Abstract
Little is known about the use of guideline‐directed medical therapy (GDMT) in outpatients with heart failure with reduced left ventricular ejection fraction (HFrEF; ≤40%) in India. Our objective was to understand the use of GDMT in outpatients with HFrEF in India. The Practice Innovation And Clinical Excellence (PINNACLE) India Quality Improvement Program (PIQIP) is a registry for cardiovascular quality improvement in India supported by the American College of Cardiology Foundation. Between January 2008 and September 2014, we evaluated documentation of use of angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) and β‐blockers, or both, among outpatients with HFrEF seeking care in 10 centers enrolled in the PIQIP registry. Among 75 639 patients in the PIQIP registry, 34 995 had EF reported, and 15 870 had an EF ≤40%. The mean age was 56 years; 23% were female. Hypertension, diabetes, coronary artery disease, and myocardial infarction were present in 37%, 23%, 27%, and 17%, respectively. Use of ACEIs/ARBs, β‐blockers, and both were documented in 33.5%, 34.9%, and 29.6% of patients, respectively. The documentation of GDMT was higher in men, in patients age ≥65 years, and in those with presence of hypertension, diabetes, or coronary artery disease. Documentation of GDMT gradually increased over the study period. Among patients enrolled in the PIQIP registry, about two‐thirds of patients with EF ≤40% did not have documented receipt of GDMT. This study is an initial step toward improving adherence to GDMT in India and highlights the feasibility of examining quality of care in HFrEF in a resource‐limited setting.
Introduction
India has an increasing burden of traditional atherosclerotic cardiovascular risk factors and cardiovascular disease (CVD).1, 2 The prevalence of heart failure (HF) in India is also expected to increase over time because of the rising prevalence of CVD, particularly coronary artery disease (CAD).3, 4 In addition, presence of nonatherosclerotic diseases such as rheumatic heart disease and a decline in competing cause of death and aging population is also expected to contribute to an increasing burden of HF in India, including HF patients with reduced ejection fraction (HFrEF; ie, a left ventricular ejection fraction ≤40%).3, 4 Guideline‐directed medical therapy (GDMT), such as the use of angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) and β‐blockers, has a class I indication for use in patients with HFrEF.5, 6 Furthermore, use of these medications is currently a performance measure for HFrEF in the United States.7
In a hospital‐based registry of 1205 HF cases in Trivandrum, Kerala, India, evidence‐based medical therapy was used in 19% and 25% of inpatients with left ventricular systolic dysfunction during hospital admission and at hospital discharge, respectively.8 Little is known about GDMT in patients with HFrEF in the outpatient setting in India and feasibility of such an evaluation in a resource‐limited setting. The purpose of this study was to determine the feasibility of outpatient quality of care assessment among patients with HFrEF in India. We examined the use of ACEIs/ARBs and β‐blockers in patients with HFrEF seeking care in outpatient cardiology practices in India, using data from the Practice Innovation And Clinical Excellence (PINNACLE) India Quality Improvement Program (PIQIP),9 which is an extension of the American College of Cardiology's PINNACLE registry in the United States.7
Methods
Data Collection
Details about data collection in the PIQIP registry have been previously described.9 Briefly, trained personnel who held a bachelor's degree in pharmacy collected the data by scanning each outpatient card, which were assigned to each patient visiting a cardiologist's clinic. The outpatient cards contain patients' demographic information, diagnoses, pertinent laboratory results, and prescriptions. Because of a lack of local standards governing patient data collection, our methods for data collection were held to the standards of the Health Insurance Portability and Accountability Act (HIPAA).
Study Population
For the purpose of this study, we included patients seen between January 1, 2008, and September 30, 2014. Participation of practices in the registry was voluntary, and all practices currently participating in the registry were included in the current analysis (N = 10 practices). All patients visiting the cardiology practices were eligible for the PIQIP registry. Patients without a documented EF or EF >40% were excluded from our analysis.
Statistical Analysis
In this descriptive study, we examined demographic variables, prevalence of comorbid conditions, and GDMT in patients with HFrEF. As each patient in the registry could have multiple encounters during the study interval, we defined GDMT for a unique patient as the documentation of prescription of any dose of these medications at any encounter during the study interval. We first assessed demographic variables, followed by GDMT documentation in all patients with HFrEF and by subgroups of age (<65 vs ≥65 years), sex, and history of hypertension (HTN), diabetes mellitus (DM), and CAD. For these analyses, the unit of analysis was based on any encounter for each patient.
We also examined quarterly trends in the documentation of GDMT. For these analyses, the unit of assessment was all encounters during each study quarter. Finally, we examined the range of medication use per site after excluding sites with <20 patients with HFrEF. All analyses were conducted with SPSS version 20 (IBM Corp., Armonk, NY).
Results
A total of 75 639 patients were enrolled in the PIQIP registry during the study period. Ejection fraction was documented in 34 995 patients (46.3%). Our study population comprised 15 870 patients with EF ≤40% based on any encounter (21.0% of total, and 45.4% of those with documented EF). The total number of patient encounters for those with HFrEF was 33 562 during the study period. The mean EF (SD) in patients with HFrEF was 31.5% ± 7.2%. There were a total of 58 cardiologists from 10 practices, with each cardiologist caring for, on average, 273.6 patients with HFrEF.
Demographic characteristics of the study cohort are presented in Table 1. The mean age of the study participants was 56 years, of which 74% were age <65 years and 23% were female. The mean systolic and diastolic blood pressure was 123 and 77 mm Hg, respectively. Hypertension and DM were prevalent in 37% and 23% of the patients, respectively. History of myocardial infarction and CAD were documented in 17% and 27%, respectively. Other conditions such as current tobacco use, dyslipidemia, atrial fibrillation, and history of stroke were documented to be present in only a small portion of the population.
Table 1.
Baseline Characteristics of Patients With HFrEF (N = 15 870)
| Characteristics | |
|---|---|
| Age, y | 56 (12.5) |
| Age <65 y | 73.9 |
| Female sex | 22.9 |
| BMI, kg/m2 | 25.7 (9.2) |
| SBP, mm Hg | 123 (20.2) |
| DBP, mm Hg | 77 (11.3) |
| HTN | 37.0 |
| Current tobacco use | 2.5 |
| Dyslipidemia | 2.5 |
| DM | 23.2 |
| History of CAD | 27.3 |
| History of MI | 17.4 |
| History of any type of stroke | 0.01 |
| History of AF | 0.7 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HFrEF, heart failure with reduced ejection fraction; HTN, hypertension; MI, myocardial infarction; SBP, systolic blood pressure; SD, standard deviation.
Data are presented as % or mean (SD).
In patients with HFrEF, receipt of ACEI/ARB, β‐blockers, and both were documented in 33.5%, 34.9%, and 29.6%, respectively (Figure 1). The GDMT was more likely to be prescribed in patients age ≥65 years than in those age <65 years; in more males than females; and in patients with history of HTN, DM, or CAD than in those without the presence of those conditions (Table 2). Documentation of receipt of both ACEI/ARB and β‐blocker therapy was 28.4% and 32.9% in patients age <65 years and age ≥65 years, respectively; 31.1% and 24.4% in males and females, respectively; 34.6% and 26.6% in patients with and without history of HTN, respectively; 41.1% and 26.1% in patients with and without history of DM, respectively; and 49.9% and 22.0% in patients with and without history of CAD, respectively (P < 0.001 for all).
Figure 1.
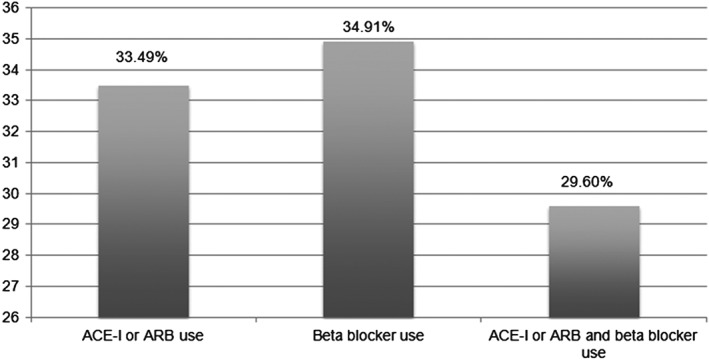
Documentation of GDMT at any time during the study period in patients with HFrEF. Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; GDMT, guideline‐directed medical therapy; HFrEF, heart failure with reduced ejection fraction.
Table 2.
Documentation of GDMT in Patients With HFrEF by Subgroups
| Age, y | Sex | HTN | DM | History of CAD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <65, N = 11 727 | ≥65, N = 4143 | Male, N = 12 236 | Female, N = 3634 | Yes, N = 5879 | No, N = 9991 | Yes, N = 3675 | No, N = 12 195 | Yes, N = 4329 | No, N = 11 541 | |
| On ACEI or ARB therapy | 32.3 | 36.9 | 34.9 | 28.6 | 38.8 | 30.4 | 44.7 | 30.1 | 53.0 | 26.2 |
| On β‐blocker therapy | 33.7 | 38.3 | 36.4 | 29.9 | 39.6 | 32.2 | 46.0 | 31.6 | 55.9 | 27.0 |
| On ACEI or ARB + β‐blocker therapy | 28.4 | 32.9 | 31.1 | 24.4 | 34.6 | 26.6 | 41.1 | 26.1 | 49.9 | 22.0 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; DM, diabetes mellitus; GDMT, guideline‐directed medical therapy; HFrEF, heart failure with reduced ejection fraction; HTN, hypertension.
Data are presented as %.
The P value comparing use of all GDMT within each subgroup was <0.001.
The documentation of GDMT gradually increased over the study period (Figure 2). Therapy with ACEI/ARB, β‐blockers, and ACEI/ARB plus β‐blockers was documented in 27.6%, 27.8%, and 24.8%, respectively, in the first study quarter (January to March 2008); and in 44.9%, 51.2%, and in 41.3%, respectively, in the last study quarter (July to September 2014).
Figure 2.
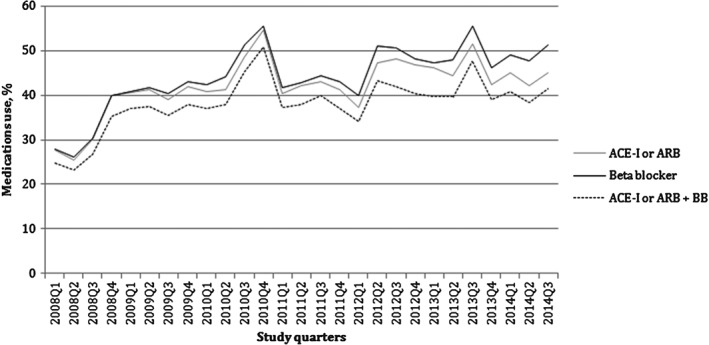
Quarterly trends in the documentation of GDMT in patients with HFrEF. Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β‐blocker; GDMT, guideline‐directed medical therapy; HFrEF, heart failure with reduced ejection fraction; Q, study quarter.
After excluding sites with ≤20 patients with HFrEF, the site‐level documentation rates among patients with HFrEF varied between 6.1% to 35.6% for ACEI/ARB, between 9.8% to 37.0% for β‐blockers, and between 4.5% to 31.8% for ACEI/ARB plus β‐blockers.
Discussion
In this study of patients seeking outpatient care in 10 cardiology practices in India, HFrEF was present in 21% of all the PIQIP registry participants and in 45.4% of patients with documented EF. Receipt of ACEI/ARB was documented in 33.5%, β‐blockers in 34.9%, and both classes of medications in 29.6% of the patients with HFrEF. The documentation of GDMT was higher in patients age ≥65 years, males, and patients with history of HTN, DM, and CAD. The documentation improved gradually during the study period.
The burden of HFrEF is expected to increase in India with increasing prevalence of CAD and its risk factors.3, 4 Indirect estimates report the prevalence of HF to be about 1.3 to 22.7 million.3, 4 In this study, we found that 21% of study participants had HFrEF. Echocardiography may be expensive for wider use in India to screen for patients with HFrEF. Using echocardiography in patients with a high pretest probability for HF, such as those with high B‐type natriuretic peptide levels or those with physical‐examination findings suggestive of systolic HF, may be an effective way to identify patients with HFrEF.
A study estimated the total HF spending in India in 2012 to be $1.18 billion, with an estimated direct cost of $80 million and indirect cost of $1.1 billion.10 Medications such as ACEIs or ARBs and β‐blockers have been shown to reduce the risk of death and hospitalizations in patients with HFrEF and are a class I guideline recommendation.5, 6 Therefore, optimal treatment of HF and examining quality of HF care in India is important from a public‐health perspective. Furthermore, participation of facilities in HF practice‐improvement programs in the United States has been associated with an improvement in evidence‐based care delivery, adherence to performance measures, and decreased length of stay in hospitalized HF patients.11, 12 Therefore, our study represents an important first step in understanding patterns of outpatient care in patients with HFrEF in India.
While assessing the feasibility of quality of care in patients with HFrEF in the PIQIP registry, several challenges were encountered. In the United States, comprehensive documentation is mandated by payers (for example, insurance providers), but in India most patients pay for their medical expenses on their own.13 Because patients are responsible for maintaining their own medical files and bringing them to and from physician visits, detailed official medical record‐keeping is not a priority in India. This is important to consider in developing a registry in a setting with high clinical workload with little emphasis on documentation. As expected, additional resources were needed for data collection and management, and there was a concern about changing physicians' clinical workflow. However, with careful planning and coordination, the study was feasible without actually affecting increasing workload, and local physicians were supportive of the study.
In a hospital‐based registry of 1205 HF cases from Trivandrum in Kerala in India,8 optimal medical therapy (defined using a combination of β‐blockers, ACEI/ARB, and aldosterone receptor blockers in patients with left ventricular EF <45%) was prescribed to 19% and 25% of patients during hospitalization and at hospital discharge, respectively. An ACEI or ARB was prescribed in 48% and 50%, and β‐blockers were prescribed in 56% and 60% in patients with EF <45% during hospitalization and at hospital discharge, respectively, which was higher than we have observed in the current study.
There are several possible reasons for lower documentation of GDMT seen in our study. There is a very high clinical demand for cardiologists in India,13 which we have also shown in the care of patients with HFrEF. On average, there were only 2 encounters per patient over 7 years, and about 1 cardiologist caring for 274 patients. This could impact continuity of care and ability to improve use of GDMT. As discussed earlier, comprehensive medical documentation for outpatient encounters is not a common practice in India. The outpatient cards usually do not have information on contraindications/intolerances to pharmacological treatment. Thus, our study may not have accounted for contraindications to the use of reported medications. It is possible that financial barriers to afford medications could also be a reason for low documentation rates for the use of GDMT in these patients. In health care systems where documentation is considered important from both quality‐measurement and reimbursement perspectives, such as in the United States, this would not restrict cardiologists from prescribing and/or documenting medications. However, because such documentation is not mandated in the Indian health care system, this phenomenon could itself lead to a lower documentation rate for the use of GDMT. We noted a trend for increasing documentation of medications over the study period, which could suggest that documentation of GDMT may increase after an initial learning phase. Apart from improved prescription and/or documentation of GDMT in HFrEF, these results could also indicate better data capture in the PIQIP registry as the experience of individuals capturing data (and understanding various brand names for medications used in patients with HFrEF) improved over time. In addition, this may reflect a secular trend of improvement in GDMT use. This increase over time may also represent benefits of participation in a quality‐improvement registry. This phenomenon of significant increase in the use of GDMT associated with participation in a quality‐improvement registry has been seen in the United States.12 Although this is possible, we could not confirm this, given the lack of control sites. Lastly, audit and feedback were not routinely provided in the current phase of the study, and we expect that documentation may improve as we employ such procedures in the future.
Study Limitations
Our study has several limitations. The intent of this study was to provide an initial glimpse of the burden of HFrEF and the documentation of use of evidence‐based medications. Our findings were based on preliminary data from a few centers and therefore may not reflect the use of evidence‐based medications in patients with HFrEF in India at large. Most of our participating sites were located in urban areas, and we expect use of GDMT in suburban and rural areas to be potentially lower. After the PIQIP expands to include practices from wider geographic regions of India and implements additional quality‐control measures, future analyses could provide important insights into geographic variation in care among patients with HFrEF. Similarly, capturing detailed patient information to allow researchers to identify contraindications to otherwise‐indicated therapy will be important to better understand the care of patients with HFrEF. Other medications used in HF, such as diuretics and aldosterone antagonists, and other proven therapies in HFrEF, can also be explored in the future. As explained earlier, we believe that different documentation practices in India (for example, compared with the United States) could also explain the lower documentation of GDMT in India. Our study is based only on cardiology practices, and a large number of HF patients in India may be cared for by noncardiologists13 with possibly even a lower frequency of GDMT. Given the data limitation, we did not evaluate whether the doses of β‐blockers or ACEI/ARBs used were consistent with those recommended by the HF guidelines.5, 6 We did not have levels of serum creatinine or serum potassium available in a great majority of these patients; therefore, any contraindication to the use of ACEIs/ARBs related to this could not be ascertained. Finally, given their participation in the PIQIP registry, the participating sites could be more motivated to improve their quality of care; therefore, our findings may not be broadly generalizable to routine cardiology practices. Despite these limitations, we show that measurement of outpatient quality of care for HFrEF is indeed feasible in a country with limited resources.
Conclusion
This is the first study to examine feasibility of quality improvement in patients with HFrEF receiving care in an outpatient setting in India. Despite several challenges, it was feasible to examine guideline‐directed medications use in outpatients with HFrEF. Further efforts to expand the PIQIP registry will facilitate a better understanding of the determinants of evidence‐based care delivery among patients with HFrEF in India.
The views expressed in this article are those of the authors and do not necessarily represent the views of the American College of Cardiology, the Department of Veterans Affairs, or the National Institutes of Health. This work was supported by the American College of Cardiology Foundation, Washington, DC. Bristol Myers‐Squibb and Pfizer, Inc. are Founding Sponsors of PIQIP.
Dr. Pokharel is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health, Award Number T32HL110837. Ms. Risch and Vicera are formal employees of the American College of Cardiology; Drs. Oetgen and Wei and Mr. Glusenkamp are current employees of the American College of Cardiology. Dr. Virani is supported by a Department of Veterans Affairs Health Services Research and Development Service (HSR&D) Career Development Award, American Heart Association Beginning Grant‐in‐Aid, American Diabetes Association Clinical Science and Epidemiology Award, and Baylor College of Medicine Center for Globalization grant.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Indrayan A. Forecasting vascular disease cases and associated mortality in India. Background papers, Burden of Disease in India. New Delhi, India: National Commission on Macroeconomics and Health, Ministry of Health and Family Welfare, Government of India; 2005. [Google Scholar]
- 2. Gupta R, Joshi P, Mohan V, et al. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. [DOI] [PubMed] [Google Scholar]
- 3. Huffman MD, Prabhakaran D. Heart failure: epidemiology and prevention in India. Natl Med J India. 2010;23:283–288. [PMC free article] [PubMed] [Google Scholar]
- 4. Pillai HS, Ganapathi S. Heart failure in South Asia. Curr Cardiology Rev. 2013;9:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 6. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2013;34:158]. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 7. Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry's PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harikrishnan S, Sanjay G, Anees T, et al. Clinical presentation, management, in‐hospital and 90‐day outcomes of heart failure patients in Trivandrum, Kerala, India: the Trivandrum Heart Failure Registry. Eur J Heart Fail. 2015;17:794–800. [DOI] [PubMed] [Google Scholar]
- 9. Kalra A, Pokharel Y, Hira RS, et al. Cardiovascular disease performance measures in the outpatient setting in India: insights from the American College of Cardiology's PINNACLE India Quality Improvement Program (PIQIP). J Am Heart Assoc. 2015;4:e001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–376. [DOI] [PubMed] [Google Scholar]
- 11. Fonarow GC, Abraham WT, Albert NM, et al; OPTIMIZE‐HF Investigators and Hospitals . Influence of a performance‐improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE‐HF). Arch Intern Med. 2007;167:1493–1502. [DOI] [PubMed] [Google Scholar]
- 12. Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence‐based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence‐Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–596. [DOI] [PubMed] [Google Scholar]
- 13. DeMaria AN. Cardiology in India. J Am Coll Cardiol. 2010;56:678–679. [DOI] [PubMed] [Google Scholar]


