LOX, lipoxygenase; MAGL, monoacylglycerol lipase; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; NADA, N-arachidonoyl dopamine; NOX, NADPH oxidase; NPs-A4/J4, A4/J4-neuroprostanes; Nrf2, nuclear factor erythroid 2; p21, cyclin-dependent kinase inhibitor 1; p62, nucleoporin p62; p-cJun, phosphorylated Jun proto-oncogene; ROS, reactive oxygen species; SBP, systolic blood pressure; SHR, spontaneously hypertensive rats; TNF-α, tumor necrosis factor alpha; TRPV1, vanilloid receptor 1; URB597, [3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate; WKY, Wistar Kyoto rats; XO, xanthine oxidase
Keywords: Hypertension, Kidney, URB597, Endocannabinoid system, Redox balance
Graphical abstract
Highlights
-
•
URB597 has tendency to decrease kidney oxidative conditions.
-
•
URB597 differentiates Nrf2 pathway response in kidney of SHR and DOCA-salt rats.
-
•
URB597 enhances level of phospholipid peroxidation products and endocannabinoids.
-
•
URB597 reduces pro-inflammatory response particularly in kidney of DOCA-salt rats.
1. Introduction
The kidneys play a crucial role in blood pressure regulation and are therefore involved in the progression of hypertension. However, the consequences of hypertension include renal oxidative stress leading to kidney damage, while renal oxidative stress resulting from an imbalance between reactive oxygen species (ROS) generation and antioxidant defense mechanisms may also be involved in the development of hypertension [1], [2]. Moreover, oxidative conditions promote inflammatory processes by activating pro-inflammatory molecules such as transcription factors (NFκB) and cytokines (TNF-α and IL-6) [3]. Oxidative stress and inflammation are strongly implicated in inducing kidney hypertrophy, end-stage renal disease, arteriosclerosis, and peripheral vascular disease, which lead to kidney failure in animal models and humans [4]. ROS, generated during hypertension, change ROS- and enzymatic-dependent phospholipid metabolism. Hypertension is frequently accompanied by hyperlipidemia, which enables ROS-mediated lipid peroxidation and the generation by oxidative fragmentation electrophilic aldehydes such as malondialdehyde (MDA) and by oxidative cyclisation prostaglandin derivatives such as 8-isoprostanes, which constrict blood vessels [5], [6], [7]. However enzymatic phospholipid metabolism leads among others to the generation of endocannabinoids that in turn are involved in the regulation of ROS and inflammatory factor levels [1], [8]. The main endocannabinoids and their receptors are present within human and animal kidneys [9], [10]. Anandamide and 2-arachidonoylglycerol (2-AG), ligands of G protein–coupled receptors (mainly CB1/2 and TRPV1), are synthesized on demand from phospholipid arachidonic acid [8]. Endocannabinoids system, mainly endocannabinoids and enzymes metabolizing them, likely is involved in the regulation of renal blood flow and hemodynamics and of tubular sodium and fluid reabsorption but also participates in modulation inflammation and redox balance [11]. It is known that cannabinoid receptors take part in the regulation of redox balance as follows: CB1 activation enhances oxidative stress and may promote tissue injury by enhanced inflammation, MAPK activation, and cell death, while CB2 and TRPV1 activation prevents ROS generation and may play a protective role in preventing renal injuries, possibly by inhibiting the inflammatory response and endothelial cell activation e.g. in hypertension [12]. Moreover the existence of the crosstalk between ROS and endocannabinoids has been proven in different organs [13]. The above data indicate that cooperation of the redox and endocannabinoid systems may be due to metabolic changes during the progression of hypertension.
The levels of endocannabinoids, particularly anandamide, are regulated by the fatty acid amide hydrolase (FAAH) enzyme, which is mainly responsible for anandamide degradation [14]. Therefore, FAAH inhibitors are postulated to be antihypertensive agents. The acute administration of FAAH inhibitors [3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate (URB597) and 5-(4-hydroxyphenyl) pentanesulfonyl fluoride (AM3506) to spontaneously hypertensive rats (SHRs) normalized the blood pressure and decreased the cardiac contractility [15]. However, the chronic administration of URB597 to rats with secondary hypertension (DOCA-salt hypertensive rats) decreased but did not normalize blood pressure and cardiac and renal hypertrophy in an age-dependent manner [16]. Moreover, the chronic administration of URB597 disturbs redox metabolism in the liver of DOCA-salt hypertensive rats [17]. However, no studies have compared the metabolic effects of chronic FAAH inhibition caused by URB597 in the kidneys of rats with two different types of hypertension.
Thus, the aim of this study was to show the effects of chronic administration of the FAAH inhibitor URB597 to rats with primary (SHRs) and secondary (DOCA-salt hypertensive rats) hypertension on changes in the redox system resulting in phospholipid metabolism in the kidney.
2. Materials and methods
2.1. Animals
The experiment was performed using rats with primary hypertension (SHRs) and rats with secondary hypertension induced by the administration of DOCA (11-deoxycorticosterone acetate) and salt. All procedures and experimental protocols were approved by the local Animal Ethics Committee in Białystok, Poland [resolution No. 4/2012 of 25.01.2012].
2.2. Spontaneously hypertensive rats
Experiments were performed on 8–10-week-old male (270–350 g) SHRs and normotensive control Wistar Kyoto (WKY) rats. The animals were housed with free access to standard pelleted rat chow and water (unless otherwise stated) and maintained under a 12-h light-dark cycle.
2.2.1. Experimental protocol
The rats were divided into following four groups of six rats each:
→ group 1A [WKY]: during the last 14 days, WKY rats were treated intraperitoneally (i.p.) with solvent for URB597 [1 mL] every 12 h;
→ group 2A [WKY+URB597]: during the last 14 days, WKY rats were treated i.p. with URB597 [1 mg/kg b.w. in 1 mL of URB597 solvent] every 12 h;
→ group 3A [SHR]: during the last 14 days, SHRs were treated i.p. with solvent for URB597 [1mL] every 12 h; and
→ group 4A [SHR+URB597]: during the last 14 days, SHRs were treated i.p. with URB597 [1 mg/kg b.w. in 1 mL of URB597 solvent] every 12 h.
Systolic blood pressure (SBP) was measured in conscious rats using the tail-cuff method before and after URB597 (or solvent) treatment. Rats with SBP values ≥ 150 mmHg were considered hypertensive. Two-week URB597 administration did not modify SBP in SHR (187 ± 15 mmHg and 191 ± 49 mmHg) and WKY (117 ± 18 mmHg and 101 ± 10 mmHg) rats before its first and the final dose, respectively. The solvent for URB597 did not modify SBP both in SHR (184 ± 34 and 205 ± 43 mmHg) and in WKY (114 ± 18 and 110 ± 13 mmHg) before the first and the final injection.
The kidney hypertrophy index values appointed after URB597 (or solvent) treatment were as follows: WKY, 3.9 ± 0.2 mg/g; WKY+URB597, 3.9 ± 0.2 mg/g; SHR, 3.9 ± 0.2 mg/g; and SHR+URB597, 3.7 ± 0.2 mg/g. There were no significant intergroup differences in the index hypertrophy values.
2.2.1.1. DOCA-salt hypertensive rats
Four- to 5-week-old (100–140 g) male Wistar rats were used in the experiment. The animals were housed with free access to standard pelleted rat chow and water (unless otherwise stated) and maintained under a 12-h light-dark cycle. The rats were anesthetized i.p. with pentobarbital (70 mg/kg b.w.) and unilaterally nephrectomized. After a 1-week recovery period, hypertension was induced for 6 weeks through subcutaneous (s.c.) injections of DOCA (25 mg/kg b.w. in 0.4 mL of N, N-dimethylformamide [DMF]/kg b.w.) twice weekly and the replacement of drinking water with a 1% NaCl solution. After 4 weeks, the DOCA-salt rats were injected i.p. with URB597 (1 mg/kg b.w. in 1 mL of URB597 solvent) every 12 h for 14 days [18], [19].
2.2.2. Experimental protocol
The rats were divided into following four groups of six rats each:
→ group 1B [Wistar]: twice weekly for 6 weeks, uninephrectomized rats were treated s.c. with 0.4 mL of DMF/kg b.w.; during the last 14 days, the rats were treated i.p. with solvent [1 mL/kg b.w.] every 12 h;
→ group 2B [Wistar+URB597]: twice weekly for 6 weeks, uninephrectomized rats were treated s.c. with 0.4 mL of DMF/kg b.w.; during the last 14 days, they were treated with URB597 [1 mg/kg b.w. in 1 mL of URB597 solvent] every 12 h;
→ group 3B [DOCA-salt]: twice weekly for 6 weeks, uninephrectomized rats were treated s.c. with 25 mg of DOCA /kg b.w. in 0.4 mL of DMF/kg b.w. and received drinking water with a 1% NaCl solution; during the last 14 days, they were treated i.p. with solvent for URB597 [1 mL/kg b.w.] every 12 h; and
→ group 4B [DOCA-salt+URB597]: twice weekly for 6 weeks, uninephrectomized rats were treated s.c. with 25 mg of DOCA /kg b.w. in 0.4 mL of DMF/kg b.w. and received drinking water with a 1% NaCl solution; during the last 14 days, the rats were treated i.p. with URB597 [1 mg/kg b.w. in 1 mL of URB597 solvent] every 12 h.
SBP was measured in conscious rats using the tail-cuff method before and after URB597 (or solvent) treatment. Rats with SBP values ≥ 150 mmHg were considered hypertensive. Two-week URB597 administration only tended to reduce SBP (from 216 ± 15 mmHg to 186 ± 34 mmHg) in DOCA-salt hypertensive rats but did not affect SBP in normotensive Wistar rats (136 ± 30 and 127 ± 8 mmHg before the first and the final dose, respectively). The solvent did not modify SBP in either the hypertensive (224 ± 35 and 218 ± 29 mmHg) or normotensive animals (129 ± 23 and 123 ± 11 mmHg before the first and the final injection, respectively) [17].
The index of kidney hypertrophy appointed after URB597 (or solvent) treatment was as follows: Wistar, 6.8 ± 0.4 mg/g; Wistar+URB597, 7.0 ± 0.5 mg/g; DOCA-salt, 13.0 ± 1.7 mg/g; and DOCA-salt+URB597, 11.3 ± 0.2 mg/g. The values for hypertensive rats and hypertensive rats treated with URB597 were significantly different compared with the Wistar and Wistar+URB597 groups, while the index of hypertrophy for the DOCA-salt+URB597 group was significantly lower than that for the hypertensive rats [16].
2.3. Tissue preparation
At the end of the experiments, the rats were anesthetized with an intraperitoneal injection of pentobarbital (70 mg/kg b.w.) and sacrificed. The kidneys were excised and prepared for biochemical examinations in three different ways:
-
•
some fresh tissue samples were used for the determination of ROS generation;
-
•
some fresh tissue samples were pulverized in liquid nitrogen for examination of their fatty acids and metabolites as well as glutathione (GSH) levels and FAAH and monoacylglycerol lipase (MAGL) activity;
-
•
some fresh tissue samples were homogenized under standardized conditions (10% homogenates obtained in 0.9% NaCl solution and centrifuged at 20,000 × g for 15 min at 4 °C) and used to estimate other parameters.
3. Biochemical studies
3.1. Determination of ROS
Total ROS generation was detected using an electron spin resonance (ESR) spectrometer e-scan (Noxygen GmbH/Bruker Biospin GmbH, Germany), where selective interaction between ROS and the spin probes CMH (1-hydroxy-3-methoxy-carbonyl-2,2,5,5-tetrame-thylpyrrolidine) led to stable nitroxide CM-radical (t1/2 = 4 h) and the kinetics of this radical accumulation was measured according to the electron spin resonance (ESR) amplitude of the low field component of ESR spectra [20].
3.2. Determination of prooxidant enzymes activity
NADPH oxidase (NOX – EC 1.6.3.1) activity was measured by the luminescence assay using lucigenin (20 µM) as a luminophore [21]. One unit of NADPH activity was defined as the amount of the enzyme which is required to release 1 nmol of O2- per minute. Enzyme specific activity is expressed in RLU (Relative Luminescence Units) per milligram protein. Xanthine oxidase (XO - EC1.17.3.2) activity was assessed by uric acid formation from xanthine by measuring the increase in absorbance at 290 nm [22]. One unit of XO activity was defined as the amount of the enzyme, which is required to release 1 μM of uric acid per minute.
3.3. Determination of antioxidant enzymes activity
Superoxide dismutase (Cu/Zn–SOD – EC.1.15.1.1) activity was measured spectrophotometrically [at 480 nm] as inhibition of adrenaline oxidation to adrenochrome [23]. One unit of SOD was defined as the amount of the enzyme which inhibits epinephrine oxidation to adrenochrome by 50%.
Catalase activity (CAT–EC 1.11.1.9) was determined spectrophotometrically by measuring the decrease of hydrogen peroxide absorbance at 240 nm [24]. One unit of CAT was defined as the amount of the enzyme required to catalyze the decomposition of 1 µmol hydrogen peroxide to water and oxygen for 1 min.
Glutathione peroxidase (GSH-Px – EC.1.11.1.6) activity was assessed spectrophotometrically by measuring the conversion of NADPH to NADP at 340 nm [25]. One unit of GSH-Px activity was defined as the amount of enzyme catalyzing the oxidation of 1 mmol NADPH min−1 at 25 °C and pH 7.4.
Glutathione reductase (GSSG-R – EC.1.6.4.2) activity was measured spectrophotometrically by the oxidation of NADPH to NADP at 340 nm [26]. One unit of GSSG-R oxidized 1 mmol of NADPH/min at 25 °C and pH 7.4.
3.4. Western blot analysis
Western blot analysis of cellular proteins (Nrf2, Keap1, TNFα, HO-1, Bach1, KAP1, p21, p62, p-cJun (pSer63), CB1, CB2, TRPV1) was performed according to Eissa and Seada [27]. Whole kidney homogenates or membrane fractions containing 30 µg proteins were mixed with sample loading buffer (Laemmle buffer containing 5% 2-mercaptoethanol), heated at 95 °C for 10 min, and separated by 10% Tris-glycine SDS-PAGE. The same procedure was used to prepare the negative control (containing pure PBS buffer) and the positive control (commercially purchased complete cell lysate - Santa Cruz Biotechnology, Santa Cruz, CA, USA). As internal loading controls, β-actin and Na+/K+ ATPase (for kidney homogenates and membrane fractions, respectively) were used. Following separation proteins were electrophoretically transferred onto nitrocellulose membranes. The blotted membranes were blocked with 5% skim milk in TBS-T buffer (5% Tween 20) for 1 h and cut into fragments corresponding to the selected molar masses of the proteins. Primary antibodies were raised against Keap1 (host: goat), p-cJun (pSer63) (host: rabbit), and Nrf2, TNFα, HO-1, β-actin, and Na+/K+ ATPase (host: mouse) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used at a concentration of 1:1000. Primary antibodies against Bach1, KAP1, p21, p62, CB1, CB2, TRPV1 (host: rabbit) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), were also used at a concentration of 1:1000. Visualized protein bands were quantitated using the Versa Doc System and Quantity One software (Bio-Rad Laboratories Inc., CA). The results are expressed as a percentage of the expression determined in control groups [1 A and 1B].”
3.5. Detection of non-enzymatic antioxidant level
The level of reduced glutathione was measured using capillary electrophoresis [28]. Samples were sonicated with a mixture containing AcN/H2O (62.5:37.5, v/v). The separation was performed on a 40 cm effective length capillary and was operated at 27 kV with UV detection at 200 nm.
High-performance liquid chromatography was used to detect the levels of vitamins C [29], A and E [30]. For determination of vitamin C samples were mixed metaphosphoric acid. Separation was performed using RP-18 column and UV detection at 250 nm. The mobile phase was phosphate buffer (pH 2.8) and water (97:3). Vitamins A and E were extracted from homogenates using hexane, dried, diluted in ethanol and detected at 294 nm.
3.6. Determination of phospholipid metabolism and mediators
The phospholipid and free fatty acids were determined by gas chromatography [31]. Lipids components were isolated from homogenates by Folch extraction using chloroform/methanol mixture (2:1, v/v) in the presence of 0.01% butylated hydroxytoluene. Using TLC free fatty acids and total phospholipids were separated with the mobile phase: heptane – diisopropyl ether – acetic acid (60:40:3, v/v/v). All lipid fractions were transmetylated to fatty acid methyl esters (FAMEs) with boron trifluoride in methanol. FAMEs were analyzed by gas chromatography with a flame ionization detector (FID) on Clarus 500 Gas Chromatograph (Perkin Elmer). Separation of FAMEs was carried out on capillary column coated with Varian CP-Sil88 stationary phase (50 m × 0.25 mm, ID 0.2 µm, Varian). Identification of FAMEs was made by comparison on their retention time with those authentic standards and quantitation was achieved using an internal standard method (nonadecanoic acid (19:0)) and 1,2-dinonadecanoyl-sn-glycero-3-phosphocholine (19:0 PC) (were used as internal standards).
Lipid peroxidation was estimated by assessing the level of 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) by GC MS/MS [32] and as F2-isoprostanes (8-isoPGF2α) and A4/J4-neuroprostanes (NPs) by LC MS/MS [33], [34]. Derivatized aldehydes were detected by selected ion-monitoring (SIM) mode. The ions used were: m/z 333.0 and 181.0 for 4-HNE-PFB-TMS, m/z 204.0 and 178.0 for MDA-PFB and m/z 307.0 for IS (benzaldehyde-D6) derivatives. 8-isoPGF2α as well as A4/J4-NPs have been isolated using solid phase extraction (SPE) method. In both cases 8-isoPGF2α–d4 as an internal standard was used. 8-isoPGF2α was analyzed in negative-ion mode using MRM mode: m/z 353.2→193.1 (for 8-isoPGF2α) and 357.2→197.1 (for 8-isoPGF2 α-d4). NPs were analyzed by selected ion monitoring (SIM) in the m/z 357.0 as a series of peaks that have molecular masses and retention times expected for NPs generated from the oxidation of DHA in vitro.
Endocannabinoids: anandamide (AEA), 2-arachidonoylglycerol (2-AG) and N-arachidonoyl dopamine (NADA) were determined using ultra-performing liquid chromatography tandem mass spectrometry (UPLC-MS/MS) [35]. Octadeuterated endocannabinoids: AEA-d8 and 2-AG-d8, NADA-d8 as internal standards were added into the homogenates and all endocannabinoids were isolated using a solid phase extraction. The samples were analyzed in positive-ion mode using multiple reaction monitoring (MRM). Transitions of the precursor to the product ion was as follows: m/z 348.3→62.1, m/z 379.3→287.2, m/z 440.0→137.0, m/z 356.3→63.1, m/z 387.0→295.0 m/z 448.0→137.0 (for AEA, 2-AG and NADA, AEA-d8 and 2-AG-d8, NADA-d8 respectively).
The activity of enzymes involved in phospholipid metabolism was examined as follows: FAAH (EC-3.5.1.99) by spectrophotometric measuring (at 410 nm) the level of m-nitroaniline (m-NA) releasing from decanoyl m-nitroaniline [14]; monoacylglycerol lipase (MAGL) (EC 3.1.1.23) spectrophotometric measuring (at 412 nm) 5'-thio-2-nitrobenzoic acid formation during 1 min reaction [36]; cytosolic phospholipase A2 (cPLA2–EC 3.1.1.4) spectrophotometric measuring (at 414 nm) DTNB bond to the free thiol releases from the arachidonoyl thioester using cPLA2 Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA) according to the company's instruction [37]; and cyclooxygenase 1 and 2 (COX1/2–EC.1.14.99.1) spectrophotometrically (at 590 nm) by monitoring the appearance of oxidized TMPD using a commercial assay kit (Cayman Chemical Company, Ann Arbor, MI, USA).
3.7. Determination of protein and DNA modifications
Protein oxidative modifications were estimated by carbonyl group and tryptophan levels [38]. Carbonyl groups were determined spectrophotometrically (370 nm) using 2,4-dinitrophenylhydrazine, while fluorescence emission/excitation at 288 nm/338 nm was used to detect tryptophan content. The protein level and the levels of the tryptophan and carbonyl groups were normalized to the protein content determined by the Bradford assay.
The level of 8-hydroxy-2’-deoxyguanosine (8-OHdG) was assayed using LC MS/MS [39]. Genomic DNA was isolated using commercial kit (Sigma's GenElute Mammalian Genomic DNA Miniprep Kit, USA). DNA concentration in the preparations was determined spectrophotometrically. The samples were analyzed in the positive ion multiple reaction monitoring (MRM) mode and the transition of the precursor to the product ions were as follows: m/z 284.1→168.1.
The results of these examinations are expressed as a fold change relative to the control groups (WKY-1A and Wistar rats-1B).
More details the details of the examinations conditions are described in our previous paper [17].
4. Statistical analysis
All analyses were performed in duplicate for six independent samples. The obtained data are expressed as mean ± SD. These data were analyzed using two-way analysis of variance followed by a post hoc Tukey test. Values of p < 0.05 were considered significant.
5. Results
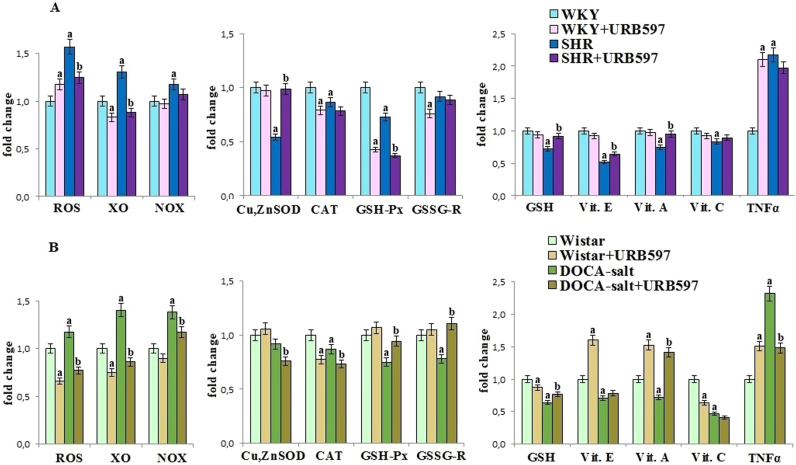
Primary and secondary hypertension led to redox disturbances in rat's kidney (Fig. 1). A significant shift into more oxidative status involving higher ROS generation and decreased antioxidant defense was observed in parallel with enhanced activity of enzymes generating superoxide anions such as xanthine and NADPH oxidases. The activities of antioxidant enzymes (Cu,Zn-SOD, CAT, GSH-Px and GSSG-R) and the levels of all non-enzymatic antioxidants (GSH, vit. E, A and C) were decreased in the kidney of both groups of hypertensive rats. However, the administration of the FAAH inhibitor, URB597, partially prevented the observed alterations, causing decrease of xanthine and NADPH oxidases activities and in turn inhibition of ROS generation in both groups of rats with hypertension. In addition after URB597 administration decrease in Cu,Zn-SOD activity as well as GSH, vitamin E and vitamin A levels were observed in kidney of SHR, while GSH-Px and GSSG-R activities and GSH level in kidney of rats with secondary hypertension were elevated. Moreover expression of pro-inflammatory cytokine - TNF-α, increased in kidney of hypertensive rats, was significantly decreased after URB597 administration to DOCA-salt rats. URB597 administration, in general, led to enhanced oxidative status in kidney of Wistar Kyoto rats and promotion of pro-inflammatory environment estimated by TNF-α level in both control groups of rats.
Fig. 1.
ROS level, ROS-generated enzymes activities [XO and NADPH oxidases] and the activities/levels of antioxidant parameters in the kidney of hypertensive rats (A-SHR; B-DOCA-salt) and hypertensive rats after URB597 administration. A. Data points represent the mean ± SD, n = 6; (a, significantly different from WKY group, p < 0.05; b, significantly different from SHR group, p < 0.05). B. Data points represent the mean ± SD; n = 6; (a, significantly different from Wistar group, p < 0.05); b, significantly different from DOCA-salt group, p < 0.05). The results are shown in comparison to the control groups [WKY rats and Wistar rats]: ROS level (7.00 ± 0.36 µM/min/g tissue for WKY rats, 4.15 ± 0.21 µM/min/g tissue for Wistar rats); NADPH oxidase activity (1246 ± 58 RLU/mg prot. for WKY rats, 1086 ± 55 RLU/mg prot. for Wistar rats); XO oxidase activity (1.18 ± 0.06 U/mg prot. for WKY rats, 0.72 ± 0.03 U/mg prot. for Wistar rats); SOD activity (3.50 ± 0.17 U/mg prot. for WKY rats, 1.71 ± 0.10 U/mg prot. for Wistar rats); CAT activity (1.44 ± 0.07 U/mg prot. for WKY rats, 2.31 ± 0.09 U/mg prot. for Wistar rats); GSH-Px activity (1004 ± 51µU/mg prot. for WKY rats, 277 ± 12 µU/mg prot. for Wistar rats); GSSG-R activity (3.47 ± 0.18 mU/mg prot. for WKY rats, 1.32 ± 0.07 mU/mg prot. for Wistar rats); glutathione level (2.73 ± 0.13 nmol/g tissue for WKY rats, 2.25 ± 0.12 nmol/g tissue for Wistar rats); vitamins level (vitamin E: 3.89 ± 0.17 µg/g tissue for WKY rats, 5.56 ± 0.25 µg/g tissue for Wistar rats; vitamin A: 1.12 ± 0.06 µg/g tissue for WKY rats, 0.63 ± 0.03 µg/g tissue for Wistar rats; vitamin C: 41.7 ± 1.8 µg/g tissue for WKY rats, 37.6 ± 1.8 µg/g tissue for Wistar rats).
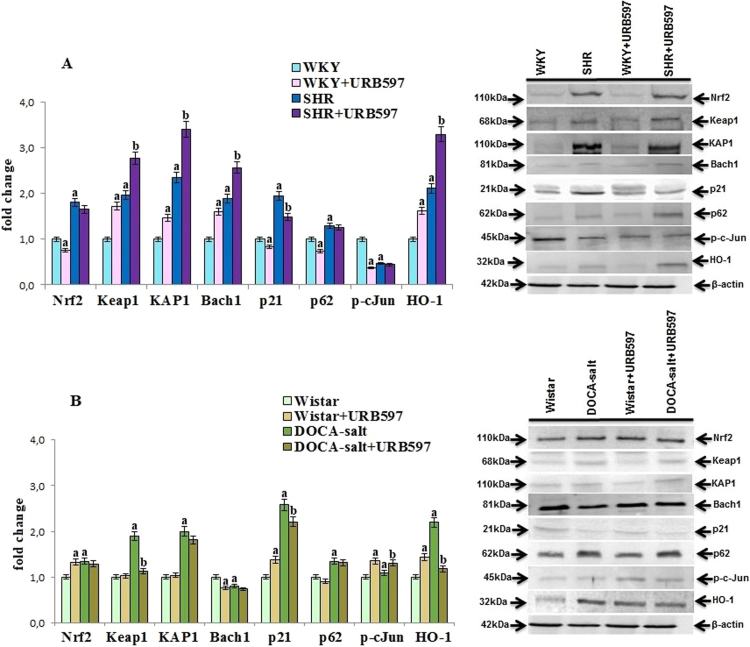
Hypertension altered also antioxidant defense at transcription level estimated by changes in transcription factor Nrf2 (responsible for antioxidant protein gene transcription) and Nrf2 activators and inhibitors activities (Fig. 2). Increased expression of the Nrf2 transcription factor and Nrf2 target protein HO-1 was observed in both hypertensive rat groups associated with enhanced expression of Nrf2 activators such as KAP1, p21, and p62. However, the expression of Keap1, that is the cytoplasmic inhibitor of Nrf2, was also increased in both groups of hypertensive rats, while, contrary to SHR, the level of the nucleic inhibitor Bach1 was decreased only in the DOCA-salt group. The administration of URB597 to SHR disturbed Nrf2 pathway by increasing the levels of Nrf2 inhibitors such as Keap1, Bach1, and activator-KAP1 as well as decreasing the Nrf2 inhibitor p21 level. The opposite effects with decreased expression of Keap1 and p21 were observed after the chronic administration of URB597 to rats with secondary hypertension. URB597 given to both groups of normotensive rats also caused opposite changes in the Nrf2 pathway response.
Fig. 2.
The levels of Nrf2 and its activators [KAP1, p21, p62, p-cJun] and inhibitors [Keap1, Bach1] as well as HO-1 in the kidney of hypertensive rats (A-SHR; B-DOCA-salt) and hypertensive rats after administration of URB597. The expression of the examined proteins is shown compared to the control groups. A. Data points represent the mean ± SD, n = 6; (a, significantly different from WKY group, p < 0.05; b, significantly different from SHR group, p < 0.05). B. Data points represent the mean ± SD; n = 6; (a, significantly different from Wistar group, p < 0.05); b, significantly different from DOCA-salt group, p < 0.05).
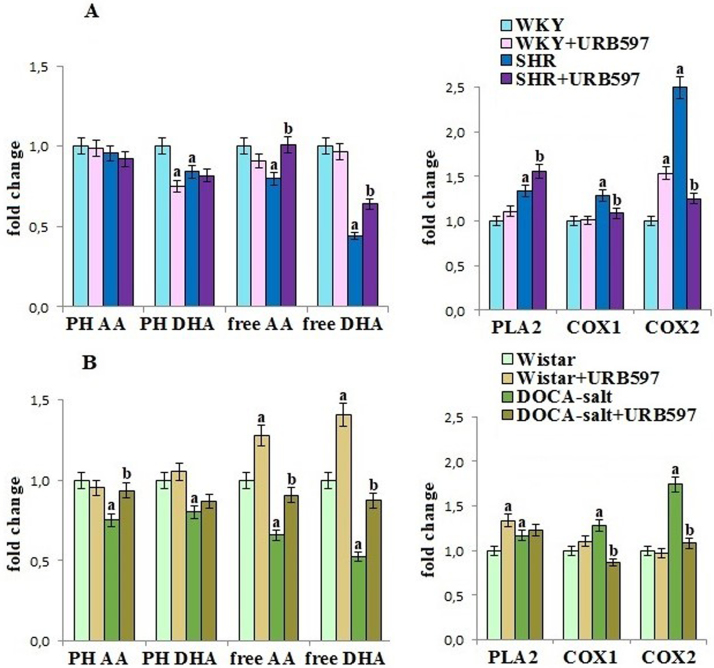
Hypertension promoted disturbances in kidney phospholipid metabolism by changes in phospholipid-metabolizing enzymes activities and fatty acids levels. The increase of PLA2, COX1 and COX2 activities in kidney of both hypertensive rat groups was observed (Fig. 3). These changes favored release of polyunsaturated fatty acids (PUFA) from kidney phospholipids, resulting in decreased level of phospholipid arachidonic acid (AA) and docosahexaenoic acid (DHA). The chronic administration of URB597 prevented increase of COX1, and particularly COX2 activity in both groups of hypertensive rats and partially protected free and phospholipid PUFA's levels. However, URB597 also caused an increase of PLA2 activity in kidney of Wistar rats, leading to increased free PUFA's level.
Fig. 3.
The levels of phospholipid and free fatty acids as well as the activities of PLA2, COX1 and COX2 in the kidney of hypertensive rats (A-SHR; B-DOCA-salt) and hypertensive rats after the administration of URB597. Data points represent the mean ± SD, n = 6; (a, significantly different from WKY group, p < 0.05; b, significantly different from SHR group, p < 0.05). B. Data points represent the mean ± SD; n = 6; (a, significantly different from Wistar group, p < 0.05); b, significantly different from DOCA-salt group, p < 0.05). The results are shown in comparison to the control groups [WKY rats and Wistar rats]: fatty acids level (PH AA: 10.68 ± 0.66 µmol/g tissue for WKY rats, 33.8 ± 1.5 µmol/g tissue for Wistar rats; PH DHA: 0.88 ± 0.05 µmol/g tissue for WKY rats, 2.74 ± 0.13 µmol/g tissue for Wistar rats; free AA: 479 ± 28 nmol/g tissue for WKY rats, 904 ± 47 nmol/g tissue for Wistar rats; free DHA: 51.5 ± 2.7 nmol/g tissue for WKY rats, 91. ± 4.9 nmol/g tissue for Wistar rats); cPLA2 activity (1.71 ± 0.08 nmol/min/mg prot. for WKY rats, 1.27 ± 0.06 nmol/min/mg prot. for Wistar rats); COX's activity (COX 1: 11.6 ± 0.7 nmol/min/mg prot. for WKY rats, 9.30 ± 0.41 nmol/min/mg prot. for Wistar rats; COX 2: 6.23 ± 0.31 nmol/min/mg prot. for WKY rats, 8.88 ± 0.44 nmol/min/mg prot. for Wistar rats).
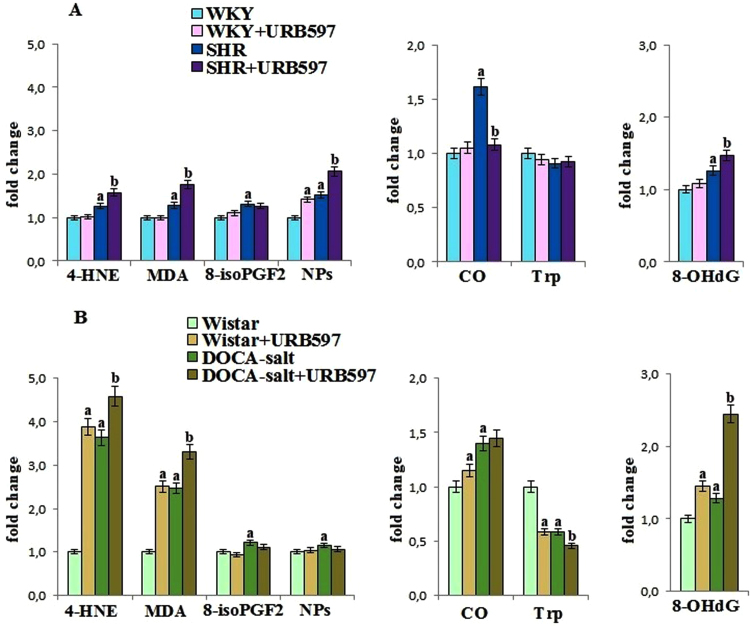
Oxidative stress favored oxidative damages to the phospholipid, but protein and DNA oxidative modifications were also observed in kidney of hypertensive rats (Fig. 4). The level of phospholipid oxidative fragmentation products, such as MDA and 4-HNE, as well as phospholipid oxidative cyclisation products, such as 8-isoprostanes and neuroprostanes, were increased in both groups of hypertensive rats. Moreover an increase in the level of these parameters was significantly higher in kidney of rats with secondary hypertension. Increased level of the carbonyl groups in all hypertensive rats and decreased level of tryptophan in DOCA-salt rats confirmed oxidative modification of kidney proteins. The level of 8-OHdG, a marker of DNA oxidative modification, was also elevated in both groups of hypertensive rats. The administration of URB597 to hypertensive rats caused further increase in the level of electrophilic lipid peroxidation products and 8-OHdG, while the level of neuroprostanes was significantly increased after URB597 administration only in kidney of SHR. The FAAH inhibitor prevented the increase in protein oxidative modifications only in SHR kidney. Moreover, URB597 administered to control – Wistar rats increased oxidative modifications in the kidneys.
Fig. 4.
The levels of lipid peroxidation products [4-HNE, MDA, 8-isoPGF2 and NPs] as well as the levels of oxidative modification products of protein [CO and Trp] and DNA [8-OH dG] in the kidney of hypertensive rats (A-SHR; B-DOCA-salt) and hypertensive rats after the administration of URB597. A. Data points represent the mean ± SD, n = 6; (a, significantly different from WKY group, p < 0.05; b, significantly different from SHR group, p < 0.05). B. Data points represent the mean ± SD; n = 6; (a, significantly different from Wistar group, p < 0.05); b, significantly different from DOCA-salt group, p < 0.05). The results are shown in comparison to the control groups [WKY rats and Wistar rats]: aldehydes concentrations (4-HNE: 691 ± 32 nmol/g tissue for WKY rats, 133 ± 6 nmol/g tissue for Wistar rats; MDA: 146 ± 8 nmol/g tissue for WKY rats, 29.7 ± 1.4 nmol/g tissue for Wistar rats); 8-isoPGF2α level (56.6 ± 2.7 ng/g tissue for WKY rats, 34.3 ± 1.5 ng/g tissue for Wistar rats); A4/J4-NPs concentrations (25.8 ± 1.1 ng/g tissue for WKY rats, 8.94 ± 0.46 ng/g tissue for Wistar rats); carbonyl groups level (3.26 ± 1.1 nmol/mg prot. for WKY rats, 6.66 ± 0.19 nmol/mg prot. for Wistar rats); tryptophan level (20.7 ± 1.1 U/mg prot. for WKY rats, 20.01 ± 1.10 U/mg prot. for Wistar rats); 8-OHdG level (10.8 ± 0.6 ng/mg DNA for WKY rats, 4.98 ± 0.22 ng/mg DNA for Wistar rats).
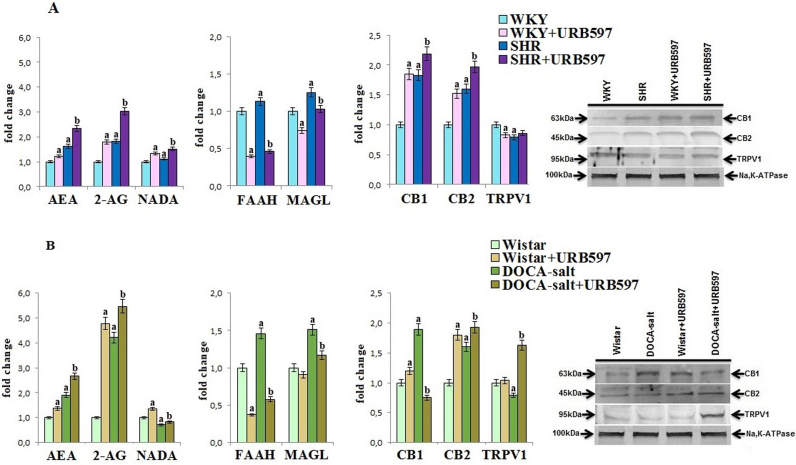
Changes in kidney phospholipid metabolism induced by hypertension were also associated with disturbances in endocannabinoid system (Fig. 5). Despite of increased activity of endocannabinoid-degrading enzymes, namely FAAH and MAGL in both groups of hypertensive rats, the levels of AEA and 2-AG were significantly increased. The NADA level was increased in the kidney of SHR rats while in DOCA-salt–treated animals was decreased. This finding was accompanied by enhanced CB1 and CB2 and decreased TRPV1 receptors expression in both groups of hypertensive rats. The administration of URB597 caused further increase in the level of AEA, 2-AG, and NADA because of the decreased activity of FAAH and MAGL in both groups of hypertensive rats. In addition, increased expression of the CB1 and CB2 receptors in SHRs and TRPV1 and CB2 receptors in DOCA-salt hypertensive rats was observed. URB597 administration to control rats also caused decrease in FAAH and MAGL activities, resulting in elevated level of AEA, 2-AG, and NADA in kidney of Wistar and Wistar Kyoto rats. Moreover, the expressions of CB1 and CB2 were significantly enhanced in the kidneys of both groups of control rats.
Fig. 5.
The levels of endocannabinoids [AEA, 2-AG and NADA] and their receptors [CB1, CB2, TRPV1] and activities of enzymes degrading endocannabinoids [FAAH, MAGL] in the kidney of hypertensive rats (A-SHR; B-DOCA-salt) and hypertensive rats after the administration of URB597. A. Data points represent the mean ± SD, n = 6; (a, significantly different from WKY group, p < 0.05; b, significantly different from SHR group, p < 0.05). B. Data points represent the mean ± SD; n = 6; (a, significantly different from Wistar group, p < 0.05); b, significantly different from DOCA-salt group, p < 0.05). The results are shown in comparison to the control groups [WKY rats and Wistar rats]: endocannabinoids concentrations (AEA: 56.1 ± 2.7 pmol/g tissue for WKY rats, 43.2 ± 2.7 pmol/g tissue for Wistar rats; 2-AG: 4.53 ± 0.22 nmol/g tissue for WKY rats, 2.47 ± 0.15 nmol/g tissue for Wistar rats; NADA: 15.8 ± 0.7 pmol/g tissue for WKY rats, 16.1 ± 0.8 pmol/g tissue for Wistar rats); FAAH activity (25.0 ± 1.2 nmol m-NA/min/mg prot. for WKY rats and 22.14 ± 1.24 nmol m-NA/min/mg prot. for Wistar rats); MAGL activity (14.7 ± 0.9 nmol TNB/min/mg prot. for WKY rats and 11.5 ± 0.8 nmol TNB/min/mg prot. for Wistar rats).
6. Discussion
ROS and endocannabinoids play a crucial role in blood pressure regulation and are involved in the development of hypertension. The endocannabinoids that level is enhanced during hypertension [40] through activation of cannabinoid receptor may modulate ROS generation [41], [42]. However, enhanced ROS generation enhances the inflammatory response and endothelial dysfunction, which can trigger the development of hypertension [3] and lead to kidney metabolic disorders [43], [44]. The analysis of the functional parameters shown in this and previous study [16] suggests that the chronic administration of URB597, a FAAH inhibitor, in contrast to acute administration, does not prevent hypertension, particularly in rats with spontaneous hypertension.
6.1. Redox and endocannabinoid systems in the kidneys of hypertensive rats
Primary and secondary hypertension is associated with kidney inflammatory and oxidative responses, perhaps as a result of enhanced xanthine and NADPH oxidase activity and consequently superoxide anion generation. Such situation favors oxidative conditions that are additionally escalated by inhibition of the antioxidant defense. The study results indicate that diminished antioxidant enzymes activity may be associated with oxidative modifications at the transcriptional and protein levels. Regulation of a broad spectrum of antioxidant protein biosynthesis results from Nrf2 transcription factor activity, which is regulated by cytoplasmic and nuclear inhibitors and activators [45]. This study's findings indicate that hypertension enhances the levels of Nrf2 and its cytosolic inhibitor Keap1. However, the biological activity of Keap1 is dependent on the availability of the functional cysteine thiol groups, which are susceptible to modifications by ROS and electrophilic aldehydes generated during phospholipid peroxidation [46], and that enhanced levels are observed in the kidneys of hypertensive rats, particularly DOCA-salt–treated rats. Moreover the enhanced expression of KAP1, p62, and p21, inhibitors of Keap1-Nrf2 complex formation prevents Nrf2 degradation and allows Nrf2 to move to the nucleus, where it binds to ARE element for transcription of antioxidant protein. However, an increased expression of Bach1 (the nuclear competitor for DNA binding) in the SHR kidney tissues may prevent Nrf2 transcriptional activity. Similar changes in Nrf2 level have been observed in lupus nephritis disease [47]. In reference to a previous paper, the Nrf2 pathway may be activated through the upregulation of ROS signaling switched on by anandamide and other FAAH substrates [48]. This activation mechanism may be cannabinoid receptor dependent or independent. Our results indicate that a receptor-dependent mechanism is involved in Nrf2 activation in both types of hypertension because levels of Nrf2 and its target protein - HO-1 correspond with higher levels of endocannabinoids and CB1 receptor, which is responsible for ROS generation.
Disturbances in antioxidant proteins synthesis in kidney of rats with primary and secondary hypertension are accompanied by inhibition of antioxidant enzymes activity. These changes may result from protein modifications by ROS as well as by electrophilic lipid peroxidation products. Increased activity of PLA2 promotes realizing free PUFAs that are oxidized by ROS with generation of aldehydes (4-HNE, MDA) that further propagate oxidative damages. These strong electrophiles easily react with nucleophilic cysteine, lysine, and histidine residues of peptides and proteins to form stable covalent adducts. Cysteine residues in proteins (e.g. GSH-Px) and GSH are particularly susceptible to electrophilic addition [49].
However, the enhanced generation of lipid peroxidation products leads to a decreased level of phospholipids and free PUFAs in both types of hypertension. The decreased levels of free PUFAs may also be dependent on the observed enhanced activity of COX1/2 in hypertensive rat kidneys, which participates in the generation of oxidative products [50]. Several lines of evidence have demonstrated that hypertension leads to an increased expression of COX-2, which plays a role in maintaining kidney functions [51], [52]. Enhanced levels of other lipid mediators, such as endocannabinoids, in the kidneys of hypertensive rats may be partially associated with the availability of the substrate arachidonic acid for biosynthesis [53], [54]. Enhanced levels of AEA and 2-AG through enhanced expression of the CB1 receptor with lower TRPV1 and CB2 receptor expressions may trigger oxidative disturbances and inflammation, which interfere with kidney metabolism and function.
6.2. Effects of URB597 on redox and endocannabinoid systems in the kidneys of hypertensive rats
Despite the slight effect of URB597 on the functional parameters of hypertensive rats, this compound affects cellular metabolism associated with the redox and endocannabinoid systems in the kidney. The chronic administration of URB597 to SHR and DOCA-salt hypertensive rats downregulates kidney FAAH and MAGL activity, resulting in additional increase in the level of AEA, 2-AG, and NADA in comparison to those in hypertensive rats. This finding confirms the effectiveness of URB597 inhibition activity. The observed effect is stronger than those in previous examinations of the livers of DOCA-salt hypertensive rats [17]. As a result, URB597 is involved in upregulating the expression of cannabinoid receptors, which are responsible for ROS level suppression, CB2 and TRPV1 in DOCA-salt hypertensive rats and CB2 in SHR as well as the downregulation expression of CB1 receptors, which promote decrease in the ROS level and inflammatory response in the kidneys of rats with secondary hypertension, but their upregulation in SHR. With respect to DOCA-salt hypertensive rats, this study confirms the findings of previous studies [19], [55] indicating that inactivation of endocannabinoid degradation enzymes is associated with reduced levels of TNF-α, but is the first to show that URB597 administration in SHRs increases CB1 and CB2 expressions in the kidney. In such situations, it is difficult to predict which metabolic pathway (pro- or antioxidative) dominates. The ROS-generated enzyme activity and, consequently, ROS level are decreased. However, superoxide dismutase activity in the kidneys of SHRs is significantly enhanced, which may explain why, despite decreased ROS levels, the degree of oxidative modifications is enhanced. Superoxide dismutase is responsible for superoxide anion dismutation into hydrogen peroxide that may oxidize the thiol groups of proteins, changing their structure and functions [56]. Hydrogen peroxide may be then metabolized into reactive hydroxyl radicals. Such a scenario is very likely because the activities of two main enzymes responsible for hydrogen peroxide decomposition (glutathione peroxidase and catalase) are decreased, particularly in the kidneys of SHRs after URB597 administration. As a consequence, hydroxyl radical activity promotes the oxidative modifications of the cellular components and redox metabolism observed in this study, while activated CB1 receptors may promote cardiovascular dysfunction and tissue injury. Therefore, it may explain the lack of functional and metabolic improvement noted after URB597 administration to SHR.
Independently, URB597 given to hypertensive rats induces additional redox disturbances in kidney at the transcription level associated with Nrf2 activity in the antioxidant system in both groups of hypertensive rats. The study results indicate the existence of opposite changes in DOCA-salt hypertensive rats and SHR kidneys regarding the Nrf2 environment. In the kidneys of rats with primary hypertension receiving URB597, oxidative stress and enhanced expression of the main Nrf2 activators (KAP1) favors the enhanced transcriptional activity of Nrf2. This is evidenced by the increased expression of HO-1 despite the increased expression of Nrf2 inhibitors (Keap1 and Bach1). However, the opposite metabolic response to URB597 is observed in kidney of rats with secondary hypertension, in which non-awaited metabolic changes affecting Nrf2 transcription activity are observed. One reasonable suggestion is that significantly higher oxidative modifications of DNA occur in the kidneys of rats with secondary hypertension compared to those with primary hypertension and may affect the transcriptional ARE region required for antioxidant protein activation, including HO-1 biosynthesis. This suggestion may be confirmed by diverse changes in antioxidant enzyme activity. Concurrently, a lower HO-1 transcription level in the kidneys of DOCA-salt rats is accompanied by decreased CB1 receptor expression. The participation of cannabinoid receptors in Nrf2 activation indicated earlier [57] is confirmed in present situation.
Changes in the oxidant and antioxidant parameters caused by URB597 in the kidneys of hypertensive rats globally lead to increased oxidative conditions, resulting in cellular macromolecules oxidative modifications. This is particularly evident in relation to the lipid peroxidation products, such as reactive aldehydes, that are generated during ROS-dependent and LOX/COX-dependent metabolism [50]. In the kidneys of DOCA-salt hypertensive rats receiving URB597, oxidative fragmentation with low molecular aldehyde generation is enhanced, while in the kidneys of SHRs, increased oxidative fragmentation as well as cyclization with neuroprostane generation are observed. Reactive aldehydes (4-HNE, MDA) may behave as secondary messengers that can propagate oxidative damages, in particularly in protein and DNA structure [49]. However, a significant decrease in the COX1/2 enzyme activities responsible for fatty acid oxidation favors the observed increase in PUFA's and endocannabinoid's levels.
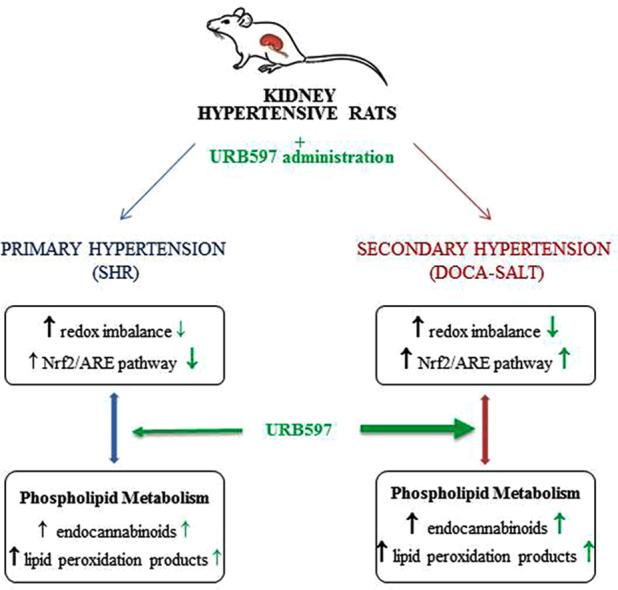
This study's findings indicate that primary and secondary hypertension modulate the endocannabinoid system, which participates in enhancing oxidative conditions and inflammatory response in rat kidneys. However, chronic URB597 treatment of hypertensive rats modifies the redox balance by enhancing the generation of phospholipid mediators including endocannabinoids. Changes caused by URB597 are dependent on model of hypertension. Administering FAAH inhibitor to rats with primary hypertension does not significantly change the pro-inflammatory or oxidative conditions caused by hypertension that result from enhanced CB1 expression. After the administration of URB597 to DOCA-salt hypertensive rats, enhanced disturbances in cross-talk among endocannabinoids, oxidants, and inflammatory factors in the kidney increased the likelihood of functional disorders. However, we suggest that the above disturbances are dependent on the primary changes caused by differently induced hypertension.
Independently of the influence of chronic URB597 administration on the metabolic changes in the kidneys of hypertensive rats, this FAAH inhibitor also affects kidney cellular metabolism of normotensive rats. URB597 given chronically to Wistar rats enhances phospholipid oxidative fragmentation and the oxidative modifications of DNA and proteins similarly to its administration to rats with secondary hypertension. However, the metabolic response of WKY rat kidneys to URB597 administration indicates enhanced oxidative conditions mainly with oxidative phospholipid cyclization but without significant enhanced oxidative modifications to DNA and proteins. Because the direction of changes in metabolic response to chronic administration of URB597 to Wistar and Wistar Kyoto rats is similar to that observed in appropriate groups of hypertensive rats, it suggests that modulation cellular metabolism by FAAH inhibitor is associated with differences in basic kidney metabolism of these two different rat strains.
In conclusion, because URB597 disturbs the kidney redox system and phospholipid ROS- and enzymatic-dependent metabolism, its chronic administration may lead to kidney disorders in hypertensive and normotensive rats. Kidneys from Wistar rats with induced hypertension and their controls are more vulnerable to URB597 action than those from SHR and their controls. Therefore, further studies should be conducted with particular care.
Acknowledgements
This study was conducted with the use of equipment purchased by Medical University of Bialystok as part of the OP DEP 2007–2013, Priority Axis I.3, contract No. POPW.01.03.00-20-022/09.
All procedures and experimental protocol were approved by the local Animal Ethics Committee in Bialystok (Poland).
Acknowledgments
Conflict of interest
The authors declare that there are no conflicts of interest.
Disclosure statement
The authors have nothing to disclose.
References
- 1.Luo W.M., Kong J., Gong Y., Liu X.Q., Yang R.X., Zhao Y.X. Tongxinluo protects against hypertensive kidney injury in spontaneously-hypertensive rats by inhibiting oxidative stress and activating forkhead Box O1 signaling. PLoS One. 2015;10:e0145130. doi: 10.1371/journal.pone.0145130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prahalathan P., Kumar S., Raja B. Morin attenuates blood pressure and oxidative stress in deoxycorticosterone acetate-salt hypertensive rats: a biochemical and histopathological evaluation. Metabolism. 2012;61:1087–1099. doi: 10.1016/j.metabol.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Crowley S.D. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid. Redox Sign. 2014;20:102–120. doi: 10.1089/ars.2013.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaziri N.D. Causal link between oxidative stress, inflammation, and hypertension. Iran. J. Kidney Dis. 2008;2:1–10. [PubMed] [Google Scholar]
- 5.Elmarakby A.A., Quigley J.E., Imig J.D., Pollock J.S., Pollock D.M. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R76–R83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasap S., Gönenç A., Sener D.E., Hisar I. Serum cardiac markers in patients with acute myocardial infarction: oxidative stress, C-reactive protein and N-terminal probrain natriuretic peptide. J. Clin. Biochem. Nutr. 2007;41:50–57. doi: 10.3164/jcbn.2007007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerman L.O., Nath K.A., Rodriguez-Porcel M., Krier J.D., Schwartz R.S., Napoli C., Romero J.C. Increased oxidative stress in experimental renovascular hypertension. Hypertension. 2001;37:541–546. doi: 10.1161/01.hyp.37.2.541. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca B.M., Costa M.A., Almada M., Correia-da-Silva G., Teixeira N.A. Endogenous cannabinoids revisited: a biochemistry perspective. Prostag. Oth. Lipid M. 2013;103:13–30. doi: 10.1016/j.prostaglandins.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Barutta F., Piscitelli F., Pinach S., Bruno G., Gambino R., Rastaldi M.P., Salvidio G., Di Marzo V., Cavallo Perin P., Gruden G. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes. 2011;60:2386–2396. doi: 10.2337/db10-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larrinaga G., Varona A., Pérez I., Sanz B., Ugalde A., Cándenas M.L., Pinto F.M., Gil J., López J.I. Expression of cannabinoid receptors in human kidney. Histol. Histopathol. 2010;25:1133–1138. doi: 10.14670/HH-25.1133. [DOI] [PubMed] [Google Scholar]
- 11.Ritter J.K., Li G., Xia M., Boini K. Anandamide and its metabolites: what are their roles in the kidney? Front. Biosci. 2016;8:264–277. doi: 10.2741/s461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Wang D.H. Aggravated renal inflammatory responses in TRPV1 gene knockout mice subjected to DOCA-salt hypertension. Am. J. Physiol.-Ren. 2009;297:F1550–F1559. doi: 10.1152/ajprenal.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipina C., Hundal H.S. Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol. 2016;6:150276. doi: 10.1098/rsob.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegmund S.V., Seki E., Osawa Y., Uchinami H., Cravatt B.F., Schwabe R.F. Fatty acid amide hydrolase determines anandamide induced cell death in the liver. J. Biol. Chem. 2006;281:10431–10438. doi: 10.1074/jbc.M509706200. [DOI] [PubMed] [Google Scholar]
- 15.Godlewski G., Alapafuja S.O., Bátkai S., Nikas S.P., Cinar R., Offertáler L., Osei- Hyiaman D., Liu J., Mukhopadhyay B., Harvey-White J., Tam J., Pacak K., Blankman J.L., Cravatt B.F., Makriyannis A., Kunos G. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem. Biol. 2010;17:1256–1266. doi: 10.1016/j.chembiol.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toczek M., Baranowska-Kuczko M., Grzęda E., Pędzińska-Betiuk A., Weresa J., Malinowska B. Age-specific influences of chronic administration of the fatty acid amide hydrolase inhibitor URB597 on cardiovascular parameters and organ hypertrophy in DOCA-salt hypertensive rats. Pharmacol. Rep. 2016;68:363–369. doi: 10.1016/j.pharep.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Biernacki M., Łuczaj W., Gęgotek A., Toczek M., Bielawska K., Skrzydlewska E. Crosstalk between liver antioxidant and the endocannabinoid systems after chronic administration of the FAAH inhibitor, URB597, to hypertensive rats. YTAAP. 2016;301:31–41. doi: 10.1016/j.taap.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Kinsey S.G., Naidu P.S., Cravatt B.F., Dudley D.T., Lichtman A.H. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol. Biochem. Be. 2011;99:718–725. doi: 10.1016/j.pbb.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy N., Cowley T.R., Blau C.W., Dempsey C.N., Noonan J., Gowran A., Tanveer R., Olango W.M., Finn D.P., Campbell V.A., Lynch, M.A M.A. The fatty acid amide hydrolase inhibitor URB597 exerts anti-inflammatory effects in hippocampus of aged rats and restores an age-related deficit in long-term potentiation. J. Neuroinflamm. 2012;9:79. doi: 10.1186/1742-2094-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuzkaya N., Weissmann N., Harrison D.G., Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitricoxide synthase. J. Biol. Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 21.Griendling K.K., Minieri C.A., Ollerenshaw J.D., Alexander R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 22.Prajda N., Weber G. Malignant transformation-linked imbalance: decreased xanthine oxidase activity in hepatomas. FEBS Lett. 1975;59:245–259. doi: 10.1016/0014-5793(75)80385-1. [DOI] [PubMed] [Google Scholar]
- 23.Sykes J.A., McCormac F.X., O’Breien T.J. Preliminary study of the superoxide dismutase content of some human tumors. Cancer Res. 1978;38:2759–2762. [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 25.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 26.Mize C.E., Langdon R.G. Hepatic glutathione reductase. Purification and general kinetic properties. J. Biol. Chem. 1962;237:1589–1595. [PubMed] [Google Scholar]
- 27.Eissa S., Seada L.S. Quantitation of bcl-2 protein in bladder cancer tissue by enzyme immunoassay: comparison with Western blot and immunohistochemistry. Clin. Chem. 1998;44:1423–1429. [PubMed] [Google Scholar]
- 28.Maeso N., Garcia-Martinez D., Ruperez F.J., Cifuentes A., Barbas C. Capillary electrophoresis of glutathione to monitor oxidative stress and response to antioxidant treatments in an animal model. J. Chromatogr. B. 2005;822:61–69. doi: 10.1016/j.jchromb.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Ivanović D., Popović A., Radulović D., Medenica M. Reversed-phase ion-pair HPLC determination of some watersoluble vitamins in pharmaceuticals. J. Pharm. Biomed. Anal. 1999;18:999–1004. doi: 10.1016/s0731-7085(98)00109-5. [DOI] [PubMed] [Google Scholar]
- 30.Vatassery G.T., Brin M.F., Fahn S., Kayden H.J., Traber M.G. Effect of high doses of dietary vitamin E on the concentrations of vitamin E in several brain regions, plasma, liver, and adipose tissue of rats. J. Neurochem. 1988;512:621–623. doi: 10.1111/j.1471-4159.1988.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 31.Christie W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In: Christie W.W., editor. Advances in Lipid Methodology – Two. Oily Press; Dundee: 1993. pp. 69–111. [Google Scholar]
- 32.Luo X.P., Yazdanpanah M., Bhooi N., Lehotay, D.C D.C. Determination of aldehydes and other lipid peroxidation products in biological samples by gas chromatography-mass spectrometry. Anal. Biochem. 1995;228:294–298. doi: 10.1006/abio.1995.1353. [DOI] [PubMed] [Google Scholar]
- 33.Coolen S.A., Van Buuren B., Duchateau G., Upritchard J., Verhagen H. Kinetics of biomarkers: biological and technical validity of isoprostanes in plasma. Amino Acids. 2005;29:429–436. doi: 10.1007/s00726-005-0229-2. [DOI] [PubMed] [Google Scholar]
- 34.Fam S.S., Murphey L.J., Terry E.S., Zackert W.E., Chen Y., Gao L., Pandalai S., Milne G.L., Roberts L.J., Porter N.A., Montine T.J., Morrow J.D. Formation of highly reactive A-ring and J-ring isoprostane-like compounds (A4/J4-neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 2002;277:36076–36084. doi: 10.1074/jbc.M205638200. [DOI] [PubMed] [Google Scholar]
- 35.Lam P.M., Marczylo T.H., El-Talatini M., Finney M., Nallendran V., Taylor A.H., Konje J.C. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma. Anal. Biochem. 2008;380:195–201. doi: 10.1016/j.ab.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 36.Ulloa N.M., Deutsch D.G. Assessment of a spectrophotometric assay for monoacylglycerol lipase activity. AAPS J. 2010;12:197–201. doi: 10.1208/s12248-010-9180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds L.J., Hughes L.L., Yu L., Dennis E.A. 1-Hexadecyl-2-arachidonoylthio-2-deoxy-sn-glycero-3-phosphorylcholine as a substrate for the microtiterplate assay of human cytosolic phospholipase A2. Anal. Biochem. 1994;217:25–32. doi: 10.1006/abio.1994.1079. [DOI] [PubMed] [Google Scholar]
- 38.Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G., Ahn B.W., Shaltiel S., Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 39.Dizdaroglu M., Jaruga P., Rodriguez, H H. Measurement of 8-hydroxy-2’-deoxyguanosine in DNA by highperformance liquid chromatography-mass spectrometry: comparison with measurement by gas chromatography-mass spectrometry. Nucleic Acids Res. 2001;29:e12. doi: 10.1093/nar/29.3.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bátkai S., Pacher P., Osei-Hyiaman D., Radaeva S., Liu J., Harvey-White J., Offertáler L., Mackie K., Rudd M.A., Bukoski R.D., Kunos G. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukhopadhyay P., Pan H., Rajesh M., Bátkai S., Patel V., Harvey-White J., Mukhopadhyay B., Haskó G., Gao B., Mackie K., Pacher P. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br. J. Pharmacol. 2010;160:657–668. doi: 10.1111/j.1476-5381.2010.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukhopadhyay P., Rajesh M., Pan H., Patel V., Mukhopadhya B., Bátkai S., Gao B., Haskó G., Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic. Biol. Med. 2010;48:457–467. doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin L., Beswick R.A., Yamamoto T., Palmer T., Taylor T.A., Pollock J.S., Pollock D.M., Brands M.W., Webb R.C. Increased reactive oxygen species contributes to kidney injury in mineralocorticoid hypertensive rats. J. Physiol. Pharmacol. 2006;57:343–357. [PubMed] [Google Scholar]
- 44.Hosohata K. Biomarkers for chronic kidney disease associated with high salt intake. Int. J. Mol. Sci. 2017;30:E2080. doi: 10.3390/ijms18102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gęgotek A., Skrzydlewska E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015;307:385–396. doi: 10.1007/s00403-015-1554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H.J., Vaziri N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Ren. 2010;298:662–671. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 47.Jiang T., Tian F., Zheng H., Whitman S.A., Lin Y., Zhang Z., Zhang N., Zhang D.D. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 2014;85:333–343. doi: 10.1038/ki.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H., Wood J.T., Whitten K.M., Vadivel S.K., Seng S., Makriyannis A., Avraham H.K. Inhibition of fatty acid amide hydrolase activates Nrf2 signalling and induces heme oxygenase 1 transcription in breast cancer cells. Br. J. Pharmacol. 2013;170:489–505. doi: 10.1111/bph.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aldini G., Dalle-Donne I., Facino R.M., Milzani A., Carini M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev. 2007;27:817–868. doi: 10.1002/med.20073. [DOI] [PubMed] [Google Scholar]
- 50.Griesser M., Boeglin W.E., Suzuki T., Schneider C. Convergence of the 5-LOX and COX-2 pathways: heme-catalyzed cleavage of the 5S-HETE-derived di-endoperoxide into aldehyde fragments. J. Lipid Res. 2009;50:2455–2462. doi: 10.1194/jlr.M900181-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Félétou M., Huang Y., Vanhoutte P.M. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br. J. Pharmacol. 2011;164:894–912. doi: 10.1111/j.1476-5381.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pérez-Girón J.V., Palacios R., Martín A., Hernanz R., Aguado A., Martínez-Revelles S., Barrús M.T., Salaices M., Alonso M.J. Pioglitazone reduces angiotensin II-induced COX-2 expression through inhibition of ROS production and ET-1 transcription in vascular cells from spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H1582–H1593. doi: 10.1152/ajpheart.00924.2013. [DOI] [PubMed] [Google Scholar]
- 53.Ambrosi S., Ragni L., Ambrosini A., Paccamiccio L., Mariani P., Fiorini R., Bertoli E., Zolese G. On the importance of anandamide structural features for its interactions with DPPC bilayers: effects on PLA2 activity. J. Lipid Res. 2005;46:1953–1961. doi: 10.1194/jlr.M500121-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu T., Ohto T., Kita Y. Cytosolic phospholipase biochemical properties and physiological roles. IUBMB Life. 2006;58:328–333. doi: 10.1080/15216540600702289. [DOI] [PubMed] [Google Scholar]
- 55.Cao Z., Mulvihill M.M., Mukhopadhyay P., Xu H., Erdélyi K., Hao E., Holovac E., Haskó G., Cravatt B.F., Nomura D.K., Pacher P. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology. 2013;144:808–817. doi: 10.1053/j.gastro.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halliwell B., Gutteridge J.M.C. fifth edition. Oxford University Press; United Kingdom: 2015. Free Radicals in Biology and Medicine. [Google Scholar]
- 57.Li H., Wood J.T., Whitten K.M., Vadivel S.K., Seng S., Makriyannis A., Avraham H.K. Inhibition of fatty acid amide hydrolase activates Nrf2 signalling and induces heme oxygenase 1 transcription in breast cancer cells. Br. J. Pharmacol. 2013;170:489–505. doi: 10.1111/bph.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]