Graphical abstract
Keywords: Military impacts, Syringodium filiforme, Trace elements, Vieques
Highlights
-
•
The United States Navy used half of Vieques, Puerto Rico and conducted military training exercises from 1947 to 2003.
-
•
We conducted a longitudinal study to compare lead Pb, Cd and Cu content in a seagrass collected at a former bombing range in Puerto Rico.
-
•
Pb, Cd, and Cu levels were consistently higher at the bombing range.
-
•
High variability in Pb and Cd content were observed with up to 14 and 17 times higher than seagrass from the control site.
-
•
Syringodium filiforme has the ability to bioaccumalate heavy metals from sediments and potentially can be used for restoration efforts.
Abstract
Trace element composition in plant biomass could be used as an indicator of environmental stress, management practices and restoration success. A longitudinal study was conducted to compare Pb, Cd, and Cu content in seagrass Syringodium filiforme collected at a former bombing range in Puerto Rico with those of a Biosphere Reserve under similar geoclimatic conditions. Trace elements were measured by atomic absorption after dry-ashing of samples and extraction with acid. In general, levels of Pb, Cd, and Cu varied during 2001, 2003, 2005–2006, and 2013–2016. Results showed that bioaccumulated concentration of these trace elements were consistently higher, but not significant, at the bombing range site. As expected in polluted areas, greater variability in Pb and Cd content were observed in the military impacted site with levels up to 14 and 17 times higher than seagrass from the reference site, respectively. Although a decrease in Pb was observed after cessation of all military activities in 2003, the concentration in plant biomass was still above levels of ecological concern, indicating that natural attenuation is insufficient for cleanup of the site.
1. Introduction
Military practices have left a legacy of pollution worldwide, representing a significant anthropogenic disturbance. In Puerto Rico, the Eastern island municipality of Vieques was used for military practices by the US Navy from mid-1940 until 2003. During the intensive training activities at the 23,000-acre site known as the Atlantic Fleet Weapons Training Facility (AFWTF), significant amounts of live ammunition was fired, including both conventional and unconventional weapons, napalm, agent orange and bullets with depleted uranium. Leaving the population with areas highly contaminated and with the potential risk of developing conditions associated with munitions-specific carcinogens [1]. After cessation of all military activities was ordered, the site was considered as a highest priority for cleanup, including large portions of surrounding waters. Therefore, the area was included in 2004 in the National Priorities List by the US Environmental Protection Agency.
In addition to explosive compounds, military activities have been linked to heavy metal pollution including lead (Pb), cadmium (Cd) and copper (Cu), which are known to be toxic elements [2], [3], [4], [5], [6]. Beyond direct exposure and toxic consequences, these elements bioaccumulate in living organisms, including humans and plants. Many preventive measures to decrease the exposure to Pb have been implemented around the world. However, Pb toxicity is still prevalent. Main sources of Pb exposure include mining, smelting, lead battery disposal, crystal and ceramic industries. Pb toxicityinduces a wide array of physiological, biochemical and behavioral dysfunctions. These effects are found in laboratory animals, as well as humans, and affect the nervous, hematopoietic, cardiovascular, and reproductive systems [7].
Cd toxicity has been studied thoroughly. These studies show that, in humans, kidneys are the most affected organs. Other affected organs in animals and humans include liver, lungs, pancreas, bones, reproductive organs, hematopoietic, nervous and cardiovascular systems [8]. Other health conditions attributed to Cd toxicity include hypertension, type 2 diabetes mellitus, thyroid function, and cancer [8], [9]. Although Cu is an essential microelement for plants and human bodies, in high concentrations it is toxic to the ecosystem and human health. A constant intake of Cu over an extended period can cause anemia, damage of the pancreas, liver, and kidney, and decrease in the levels of high-density lipoprotein cholesterol [10].
Aquatic ecosystems are more sensitive to heavy metals than terrestrial ecosystems. Specifically, seagrass can bioaccumulate essential and non essential elements from the water column or sediment material, thus compromising the marine food web. The ability of seagrass Zostera japonica to accumulate Pb, Cd and Cu to potentially enhanced environmental decontamination has been shown previously [11]. An increased concentration of Cd and Cu in seagrass increases oxidative stress and induces antioxidant defense systems against reactive oxygen species [12]. Furthermore, the accumulation of heavy metals by seagrasses can be a good indicator of a decrease of antioxidant levels due to free radicals [13]. Tolerance in plants to toxic elements has been extensively documented for over 35 years. Information on the ability of seagrass worldwide to absorb these elements is, however, still limited. The direct uptake of Pb, Cd, and Cu by aquatic plants increases the likelihood that the toxins will be transferred to marine organisms through the food web, including fish and endangered animals. For humans, it is known that prolonged consumption of fish contaminated with heavy metals can lead to biochemical disruption within organs, negatively impacting liver, kidney, cardiovascular, nervous and bone conditions [13], [14].
In Vieques, a shallow bed of Syringodium filiforme is the dominant seagrass species along Carrucho Beach at the south coast of the former bombing range. Tribble [15] demonstrated that coral reef fish have a preference for Syringodium rather than other marine plants such as Thalassia. Their distribution in coral reefs is usually limited by selective grazing activity. Furthermore, this species is very common in the Caribbean and has been associated to high resistance to storm disturbances and perhaps to a variety of other environmental disturbances. Physiological differences and ecotypes of S. filiforme have been reported, including specific adaptation to light histories among others [16].
Because seagrasses represent a key species in the marine ecosystem, for 15 years we evaluated accumulation of Pb, Cd and Cu of acid digested samples from AFWTF and Guánica State Dry Forest (GSDF) as a reference location. These observations provide insights on the ecological consequences of anthropogenic disturbances and the potential transfer of pollutants through the open ecosystem.
2. Materials and methods
Our reference location to collect samples of S. filiforme was Tamarindo Beach at Guánica State Dry Forest (GSDF). It’s geology and environmental conditions resemble those observed at the eastern part of Vieques;both sites are under the direct influence of the Caribbean Sea (Fig. 1). This forest encompasses great diversity, including endangered species, encompassing almost 1000 acres of land. Due to its ecological importance, it was designated in 1981 as a United Nations International Biosphere Reserve. Currently, this location is considered the best-preserved subtropical dry forest, as well as a great representative of dry forest in the Caribbean [17].
Fig. 1.
Sampling site in Carrucho Beach at the former Atlantic Fleet Weapons Training Facility in Vieques, Puerto Rico. The reference location was located southwest mainland Puerto Rico in Tamarindo Beach at the Guánica State Dry Forest.
Samples of S. filiforme were manually collected from a one square meter plot at Carrucho Beach in the former AFWTF (18°08.32N, 65°18.10W) in the island of Vieques, Puerto Rico (Fig. 1). Replicate plots along the coastal line were sampled several times during 2001, 2003, 2005–2006, and 2013–2016. Samples were similarly taken from GSDF reference location at Tamarindo Beach (17°57.21N, 66°50.971W). After collection, samples were placed in large plastic bags and immediately transported to the laboratory. Samples were handled only with plastic, glass, or porcelain tools. Field and blank controls were included during sampling campaigns. Pb, Cd, and Cu concentrations were below detection limits in these controls.
Analyses of heavy metals followed Montgomery et al. [18] and Thompson [19]. Samples were rinsed thoroughly with deionized water, shaken to remove most of the water, allowed to air dry, and grounded in a ceramic mortar. Approximately 3 g of finely cut material was weighed in a porcelain dish previously heated at 600 °C for 2 h. Samples were then dried in an oven at 65 °C for 24 h, allowed to cool in a desiccator, weighed, and incinerated in a muffle furnace for 2–3 h at 575 °C. Ashes were dissolved in 5 ml of 20% HCl and filtered. The concentration of acid-extractable elements was determined by air-acetylene flame detection in an atomic absorption spectrophotometer (Perkin Elmer Model AA100).
We compared Pb, Cd, and Cu concentrations at AFWTF using an Analysis of Variance with Multiple Comparisons. To compare Pb, Cd, and Cu concentrations at AFWTF with the concentration at GSDF we used t-tests. In addition we compared the slopes of the heavy metals concentration lines at ASWTF versus GSDF across years (Fig. 2).
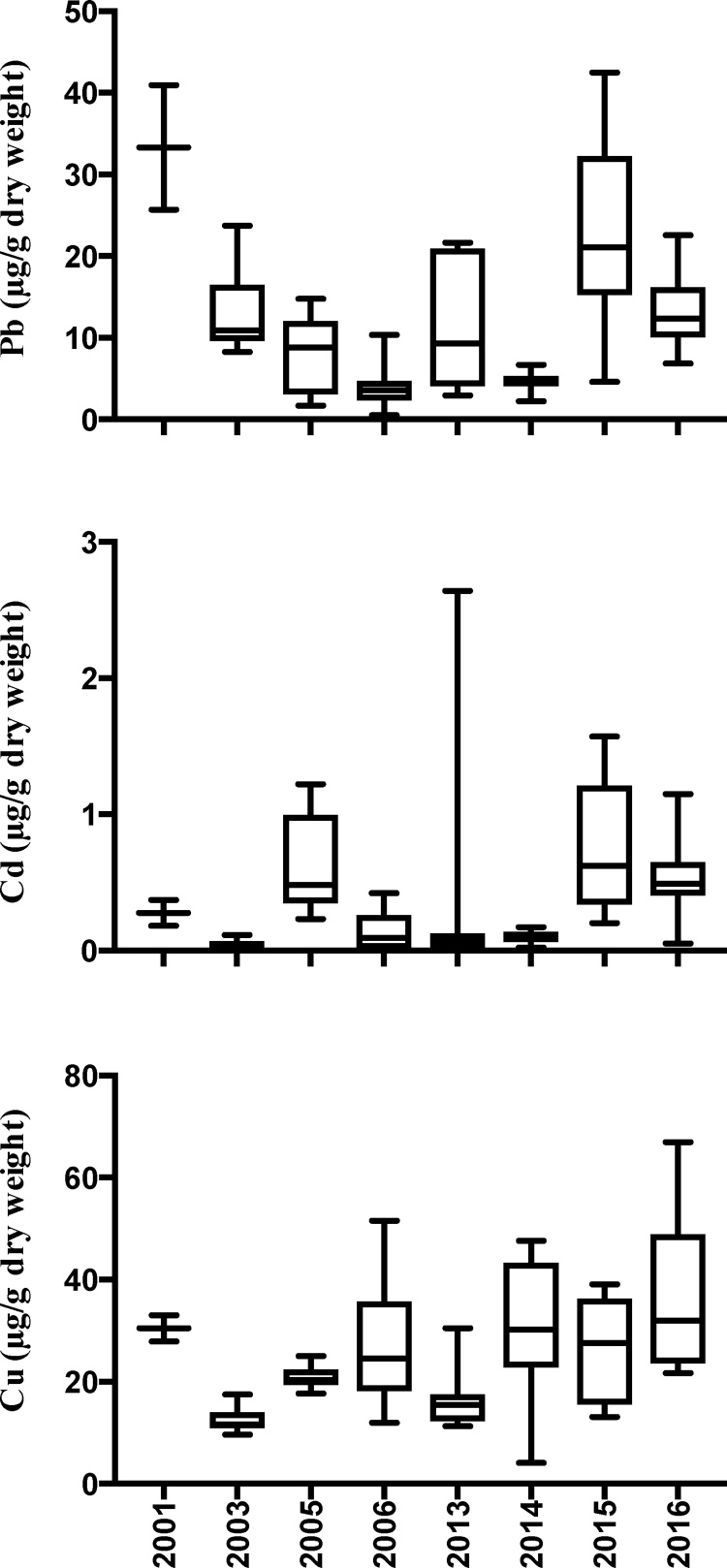
Fig. 2.
Lead (F7,75 = 14.45, p < 0.01), cadmium (F7,75 = 5.16, p < 0.01), and copper (F7,75 = 6.56, p < 0.01) concentrations at the former Atlantic Fleet Weapons Training Facility in Vieques, Puerto Rico during 2001, 2003, 2005–2006, and 2013–2016.
3. Results
Differences in the concentration of Pb, Cd, and Cu were observed during the specified observation period (F7,75 = 14.45, p < 0.01, F7,75 = 5.16, p< 0.01, F7,75 = 6.56, p < 0.01). For Pb, 2003 presented itself as the period with the most erratic results. For both Cd and Cu, 2011 presented itself as a period of wide-ranging results. Pb concentrations were highly variable among and within both time frames. For example, Pb minimum concentration was 0.54 μg/g dry weight in 2006 and 42.47 μg/g dry weight in 2015. Within 2015 it ranged from 4.60 to 42.47 μg/g dry weight. Pb levels at the former AFWTF sampling resulted up to 14 times more than reference samples from GSDF in 2001 when AFWTF was still operational. Cd concentration ranged from 0.01 to 2.64 μg/g dry weight. However, most concentrations were under 0.50 μg/g dry weight. The most variable year was 2013 with minimum concentration below detection levels and a maximum level of 2.64 μg/g dry weight. Cu concentrations ranged from 4.11 to 66.94 μg/g dry weight. The years with the minimal amount of statistical variances were 2001, 2003, and 2005. When we divided the data in two sampling campaigns (2001–2006, and 2013–2016), comparisons of Pb, Cd and Cu total concentrations vs. controls could not be made because unequal sampling among years.
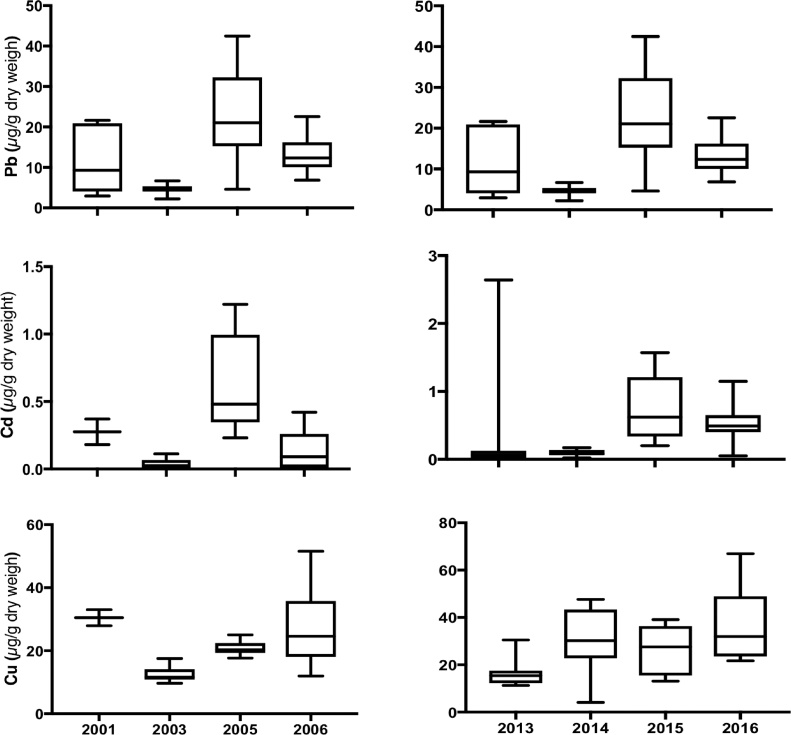
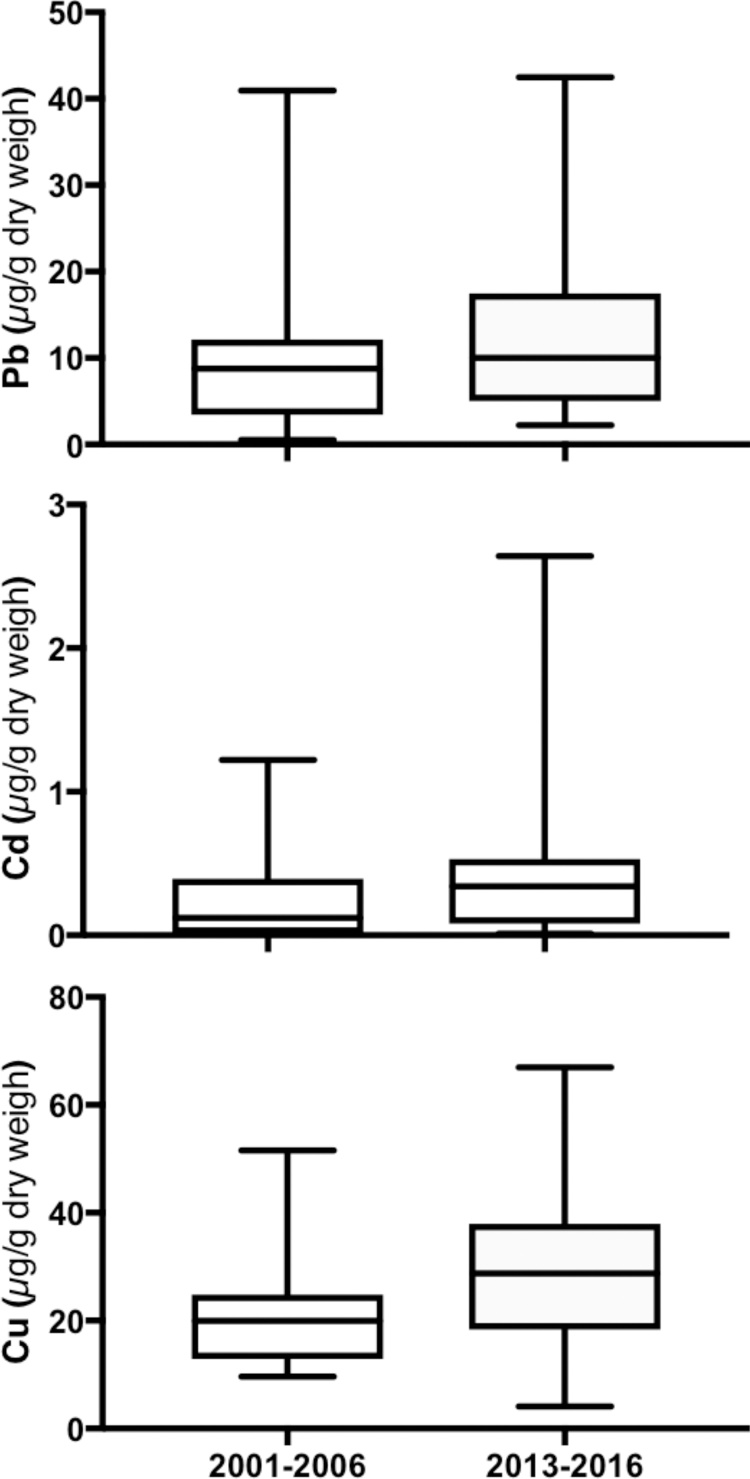
However, Pb concentrations between 2001 and 2006 were different (Fig. 3; F3,28 = 24.63, p < 0.01), 2001 was different from 2003, 2005 and 2006, and 2003 was different from 2006 (Tukey’s posthoc test). Similarly Pb concentrations between 2013 and 2016 were different (Fig. 3; F3,47 = 13.86, p < 0.01), 2013 was different from 2015 and 2014 was different from 2015 and 2016 (Tukey’s posthoc test). Cadmium concentrations between 2001 and 2006 were different (Fig. 3; F3,28 = 13.44, p < 0.01), 2003 was different from 2005 and 2005 was different from 2006 (Tukey’s posthoc test). Similarly Cd concentrations between 2013 and 2016 were different (F3,47 = 1.897, p < 0.01), 2014 was different from 2015 (Tukey’s posthoc test). Copper concentrations between 2001 and 2006 were different (F3,30 = 8.166, p < 0.01), 2001 was different from 2003 (Tukey’s posthoc test). Similarly Cu concentrations between 2013 and 2016 were different (F3,45 = 5.805, p < 0.01), 2013 was different from 2016 (Tukey’s posthoc test). When we analyzed Pb, Cd and Cu grouped concentrations from at the former Atlantic Fleet Weapons Training Facility in Vieques, Puerto Rico from 2001 to 2006 compared to grouped concentrations from 2013 to 2016, we only find differences in Cu concentrations (Fig. 5; t = 2.903, df = 81, p < 0.01).
Fig. 3.
Lead (F3,28 = 24.63, p < 0.01), cadmium (F3,47 = 13.86, p< 0.01), and copper (F3,30 = 8.17, p < 0.01) concentrations at the former Atlantic Fleet Weapons Training Facility in Vieques, Puerto Rico during two sampling campaigns (2001–2006, and 2013–2016).
Fig. 5.
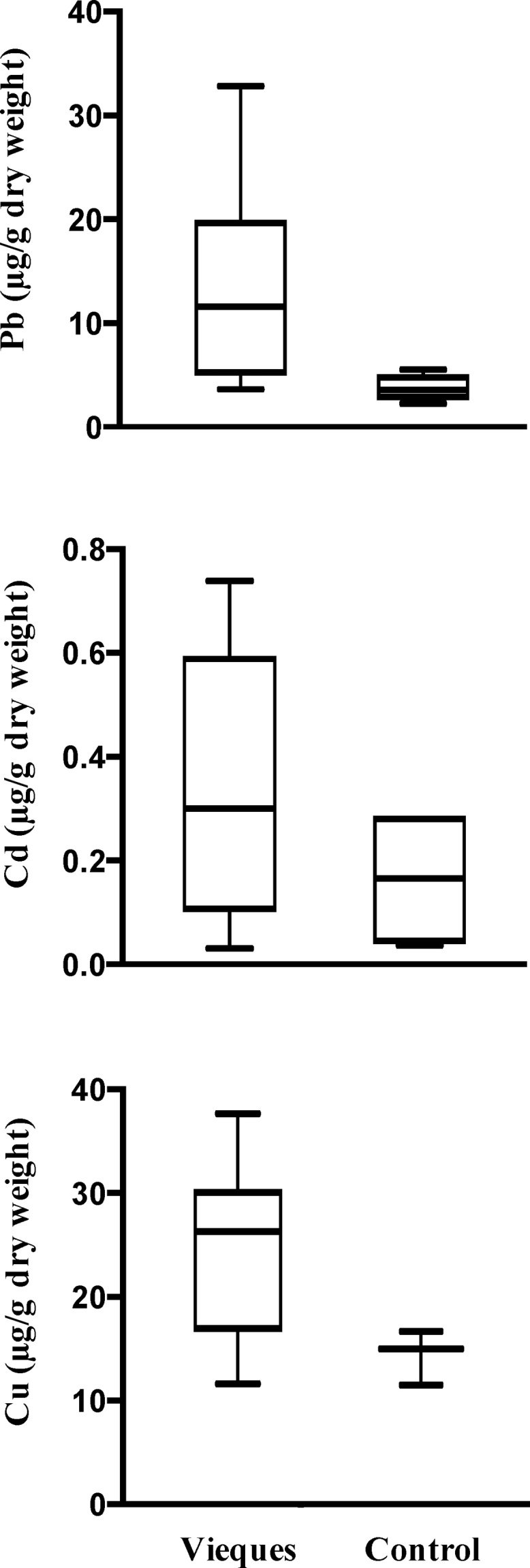
Lead (t = 1.97, p > 0.05), cadmium (t = 1.30, p > 0.05), and copper (t = 2.11, p > 0.05) concentrations at the former Atlantic Fleet Weapons Training Facility in Vieques, Puerto Rico compared to control values obtained at Tamarindo Beach at Guánica State Dry Forest during 2001, 2003, 2005–2006, and 2013–2016.
Neither the US Environmental Protection Agency nor the US Department of Agriculture has established safety standards for these elements in plant biomass [20]. However, the Council of the European Union has specific criteria for feedstock [21]. If this seagrass were used as feedstock, Pb content would be consistently above this safety guideline. Although levels of Cd were not of concern for most of the sampling period, in 2015 its concentration reached significant ecological levels above the 1.2 μg/g dry weight and 17 times higher than reference biological samples.
When we compared AFWTF against GSDF, in all instances, Pb, Cd and Cu concentration was higher in samples collected offshore from the bombing range, but was not significant (Fig. 4; t = 1.97, p > 0.05, t = 1.30, p > 0.05, t = 2.11, p > 0.05). The variance of Pb concentrations was higher (values ranged from 4.11 to 33.32 μg/g dry weight) in AFWTF than in GSDF (values ranged from 2.33 to 3.55 μg/g dry weight). When comparing Pb concentration across years in AFWTF and GSDF slopes were identical, there is a 66.84% chance of randomly choosing data points with slopes this different. The difference between slopes was not significant (F1,8 = 0.20, p > 0.05). Similarly, Cd concentrations in AFWTF ranged from 0.03 to 0.74 μg/g dry weight and GSDF ranged from 0.03 to 0.28 μg/g dry weight. When we compared if Cd concentration across years in AFWTF and GSDF were identical, there was a 22.44% chance of randomly choosing data points with slopes this different, difference between slopes was not significant (F1,8 = 1.73, p > 0.05). We observed a high variance of Cu concentrations in AFWTF (values ranged from 12.74–30.48 μg/g dry weight) but not in GSDF (values ranged from 12.16–16.95). When we compared slopes for Cu concentration across years in AFWTF and GSDF, there is a 72.92% chance of randomly choosing data points with slopes this different, difference between slopes was not significant (F1,8 = 3.61, p > 0.05).
Fig. 4.
Lead (t = 1.53, p > 0.05), cadmium (t = 1.79, p > 0.05), and copper (t = 2.09, p < 0.05) grouped concentrations from at the former Atlantic Fleet Weapons Training Facility in Vieques, Puerto Rico from 2001 to 2006 compared to grouped concentrations from 2013 to 2016.
4. Discussion
Military activities conducted on land and at seahave been associatedwith the release of toxic elements into the environment [6]. In our study, Pb in above-ground tissue samples of S. filiforme from the AFWTF could indicate greater anthropogenic impact than on other seagrass habitats offshore from the Guánica Dry State Forest. The levels of Pb, Cd and Cu found in this study were within concentrations previously reported for marine plants and algae exposed to heavy metals elsewhere [11], [12]. Differences were higher for Pb at the AFWTF, but not in general for Cu and Cd. The level of Pd in plant biomass from the former bombing range could lead to further dispersion and perhaps might indicate the transfer of other military associated elements throughout the marine food web.
Although Pb can be found in the environment, its accumulation by seagrass cannot be explained solely as a result of natural processes. The water pH (approximately 8 ± 0.5) limits the solubility of many metals, including Pb and Cd. These metals must be dissolved in order to be available for uptake by marine plants. During active military training in Vieques, water quality data was collected by US Environmental Protection Agency. Discharge Monitoring Reports from 1984 to 1999 revealed elevated concentrations of Pb and other military associated pollutants in seawater, as well as a variation in pH [22]. For example, Pb was reported at levels as high as 5 mg/L at Carrucho Beach when bombs exploded offshore near the range. Changes in the water chemistry during military maneuvers, including resuspension of sediment material, could result in enhancement of metal bioavailability and uptake by marine life.
In May 2003, military operations ceased at the AFWTF. Samples obtained in 2004 of S. filiforme showed lower concentrations of Pb to those observed previously. However, levels remained higher than at a control site in Puerto Rico. Currently, removal of unexploded ordnance is in progress. By 2015, more than 44,000 bombs were removed from the terrestrial ecosystem; possible contamination in the marine ecosystem has yet to be assessed [23]. On land, the main ordinance removal strategy consists of open burning of terrestrial plants and open detonation. Even though a direct connectivity exists between the former bombing range and the marine ecosystem, efforts to control water runoff and prevent erosion into the seahave not been implemented. In addition, a long water channel that connects Anones lagoon to the sampling sites in Carrucho Beach was reopened to the sea during late 2004. Anones lagoon is located in the middle of the range; storm waters mainly drain to this point. Perhaps, the lack of water management (i.e. containment) practices has facilitated the leaching of pollutants to the Caribbean Sea. This migration of toxins has continued even though the range is currently inactive.
Regardless of the source of exposure, anthropogenic or natural exposure to resuspended sediments during riptides, the levels of Pb and Cd in S. filiforme demonstrate the potential for dispersion, resulting in a dangerous bioaccumulation, posing a threat to human health via fish or crustacean consumption. Once toxic elements reach the base of the food web, the problem crosses commonly recognizedlocal boundaries and becomes a more significant regional issue. Many of these elements could adversely compromise reproductive success rates of marine species, as well as ecosystem productivity.
For example, biomonitoring of trace elements such as Pb, Cd and Cu in fish from the Mediterranean Region [13] or heavy metals in southern India [14] has shown the regional implications and threat to marine organisms. In Puerto Rico, fish, crustaceans, and manatees directly or indirectly consume S. filiforme. The US Fish and Wildlife Service reported manatees feeding in Vieques and most intensively near the former AFWTF. A better understanding of trace elements and other pollutants in this environment is vital to establish management practices intended to prevent further exposure, to and reduce threats to humans and endangered species.
In conclusion, our results demonstrate the capacity of S. filiforme to bioaccumulate Pb, Cd and Cu from seawater and/or marine sediments. These results confirm the need to address links between past military impacts and the environment at this and other abandoned military sites. Furthermore, this commonly found and abundant seagrass has proven to be useful for monitoring environmental health, which, in turn, could be useful when developing policies and procedures leading to effective, sustained ecological reclamation and restoration.
Acknowledgements
This project was funded in part by Casa Pueblo de Adjuntas. We thank Cacimar Zenón, Ernie Pérez, Manuel Berrios and Lizbeth Dávila for their help during sampling activities and Larry Forney for his contribution to the manuscript. We are grateful to José R. Almodóvar who developed the graphic abstract and two anonymous reviewers.
References
- 1.Sanderson H., fauser P., Stauber R.S., Christensen J., Løfstrøm P., Becker T. Civilian exposure to munitions-specific carcinogens and resulting cancer risks for civilians in the Puerto Rico island of Vieques following military execises. Global Secur.: Helath Sci. Policy. 2017;2:39–60. [Google Scholar]
- 2.Jenkins T.F., Pennington J.C., Ranney T.A., Berry T.E., Jr., Miyares P.H., Walsh M.E., Hewitt A.D., Perron N.M., Parker L.V., Hayes C.A., Wahlgren E.G. U.S Army Engineer Research and Development Center Cold Regions Research and Engineering Laboratory; 2001. Characterization of Explosives Contamination at Military Firing Ranges. [Google Scholar]
- 3.Lawrence M.J., Stemberger H.L.J., Zolderdo A.J., Struthers D.P., Cook S.J. The effects of modern war and military activities on biodiversity and the environment. Environ. Rev. 2015;23:443–460. [Google Scholar]
- 4.Young G.A. Naval Surface Weapons Center; 1978. Environmental Dispersion of the Products of Explosions of Conventional Ordnance at Vieques Island. [Google Scholar]
- 5.Massol-Deyá A., Pérez D., Pérez E., Berrios M., Díaz E. Trace elements analysis in forage samples from a US Navy bombing range (Vieques, Puerto Rico) Int. J. Environ. Res. Public Health. 2005;2:263–266. doi: 10.3390/ijerph2005020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gębka K., Bełdowska M., Bełdowski J. The impact of military practices on the concentration of mercury in soils of military training grounds and marine sediments. Environ. Sci. Pollut. Res. 2016;23:23103–23113. doi: 10.1007/s11356-016-7436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agency for Toxic Substances Disease Registry . U.S. Department of health and Human Services, Public Health Service; Atlanta, GA: 2007. Toxicological Profile for Lead. [Google Scholar]
- 8.Matović V., Buha A., Dukić-Ćosić D., Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015;78:130–140. doi: 10.1016/j.fct.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Buha A., Antonijević B., Bulat Z., Jaćević V., Milovanović V., Matović V. The impacted of prolonged cadmium exposure and co-exposure with polychlorinated biphenyls on thyroid function in rats. Toxicol. Lett. 2013;221:83–90. doi: 10.1016/j.toxlet.2013.06.216. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Toxic Substances Disease Registry . U.S. Department of health and Human Services, Public Health Service; Atlanta, GA: 2005. Toxicological Profile for Cupper. [Google Scholar]
- 11.Lin H., Sun T., Zhou Y., Zhang X. Anti-oxidative feedback and biomarkers in the intertidal seagrass Zostera japonica induced by exposure to copper, lead and cadmium. Mar. Pollut. Bull. 2016;109:325–333. doi: 10.1016/j.marpolbul.2016.05.062. [DOI] [PubMed] [Google Scholar]
- 12.Yokesh Babu M., Palanikumar L., Nagarani N., Janaki Devi V., Ramesh Kumar S., Ramakritinan C.M., Kumaraguru A.K. Cadmium and copper toxicity in three marine macroalgae: evaluation of the biochemical responses and DNA damage. Environ. Sci. Pollut. Res. 2014;21:9604–9616. doi: 10.1007/s11356-014-2999-0. [DOI] [PubMed] [Google Scholar]
- 13.Renieri E.A., Alegakis A.K., Kiriakakis M., Vinceti M., Ozcagli E., Wilks M.F., Tsatkis Cd A.M. Pb and Hg Biomonitoring in fish of the Mediterranean region and risk estimation on fish consumption. Toxics. 2014;2:417–442. [Google Scholar]
- 14.Karunanidhi K., Rajendran R., Pandurangan D., Arumugam G. First report on distribution of heavy metals and proximate analysis in marine edible puffer fishes collected from Gulf of Mannar Marine Biosphere reserve, South India. Toxicol. Rep. 2017;2:319–327. doi: 10.1016/j.toxrep.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tribble G.W. Reef-based herbivores and the distribution of two seagrasses (Syringodiumfiliforma and Thalassia testudinum) in the San Blas Island (Western Caribbean) Mar. Biol. 1981;65:277–281. [Google Scholar]
- 16.Steiner S.C., Macfarlane K.J., Price L.M., Willette D.A. The distribution of seagrasses in Dominica, Lesser Antilles. Rev. Biol. Trop. 2010;58(Suppl. 3):89–98. [PubMed] [Google Scholar]
- 17.Ewel J.J., Whitmore J.L. Forest Service Institute of Tropical Forestry; Rio Piedras, Puerto Rico: 1973. The Ecological Life Zones of Puerto Rico and the U.S. Virgin Islands. ITF-18, USDA. [Google Scholar]
- 18.J.R. Montgomery The release of cadmium, chromium, copper, nickel and zinc by sewage sludge and the subsequent uptake by members of a turtle grass (Thalassia testudinum) ecosystem. Center for Energy and Environmental Research: Mayagüez, PR; In ERDA No. 40–468-74 and EPA No. IAG-D4-0541, 1977.
- 19.Thompson M.H. Analysis of fish and other marine products. J. Ass. Offic. Anal. Chem. 1969;52:55. [Google Scholar]
- 20.US Department of Agriculture, Soil Conservation Service . US Department of Agriculture, Soil Conservation Service; Hyattsville, MD: 1973. Soil Conservation Service Plants Used as Bird Food. [Google Scholar]
- 21.Council of the European Union. Council Directive April 1999/29/EC of 22 April 1999 on the undesirable substances and products in animal nutrition. Off. J. Europ. Com. 1999;L115:32–46. [Google Scholar]
- 22.US Environmental Protection Agency, Discharge Monitoring Reports for the AFWTF, 1984–1999.
- 23.US Environmental Protection . Environmental Protection Agency; 2013. Agency. May 2013 Vieques Activities Update. [Google Scholar]