Highlights
-
•
Ectopic Cushing’s syndrome is a form of Cushing’s in which a tumor outside the pituitary gland produces a hormone called (ACTH).
-
•
Small cell carcinoma and carcinoid of the lung comprises half of its cases.
-
•
Ectopic cushing syndrome is a complicated medical problem especially when it comes to identification of the ectopic spot.
-
•
The surgical treatment could be curative when the spot is determined.
Keywords: Case report, Ectopic Cushing’s, Carcinoid tumor
Abstract
Introduction
Ectopic Cushing syndrome is a form of Cushing’s in which a tumor outside the pituitary gland produces adrenocorticotropic hormone (ACTH). Small cell carcinoma and carcinoid of the lung comprises half of its cases. The main purpose of this study is to present a case of ectopic Cushing syndrome caused by a hidden lung carcinoid and how to manage it.
Presentation of case
Here we present a case of a 26 year old young male complains of increased weight and appetite, proximal muscle weakness, easy bruising and appearing of purple striae on his abdomen, with a final diagnosis of ACTH secreting lung carcinoid.
Discussion
The diagnosis was made by non-invasive radiological procedures (CT scan and MRI) and serological tests. The management consisted of medical treatment which was not useful, then bilateral adrenalectomy to limit the patient symptoms. The ectopic spot was finally detected and excised surgically through thoracotomy. After six months of follow up there was no recurrence, signs and symptoms of Cushing syndrome begin to disappear.
Conclusion
Ectopic cushing syndrome is a complicated medical problem especially when it comes to identification of the ectopic spot. The surgical treatment could be curative when the spot is determined.
1. Introduction
The present work has been reported in line with the SCARE criteria [1].
Cushing’s syndrome is caused by excess amounts of glucocoticosteroids in the blood which can be exogenous or endogenous in origin [2]. The most suggestive symptoms of hypercortisolism include: facial plethora, proximal muscle weakness, bruising without an obvious trauma, accumulation of fat pads in the abdomen and supraclavicular fossa [3]. Increased endogenous glucocorticosteroids can be either from the pituitary (the most common cause [4]), adrenal glands or from ectopic secretion of ACTH or CRH which will eventually lead to elevated blood cortisol. Ectopic Adrenocorticotropic hormone (ACTH) secretion is primarily from small cell carcinoma and carcinoid of the lung, these two comprises half of cases [5], others are mostly from tumors of the thymus, and the pancreas [6], therefore the majority of ectopic secretion cases originate from the lungs which make them the first place to be searched to find the ectopic spot. Initial failed localization of the ACTH secreting spot is common and suggests pulmonary carcinoid as a cause [6]. Here we describe a case of a young male adult who presents with Cushing’s syndrome type of symptoms that appeared afterwards to be originated from ACTH secreting small pulmonary carcinoid. Highlighting how difficult it might be to determine the source of ACTH secretion and how to manage such a case.
The patient approved on all the medical and surgical procedures and was adherent.
The patient consent was obtained before writing the case.
2. Case presentation
A 26 year old young man presented to our institute with the complaint of increasing weight and appetite, his problem started a year ago when he noticed that his appetite has gone up and he started to put on weight rapidly, this was associated with headache, fatigue, proximal muscle weakness and easy bruising and purple striae appeared on his abdomen. There was no previous medical or surgical history, no family history for endocrine disease. The patient is a smoker 30 pack- year and alcoholic from the age of 14.
Physical exam showed multiple purple striae on the abdomen and weakness in the proximal muscle groups in the four limbs, the rest of the exam was within normal limits. Cushing’s syndrome was suspected, the patient was admitted to the endocrinology department, initial work up showed: cortisol 8 a.m. = 25 μg/dL (RR = 13–24 μg/dL), cortisol 11 p.m. = 23 μg/dL (RR = 1–4 μg/dL), early morning ACTH− = 106 (RR = 10–50 pg/mL) full serology of the patient is demonstrated in (Table 1). No suppression of cortisol after high dose (8 mg) dexamethasone. The diagnoses of ectopic ACTH dependent Cushing’s syndrome was established, imaging studies was done to determine the source: multi-slice computed tomography (MSCT) for the chest and abdomen was normal, brain MRI was normal.
Table 1.
Illustrates serology at admission.
| test | Patient value | Reference range |
|---|---|---|
| WBC | 9.15 × 109/L | 4.00–11.0 × 109/L |
| neutrophils | 73% | 40–75% |
| hemoblobin | 16 g/dL | 13–18 g/dL |
| PT | 100% | 60–100% |
| urea | 19 mg/dL | 7–20 mg/dL |
| creatinine | 0,88 mg/dL | 0.6–1.2 mg/dL |
| ALT | 40 units/L | 7–56 units/L |
| Total protein | 6.5 g/dL | 6–8.3 g/dL |
| Albumin | 4.1 g/dL | 3.5–5.5 g/dL |
| glucose | 120 mg/dL | 72–108 mg/dL |
| Total cholesterol | 193 mg/dL | less than 200 mg/dL |
| TG | 163 mg/dL | Less than 150 mg/dL |
| sodium | 140 mEq/L | 135–145 mEq/L |
| potassium | 3.6 mEq/L | 3.5–5.0 mEq/L |
| calcium | 9.7 mg/dL | 8.5–10.2 mg/dL |
| Cortisol 8 a.m. | 23 μg/dL | 13–24 μg/dL |
| Cortisol 11 p.m. | 22.9 μg/dL | 1–4 μg/dL |
| ACTH | 106 pg/mL | 10–50 pg/mL |
| TSH | 0.45 mIU/L | 0.4–4.0 mIU/L |
Bronchoscopy and lavage was done and nothing was detected. The patient was discharged on ketoconazole 200 mg PO once daily, calcium and vitamin D and was asked to revisit the hospital clinic after 3 months.
In the follow up visit the patient condition hadn’t got any better and imaging studies still showed nothing. To limit the patient symptoms and prevent Cushing’s complications, our general surgery team decided to perform bilateral transabdominal endoscopic adrenalectomy and the patient agreed. Four trocars were placed in the subcostal area and the midline to resect the right then the left adrenals. After surgery, the patient was discharged on prednisolone 5 mg PO once daily, fludrocortisone 0.1 mg PO once daily, calcium and vitamin D. After 18 months the patient was re-admitted to the hospital with deterioration of his state. He complained of general malaise, fever, dysphagia and occipital headache that responds to OTC analgesics, physical exam of the neck showed painful swelling with redness under the right mandibular angel and a soft lump in the posterior triangle of the neck that measures 2 cm, lab results was: WBC = 18,000, CRP = 214 the rest of the patient’s serology is shown in (Table 2). The patient was diagnosed with lymphadenitis, blood sample was drawn for culture and the patient received empirical antibiotics.
Table 2.
illustrates serology at the second admission.
| test | Patient value | Reference range |
|---|---|---|
| WBC | 18.0 × 109/L | 4.00–11.0 × 109/L |
| neutrophils | 84% | 40–75% |
| hemoglobin | 16 g/dL | 13–18 g/dL |
| PT | 95% | 60–100% |
| ESR | 150 mm/h | 0–22 mm/h |
| CRP | 214 mg/dL | Less than 3.0 mg/dL |
| urea | 19 mg/dL | 7–20 mg/dL |
| creatinine | 1,2 mg/dL | 0.6–1.2 mg/dL |
| ALT | 49 units/L | 7–56 units/L |
| Total protein | 6.6 g/dL | 6–8.3 g/dL |
| Albumin | 5.0 g/dL | 3.5–5.5 g/dL |
| glucose | 130 mg/dL | 72–108 mg/dL |
| sodium | 140 mEq/L | 135–145 mEq/L |
| potassium | 3.9 mEq/L | 3.5–5.0 mEq/L |
| calcium | 9.3 mg/dL | 8.5–10.2 mg/dL |
| Cortisol 8 a.m. | 17,11 μg/dL | 13–24 μg/dL |
| ACTH | 337 pg/mL | 10–50 pg/mL |
blood culture were sterile, reassessment of the ectopic spot through MSCT for the neck,chest and abdomen was done and this time showed a 2 cm mass in the right middle lobe (Fig. 1) and the adrenals reappeared in both sides (Fig. 2). Thoracic surgery team of our institute decided to remove the mass after the patient condition was stabilized and the patient agreed to undergo the procedure. After a week on empiric antibiotic the patient’s lab results returned to normal, the patient was prepared with 100 mg hydrocortisone before surgery to match the increased need for cortisol in stressful situations such as surgical operation, a right thoracotomy on the fifth intercostal space was made, 2 cm mass in the right middle lobe was found and a wedge shaped resection of the mass was done (Figs. 3 ) and a sample from mediastinal lymph nodes was taken followed by proper closure and a chest tube in place. The mass was sent to the pathology lab. The result was a typical carcinoid tumor (according to WHO classification 2015) which was strongly positive for ACTH immunostaining, and one mediastinal node was positive. Six months after the operation the patient appeared stable with no complaints and CT scan for the chest and abdomen showed no abnormalities.
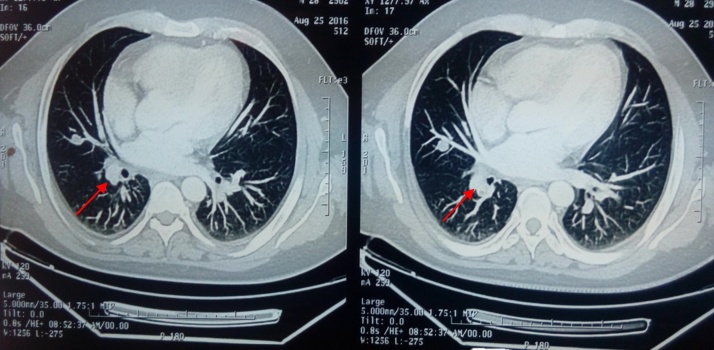
Fig. 1.
CT of the chest shows 1.5 cm mass in the right middle lobe (red arrows).
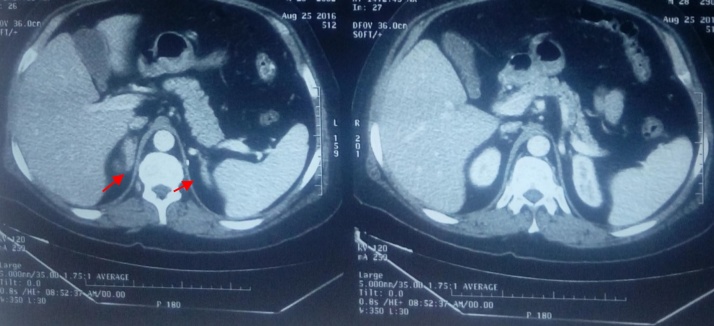
Fig. 2.
CT of the abdomen shows reappearance of adrenal glands in both sides(red arrows).
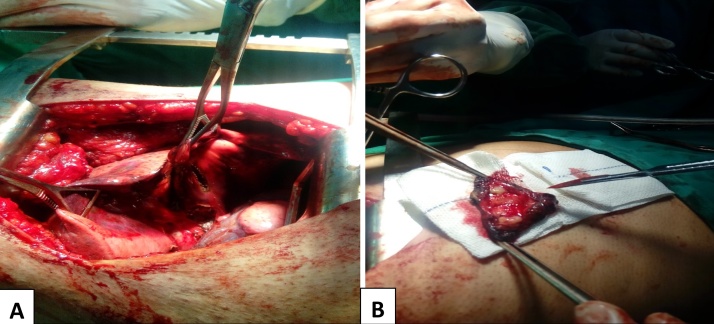
Fig. 3.
Intraoperative pictures of the resected nodule: A: demonstrates dissection of the nodule from adjacent structures, B: The resection is complete with adequate surgical margin.
3. Discussion
our patient had Cushing’s syndrome initially treated medically, then through bilateral adrenalectomy because we couldn’t find the source of ACTH, it took approximately 2 years to identify that a small carcinoid tumor was responsible of the case.
The first priority in this patient was to establish the cause of Cushing which was done through high dose dexamethasone suppression test, pituitary adenomas would be suppressed, however most of ectopic secreting spots wouldn’t [7].
Ectopic ACTH syndrome accounts for 10–20% of ACTH dependent Cushing cases [8], small cell lung carcinoma and lung carcinoids causes half of cases [9], the source should be established because the excision of the tumor can be curative [10], [11], so we focused on the lung to find the source of ACTH (because it contains most of ACTH secreting spots) by doing MSCT and bronchoscopy but at first nothing was abnormal. In patients with ectopic ACTH in whom tumor cannot be identified adrenal enzyme inhibitors or bilateral adrenalectomy can be used to control Cushing’s symptoms, Such patients should be followed with CT, MRI or scintigraphy for several years if necessary until the tumor can be located and treated [6], [12]. Initial failed localization is common in ectopic ACTH syndrome and it is usually due to carcinoid [6]. Carcinoid tumor identification is challenging because they are small, relatively slow growing and can be confused with pulmonary vasculature because of increased blood supply, conventional imaging studies such as CT,MRI detect tumors in only 50% of cases [10], [11], [13].
It is recommended to use a modern multi detector high resolution CT with 16–24 slices per second and 2.5 mm slices from lung apex to iliac crests [14]. Scintigraphy and PET scan can be used when no tumors are detectable with CT or MRI and when metastatic disease is suspected [15].
About ¾ of carcinoids are centrally located, so bronchoscopy can be of diagnostic benefit, cytological study of bronchial brushings have low overall diagnostic importance because the intact bronchial mucosa lining carcinoid prevents tumor cells from exfoliating [16].
The preferred treatment of localized lung carcinoid is surgical resection assuming adequate pulmonary reserve – if possible – with mediastinal lymph node sampling or dissection, for patients whose condition doesn’t permit surgical resection trans-bronchial resection may be an alternative [17].
For small (less than 2 cm) typical carcinoids, wedge resection with surgical margin as narrow as 5 mm is adequate in addition to mediastinal nodes sampling or dissection [17], [18], [19], whereas atypical or poorly- differentiated tumors may require more extensive surgery like lobectomy or even pneumonectomy [17]. There is very little evidence regarding the benefits of postoperative surveillance for patients with negative mediastinal nodes metastasis [17], [20].
Patients with positive mediastinal or atypical tumors nodes needs follow up through taking history, physical exam and CT scan of the chest and abdomen every six months for 2 years and once a year afterwards [17]. Prognosis of carcinoid depends on the histologic pattern, typical carcinoid have an excellent prognosis with a five year survival of 87–100% and ten years survival of 82–87% [17], [21], [22] and 3% recurrence rate after surgical resection [20], however atypical carcinoids have wide five year survival rate 30–95% and ten year survival of 35–56% and have more tendency to metastasize 16–23% [17], [21], [22] and local recurrence 3–25% [20].
4. Conclusion
ACTH secreting tumors can be very hard to detect, in such cases we should first aim to lower blood cortisol medically or through bilateral adrenalectomy to avoid Cushing’s complications such as severe infections, such patients should then be followed up through imaging studies (CT, MRI, scintigraphy or PET) to detect the tumor and resect it which Is the definitive treatment of these patients.
Conflict of interest
None.
Funding
None.
Ethical approval
We belong to university hospital in which patients accept to enter in researches, So ethical approval from a special committee is not required by our institution.
Consent
Informed consent was obtained from the patient for publication of this case report and accompanying images.
Authors contribution
Ghanem Aljassem: study concept, writing the paper.
Hazem Aljasem: study concept, writing the paper, data collection.
Guarantors
Hazem Aljasem, Ghanem Aljasem.
References
- 1.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., SCARE Group The SCARE statement: consensus-based surgical case reportguidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Nieman L.K. In: Epidemiology and Clinical Manifestations of Cushing’s Syndrome. UpToDate, Post T.W., editors. UpToDate; Waltham, MA: 2017. (Accessed on 10 April 2017) [Google Scholar]
- 3.Nieman L.K. Cushing’s syndrome: update on signs, symptoms and biochemical screening. Eur. J. Endocrinol. 2015;173(October (4)):M33–M38. doi: 10.1530/EJE-15-0464. Epub 2015 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieman L.K. In: Causes and Pathophysiology of Cushing’s Syndrome. UpToDate, Post T.W., editors. UpToDate; Waltham, MA: 2017. (Accessed on 10 April 2017) [Google Scholar]
- 5.Beuschlein F., Hammer G.D. Ectopic pro-opiomelanocortin syndrome. Endocrinol. Metab. Clin. N. Am. 2002;31(1):191–234. doi: 10.1016/s0889-8529(01)00025-1. [DOI] [PubMed] [Google Scholar]
- 6.Ilias I., Torpy D.J., Pacak K., Mullen N., Wesley R.A., Nieman L.K. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J. Clin. Endocrinol. Metab. 2005;90(August (8)):4955–4962. doi: 10.1210/jc.2004-2527. Epub 2005 May 24. [DOI] [PubMed] [Google Scholar]
- 7.Invitti C., Pecori Giraldi F., de Martin M., Cavagnini F. Diagnosis and management of Cushing’s syndrome: results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J. Clin. Endocrinol. Metab. 1999;84(February (2)):440–448. doi: 10.1210/jcem.84.2.5465. [DOI] [PubMed] [Google Scholar]
- 8.Ilias I., Torpy D.J., Pacak K., Mullen N., Wesley R.A., Nieman L.K. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J. Clin. Endocrinol. Metab. 2005;90(8):4955–4962. doi: 10.1210/jc.2004-2527. [DOI] [PubMed] [Google Scholar]
- 9.Beuschlein F., Hammer G.D. Ectopic pro-opiomelanocortin syndrome. Endocrinol. Metab. Clin. N. Am. 2002;31:191–234. doi: 10.1016/s0889-8529(01)00025-1. [DOI] [PubMed] [Google Scholar]
- 10.Tani Y., Sugiyama T., Hirooka S., Izumiyama H., Hirata Y. Ectopic ACTH syndrome caused by bronchial carcinoid tumor indistinguishable from Cushing’s disease. Endocr. J. 2010;57(8):679–686. doi: 10.1507/endocrj.k10e-129. [DOI] [PubMed] [Google Scholar]
- 11.Zemskova M.S., Gundabolu B., Sinaii N., Chen C.C., Carrasquillo J.A., Whatley M., Chowdhury I., Gharib A.M., Nieman L.K. Utility of various functional and anatomic imaging modalities for detection of ectopic adrenocorticotropin-secreting tumors. J. Clin. Endocrinol. Metab. 2010;95(3):1207–1219. doi: 10.1210/jc.2009-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isidori A.M., Kaltsas G.A., Pozza C., Frajese V., Newell-Price J., Reznek R.H., Jenkins P.J., Monson J.P., Grossman A.B., Besser G.M. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J. Clin. Endocrinol. Metab. 2006;91(February (2)):371–377. doi: 10.1210/jc.2005-1542. Epub 2005 Nov 22. [DOI] [PubMed] [Google Scholar]
- 13.Boscaro M., Arnaldi G. Approach to the patient with possible Cushing’s syndrome. J. Clin. Endocrinol. Metab. 2009;94(9):3121–3131. doi: 10.1210/jc.2009-0612. [DOI] [PubMed] [Google Scholar]
- 14.Isidori A.M., Kaltas G.A., Pozza C. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management and long-term follow-up. J. Clin. Endocrinol. Metab. 2006;91:371–377. doi: 10.1210/jc.2005-1542. [DOI] [PubMed] [Google Scholar]
- 15.Strosberg J.R. In: Diagnosis of the Carcinoid Syndrome and Tumor Localization. UpToDate, Post T.W., editors. UpToDate; Waltham, MA: 2017. (Accessed on 15 April 2017) [Google Scholar]
- 16.Thomas C.F., Jett J.R., Strosberg J.R. In: Bronchial Neuroendocrine (carcinoid) Tumors: Epidemiology, Risk Factors, Classification, Histology, Diagnosis, and Staging. UpToDate, Post T.W., editors. UpToDate; Waltham, MA: 2017. (Accessed on 15 April 2017) [Google Scholar]
- 17.Thomas C.F., Jett J.R., Strosberg J.R. In: Bronchial Neuroendocrine (carcinoid) Tumors: Treatment and Prognosis. UpToDate, Post T.W., editors. UpToDate; Waltham, MA: 2017. (Accessed on 15 April 2017) [Google Scholar]
- 18.El Jamal M., Nicholson A.G., Goldstraw P. The feasibility of conservative resection for carcinoid tumours: is pneumonectomy ever necessary for uncomplicated cases? Eur. J. Cardiothorac. Surg. 2000;18(September (3)):301–306. doi: 10.1016/s1010-7940(00)00519-4. [DOI] [PubMed] [Google Scholar]
- 19.Lucchi M., Melfi F., Ribechini A., Dini P., Duranti L., Fontanini G., Mussi A. Sleeve and wedge parenchyma-sparing bronchial resections in low-grade neoplasms of the bronchial airway. J. Thorac. Cardiovasc. Surg. 2007;134(August (2)):373–377. doi: 10.1016/j.jtcvs.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Lou F., Sarkaria I., Pietanza C., Travis W., Roh M.S., Sica G., Healy D., Rusch V., Huang J. Recurrence of pulmonary carcinoid tumors after resection: implications for postoperative surveillance. Ann. Thorac. Surg. 2013;96(October (4)):1156–1162. doi: 10.1016/j.athoracsur.2013.05.047. Epub 2013 Jul 31. [DOI] [PubMed] [Google Scholar]
- 21.Fiala P., Petrásková K., Cernohorský S., Kinkor Z., Krepela E., Zatloukal P. Bronchial carcinoid tumors: long-term outcome after surgery. Neoplasma. 2003;50(1):60–65. [PubMed] [Google Scholar]
- 22.Rea F., Rizzardi G., Zuin A., Marulli G., Nicotra S., Bulf R., Schiavon M., Sartori F. Outcome and surgical strategy in bronchial carcinoid tumors: single institution experience with 252 patients. Eur. J. Cardiothorac. Surg. 2007;31(February (2)):186–191. doi: 10.1016/j.ejcts.2006.10.040. Epub 2006 Nov 30. [DOI] [PubMed] [Google Scholar]