Abstract
Amplitudes of auditory evoked potentials (AEP) increase with the intensity/loudness of sounds (loudness-dependence of AEP, LDAEP), and the time between adjacent sounds (time-dependence of AEP, TDAEP). Both, blunted LDAEP and blunted TDAEP are markers of altered auditory function in schizophrenia (SZ). However, while blunted LDAEP has been attributed to altered serotonergic function, blunted TDAEP has been linked to altered NMDA receptor function. Despite phenomenological similarities of the two effects, no common pharmacological underpinnings have been identified. To test whether LDAEP and TDAEP are both affected by NMDA receptor blockade, two rhesus macaques passively listened to auditory clicks of 5 different intensities presented with stimulus-onset asynchronies ranging between 0.2 and 6.4 seconds. 8 AEP components were analyzed, including the N85, the presumed human N1 homolog. LDAEP and TDAEP were estimated as the slopes of AEP amplitude with intensity and the logarithm of stimulus-onset asynchrony, respectively. On different days, AEPs were collected after systemic injection of MK-801 or vehicle. Both TDAEP and LDAEP of the N85 were blunted by the NMDA blocker MK-801 and recapitulate the SZ phenotype. In summary, LDAEP and TDAEP share important pharmacological commonalities that may help identify a common pharmacological intervention to normalize both electrophysiological phenotypes in SZ.
1. Introduction
Individuals with schizophrenia (SZ) exhibit auditory deficits (Javitt and Sweet, 2015; Leitman et al., 2010) that manifest, for example, as impaired performance in delayed pitch-discrimination tasks (Javitt et al., 1997; March et al., 1999; Rabinowicz et al., 2000; Strous et al., 1995), or impaired extraction of prosody from speech (Kantrowitz et al., 2013). These behavioral deficits go along with altered auditory evoked potentials in several passive listening tasks. Relative to healthy controls, SZ exhibit a reduced dynamic range of N1-P2 amplitude in response to sounds of different intensity (loudness-dependence of auditory evoked potential, LDAEP) (Gudlowski et al., 2009; Juckel et al., 2003; 2008a; Park et al., 2010). Similarly, SZ exhibit a reduced dynamic range of P1 and N1 amplitude in response to sounds preceded by different amounts of silence (time-dependence of auditory evoked potentials, TDAEP) (Erwin et al., 1991; 1994; Roth et al., 1991; 1980; Shelley et al., 1999).
Both LDAEP and TDAEP are most evident for the N1 component, and may thus reflect activity of the same neural generators. Both are blunted in SZ, and in both cases, this blunting is caused by reduction of peak amplitudes that are observed for the loudest tones and for tones preceded by longest periods of silence. These similarities support the notion of a common underlying pathology. In particular, they are both consistent with the hypothesis that structural and molecular alterations in the disease prevent the generation of maximal post-synaptic currents/potentials in pyramidal cells of auditory cortex (Javitt et al., 1996; Lewis and Sweet, 2009).
Work in monkeys and humans has shown that non-competitive NMDA receptor antagonists such as ketamine or PCP mimic blunted TDAEP observed in SZ (Boeijinga et al., 2007; Javitt et al., 2000). However, to date it is not known if NMDA receptor blockade also mimics blunted LDAEP as would be expected if both phenotypes reflect the same pathology, and if this pathology is accurately modeled by NMDA receptor blockade. This question is particularly relevant since other work has implicated altered serotonergic neuro-transmission as the reason for blunted LDAEP in SZ (Gudlowski et al., 2009; Juckel et al., 2008a; 2003; Park et al., 2010).
To answer this question we developed an auditory paradigm to simultaneously measure LDAEP and TDAEP in the non-human primate, and tested if both are affected by MK-801, a highly selective non-competitive NMDA antagonist. The results show that both, LDAEP and TDAEP, are blunted by MK-801. This finding supports the notion that both phenotypes are caused by a common pathological mechanism that can be modeled in the non-human primate by NMDA receptor blockade.
2. Materials and methods
2.1 Subjects
Experiments were performed on 2 adult male macaque monkeys (Macaca mulatta, animals S and W). The treatment of the monkeys was in accordance with the guidelines set by the U.S. Department of Health and Human Services (National Institutes of Health) for the care and use of laboratory animals. All methods were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. All animals have previously been exposed to similar passive listening paradigms in previous studies (Teichert, 2016; Teichert et al., 2016).
2.2 Cranial EEG recordings
The rhesus EEG recording system was designed to be as similar as possible to human scalp recordings, while reducing setup times and enabling long-term recordings over the period of many months. Details of the EEG recording system were reported previously (Teichert, 2016; Teichert et al., 2016). Briefly, animals had 33 electrodes implanted into 1 mm deep holes in the cranium covering roughly the same anatomy as the international 10-20 system (Teichert, 2016).
2.3 Experimental Setup
Experiments were performed in a small (4′ wide by 4′ deep by 8′ high) sound-attenuating and electrically insulated recording booth (Eckel Noise Control Technology). Animals were positioned and head-fixed in custom-made primate chairs (Scientific Design). Cranial EEG potentials were recorded with a 32-channel digital amplifier system (RHD2000, Intan). Experimental control was handled by a windows PC running an in-house modified version of the Matlab software-package monkeylogic and presented by routines of the Matlab package Psychtoolbox. Sounds were presented using a single element 4 inch full-range driver (Tang Band W4-1879) located 8 inches in front of the animals.
2.4 Stimuli and Experimental Design
The auditory paradigm was a modification of a paradigm we used previously (Teichert, 2016; Teichert et al., 2016). In this variant of the paradigm, animals passively listened to 0.1 ms long bi-phasic clicks of 5 different intensities (62, 68, 74, 80, 86 dB SPL) (Fig 1). Times between individual clicks (stimulus-onset asynchrony, SOA) were drawn from an exponential distribution truncated at 12.8 seconds and a constant offset of 250 ms. Click intensity and SOA remained constant 90% percent of the time leading to sequences of clicks with identical intensity and timing.
Figure 1. Joint TDAEP and LDAEP paradigm.
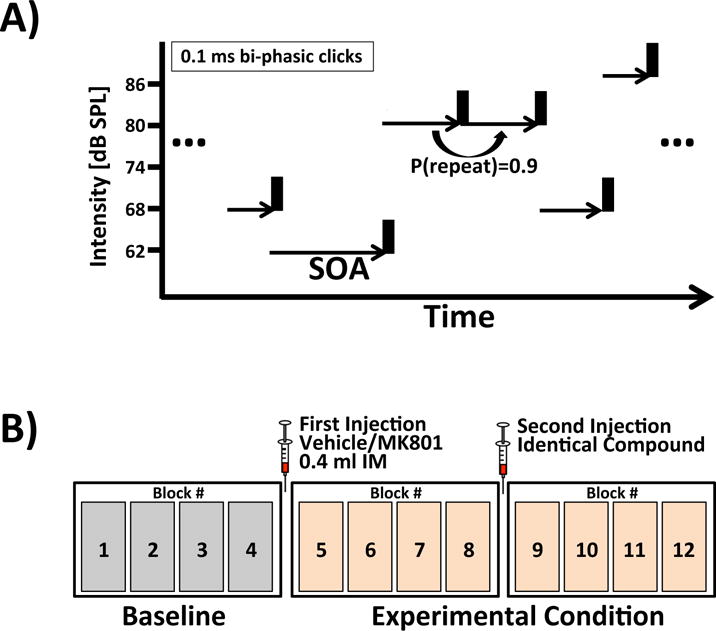
(A) Subjects passively listen to regular sequences of bi-phasic clicks with 5 different intensities and stimulus-onset asynchronies covering a range of five octaves from 0.200 to 6.4 seconds. (B) Tone presentations are structured into 12 blocks of 9-12 minutes duration. Injection of MK-801 or vehicle occurred after blocks 4 and 8.
Click presentations were structured into blocks between 9 and 12 minutes duration. Each recording session consisted of 12 blocks. After block number four, the subjects were given a 0.4 mL intramuscular injection of either MK-801 (0.1 mg/kg) or vehicle. The same injection was repeated after block number 8 to maintain an approximately constant concentration of MK-801. The control and experimental condition occurred on alternating days, with the experimental condition never occurring more than once a week.
2.5 Auditory evoked potentials
Raw data was down-sampled from 5000 to 500 Hz and filtered with a 70 Hz low-pass filter. The filtered data was cut into short epochs around the onset of each sound (−150 to 750 ms). A subtraction method was used to reduce AEP superposition for tones with short SOAs (Teichert et al., 2016). The data was then exported for use with the statistics software R (R Development Core Team, 2009). Trials with peak-to-peak amplitudes above 1500 μV were excluded to minimize motion artifacts. The remaining trials were sorted into bins of SOA with a width of 1 octave (0.2-0.4 s, 0.4-0.8 s, etc) and averaged.
2.6 Quantifying LDAEP and TDAEP
Previous work in the same animals identified 8 distinct middle and long-latency components (Teichert, 2016). Most components could readily be identified in all animals despite inter-individual differences in timing and topography. For each animal, each component was associated with a time-window and a list of channels. Component amplitudes on each trial were estimated by averaging activity across the corresponding channels and time-bins.
For each recording session, a simple linear model was used to quantify LDAEP and TDAEP.
Here L refers to the intensity of the clicks measured in dB SPL, and T refers to the time between tones, i.e., SOA, measured on a log2-scale. λ is the estimate of LDAEP, and τ is the estimate of TDAEP.
For each animal and AEP component, a linear model was used to determine whether λ and τ are significantly different from zero on days with vehicle injection. Rejection of the corresponding null-hypothesis indicated that a particular component was significantly modulated by intensity, SOA or both. A similar approach was used to test if the MK-801 significantly altered the relationship between intensity or SOA and AEP amplitude. To account for potential gradual changes of λ or τ over the course of successive recording sessions, we included session number as an additional predictor. Effect of drug and session number on λ and τ was tested using type-II sums-of-squares to account for the fact that session number and drug condition were not balanced.
3. Results
High-density tone-evoked cranial EEG responses were measured in two male macaque monkeys while they passively listened to sequences of bi-phasic clicks presented at 5 different intensities (62, 68, 74, 80, 86 dB SPL) and SOAs between 0.2 and 6.4 seconds. The present work focuses on the monkey N85 AEP that is believed to be homolog to the human N1. In addition, we also report results from other previously identified AEP components referred to by polarity and latency as P14, P21, P31, N43, P55, N85, P135 and N170 (27). Earlier work has shown that all 8 components exhibit TDAEP (Teichert et al., 2016), and that TDAEP can be blunted by non-competitive NMDA antagonists such as ketamine and MK-801 (under review). The aim of the current experiments was to determine whether components also show LDAEP, and whether MK-801 would simultaneously blunt both LDAEP and TDAEP.
Figure 2 shows AEPs averaged across 6 fronto-central electrodes as a function of the 5 different intensities and 5 different SOA-bins on days following injection of vehicle (black) or 0.1 mg/kg MK-801 (red). The data for this representative animal highlights all key findings that will be quantified in more detail below. On vehicle days, the data clearly reveal LDAEP as well as TDAEP. Both effects are especially pronounced for the N85. On days following MK-801 injection, LDAEP and TDAEP are clearly blunted, mostly due to reduced peak amplitudes for the loudest tones preceded by the longest periods of silence.
Figure 2. MK801 reduces peak potentials of auditory evoked potentials.
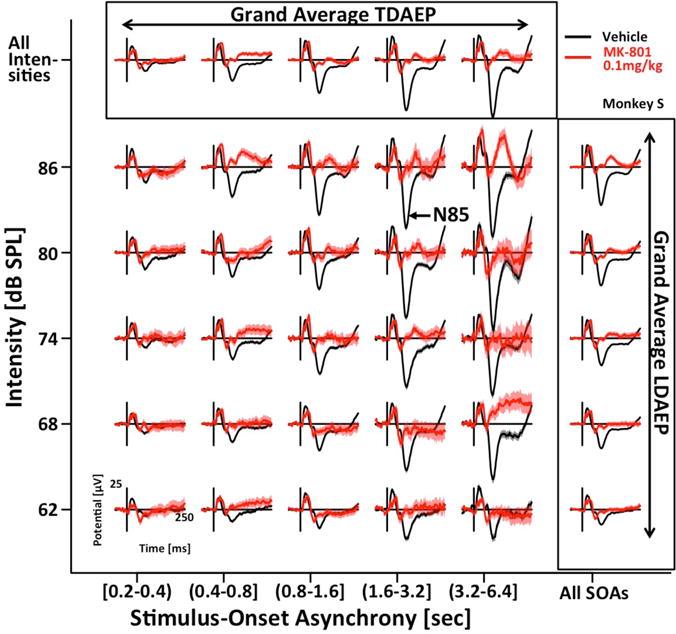
Click-evoked EEG-responses of a representative example subject on vehicle (black), and MK801 (red) days are displayed for five different intensities and ranges of stimulus-onset asynchrony (activity averaged over 6 fronto-central channels). On vehicle days, responses scale with SOA (TDAEP) and intensity (LDAEP). On days with MK801 injection the peak potentials are reduced.
3.1 Quantifying LDAEP and TDAEP
To established that both intensity and SOA have a significant effect on AEP amplitude, we estimated for each component and recording session the regression coefficients λ and τ that quantified the effect of intensity and SOA on AEP amplitude (section 2.6). A linear model determined if λ and τ are significantly different from 0 for both animals and all 8 components separately. The results of these tests are summarized in the top half of Table 1. In line with earlier work from our lab, most components were modulated by SOA. In line with work from humans, many AEP components in the monkey scaled with intensity. In particular, our data established that the N85 is significantly modulated by both SOA and intensity in both animals (p<0.001 in all cases). LDAEP and TDAEP of the N85 was quantified as the average increase of N85 amplitude for each doubling of SOA (referred to as octaves in units of seconds) or intensity (corresponding to an increase of 6 dB SPL). TDAEP and LDAEP had average values of 5.3±0.7 μV/octave and 2.6±0.1 μV/6dB, respectively.
Table 1.
Summary of tests for significant TDAEP and LDAEP (top half), as well as their interaction with MK-801 (bottom half). ‘S’ and ‘W’ corresponds to the results of the two animals. P14 through N170 indicate all 8 AEP components tested. Highlighted in light gray are the 5 components visualized in Figre 3. Highlighted in dark gray is the N85, the putative monkey homolog of the N1.
| P14 | P21 | P31 | N43 | P55 | N85 | P135 | N170 | ||
|---|---|---|---|---|---|---|---|---|---|
| TD | S | *** | *** | ** | * | *** | ** | *** | |
| W | *** | *** | *** | * | *** | *** | *** | * | |
| LD | S | *** | *** | *** | *** | ** | *** | ||
| W | *** | ** | *** | . | *** | *** | *** | ||
| TD*Drug | S | ** | . | . | *** | ** | |||
| W | ** | *** | |||||||
| LD*Drug | S | *** | *** | *** | |||||
| W | . | . | *** | * |
Legend: p<0.1: ‘.’; p<0.05:‘*’; p<0.01:‘**’; p<0.001:‘***’.
3.2 Quantifying the effect of MK-801 on LDAEP and TDAEP
The goal was to determine whether both LDAEP and TDAEP of the N85 are blunted by MK-801. This question was answered using a linear model (Section 2.6) to test if the slope of AEP amplitude with intensity (λ) and SOA (τ) was reduced on days with MK801 compared to vehicle administration. The bottom half of Table 1 shows the results of these tests. Most importantly, the analyses show that both LDAEP and TDAEP of the N85 were significantly reduced by MK-801. Averaged across both animals, LDAEP of the N85 was reduced by 93±25%, and TDAEP was reduced by 87±15%. Figure 3 visualizes this effect of MK-801 on the scaling of AEP amplitude with intensity and SOA. LDAEP and TDAEP of the P21 and P31 were not affected by MK-801. In contrast, LDAEP and TDAEP were clearly reduced for the N85 and the N170, even if the effect of the N170 does not reach significance for both animals (Table 1).
Figure 3. MK-801 blunts time- and intensity-dependence of the N85.
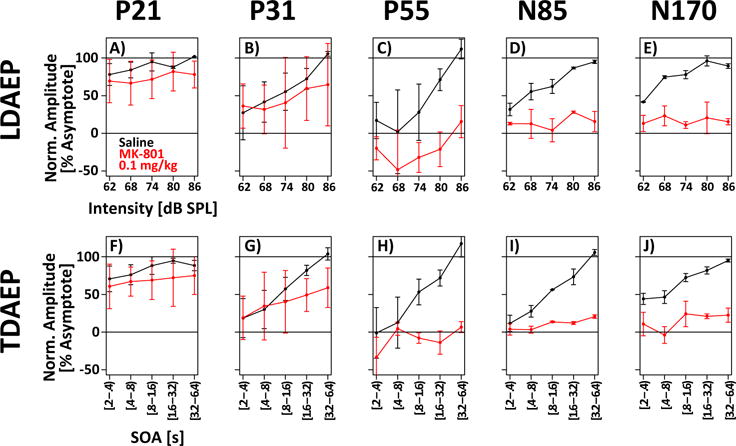
Normalized component amplitude is plotted as a function of intensity (top row) or SOA (bottom row) for five different AEPs (columns). On vehicle days (black), many components scale with intensity and SOA. On MK-801 days, this scaling is blunted. The blunting is most evident for the N85 and N170 components. Statistics for individual subjects and AEP components are presented in Table 1.
4. Discussion
The blunting of loudness (LDAEP) and time-dependence (TDAEP) of auditory evoked potentials are two important markers of auditory cortex pathology in SZ. The presented work establishes a new paradigm to simultaneously study LDAEP and TDAEP in non-human primates, and shows that both are blunted by NMDA receptor blockade. LDAEP and TDAEP are thus mediated by partially overlapping pharmacological mechanisms and this shared mechanism may make both vulnerable to the same pathological process in SZ.
In particular, the results show that LDAEP and TDAEP can be blunted by reducing glutamatergic neurotransmission at the NMDA receptor. We proposed the following mechanism to account for this finding: if earlier depolarizing input has already removed the voltage-dependent Mg2+ block from the NMDA receptor pore, NMDA antagonists will block the fraction of the depolarizing currents carried by the NMDA receptors, thus blunting the stimulus response. Such a fractional reduction would then manifest in a reduced slope of LDAEP and TDAEP. Blunted LDAEP and TDAEP in SZ may thus be markers of reduced excitatory function caused by pyramidal cell pathology in auditory cortex (Sweet et al., 2007; 2004; 2009).
However, earlier work has argued that LDAEP is a marker of serotonergic innervation of layer 4 of primary auditory cortex (Hegerl and Juckel, 1993; Juckel et al., 1999; 1997). Consequently, blunted LDAEP in SZ has been suggested to reflect increased serotonergic tone in the disease (Gudlowski et al., 2009; Juckel et al., 2008a; 2003; Park et al., 2010). Hence, is important to consider the possibility that MK-801 blunts LDAEP, and potentially also TDAEP, indirectly by increasing serotonergic tone in primary auditory cortex. Indeed, MK-801 administration has been shown to increase serotonin concentration in rat hippocampus and striatum (Whitton et al., 1992). This increase may either be caused indirectly by downstream effects of MK-801-mediated NMDA receptor antagonism, or directly via blockade of the serotonin-reuptake transporter (SERT) by MK-801 (Löscher and Hönack, 1992; Nishimura et al., 1998; Whitton et al., 1992). Based on these and other findings, it has been suggested that SZ-like positive and cognitive symptoms that are induced by non-competitive NMDA receptor antagonists may to some degree be mediated via downstream effects on the serotonergic system (Meltzer et al., 2011). So it is certainly worth considering that the SZ-like sensory deficits, e.g., blunted LDAEP and TDAEP, that are induced by noncompetitive NMDA antagonists could also be mediated by downstream serotonergic action.
There are, however, some arguments against this notion that MK-801 affects LDAEP and TDAEP indirectly by increasing serotonin concentration in primary auditory cortex: (1) While genetic association studies have repeatedly implicated the serotonin system in LDAEP (Juckel et al., 2008b; 2010; Kawohl et al., 2008), acute manipulations of serotonergic tone are less conclusive. The selective serotonin reuptake inhibitor (SSRI) citalopram has contradictory effects on LDAEP in humans: one study reported the expected blunting (Nathan et al., 2006), while a second study found some evidence of enhancement (Uhl et al., 2006). (2) In Wistar rats, citalopram leads to the expected increase of cortical serotonin levels but without the expected decrease of LDAEP (Wutzler et al., 2008). (Note that there was a correlation between the change in 5-HT and the change in LDAEP, but no main effect of citalopram on LDAEP). These negative findings in humans and rodents suggest that the acute effects of serotonin on LDAEP may be small and somewhat unreliable. (3) Lastly, serotonergic innervation specifically targets thalamic input layers of primary auditory cortex (Hegerl and Juckel, 1993; Juckel et al., 1997; 1996). In contrast, MK-801 had the strongest effect on LDAEP of the N85 component which is most likely not generated in layer 4, and receives substantial contribution from non-primary auditory cortex (Arezzo et al., 1975). Consequently, it is not clear whether an acute increase of cortical serotonin levels, if indeed it were caused by MK-801, would be expected to lead to the strong blunting of the loudness-dependence of the N85.
To put our findings in context it is important to note certain limitations of this study. In particular, the current work did not test the effects of other transmitter systems such serotonin or GABA on LDAEP and TDAEP. Thus, it remains an open question to which degree the observed effects are specific to NMDA blockade and to which degree they speak to the NMDA hypothesis of SZ. Furthermore, the current study used systemic rather than local drug administration. Thus, it remains an open question to which degree MK-801 acted in auditory cortex or other brain regions such as prefrontal cortex that contribute to the N85 (Arezzo et al., 1975).
In summary, our results establish NMDA receptor blockade as a common pharmacological intervention to mimic both blunted LDAEP and TDAEP observed in SZ. Future work needs to establish if blunted LDAEP and TDAEP in SZ reflect a shared pathology, and if so, whether it is more closely linked to reduced glutamatergic function or increased serotonergic tone. Future work in non-human primates can help address these issues by answering several important questions: (1) Can SSRIs or serotonergic agonists/antagonists directly affect LDAEP and TDAEP? (2) Does the systemic injection of MK-801 lead to increased serotonin levels in the auditory cortex? (3) If so, is the increase of serotonin correlated with blunted LDAEP and TDAEP? (4) Can the MK-801-induced blunting of LDAEP/TDAEP be exacerbated or rescued by serotonergic interventions as previously shown for SZ-like positive and cognitive symptoms in the rodent model (Meltzer et al., 2011)?
Acknowledgments
This work was supported by MH113041-01 to TT. I want to acknowledge the help of Kate Gurnsey during data collection, and feedback to an earlier version of the manuscript by members of the SONARS group at the University of Pittsburgh.
References
- Arezzo J, Pickoff A, Vaughan HG., Jr The sources and intracerebral distribution of auditory evoked potentials in the alert rhesus monkey. Brain Res. 1975;90:57–73. doi: 10.1016/0006-8993(75)90682-4. [DOI] [PubMed] [Google Scholar]
- Boeijinga PH, Soufflet L, Santoro F, Luthringer R. Ketamine effects on CNS responses assessed with MEG/EEG in a passive auditory sensory-gating paradigm: an attempt for modelling some symptoms of psychosis in man. Journal of Psychopharmacology. 2007;21:321–337. doi: 10.1177/0269881107077768. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Mawhinney-Hee M, Gur RC, Gur RE. Midlatency auditory evoked responses in schizophrenia. Biol Psychiatry. 1991;30:430–442. doi: 10.1016/0006-3223(91)90304-5. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Shtasel D, Gur RE. Effects of medication history on midlatency auditory evoked responses in schizophrenia. Schizophr Res. 1994;11:251–258. doi: 10.1016/0920-9964(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Gudlowski Y, Ozgürdal S, Witthaus H, Gallinat J, Hauser M, Winter C, Uhl I, Heinz A, Juckel G. Serotonergic dysfunction in the prodromal, first-episode and chronic course of schizophrenia as assessed by the loudness dependence of auditory evoked activity. Schizophr Res. 2009;109:141–147. doi: 10.1016/j.schres.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Juckel G. Intensity dependence of auditory evoked-potentials as an indicator of central serotonergic neurotransmission - a new hypothesis. Biol Psychiatry. 1993;33:173–187. doi: 10.1016/0006-3223(93)90137-3. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Jayachandra M, Lindsley RW, Specht CM, Schroeder CE. Schizophrenia-like deficits in auditory P1 and N1 refractoriness induced by the psychomimetic agent phencyclidine (PCP) Clin Neurophysiol. 2000;111:833–836. doi: 10.1016/s1388-2457(99)00313-2. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: Implications for schizophrenia. Proc Natl Acad Sci USA. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. Journal of Abnormal Psychology. 1997;106:315–324. doi: 10.1037/0021-843X.106.2.315. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015;16:535–550. doi: 10.1038/nrn4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Csepe V, Molnar M, Hegerl U, Karmos G. Intensity dependence of auditory evoked potentials in behaving cats. Electroencephalogr Clin Neurophysiol. 1996;100:527–537. doi: 10.1016/s0168-5597(96)95534-3. [DOI] [PubMed] [Google Scholar]
- Juckel G, Gallinat J, Riedel M, Sokullu S, Schulz C, Möller HJ, Müller N, Hegerl U. Serotonergic dysfunction in schizophrenia assessed by the loudness dependence measure of primary auditory cortex evoked activity. Schizophr Res. 2003;64:115–124. doi: 10.1016/S0920-9964(03)00016-1. [DOI] [PubMed] [Google Scholar]
- Juckel G, Gudlowski Y, Mueller D, Oezguerdal S, Bruene M, Gallinat J, Frodl T, Witthaus H, Uhl I, Wutzler A, Pogarell O, Mulert C, Hegerl U, Meisenzahl E-M. Loudness dependence of the auditory evoked N1/P2 component as an indicator of serotonergic dysfunction in patients with schizophrenia - A replication study. Psychiatry Res. 2008a;158:79–82. doi: 10.1016/j.psychres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Juckel G, Hegerl U, Giegling I, Mavrogiorgou P, Wutzler A, Schuhmacher C, Uhl I, Brüne M, Mulert C, Pogarell O, Rujescu D. Association of 5 HT1B receptor polymorphisms with the loudness dependence of auditory evoked potentials in a community based sample of healthy volunteers. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008b;147B:454–458. doi: 10.1002/ajmg.b.30628. [DOI] [PubMed] [Google Scholar]
- Juckel G, Hegerl U, Molnar M, Csepe V, Karmos G. Auditory evoked potentials reflect serotonergic neuronal activity - A study in behaving cats administered drugs acting on 5-HT1A autoreceptors in the dorsal raphe nucleus. Neuropsychopharmacology. 1999;21:710–716. doi: 10.1016/S0893-133X(99)00074-3. [DOI] [PubMed] [Google Scholar]
- Juckel G, Molnár M, Hegerl U, Csépe V, Karmos G. Auditory-evoked potentials as indicator of brain serotonergic activity first evidence in behaving cats. Biol Psychiatry. 1997;41:1181–1195. doi: 10.1016/s0006-3223(96)00240-5. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schumacher C, Giegling I, Assion H-J, Mavrogiorgou P, Pogarell O, Mulert C, Hegerl U, Norra C, Rujescu D. Serotonergic functioning as measured by the loudness dependence of auditory evoked potentials is related to a haplotype in the brain-derived neurotrophic factor (BDNF) gene. Journal of Psychiatric Research. 2010;44:541–546. doi: 10.1016/j.jpsychires.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Leitman DI, Lehrfeld JM, Laukka P, Juslin PN, Butler PD, Silipo G, Javitt DC. Reduction in tonal discriminations predicts receptive emotion processing deficits in schizophrenia and schizoaffective disorder. Schizophr Bull. 2013;39:86–93. doi: 10.1093/schbul/sbr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawohl W, Giegling I, Mavrogiorgou P, Pogarell O, Mulert C, Möller HJ, Hegerl U, Rujescu D, Juckel G. Association of functional polymorphisms in NOS1 and NOS3 with loudness dependence of auditory evoked potentials. Int J Neuropsychopharmacol. 2008;11:477–483. doi: 10.1017/S1461145708008420. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Hönack D. The behavioural effects of MK-801 in rats: involvement of dopaminergic, serotonergic and noradrenergic systems. Eur J Pharmacol. 1992;215:199–208. doi: 10.1016/0014-2999(92)90029-4. [DOI] [PubMed] [Google Scholar]
- March L, Cienfuegos A, Goldbloom L, Ritter W, Cowan N, Javitt DC. Normal time course of auditory recognition in schizophrenia, despite impaired precision of the auditory sensory (“echoic”) memory code. Journal of Abnormal Psychology. 1999;108:69–75. doi: 10.1037//0021-843x.108.1.69. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Horiguchi M, Massey BW. The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology. 2011;213:289–305. doi: 10.1007/s00213-010-2137-8. [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Segrave R, Phan KL, O’Neill B, Croft RJ. Direct evidence that acutely enhancing serotonin with the selective serotonin reuptake inhibitor citalopram modulates the loudness dependence of the auditory evoked potential (LDAEP) marker of central serotonin function. Hum Psychopharmacol. 2006;21:47–52. doi: 10.1002/hup.740. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Schloss P, Shimada S, Tohyama M. MK 801 blocks monoamine transporters expressed in HEK cells. FEBS Letters. 1998;423:376–380. doi: 10.1016/S0014-5793(98)00126-4. [DOI] [PubMed] [Google Scholar]
- Park Y-M, Lee S-H, Kim S, Bae S-M. The loudness dependence of the auditory evoked potential (LDAEP) in schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, and healthy controls. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:313–316. doi: 10.1016/j.pnpbp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2009. [Google Scholar]
- Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia - Imprecision or distractibility? Arch Gen Psychiatry. 2000;57:1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- Roth WT, Goodale J, Pfefferbaum A. Auditory event-related potentials and electrodermal activity in medicated and unmedicated schizophrenics. Biol Psychiatry. 1991;29:585–599. doi: 10.1016/0006-3223(91)90094-3. [DOI] [PubMed] [Google Scholar]
- Roth WT, Horvath TB, Pfefferbaum A, Kopell BS. Event-related potentials in schizophrenics. Electroencephalogr Clin Neurophysiol. 1980;48:127–139. doi: 10.1016/0013-4694(80)90299-0. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37:65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- Strous RD, Cowan N, Ritter W, Javitt DC. Auditory sensory (“echoic”) memory dysfunction in schizophrenia. Am J Psychiatry. 1995 doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert T. Tonal frequency affects amplitude but not topography of rhesus monkey cranial EEG components. Hear Res. 2016;336:29–43. doi: 10.1016/j.heares.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Teichert T, Gurnsey K, Salisbury DF, Sweet RA. Contextual processing in unpredictable auditory environments: The limited resource model of auditory refractoriness in the rhesus. Journal of Neurophysiology. 2016 doi: 10.1152/jn.00419.2016. jn.00419.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl I, Gorynia I, Gallinat J, Mulert C, Wutzler A, Heinz A, Juckel G. Is the loudness dependence of auditory evoked potentials modulated by the selective serotonin reuptake inhibitor citalopram in healthy subjects? Human Psychopharmacology: Clinical and Experimental. 2006;21:463–471. doi: 10.1002/hup.803. [DOI] [PubMed] [Google Scholar]
- Whitton PS, Biggs CS, Pearce BR, Fowler LJ. MK 801 increases extracellular 5 hydroxytryptamine in rat hippocampus and striatum in vivo. J Neurochem. 1992;58:1573–1575. doi: 10.1111/j.1471-4159.1992.tb11381.x. [DOI] [PubMed] [Google Scholar]
- Wutzler A, Winter C, Kitzrow W, Uhl I, Wolf RJ, Heinz A, Juckel G. Loudness dependence of auditory evoked potentials as indicator of central serotonergic neurotransmission: simultaneous electrophysiological recordings and in vivo microdialysis in the rat primary auditory cortex. Neuropsychopharmacology. 2008;33:3176–3181. doi: 10.1038/npp.2008.42. [DOI] [PubMed] [Google Scholar]


